Abstract
Introduction:
To compare the efficacy and safety of stereotactic body radiation therapy with or without transcatheter arterial chemoembolization for patients with small hepatocellular carcinoma who were ineligible for resection or ablation therapies.
Methods:
A total of 150 patients with 185 hepatocellular carcinoma (≤3 nodules, Child-Turcotte-Pugh class A or B, and no vascular or extrahepatic metastases) were treated with stereotactic body radiation therapy. In principle, transcatheter arterial chemoembolization was combined before stereotactic body radiation therapy (combination group), but some patients were treated with stereotactic body radiation therapy alone. The prescribed dose of stereotactic body radiation therapy was 48 Gy in 4 fractions at the isocenter and 40 Gy in 4 or 5 fractions at the dose covering 95% of the planning target volume. The overall survival, progression-free survival, local progression free survival, and complication rates were retrospectively compared between the groups. Local progression was defined as irradiated tumor growth in dynamic computed tomography follow-up. Tumor responses were assessed according to the Modified Response Evaluation Criteria in Solid Tumors. Treatment-related toxicities were evaluated according to the Common Terminology Criteria for Adverse Events version 4.0.
Results:
Twenty-eight and 122 patients were enrolled in the stereotactic body radiation therapy alone and combination groups, respectively. The median follow-up periods were 16 and 29 months, respectively. The 2-year overall, progression-free, and local progression-free survival times in stereotactic body radiation therapy alone and combination groups were 78.6% and 80.3% (P = .6583), 49.0% and 42.9% (P = .188), and 71.4% and 80.8% (P = .9661), respectively. The incidence of ≥grade 3 toxicities was 17.9% in stereotactic body radiation therapy alone group and 18.9% in combination group (P = .903).
Conclusions:
Stereotactic body radiation therapy alone may be a good treatment option for patients with small hepatocellular carcinoma who were ineligible for resection or ablation therapies.
Keywords: hepatocellular carcinoma (HCC), stereotactic body radiation therapy (SBRT), transarterial chemoembolization (TACE), resection, ablative therapies
Introduction
Guidelines from the National Comprehensive Cancer Network (NCCN) and the Barcelona Clinic Liver Cancer system1,2 recommend transcatheter arterial chemoembolization/ transarterial chemoembolization (TACE) for patients with hepatocellular carcinoma (HCC) ineligible for resection or ablation therapies. However, the treatment results of TACE are unsatisfactory for reasons including incomplete necrosis due to hypovascularity, dual blood supply around the HCC capsule, multiple collateral feeding circulation, and others.3 Recently, stereotactic body radiotherapy (SBRT), which delivers high radiation doses to focal HCC, has been considered as an alternative locoregional therapy to resection, ablation, and TACE when these therapies have failed.1 Several studies have reported good treatment outcomes using SBRT for HCC with or without TACE.4–10 Patients with HCC in our institution routinely undergo TACE before SBRT. However, whether the combined therapy of SBRT with TACE is superior to TACE or SBRT alone is still unknown. Our multi-institutional retrospective study aimed to compare the efficacy and safety of SBRT with or without TACE in patients with small HCC who were ineligible for resection or ablation therapies.
Materials and Methods
Patient Eligibility
From December 2008 to August 2017, 182 patients with 229 tumors underwent SBRT at Hiroshima University Hospital and Hiroshima High-precision Radiotherapy Cancer Center (HIPRAC). The details of the criteria for SBRT were previously reported.11 The following inclusion criteria for curative SBRT were used: (1) over 20 years old; (2) an Eastern Cooperative Oncology Group Performance Status (PS) of 0 to 2; (3) Child-Turcotte-Pugh Class A or B; (4) <3 HCC nodules, each up to 50 mm in diameter without portal venous thrombosis or extrahepatic metastases; (5) inoperability because of poor general condition or surgery refusal; and (6) unsuitability for radiofrequency ablation (RFA) because of tumor location (on the liver surface, particularly high risk of pneumothorax, and near the porta hepatis), tumor invisibility on ultrasonography, or bleeding tendencies (platelet count ≦50 000/mL, prothrombin activity ≦ 50%). The study protocol was approved by the Human Ethics Review Committee of Hiroshima University, and a signed consent form was obtained from each participant.Hepatocellular carcinoma was diagnosed by its characteristic appearance of early arterial phase enhancement and portal venous phase hypodensity, which were revealed in most patients in dynamic computed tomography (CT) or combined angiography-CT.
