Abstract
Objectives:
To synthesize novel compounds belonging to Benzodioxane, Naphthalene diimide, Aminophenol derivatives and Porphyrin classes and test their potential anticancer properties.
Methods:
Several compounds were synthesized and their molecular identity was confirmed using nuclear magnetic resonance. Potential anticancer properties were determined using cytopathogenicity assays and growth inhibition assays using cervical cancer cells (HeLa). Cells were incubated with different concentrations of compounds belonging to Benzodioxane, Naphthalene diimide, Aminophenol derivatives and Porphyrins and effects were determined. HeLa cells cytopathogenicity was determined by measuring lactate dehydrogenase release using cytotoxicity detection assay. Growth inhibition assays were performed by incubating 50% semi-confluent HeLa cells with Benzodioxane, Naphthalene diimide, Aminophenol derivatives and Porphyrin compounds and HeLa cell proliferation was observed. Growth inhibition and host cell death were compared in the presence and absence of drugs.
Results:
Cytopathogenicity assays showed that the selected compounds were cytotoxic against HeLa cells, killing up to 90% of cells. Growth inhibition assays exhibited 100% growth inhibition. These effects are likely via oxidative stress, production of reactive oxygen species, changes in cytosolic and intracellular calcium/adenine nucleotide homeostasis, inhibition of ribonucleotide reductase/cyclooxygenase and/or glutathione depletion.
Conclusions:
Benzodioxane, Naphthalene diimide, Aminophenol derivatives and Porphyrins exhibited potent anticancer properties. These findings are promising and should pave the way in the rationale development of anticancer drugs. Using different cancer cell lines, future studies will determine their potential as anti-tumour agents as well as their precise molecular mode of action.
Keywords: Cancer, therapy, HeLa cells, cytotoxicity, growth inhibition
Background
According to the International Agency for Research on Cancer (IARC), there were 14.1 million new cancer cases, 32.6 million pre-existing cancer patients and 8.2 million deaths worldwide due to cancer in 2012.1,2 The alarming cancer statistics shows that the mortality and morbidity due to cancer remain significant despite advance in therapeutic approaches and will continue to rise, especially in developing countries.2 By 2030, the global cancer burden is expected to nearly double, growing to 21.4 million cases and 13.2 million deaths.3 Chemotherapy remains one of the principal modes of treatment for cancer, despite the non-selective nature of compounds associated with unwanted host tissue damage. Furthermore, cancer drug resistance is often observed partly due to (1) drug efflux, (2) alteration of drug targets, (3) drug inactivation, (4) DNA damage repair and (5) epigenetic effects leading to inhibition of cell death that necessitates the identification of new compounds.4 This contributes to the failure of cancer patients with successful treatment, resulting in a higher number of cancer cases. The current challenges in cancer treatment highlight the importance of identifying novel strategies for cancer therapeutics. The search to identify new compounds with anticancer properties has remained a primary research topic in the modern medicinal chemistry. Among other compounds, Naphthalimides group (growth inhibiting agents that intercalate DNA) have shown promise. Anti-tumour activities of Naphthalimides were enhanced by synthesizing bis-intercalating agents like Naphthalene diimides to improve the interactions with the phosphate groups of DNA, which resulted in anticancer properties against several human cancer cell lines.5–7 Other groups of compounds include (1) Benzodioxane derivatives that exhibit a range of activities including anti-tumour, anti-microbial and antioxidant activities;8–10 (2) Acetamol derivatives that have been implicated in cytotoxicity and/or apoptosis of host cells,11 albeit they have been used commonly to treat pain and fever,12,13 and (3) Porphyrins that have been used extensively in the context of photodynamic therapy (PDT). The basis of PDT is that the light of appropriate wavelength activates the photosensitising compound, resulting in production of singlet oxygen and other reactive oxygen species (ROS) to induce cell death in the target tissue.14,15 In this study, substituted derivatives of aforementioned groups of compounds (Benzodioxane, Naphthalene diimide, Porphyrin and Aminophenol derivatives) were synthesized. The newly synthesized compounds were examined for their in vitro cytotoxicity as well as anti-proliferative activities against HeLa cells.
Methods
Synthesis and characterization of compounds
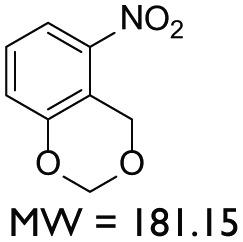
AYNBDO = 5-Nitro-1,3-benzodioxine (C8H7NO4) ( 2): 5 g (36 mmol) of 3-Nitrophenol (1) was melted in a round bottom flask at 120°C with constant stirring followed by the addition of 6 mL 1:1 (v/v) mixture of 37% solution of commercial formaldehyde and 12.6 M concentrated HCl (Scheme 1). The reaction mixture was refluxed for 2 h yielding yellow precipitates, while the progress of reaction was monitored with thin layer chromatography (TLC). After completion of reaction, 50 mL of 5 M sodium hydroxide (NaOH) was added in the reaction mixture for quenching. The crude product was extracted with EtOAc (50 mL thrice) and dried at reduced pressure to give pure yellow powdered product 2. The yield of the product was 3.6 g (55%). Electron ionization–mass spectrometry (EI-MS) m/z 181.04. 1H nuclear magnetic resonance (NMR) in CDCl3, 7.81 (d, 1H, Ar), 7.29 (t, 1H, Ar), 7.17 (d, 1H, Ar), 5.27 (s, 2H, Ar–CH2–O), 5.22 (s, 2H, O–CH2–O).