Treatment Procedure
TACE
If they were eligible and agreed, the patients underwent TACE with iodized oil (Lipiodol, Guerbet, Japan, Tokyo) 1 to 2 months prior to SBRT. Transcatheter arterial chemoembolization was performed through the femoral artery using the Seldinger technique under local anesthesia. A coaxial microcatheter was selectively inserted into the hepatic feeding artery of a segment or subsegments containing the target tumor. Anticancer chemotherapies, such as cisplatin (7-70 mg/body at a concentration of 10 mg/mL; Randa, Nippon Kayaku, Tokyo, Japan), miriplatin (20-80 mg/body at a concentration of 20 mg/mL; Miriplatin Hydrate, Dainippon Sumitomo Pharma Co, Tokyo, Japan), and epirubicin (Nippon Kayaku) were mixed with iodized oil (Laboratoire Guerbet, Villepinte, France) and administered by injecting the drug into the hepatic artery feeding the target tumor segment or subsegments. We generally used cisplatin until 2010 and have used miriplatin since that time. We also used epirubicin for patients with drug allergies or if the treatment effect was insufficient. Finally, a small amount of gelatin sponge particles was used to induce embolization until the feeding artery flow was markedly decreased.
SBRT
Stereotactic body radiotherapy was conducted within 1 to 2 months after TACE. Stereotactic body radiotherapy was administered even if a complete response was achieved by TACE because, in the long term, the capacity to keep the cancer under control using TACE alone was lost and local control after TACE was unsatisfactory, as mentioned earlier. Respiratory motion was coordinated by voluntary breath-holding at the end of the inspiratory phase with an Abches (APEX Medical, Tokyo, Japan), a device that allows patients to control their chest and abdominal respiratory motion. For simulations, dynamic CT scans (Lightspeed QX/I; GE Medical Systems, Waukesha, Wisconsin) including the noncontrast enhancement, arterial, portal, and venous phases were performed by administering a bolus injection of nonionic iodinated contrast material (100 mL at a rate of 3 mL/s). Arterial phase CT volume data were transferred to a 3-D treatment planning system (Pinnacle3 version 9.6; Phillips Medical Systems, Fitchburg, Wisconsin or Eclipse; Varian Medical Systems, Palo Alto, California). The gross tumor volume (GTV) was defined as the tumor volume containing the remains of iodized oil from TACE and early enhancement in the arterial phase of dynamic CT. The clinical target volume margin was usually defined as 0 to 3 mm around the GTV. Typically, a planning target volume (PTV) margin of 5 to 8 mm, including the respiratory motion reproducibility and setup error, was usually added. Stereotactic body radiotherapy was carried out according to the 3D conformal radiation therapy (3D-CRT) method or volumetric modulated arc therapy (VMAT). Eight noncoplanar ports in 3D-CRT and 1 or 2 arcs in VMAT were selected in all patients, including a beam direction that avoided the critical organs, when possible.
The prescribed doses and fractions were evaluated at the isocenter or the dose covering 95% of the PTV (D95%). From December 2008 to February 2014, a total dose of 48 Gy in 4 fractions at the isocenter was selected; after March 2014, 40 Gy in 4 (80% isodose) or 5 (70% isodose) fractions at the D95% of the PTV was selected. From December 2008 to November 2013, 60 Gy in 8 fractions at the isocenter was selected for centrally located HCC located within 5 mm of the major vessels such as the aorta, portal vein, and inferior vena cava. After December 2013, the same dose-fraction schedule was used for peripheral and centrally located HCC. For HCC that was close to the gastrointestinal tract, decreased dose fractions were selected to maintain the dose constraints of the gastrointestinal tract (<20 Gy in 4 fractions in 5 mL). Photon beams of 6 to 10 MV were delivered by a linear accelerator (CLINAC 2300 C/D, iX or TrueBeam; Varian Medical Systems).
Evaluation
All patients were examined and underwent follow-up dynamic CT or magnetic resonance imaging (MRI) every 3 to 6 months after SBRT completion. In addition, serum HCC-specific tumor markers were investigated every 2 months. If the tumor marker levels were significantly increased, additional dynamic CT or MRI was performed. Tumor responses were assessed according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST), with tumor necrosis recognized based on nonenhanced areas.12 Local tumor progression was defined as progressive disease on the mRECIST, while local control was defined as free of local progression. Treatment-related toxicities were evaluated according to the Common Terminology Criteria for Adverse Events, version 4.0. Levels of total bilirubin, alanine aminotransferase, aspartate aminotransferase, platelets, albumin, and ascites were evaluated for 1 year after SBRT because they were strongly affected by the progress of HCCs, cirrhosis, and/or treatments for recurrent lesions after SBRT. Portal vein thrombosis, bile duct stenosis, radiation pneumonitis, gastrointestinal disorders, and ulcers were evaluated without limiting the period. After SBRT, we carefully checked the irradiated area and evaluated patients for the presence of SBRT-induced toxicities.
Statistical Methods
Univariate analyses (UVAs) using the Mantel-Haenszel χ2 or t tests and multivariate analyses (MVAs) using logistic regression were performed to assess the statistical significance of the differences in responses. The Kaplan-Meier method was used to calculate the overall survival (OS), progression-free survival (PFS), local PFS (LPFS), and local control rates (LC). Overall survival was calculated from the starting date of SBRT until the date of the final follow-up or death. Progression-free survival and LPFS were estimated from the starting date of SBRT until the date of progression/relapse or death and local progression/relapse or death from any cause, respectively. Local control rates were estimated from the starting date of SBRT until the date of local progression/relapse. StatMate for Windows (Version 4.01; ATMS, Tokyo, Japan) was used to perform all statistical analyses. Statistical significance was defined as P values <.05, and all tests were 2-tailed. In this study, the survival time was defined as the period from the date of SBRT to the date of death or the last follow-up.
Results
Patient Characteristics
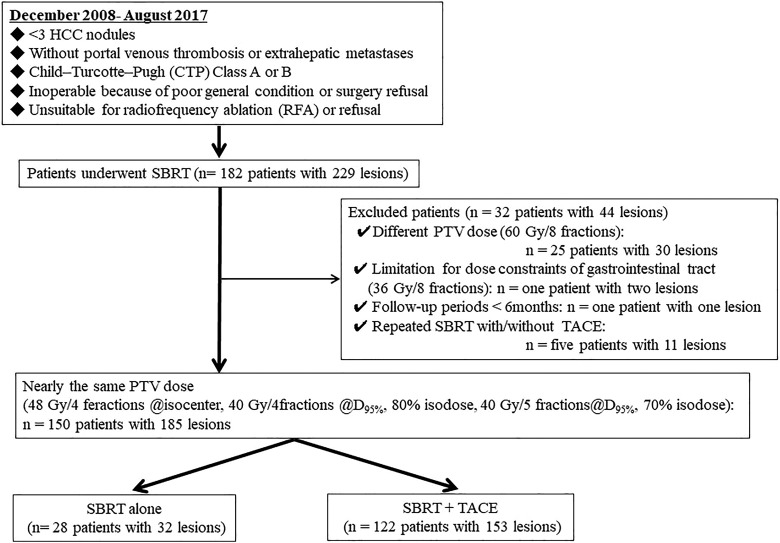
Of 182 patients with 229 tumors who underwent SBRT, 150 patients with 185 lesions who received 48 Gy in 4 fractions at the isocenter or 40 Gy in 4 or 5 fractions at the D95% of the PTV were analyzed. The consolidated standards of reporting trials (CONSORT) diagram of this study is summarized in Figure 1. In these dose fraction schedules, PTV mean dose was almost the same. Thirty-two patients with 44 lesions, including 25 patients with 30 centrally located HCC who received 60Gy in 8 fractions, 1 patient with 2 lesions who had dose constraints for gastrointestinal tract exposure, 1 patient with 1 lesion who had short follow-up periods (<6 months), and 5 patients with 11 lesions who received repeated SBRT with and without TACE were excluded from the analysis. In particular, the reason the patients who were received 60 Gy in 8 fractions were excluded was that the mean PTV dose differed from that of the other dose fraction schedules. In this patient group, some patients were treated with SBRT alone for the following reasons: refusal of TACE (11 patients), tumor hypovascularity (5 patients), difficulty in catheterization (4 patients), difficulty with usage of contrast-enhanced agent due to allergy or poor renal function (3 patients), poor PS (2 patients), bleeding tendency (2 patients), and poor liver function (1 patient; SBRT alone group: 28 patients with 32 lesions). The other patients underwent TACE combined before SBRT (SBRT + TACE group: 122 patients with 153 lesions). The clinical characteristics of the patients in both groups are summarized in Table 1. Stereotactic body radiotherapy-alone group included a significantly larger number of naive patients and longer follow-up period than those in the SBRT + TACE group.
Figure 1.