Scheme 1.

Synthesis of 5-Nitro-1,3-benzodioxine (2).
AYNH2 = 5-Amino-1,3-benzodioxine (C8H9NO2) ( 3): Product 2 (1.67 g, 10 mmol) and SnCl2 dihydrate (11.275 g, 50 mmol) were dissolved in EtOH in a round bottom flask. The reaction mixture was heated at 70°C with continuous stirring for half hour and the progress of the reaction was monitored with TLC (Scheme 2). The reaction was brought to room temperature and then was basified with 5% NaOH aqueous solution to attain neutral pH. Product 3 was achieved as yellow powder after extracting the reaction mixture with EtOAc (50 mL × 3) followed by removal of solvents in vacuum and purification by silica gel column chromatography with gradient elution of 1:1 mixture of Hexane:Dichloromethane (DCM) to pure DCM. The yield of the product was 0.97 g (70%). EI-MS m/z 151.0. 1H NMR in CDCl3, 6.94 (t, 1H, Ar), 6.33 (d, 1H, Ar), 6.28 (d, 1H, Ar), 5.18 (s, 2H, Ar–CH2–O), 4.71 (s, 2H, O–CH2–O), 3.41 (s, 2H, –NH2).
Scheme 2.
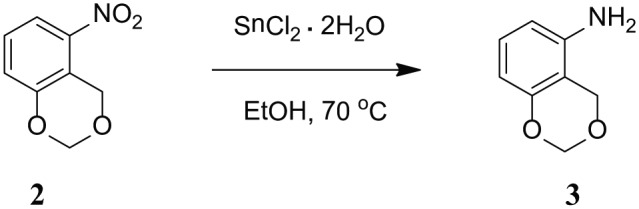
Synthesis of 5-Amino-1,3-benzodioxine (3).
AY80 = N-(4-((dimethylamino)methyl)-3-hydroxyphenyl)acetamide (C11H16N2O2) ( 5): To a solution of 3-Acetaminophenol (4) (5 g, 33 mmol) in 40% Dimethyl amine (4.5 g, 40 mmol) and MeOH (4 mL) was added 37% formaldehyde (2.68 g, 33 mmol), and the reaction mixture was placed in an ice bath (Scheme 3). After 15 min, the reaction mixture turned into solid precipitates, which were filtered and washed with ice cold water (100 mL). These precipitates were oven dried to obtain pure product 5 as white powder. The yield of the product was 6.2 g (90%). EI-MS m/z 208.26. 1H NMR in dimethyl sulfoxide (DMSO), 7.17 (s, 1 H, –NH–), 7.00 (d, 1H, Ar), 6.84 (d, 2H, Ar), 3.57 (s, 2H, Ar–CH2–N), 2.28 (s, 6H, N–CH3), 2.12 (s, 3H, –CO–CH3).
Scheme 3.

Synthesis of N-(4-((dimethylamino)methyl)-3-hydroxyphenyl)acetamide (5).
AYZHC = 4-amino-2-(hydroxymethyl)phenol (C7H9NO2) ( 7): 109 mg (1 mmol) of 4-Aminophenol (6) was treated with 0.1 mL Formaldehyde and 0.1 mL concentrated HCl with same procedure as compound 2 to afford product 7 as light yellow powder (Scheme 4). The yield of the product was 56 mg (40%). EI-MS m/z 139.15. 1H NMR in DMSO, 11.09 (s, 1H, OH), 8.19 (appeared as d, 1H, Ar), 7.99 (dd, 1H, Ar), 6.91 (d, 1H, Ar), 4.49 (s, 2H, Ar–CH2–OH).
Scheme 4.
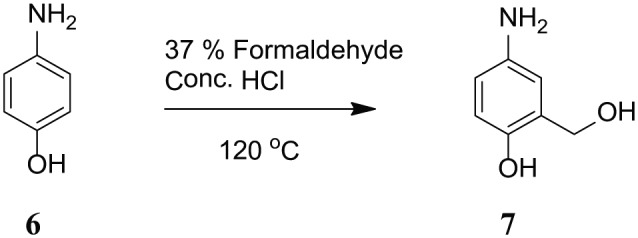
Synthesis of 4-amino-2-(hydroxymethyl)phenol (C7H9NO2) (7).