The consolidated standards of reporting trials diagram of this study of 182 patients with 229 tumors who underwent SBRT, 32 patients with 44 lesions, including 25 patients with 30 centrally located HCC who were selected 60 Gy dose in 8 fractions, 1 patients with 2 lesions who had dose constraints for gastrointestinal tract exposure, 1 patients with 1 lesion who was short follow-up periods (<6 months), and 5 patients with 11 lesions who received repeated SBRT with and without TACE were excluded from the analysis. Finally, 150 patients with 185 lesions who received 48 Gy in 4 fractions at the isocenter or 40 Gy in 4 or 5 fractions at the D95% of the PTV were analyzed. D95% indicates the dose covering 95% of the PTV; HCC, hepatocellular carcinoma; PTV, planning target volume; SBRT, stereotactic body radiotherapy.
Table 1.
Patient Background.
| SBRT Alone (n = 28) | SBRT + TACE (n = 122) | P Value | |
|---|---|---|---|
| Age, years | 77 (58-90) | 73 (38-93) | .0587 |
| Gender (male/female) | 17/11 | 82/40 | .5127 |
| Performance status (0/1 ≧) | 21/7 | 107/15 | .086 |
| Tumor size, mm | 18.5 (8-55) | 16 (5-63) | .2425 |
| T-stage (T1/T2) | 24/4 | 89/33 | .1577 |
| BCLC stage (0/A) | 17/11 | 63/59 | .3854 |
| CTP class (A/B) | 23/5 | 104/18 | .6811 |
| Etiology (HBV/HCV/NBNC) | 4/18/6 | 18/91/13 | .2987 |
| Location (peripheral/central) | 22/4 | 113/9 | .1903 |
| Initial case/recurrent case | 13/15 | 23/99 | .0021 |
| Previous treatment—surgery (−/+) | 9/19 | 52/70 | .3086 |
| Previous treatment—RFA/PEI (−/+) | 6/22 | 35/87 | .4369 |
| Follow-up period, months | 16 (6-64) | 29 (6-88) | .0063 |
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; HBV/HCV/NBNC, hepatitis B virus/hepatitis C virus/nonhepatitis B nonhepatitis C virus; RFA/PEI, radiofrequency ablation/percutaneous ethanol injection; TACE, transcatheter arterial chemoembolization; SBRT, stereotactic body radiotherapy.
Treatment Outcomes
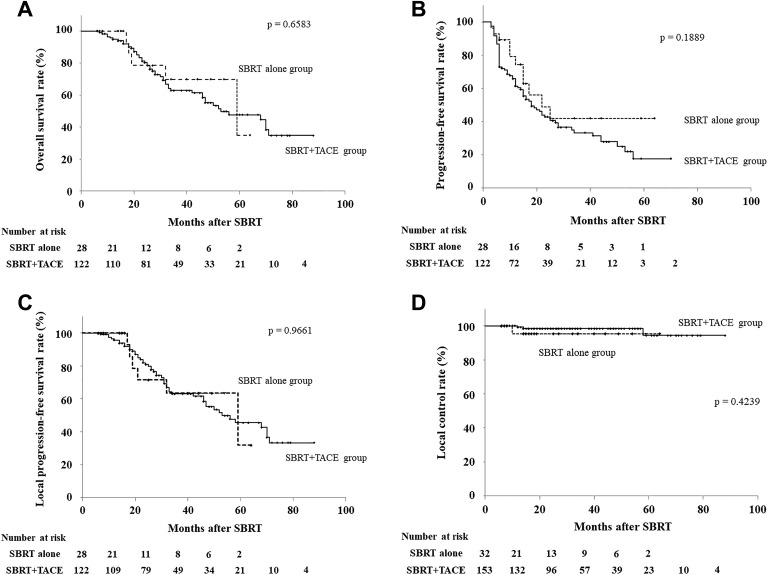
Figure 2A-D shows the OS, PFS, LPFS, and LC for both the groups. The 1- and 2-year OS rates were 100% (95% confidence interval [CI], 100%] and 78.6% (95% CI, 57.1%-100%) in the SBRT-alone group and 94.8% (95% CI, 90.8%-98.9%) and 80.3% (95% CI, 72.7%-87.9%) in the SBRT + TACE group, respectively (P = .6583). The 1- and 2-year PFS rates were 74.4% (95% CI, 56.3%-92.5%) and 49.0% (95% CI, 25.2%-72.7%) in the SBRT-alone group and 61.3% (95% CI, 52.5%-70.1%) and 42.9% (95% CI, 33.4%-52.3%) in the SBRT + TACE group, respectively (P = .1889). The 1- and 2-year LPFS rates were 100% (95% CI, 100%) and 71.4% (95% CI, 47.8%-95.1%) in the SBRT-alone group and 95.6% (95% CI, 91.8%-99.4%) and 80.8% (95% CI, 73.3%-88.4%) in the SBRT + TACE group, respectively (P = .9661). The 1- and 2-year local control rates were both 95.4% (95% CI, 86.1%-100%) in the SBRT-alone group and 99.2% (95% CI, 97.8%-100%) and 98.5% (95% CI, 96.3%-100%) in the SBRT + TACE group, respectively (P = .4239). There were no significant differences in OS, PFS, LPFS, and LC between the groups.