AYTHPP = 5,10,15,20-Tetrakis(4-hydroxyphenyl)-21 H,23 H-porphine (C44H30N4O4) ( 10): Compound 10 was synthesized as follows: 50 mL Propionic acid was heated at 90°C in a round bottom flask fitted with water condensor, and a solution of 4-Hydroxybenzaldehyde (8) (2 g, 16.37 mmol) dissolved in 2.5 mL propionic acid was added very slowly in hot propionic acid. After continuous heating and constant stirring of the solution at reflux temperature for half an hour, pyrrole (9) (1.2 mL, 16.37 mmol) solution in 2.5 mL propionic acid was added in the above-mentioned reaction mixture dropwise for half an hour. The reaction was refluxed and monitored with TLC (Scheme 5). After completion (about 2 h), the reaction mixture was cooled to room temperature followed by addition of 50 mL of 2.5 M NaOH for quenching. Crude products containing a mixture of different oligomers and tar were obtained by solvent extraction with EtOAc (50 mL × 3) and evaporating the EtOAc at reduced pressure. Compound 10 was purified from the crude product through silica gel column chromatography by using EtOAc and Hexane as mobile phase (3:1 to pure EtOAc). The yield of pure product was 1.1 g (10%). EI-MS m/z 678.1. 1H NMR in DMSO, 9.94 (s, 4H, OH), 8.85 (s, 8H, pyrrole), 7.97 (d, 8H, Ar), 7.18 (d, 8H, Ar).
Scheme 5.

Synthesis of 5,10,15,20-Tetrakis(4-hydroxyphenyl)-21H,23H-porphine (10).
AYTPyP = 5,10,15,20-Tetra(4-pyridyl)-21H,23H-porphine (C40H26N8) ( 12): Compound 12 was synthesized with same procedure as compound 10 at 18.6 mmol amount level, with minor variation in the workup of reaction mixture (Scheme 6). After completion of reaction (45 min), the reaction mixture was poured into a beaker containing 75 mL 10% sodium acetate aqueous solution to approximately attain the pH 3. The reaction mixture was precipitated at cooling to give crude product 12, which was filtered and washed with ice cold water (100 mL) and oven dried. Finally, it was passed through silica gel column chromatography with DCM as mobile phase for purification. The yield of the product was 0.9 g (8%). ESI-MS 619.22. 1H NMR in CDCl3, 9.03 (d, 8H, Ar), 8.85 (s, 8H, pyrrole), 8.13 (d, 8H, Ar).
Scheme 6.

Synthesis of 5,10,15,20-Tetra(4-pyridyl)-21 H,23 H-porphine (12).
AY102, 103 and 127 all were synthesized with similar procedure as given in the following.
102 = N,N″-Di-(3′-methylthio phenyl)-1,4,5,8-naphthalene diimide (C28H18N2O4S2) ( 15): Naphthalene dianhydride (13) (268 mg, 1 mmol) is suspended in 13 mL acetic acid at 140°C for 1 h in a round bottom flask. 3-Mehtylthioaniline (14) (1.2 mL, 10 mmol) is added in above suspension, and the reaction mixture is refluxed for 5 h (Scheme 7). Progress of the reaction is checked with TLC, and after completion, the reaction mixture is concentrated to 3 mL solution. This solution is poured into a beaker containing 13 mL MeOH to give precipitates of crude product 15. The precipitates are filtered, dried and recrystallized with 50 mL acetic acid to give pure compound 15. The yield of the product was 505 mg (99%). EI-MS 509.9. 1H NMR in CDCl3, 8.829 (s, 4H, Ar-naphth.), 7.45 (t, 2H, Ar), 7.37 (d, 2H, Ar), 7.18 (s, 2H, Ar), 7.07 (d, 2H, Ar).
Scheme 7.

Synthesis of N,N″-Di-(3′-methylthio phenyl)-1,4,5,8-naphthalene diimide (15).
103 = N,N″-Di-(4,4′-phenyl disulfide)-1,4,5,8-naphthalene diimide (C26H12N2O4S2) ( 17): The yield of the product was 60%. EI-MS 480.5. 1H NMR in CDCl3, 7.66 (d, 4H, Ar), 7.54 (d, 4H, Ar), 7.41 (s, 4H, Ar-naphth.).
Scheme 8.

Synthesis of N,N″-Di-(4,4′-phenyl disulfide)-1,4,5,8-naphthalene diimide (17).
127 = N,N″-Di-(propargyl)-1,4,5,8-naphthalene diimide (C20H10N2O4) ( 19): The yield of the product was 99%. EI-MS 342.0. 1H NMR in DMSO, 8.72 (s, 4H, Ar-naphth.), 4.80 (s, 4H, -N-CH2), 3.20 (s, 2H, alkyne).
Scheme 9.

Synthesis of N,N″-Di-(propargyl)-1,4,5,8-naphthalenediimide (19).
HeLa (Henrietta Lacks) cell lines and culture conditions. HeLa cervical cancer cells were obtained from ATCC (ATCC® CCL-2™, Singapore) and cultured in RPMI-1640 supplemented with 10% foetal bovine serum, 1% l-glutamine, 1% minimal essential media non-essential amino acid (MEM NEAA) and 1% Penicillin–Streptomycin (Life Technologies, USA). The cells were maintained in a 5% CO2 incubator at 37°C. For cytotoxicity assays, HeLa cells were cultured in 24-well plates by inoculating 5 × 105 cells per well per millilitre and incubating at 37°C in a 5% CO2 incubator, which resulted in the formation of complete monolayers within 48 h.
Cytotoxicity assay
Cytotoxicity assays were accomplished as previously described.16,17 Briefly, AY series compounds were incubated with confluent HeLa monolayers in RPMI-1640. Plates were incubated at 37°C for 24 h in a 5% CO2 incubator and observed for cytotoxic effects. At the end of this incubation period, supernatants were collected and cytotoxicity was detected through measuring lactate dehydrogenase (LDH) release (Cytotoxicity Detection kit; Roche Diagnostics, Indianapolis, IN, USA). Briefly, conditioned media of the cultures were collected and cytotoxicity detected as follows: % cytotoxicity = (sample value – control value)/total LDH release – control value) × 100. Control values were obtained from host cells incubated in RPMI-1640 alone. Total LDH release was determined from HeLa treated with 0.1% Triton X-100 for 30 min at 37°C. The basis of this assay is that cell supernatant containing LDH catalyzes the conversion of lactate to pyruvate, generating NADH and H+. In the second step, the catalyst (diaphorase, solution from kit) transfers H and H+ from NADH and H+ to the tetrazolium salt p-iodo-nitrotetrazolium violet (INT), which is reduced to formazon (dye), and absorbance is read at 492 nm.
Growth inhibition assays
Growth inhibition assays were performed to determine the effects of AY series compounds on HeLa cell growth inhibition. Briefly, HeLa cells were grown to 50% confluency in 96-well plates as described above. The AY series compounds were added to 50% confluent monolayers of HeLa cells. Plates were incubated for 24 h at 37°C in a 5% CO2 incubator and observed for growth inhibitory effects. Following this incubation, the number of viable HeLa cells were determined by adding 0.4% Trypan blue (Merck Millipore, Germany) exclusion staining (cells stained blue were considered nonviable while live cells were unstained). The numbers of HeLa cells were enumerated by haemocytometer counting.18
Results
Synthesis of compounds belonging to Benzodioxane, Naphthalene diimide, Aminophenol derivatives and Porphyrin class
In total, nine compounds were synthesized in this study. Two compounds belonged to the Benzodioxane class, namely, AYNBDO and AYNH2, with molecular weights of 181.15 and 151.16, respectively. The compound AYNH2 was linearly synthesized from AYNBDO by simple reduction of nitro functionality into amino group. Both of these compounds were soluble in chloroform and were characterized by EI-MS and 1H NMR spectroscopies, which also suggest the purity of the products. The Aminophenol class included two compounds, AY80 and AYZHC with molecular weights of 208.26 and 139.15, respectively, confirmed by EI-MS and also found to be soluble in chloroform. Their 1H NMR also provided the evident measure of product purity and compound structures. Compound AY80 was obtained by the Mannich reaction of metacetamol with Formaldehyde and Dimethyl amine to afford the Mannich base (AY80). However, AYZHC was rather an unusual product of the similar reaction as employed for the synthesis of compound 2 in Scheme 1, but the 1H NMR analysis confirmed the formation of the product as depicted in Scheme 4. Since the Nitro and Amino groups are inversely electron conjugation groups to each other, and furthermore, the change in the position of these groups in both compounds suggests the different outcomes, and the reaction in Scheme 4 is considered as more plausible than the ring closure as for compound 2 in Scheme 1. Two compounds belonged to the Porphyrin class: AYTHPP and AYTPyP with molecular weights of 678.73 and 618.69 confirmed by EI-MS and electrospray ionization (ESI)-MS, respectively. Both of these compounds were soluble in methanol and found to be stable and pure as suggested by 1H NMR. These two compounds are also commercially available, but their stability and cost are of major concern. Therefore, a simple and efficient synthetic procedure for the cyclic condensation of respective aldehyde and Pyrrole was adapted to produce these porphyrinic compounds in our own laboratory. The remaining three compounds belonged to the Naphthalene diimide class and included AY102, AY103 (molecular weights of 510.58 and 480.51, respectively) and AY127 with molecular weight of 342.30. These compounds were synthesized by identical reaction methodologies by altering the Amino analogues. The molecular weight determination for these compounds was carried out by EI-MS, while 1H NMR analysis confirmed their structure as well as the purity. Among these, AY102 and AY103 were solubilized in chloroform, while AY127 was found to be solubilized in DMSO only due to its highly nonpolar structure. A complete list of compounds synthesized in this study together with their molecular structure, molecular weight, class of compounds and their solubilities is indicated in Table 1.
Table 1.