Figure 2.
Treatment results of stereotactic body radiotherapy for hepatocellular carcinoma. A, Overall survival rates. The 1- and 2-year OS rates were 100% and 78.6% in the SBRT alone group and 94.8% and 80.3%, respectively, in the SBRT + TACE Group (P = .6583). B, Progression-free survival rates. The 1- and 2-year PFS rates were 74.4% and 49.0% in the SBRT-alone group and 61.3% and 42.9%, respectively, in the SBRT + TACE group (P = .1889). C, Local progression-free survival rates. The 1- and 2-year LPFS rates were 100% and 71.4% in the SBRT-alone group and 95.6% and 80.8%, respectively, in the SBRT + TACE group, (P = .9661). D, Local control rates. The 1- and 2-year local control rates were both 95.4% in the SBRT-alone group and 99.2% and 98.5%, respectively, in the SBRT + TACE group (P = .4239). There were no significant differences between the groups in OS, PFS, LPFS, and local control. LPFS, local progression-free survival; OS, overall survival; PFS, progression-free survival; SBRT, stereotactic body radiotherapy.
Dose–Volume Histogram Analysis of Normal Liver
The median mean uninvolved liver (total liver minus GTV) dose was 6.7 Gy (range, 2.0-15.1 Gy) in the SBRT-alone group and 6.2 Gy (range, 2.7-13.1 Gy) in the SBRT+TACE group (P = .7513). The percentages of uninvolved liver volume exceeding 10 (V10 Gy), 20 (V20 Gy), and 30 Gy (V30 Gy) in the SBRT-alone and SBRT + TACE groups were 19.1% (range, 6.0%-63.0%) and 21.7% (range, 7.5%-52.1%; P = .8585), 7.8% (range, 2.5%-26.0%) and 7.5% (range, 2.0%-23.9%; P = .8481), and 3.9% (range, 1.4%-13.0%) and 4.0% (range, 1.0%-14.3%; P = .9983), respectively.
Treatment-Related Toxicities
Table 2 shows the baseline, post-TACE, and post-SBRT liver toxicities that exceeded grade 3 in both the groups. In the SBRT-alone group, grade 3 or 4 toxicities at baseline and after SBRT completion (post-SBRT) were observed in 2 (7.1%) and 5 (17.9%) patients, respectively. A grade 4 decreased platelet count was observed in 1 patient. In the SBRT + TACE group, grade 3 or 4 toxicities at baseline, after TACE completion (post-TACE), and post-SBRT were observed in 10 (8.2%), 31 (25.4%), and 23 (18.9%) patients, respectively. Grade 4 decreased platelet counts were observed in 2 patients. The rate of grade 3 or 4 toxicities post-TACE was slightly high, but these spontaneous toxicities were cured before SBRT. The incidence of ≥grade 3 toxicities post-SBRT did not differ significantly between the groups (P = .9030). No patient experienced gastrointestinal toxicity. Table 3 shows the prognostic factors for ≥grade 3 adverse effects in all patients and both the groups. For MVA, female sex in all patients and the SBRT + TACE group was the only significant prognostic factor (P = .0202 and .0096, respectively).
Table 2.
The Reasons of Contraindication for Resection and Ablation Therapy.
| SBRT Alone (n = 28) | SBRT + TACE (n = 122) | |
|---|---|---|
| Contraindications for resection | ||
| Insufficient postoperative liver function | 5 | 52 |
| Other comorbidities (heart failure, renal failure, brain infarction, bleeding tendency, COPD) | 9 | 32 |
| Old age (>75) | 7 | 20 |
| Reject | 7 | 19 |
| Contraindications for ablation therapy | ||
| HCC adjacent to or invading main vessel or biliary system | 5 | 30 |
| HCC abutting the diaphragm | 6 | 46 |
| HCC abutting the intestine | 1 | 6 |
| Ultrasound invisible | 2 | 11 |
| Insufficient postoablative liver function | 0 | 7 |
| Old age (>75) | 2 | 2 |
| Other comorbidities (heart failure, renal failure, brain infarction, bleeding tendency, COPD) | 7 | 16 |
| Reject | 5 | 4 |
Abbreviations: HCC, hepatocellular carcinoma; COPD, chronic obstructive pulmonary disease; TACE, transcatheter arterial chemoembolization; SBRT, stereotactic body radiotherapy.