List of compounds synthesized in this study.
| Name | Class of compounds | Structures | Solvent | Purity checked on the basis of |
|---|---|---|---|---|
| AYNBDO | Benzodioxane |
|
Chloroform | Thin layer chromatography (TLC) Nuclear magnetic resonance (NMR) |
| AYNH2 | Benzodioxane |

|
Chloroform | – |
| AY80 | Acetamol derivatives |

|
Chloroform | – |
| AYZHC | Acetamol derivatives |

|
Chloroform | – |
| AYTHPP | Porphyrin |
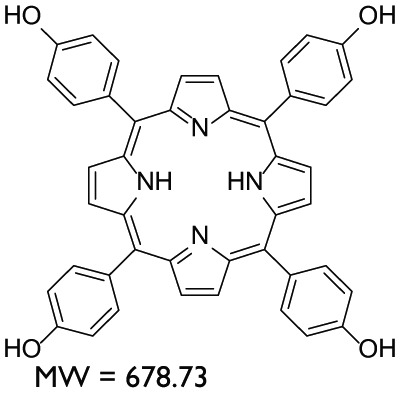
|
Methanol | – |
| AYTPyP | Porphyrin |
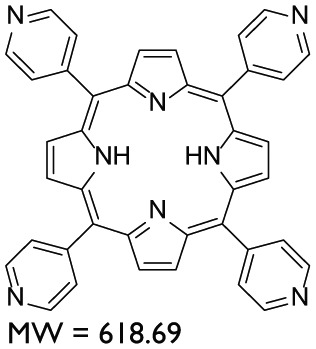
|
Methanol | – |
| AY102 | Naphthalene diimide |

|
Chloroform | – |
| AY103 | Naphthalene diimide |

|
Chloroform | – |
| AY127 | Naphthalene diimide |
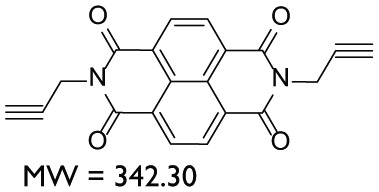
|
DMSO | – |
DMSO: dimethyl sulfoxide; MW: molecular weight.
Naphthalene diimide (AY103) and Aminophenol derivatives (AYZHC) show significant cytotoxicity against HeLa cells
To ascertain the cytotoxic effects of AY series compounds on HeLa cells, cytotoxicity assays were performed by incubating HeLa cells in the presence or absence of various concentrations of AY series compounds (100 µM) or respective solvents. At 100 µM, Aminophenol derivative (AYZHC) caused significant cytotoxic effects on HeLa cells (60%) compared with the solvent control (p > 0.05 using independent T test, two-tailed distribution; Table 2 and Figure 1(a)). Similarly, Porphyrin (AYTHPP) and Naphthalene diimide (AY103) showed cytotoxic effects against HeLa cells (Table 2 and Figure 1(a)). However, the other Aminophenol derivatives (AY80), Naphthalene diimide (AY102, AY127), Benzodioxane (AYNH2, AYNBDO) and Porphyrin (AYTPyP) did not have significant cytotoxic effects on HeLa cells when compared with their respective solvents alone (p > 0.05 using independent T test, two-tailed distribution; Table 2, Figure 1(a) and (b)).
Table 2.
Cytotoxic efficacy of Benzodioxane, Naphthalene diimide, Porphyrin and Acetamol derivatives against HeLa cells.
| Solvent | Compound | Cytotoxicity (%) |
|---|---|---|
| Chloroform (2 µL) | AYNBDO | 0 |
| AYNH2 | 0 | |
| AY80 | 0 | |
| AYZHC | 60 ± 6.7 | |
| AY102 | 0 | |
| AY103 | 45 ± 3.2 | |
| Methanol (2 µL) | AYTHPP | 41 ± 6.7 |
| AYTPyP | 0 | |
| DMSO (2 µL) | AY127 | 0 |
The results represent the mean ± standard error of three different experiments performed in duplicates.
Figure 1.
Representative cytotoxic effects of compounds on HeLa cells. (a) The cytotoxic efficacy of Aminophenol derivatives (AYZHC, AY80), Benzodioxane (AYNBDO, AYNH2) and Porphyrin (AYTHPP, AYTPyP). Briefly, HeLa cells (passage 43–46) were incubated with the respective drugs at 100 µM of concentration 37°C for 24 h and their respective solvent. Note that only Aminophenol derivatives (AYZHC) and Porphyrin (AYTHPP) exhibited cytotoxicity against HeLa cells as compared to the control (X250). (b) HeLa cells were incubated with 100 µM of Naphthalene diimide (AY102, AY103 and AY127) as well as their respective solvents and incubated at 37°C for 24 h. Note that only Naphthalene diimide (AY103) showed significant cytotoxicity against HeLa cells as compared to the solvent control (250×).
Benzodioxane (AYNBDO), Naphthalene Diimide (AY102, AY103), Aminophenol derivatives (AYZHC) and Porphyrin (AYTHPP, AYTPyP) inhibit HeLa cell growth
Growth inhibition assays were performed to determine the effects of AY series compounds on HeLa cell growth inhibition. When 50% semi-confluent monolayers of HeLa were incubated with RPMI, the number of viable cells increased from 1.11 × 105 ± 2.00 × 103 to 2.033 × 105 ± 2.51 × 103. In contrast, HeLa monolayers incubated with Benzodioxane (AYNBDO) exhibited significant growth inhibition effects at concentration of 100 µM exhibiting reduced number of cells, that is, the number of cells remained at 1.14 × 105 ± 1.15 × 103, respectively (Figure 2(a) and (b)). Moreover, for Naphthalene diimide (AY103), potent growth inhibition of HeLa cell was observed, that is, no cell growth at concentration of 100 µM (Figure 2(c) and (d)). For Naphthalene diimide (AY127), the number of cells was reduced to 9.23 × 104 at 100 µM concentration (Figure 2(c) and (d)). For Porphyrin (AYTHPP) at the concentration of 100 µM, the growth of HeLa cells was abolished (Figure 2(a) and (b)). In contrast, for Porphyrin (AYTPyP), the number of viable cells was inhibited moderately from 2.00 × 105 ± 4.93 × 103 in solvent control to 1.13 × 105 ± 4.04 × 103 (p < 0.05 using independent T test, two-tailed distribution; Figure 2(a) and (b)). Conversely, for Naphthalene diimide (AY127), there was no significant effect on the growth inhibition at 100 µM, when compared to the solvent control of DMSO (p < 0.05 using independent T test, two-tailed distribution; Figure 2(c) and (d)). Next, minimum inhibitory concentration 50 (MIC50) of effective compounds was determined. The findings revealed that Benzodioxane compound (AYNBDO), Aminophenol derivatives (AY80; AYZHC), Porphyrin (AYTyPP) and Naphthalene diimide (AY102) showed MIC50 at 100 µM. In contrast, Porphyrin (AYTHPP) and Naphthalene diimide (AY103) showed MIC50 at 50 µM.
Figure 2.
Growth inhibitory effects of compounds on HeLa cells. (a) The growth inhibition effects of Aminophenol derivatives (AYZHC, AY80), Benzodioxane (AYNBDO, AYNH2) and Porphyrin (AYTHPP, AYTPyP). Briefly, semi-confluent HeLa cells (passage 49–52) were incubated with the respective drugs or their respective solvent controls at 100 µM of concentration 37°C for 24 h as described in Materials and Methods. Next day, viable cells were counted using Trypan blue exclusion assay. Note that only Benzodioxane (AYNBDO), Aminophenol derivatives (AYZHC) and Porphyrin (AYTHPP, AYTPyP) exhibited significant anti-proliferation effects against HeLa cells in a concentration-dependent manner (p < 0.05 using independent T test, two-tailed distribution). The results represent the mean ± standard error of three different experiments performed in duplicates. (b) Following incubation with drugs indicated in (a), cells were visualized under an inverted microscope and images captures. X250. (c) HeLa cells were incubated with 100 µM of Naphthalene diimide (AY102, AY103 and AY127) as well as their respective solvents and incubated at 37°C for 24 h. Next day, viable cells were counted using Trypan Blue exclusion assay. Interestingly, Naphthalene diimide (AY102, AY103 and AY127) demonstrated significant growth inhibitory effects against HeLa cells as compared to the solvent control (p < 0.001 using independent T test, two-tailed distribution). The results represent the mean ± standard error of three different experiments performed in duplicates. (d) Following incubation with drugs indicated in (b), cells were visualized under an inverted microscope and images captures (250×).
Discussion
In this study, several Naphthalene diimide derivatives were tested, but only AY103 showed HeLa cell toxicity. However, when tested for anti-proliferative activity, both AY102 and AY103 but not AY124 and AY127 showed growth inhibitory effects suggesting their potential therapeutic value. Although the molecules reported here are novel, this is consistent with previous studies which showed that other Naphthalene diimide derivatives are promising candidates in the treatment of cancer.5,19 The mode of action of their anti-proliferative activity was attributed to four pathways including (1) DNA damage response and senescence subsequent to telomere uncapping, (2) suppression of oncogenes, (3) mitotic inhibition and apoptosis and (4) chromosomal instability through telomere aggregation or via synergistic effects of aforementioned pathways. By modifying their synthesis to enhance the interactions with the phosphate groups of DNA6 and combining structural components of anticancer molecules into a single molecule can potentially target different cancer cell lines. Among various molecules tested, AN169 showed anticancer activity against 60 human cancer cell lines. It was characterized by a side chain length of three methylene units and by two 2,3,4-trimethoxy benzyl groups.7 These studies suggested that Naphthalene diimide derivatives may be used in combination therapy together with other anticancer agents such as anti-mitotic agents to counter tumour growth or to impede occurrence of cancer resistance.