Table 3.
Treatment-Related Toxicities (Grade 3 or 4).
| Toxicity | SBRT Alone (n = 28) | SBRT + TACE (n = 122) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post-SBRT | Baseline | Post-TACE | Post-SBRT | ||||||
| 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | |
| Elevated total bilirubin | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 2 | 0 |
| Elevated AST/ALT | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 0 | 1 | 0 |
| Decreased platelet count | 2 | 0 | 3 | 1 | 9 | 0 | 17 | 0 | 16 | 2 |
| Decreased albumin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Ascites | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 |
| Portal vein thrombosis | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Radiation pneumonitis | – | – | 0 | 0 | – | – | – | – | 0 | 0 |
| Other toxicitya | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 0 |
| Total | 2 | 0 | 4 | 1 | 10 | 0 | 31 | 0 | 21 | 2 |
Abbreviations: AST/ALT, aspartate aminotransferase/alanine aminotransferase; TACE, transcatheter arterial chemoembolization; SBRT, stereotactic body radiotherapy.
a Include elevated γglutamyl transpeptidase, bile duct stenosis, and hepatic encephalopathy.
Prognostic Factors
Tables 4 –7 show the UVA and MVA of the OS, PFS, and LPFS in all patients and the SBRT-alone and SBRT + TACE groups. Adverse effects (≥grade 3) were a significant prognostic factor for longer OS in MVA of all patients and the SBRT + TACE group but was not significant in the TACE combination group. Viral infection (hepatitis C virus negative) and T-stage (T1) were significant prognostic factors for longer PFS in MVA of all patients and the SBRT + TACE group but not in the TACE combination group. There were no significant prognostic factors for longer LPFS in MVA, including the combination of TACE.
Table 4.
Prognostic Factors for ≧Grade 3 Adverse Effects; Univariate Analysis and Multivariate Analysis.
| Prognostic Factor | All Patients (n = 150) | UVA | MVA | SBRT Alone (n = 28) | UVA | MVA | SBRT + TACE (n = 122) | UVA | MVA | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≧ Grade 3 | < Grade 3 | P Value | P Value | ≧ Grade 3 | < Grade 3 | P Value | P Value | ≧ Grade 3 | < Grade 3 | P Value | P Value | ||
| Age, years | ≧75 | 9 | 64 | .0524 | − | 1 | 15 | .0641 | − | 8 | 49 | .2027 | − |
| <75 | 19 | 58 | 4 | 8 | 15 | 50 | |||||||
| Gender | Male | 13 | 86 | .0153 | .0202 | 3 | 14 | .9712 | − | 10 | 72 | .0071 | .0096 |
| Female | 15 | 36 | 2 | 9 | 13 | 27 | |||||||
| Performance status | 0 | 26 | 102 | .2121 | − | 5 | 16 | .1543 | − | 21 | 86 | .5595 | − |
| 1 or 2 | 2 | 20 | 0 | 7 | 2 | 13 | |||||||
| CTP class | A | 20 | 107 | .0311 | .087 | 3 | 20 | .1538 | − | 17 | 87 | .0889 | − |
| B | 8 | 15 | 2 | 3 | 6 | 12 | |||||||
| Viral infection | HCV | 18 | 91 | .2699 | − | 2 | 16 | .2111 | − | 16 | 75 | .5389 | − |
| Non-HCV | 10 | 31 | 3 | 7 | 7 | 24 | |||||||
| BCLC stage | 0 | 15 | 65 | .9777 | − | 2 | 15 | .2954 | − | 13 | 50 | .603 | − |
| A | 13 | 57 | 3 | 8 | 10 | 49 | |||||||
| T stage | T1 | 20 | 93 | .5951 | − | 4 | 20 | .687 | − | 16 | 73 | .6849 | − |