Among Aminophenol derivatives tested in this study, AYZHC but not AY80 showed cytotoxic effects. AYZHC proved to be an outstanding derivative because of its remarkable in vitro cytotoxic activity. When tested for anti-proliferative activity, Aminophenol derivatives AYZHC but not AY80 showed significant inhibition of HeLa cells growth. Overall, AYZHC showed both anti-proliferative as well as cytotoxic effects against HeLa cells. The molecular mechanisms associated with Aminophenol derivative–mediated cytotoxicity have been reported as binding to thiol groups of cellular proteins and/or due to oxidative stress via production of ROS that leads to apoptosis20 or via chromosomal damage at high concentrations21 through inhibition of ribonucleotide reductase,22 increase in cytosolic and intranuclear calcium levels,23 glutathione depletion11 or inhibition of cyclooxygenase (COX-2).24 Other studies suggested that oxidative stress leads to lipid peroxidation which in turn leads to changes in the intracellular adenine nucleotide and calcium homeostasis. Although increase in intracellular calcium can lead to apoptosis via endonuclease activation and DNA fragmentation, collectively all processes aforementioned can lead to cytotoxicity. This is consistent with the findings that high concentration of Acetamol leads to increased cytotoxicity dependent on intracellular calcium increase.25 Importantly, Acetamol derivatives does not induce gene mutations in bacteria or mammalian cells,11 suggesting their potential value for further investigations. Notably, neither of the Benzodioxine derivatives used in this study (AYNBDO and AYNH2) showed any cytotoxic effects. In contrast, Benzodioxine derivative, AYNBDO, exhibited anti-proliferative activities. Although Benzodioxine derivatives used in the present had limited effects against HeLa cells, they do possess a desirable synthetic targets in versatile reactions to provide the 1,4-benzodioxine scaffold. Their structures provide a number of synthetic routes, and how different groups approach these targets can be employed for the synthesis of effective molecules in the future studies.
Porphyrins derivatives are widely used as photosensitizers (PSs) for PDT against cancer.26 With targeted therapy and reduced toxicity, Porphyrin derivatives are promising agents in PDT against cancer.27 Among Porphyrin derivatives tested in this study, AYTHPP but not AYTPyP exhibited cytotoxic effects against HeLa cells. However, when tested for anti-proliferative activity, both Porphyrin derivatives, AYTHPP and AYTPyP, exhibited growth inhibitory effects against HeLa cells suggesting their potential anticancer properties. Although these findings are significant and showed the effects of several novel compounds on cell growth as well as LDH release, a marker of cell damage, future studies are needed to dissect molecular mechanisms associated with cell damage and also effects on several cell lines. In addition, several cancer cell lines need to be tested to validate these findings as well as in vivo studies to establish translational value of these findings.
Conclusion
In conclusion, among different classes of compounds synthesized and tested in this study, including Benzodioxane, Naphthalene diimide, Porphyrin and Aminophenol derivatives, several agents were identified with anti-proliferative and cytotoxic effects against HeLa cells. In addition to the synthesis of additional derivatives, future studies will determine the effects of these agents against a variety of cancer cell lines and determine the structure–activity relationship and the precise mode of action at the molecular level to establish their translational value.
Supplemental Material
Supplemental material, supplementary_file for Cytotoxic effects of Benzodioxane, Naphthalene diimide, Porphyrin and Acetamol derivatives on HeLa cells by Shareni Jeyamogan, Naveed Ahmed Khan, Ayaz Anwar, Muhammad Raza Shah and Ruqaiyyah Siddiqui in SAGE Open Medicine
Acknowledgments
N.A.K. and R.S. conceived the project. A.A. and M.R.S. synthesized compounds. R.S. and N.A.K. contributed to project design. S.J. performed biological experiments under the supervision of N.A.K. and R.S. S.J. and R.S. prepared the first draft of the manuscript, while N.A.K. finalized the manuscript while revising it critically. All authors read and approved the final manuscript. S.J. and N.A.K. contributed equally to this manuscript.
Footnotes
Availability of data and materials: The datasets used during this study are available from the corresponding author on reasonable request.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors gratefully acknowledge financial support from the Sunway University (2016-05).