| T2 | 8 | 29 | 1 | 3 | 7 | 26 | |||||||
| Greatest tumor dimensions | ≧20 mm | 12 | 51 | .9188 | − | 3 | 13 | .8867 | − | 9 | 38 | .9472 | − |
| <20 mm | 16 | 71 | 2 | 10 | 14 | 61 | |||||||
| Tumor location | Central | 4 | 9 | .2413 | − | 2 | 2 | .0698 | − | 2 | 7 | .7883 | − |
| Periferal | 24 | 113 | 3 | 21 | 21 | 92 | |||||||
| Diagnosis history | Initial | 10 | 26 | .1075 | − | 2 | 11 | .7505 | − | 8 | 15 | .031 | .3269 |
| Recurrence | 18 | 96 | 3 | 12 | 15 | 84 | |||||||
| Previous treatment (surgery) | Yes | 9 | 52 | .3086 | − | 3 | 6 | .1411 | − | 6 | 46 | .075 | − |
| No | 19 | 70 | 2 | 17 | 17 | 53 | |||||||
| Previous treatment (RFA/PEI) | Yes | 5 | 36 | .2122 | − | 1 | 5 | .9315 | − | 4 | 31 | .1836 | − |
| No | 23 | 86 | 4 | 18 | 19 | 68 | |||||||
| Mean liver dose | ≧7 Gy | 11 | 52 | .7469 | − | 3 | 11 | .6217 | − | 8 | 41 | .5589 | − |
| <7 Gy | 17 | 70 | 2 | 12 | 15 | 58 | |||||||
| Liver V20Gy | ≧10% | 8 | 48 | .2878 | − | 2 | 10 | .8867 | − | 6 | 38 | .2686 | − |
| <10% | 20 | 74 | 3 | 13 | 17 | 61 | |||||||
| Combination with TACE | Yes | 23 | 99 | .903 | − | ||||||||
| No | 5 | 23 | |||||||||||
Abbreviation: CTP, Child-Turcotte-Pugh; BCLC, Barcelona Clinic Liver Cancer; HCV, hepatitis C virus; MVA, multivariate analyses; RFA/PEI, radiofrequency ablation/ percutaneous ethanol injection; SBRT, stereotactic body radiotherapy; TACE, transcatheter arterial chemoembolization; UVA, univariate analyses; V20Gy, percentages of uninvolved liver volume exceeding 20 Gy.
Table 5.
Prognostic Factors of 2-Year Overall Survival; Univariate Analysis and Multivariate Analysis.
| Prognostic Factor | All Patients (n = 150) | SBRT Alone (n = 28) | SBRT + TACE (n = 122) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | 2-Year OS (%) | UVA, P Value | MVA, P Value | Number | 2-year OS (%) | UVA, P Value | MVA, P Value | Number | 2-Year OS (%) | UVA, P Value | MVA, P Value | ||
| Age (years) | ≧75 | 73 | 82.6 | .4302 | − | 16 | 85.7 | .6658 | − | 57 | 81.6 | .4401 | − |
| <75 | 77 | 78.3 | 12 | 71.4 | 65 | 79.2 | |||||||
| Gender | Male | 99 | 78.6 | .9496 | − | 17 | 66.7 | .1239 | − | 82 | 80.2 | .5085 | − |
| Female | 51 | 83.5 | 11 | 100 | 40 | 80.9 | |||||||
| Performance status | 0 | 128 | 79.4 | .9229 | − | 21 | 75 | .8453 | − | 107 | 79.8 | .8667 | − |
| 1 or 2 | 22 | 87.8 | 7 | 100 | 15 | 84.6 | |||||||
| CTP class | A | 127 | 81.7 | .9236 | − | 23 | 75 | .4205 | − | 104 | 82.4 | .6846 | − |
| B | 23 | 72 | 5 | 100 | 18 | 68 | |||||||
| Viral infection | HCV | 109 | 81.3 | .3823 | − | 18 | 87.5 | .823 | − | 91 | 80.5 | .4614 | − |
| non-HCV | 41 | 77 | 10 | 66.7 | 31 | 79.1 | |||||||
| BCLC stage | 0 | 80 | 83.8 | .08 | − | 17 | 85.7 | .6979 | − | 63 | 83.3 | .043 | .6999 |
| A | 70 | 75.6 | 11 | 71.4 | 59 | 76 | |||||||
| T stage | T1 | 113 | 83.5 | .0492 | .1718 | 24 | 80 | .8522 | − | 89 | 83.7 | .0291 | .5556 |
| T2 | 37 | 70.4 | 4 | 75 | 33 | 69.8 | |||||||
| Greatest tumor dimensions | ≧20 mm | 63 | 83 | .0872 | − | 12 | 71.4 | .9944 | − | 47 | 84.4 | .0541 | − |
| <20 mm | 87 | 78.5 | 12 | 83.3 | 75 | 77.9 | |||||||
| Tumor location | Central | 13 | 77.9 | .8744 | − | 4 | 66.7 | .9239 | − | 9 | 85.7 | .7532 | − |