ORCID iD: Naveed Ahmed Khan  https://orcid.org/0000-0001-7667-8553
https://orcid.org/0000-0001-7667-8553
References
- 1. Cancer epidemiology. International Agency for Research on Cancer (IARC), http://www.iarc.fr/en/publications/pdfs-online/epi/index.php
- 2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5): E359–E386. [DOI] [PubMed] [Google Scholar]
- 3. Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127(12): 2893–2917. [DOI] [PubMed] [Google Scholar]
- 4. Gatti L, Zunino F. Overview of tumor cell chemoresistance mechanisms. Methods Mol Med 2005; 111: 127–148. [DOI] [PubMed] [Google Scholar]
- 5. Ingrassia L, Lefranc F, Kiss R, et al. Naphtalimide and Azonafides as promising anti-cancer agents. Curr Med Chem 2009; 16(10): 1192–1213. [DOI] [PubMed] [Google Scholar]
- 6. Tumiatti V, Milelli A, Minarini A, et al. Design, synthesis, and biological evaluation of substituted naphthalene imides and diimides as anticancer agent. J Med Chem 2009; 52: 7873–7877. [DOI] [PubMed] [Google Scholar]
- 7. Milelli A, Tumiatti V, Micco M, et al. Structure-activity relationships of novel substituted naphthalene diimides as anticancer agents. Eur J Med Chem 2012; 57: 417–428. [DOI] [PubMed] [Google Scholar]
- 8. Kren V, Walterova D. Silybin and silymarin – new effects and applications. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2005; 149: 29–41. [DOI] [PubMed] [Google Scholar]
- 9. Gazak R, Walterova D, Kren V. Silybin and silymarin–new and emerging applications in medicine. Curr Med Chem 2007; 14: 315–338. [DOI] [PubMed] [Google Scholar]
- 10. AbouZid S. Silymarin, natural flavonolignans from milk thistle. In: Rao V. (ed.) Phytochemicals – a global perspective of their role in nutrition and health. Rijeka: InTech Publishers, 2012, pp. 255–272. [Google Scholar]
- 11. Bergman K, Müller L, Weberg Teigen S. Series: current issues in mutagenesis and carcinogenesis, No. 65. The genotoxicity and carcinogenicity of paracetamol: a regulatory (re)view. Mutat Res 1996; 349: 263–288. [DOI] [PubMed] [Google Scholar]
- 12. Scottish Intercollegiate Guidelines Network (SIGN). Guideline: Control of pain in adults with cancer (PDF). Edinburgh: National Health Service (NHS), 2008. [Google Scholar]
- 13. American Society of Health-System Pharmacists, http://www.ashp.org/
- 14. Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci 2004; 3: 436–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang L, Dai T, Hamblin MR. Antimicrobial photodynamic inactivation and photodynamic therapy for infections. Methods Mol Biol 2010; 635: 155–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sissons J, Kim KS, Stins M, et al. Acanthamoeba induces host cell death via a phosphatidylinositol 3-kinase (PI3K)-dependent mechanism. Infect Immun 2005; 73: 2704–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khan NA, Siddiqui R. Acanthamoeba affects the integrity of human brain microvascular endothelial cells and degrades the tight junction proteins. Int J Parasitol 2009; 39: 1611–1616. [DOI] [PubMed] [Google Scholar]
- 18. Abjani F, Khan NA, Yousuf FA, et al. Targeting cyst wall is an effective strategy in improving the efficacy of marketed contact lens disinfecting solutions against Acanthamoeba castellanii cysts. Cont Lens Anterior Eye 2016; 39(3): 239–243. [DOI] [PubMed] [Google Scholar]
- 19. Hampel SM, Pepe A, Greulich-Bode KM, et al. Mechanism of the antiproliferative activity of some naphthalene diimide G-quadruplex ligands. Mol Pharmacol 2013; 83(2): 470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buttke TM, Sandstrom PA. Oxidative stress as a mediator of apoptosis. Immunol Today 1994; 15(1): 7–10. [DOI] [PubMed] [Google Scholar]
- 21. Sawada M, Matsuoka A, Nohmi T, et al. Mutagenicity tests on phenacetin-related compounds in cultured mammalian cells. Mutat Res: Envir Muta 1985; 147(5): 273. [Google Scholar]
- 22. Hongslo JK, Bjørge C, Schwarze PE, et al. Paracetamol inhibits replicative DNA synthesis and induces sister chromatid exchange and chromosomal aberrations by inhibition of ribonucleotide reductase. Mutagenesis 1990; 5(5): 475–480. [DOI] [PubMed] [Google Scholar]
- 23. Shen W, Kamendulis LM, Ray SD, et al. Acetaminophen-induced cytotoxicity in cultured mouse hepatocytes: correlation of nuclear Ca2+ accumulation and early DNA fragmentation with cell death. Toxicol Appl Pharmacol 1991; 111(2): 242–254. [DOI] [PubMed] [Google Scholar]
- 24. Hinz B, Cheremina O, Brune K. Acetaminophen (Paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J 2008; 22(2): 383–390. [DOI] [PubMed] [Google Scholar]
- 25. Satorres J, Pérez-Mateo M, Mayol MJ, et al. Protective effect of diltiazem against acetaminophen hepatotoxicity in mice. Liver 1995; 15(1): 16–19. [DOI] [PubMed] [Google Scholar]
- 26. Shi JY, Liu TWB, Chen J, et al. Transforming a targeted porphyrin theranostic agent into a PET imaging probe for cancer. Theranostics 2011; 1: 363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ferreira DP, Conceic DS, Calhelha RC, et al. Porphyrin dye into biopolymeric chitosan films for localized photodynamic therapy of cancer. Carbohydr Polym 2016; 151: 160–171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, supplementary_file for Cytotoxic effects of Benzodioxane, Naphthalene diimide, Porphyrin and Acetamol derivatives on HeLa cells by Shareni Jeyamogan, Naveed Ahmed Khan, Ayaz Anwar, Muhammad Raza Shah and Ruqaiyyah Siddiqui in SAGE Open Medicine