| Peripheral | 137 | 80.4 | 24 | 81.8 | 113 | 80.1 | |||||||
| Diagnosis history | Initial | 36 | 81.5 | .662 | − | 13 | 85.7 | .3214 | − | 23 | 79.6 | .8784 | − |
| Recurrence | 114 | 79.9 | 15 | 71.4 | 99 | 80.5 | |||||||
| Previous treatment (surgery) | Yes | 61 | 81 | .664 | − | 9 | 50 | .1394 | − | 52 | 83.5 | .8899 | − |
| No | 89 | 79.7 | 19 | 90 | 70 | 77.6 | |||||||
| Previous treatment (RFA/PEI) | Yes | 41 | 81.6 | .502 | − | 5 | 66.7 | .791 | − | 35 | 83.4 | .5137 | − |
| No | 109 | 79.8 | 23 | 81.8 | 87 | 79.3 | |||||||
| Adverse effects | <Grade 3 | 122 | 83.4 | .0222 | .0391 | 23 | 80 | .537 | − | 99 | 83.6 | .0085 | .0261 |
| ≧Grade 3 | 28 | 68.7 | 5 | 75 | 23 | 67.2 | |||||||
| Combination with TACE | Yes | 28 | 78.6 | .6583 | − | ||||||||
| No | 122 | 80.3 | |||||||||||
Abbreviations: BCLC; Barcelona Clinic Liver Cancer; CTP; Child-Turcotte-Pugh; HCV; hepatitis C virus; MVA, multivariate analyses; OS, overall survival; RFA/PEI; radiofrequency ablation/percutaneous ethanol injection; SBRT, stereotactic body radiotherapy; TACE, transcatheter arterial chemoembolization; UVA, univariate analyses
Table 6.
Prognostic Factors of 2-Year Progression-Free Survival; Univariate Analysis and Multivariate Analysis.
| Prognostic Factor | All Patients (n = 150) | SBRT Alone (n = 28) | SBRT + TACE (n = 122) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | 2-Year OS (%) | UVA, P Value | MVA, P Value | Number | 2-Year OS (%) | UVA, P Value | MVA, P Value | Number | 2-Year OS (%) | UVA, P Value | MVA, P Value | ||
| Age, years | ≧75 | 73 | 35.9 | .1027 | − | 16 | 63.6 | .9466 | − | 57 | 30.7 | .0643 | − |
| <75 | 77 | 51.1 | 12 | 39.3 | 65 | 52.4 | |||||||
| Gender | Male | 99 | 42 | .825 | − | 17 | 24.5 | .1219 | − | 82 | 44.1 | .5285 | − |
| Female | 51 | 47.5 | 11 | 80.8 | 40 | 39.9 | |||||||
| Performance status | 0 | 128 | 40.9 | .1242 | − | 21 | 40.1 | .0826 | − | 107 | 40.9 | .5434 | − |
| 1 or 2 | 22 | 68.7 | 7 | 100 | 15 | 57 | |||||||
| CTP class | A | 127 | 46.6 | .0936 | − | 23 | 52.3 | .8956 | − | 104 | 45.3 | .0484 | .028 |
| B | 23 | 31.9 | 5 | 37.5 | 18 | 29.3 | |||||||
| Viral infection | HCV | 109 | 39.7 | .0025 | .0017 | 18 | 58.2 | .8181 | − | 91 | 36.7 | .0017 | .001 |
| Non-HCV | 41 | 56.3 | 10 | 38.6 | 31 | 62.4 | |||||||
| BCLC stage | 0 | 80 | 48.8 | .2006 | − | 17 | 40.1 | .4716 | − | 63 | 50 | .0946 | − |
| A | 70 | 38.8 | 11 | 62.3 | 59 | 35 | |||||||
| T stage | T1 | 113 | 52.3 | .001 | .0203 | 24 | 50.9 | .6021 | − | 89 | 52.3 | <.0001 | .012 |
| T2 | 37 | 18.3 | 4 | 50 | 33 | 13.5 | |||||||
| Greatest tumor dimensions | ≧20 mm | 63 | 42.5 | .8397 | − | 12 | 49.1 | .972 | − | 47 | 38.6 | .8236 | − |
| <20 mm | 87 | 45.4 | 12 | 43 | 75 | 45.4 | |||||||
| Tumor location | Central | 13 | 49.4 | .32 | − | 4 | 33.3 | .4061 | − | 9 | 40.6 | .1224 | − |
| Periferal | 137 | 43.5 | 24 | 61.9 | 113 | 77.8 | |||||||
| Diagnosis history | Initial | 36 | 62 | .0194 | .0153 | 13 | 72.9 | .0396 | − | 23 | 56.6 | .1365 | − |
| Recurrence | 114 | 38.7 | 15 | 28.4 | 99 | 39.6 | |||||||
| Previous treatment (surgery) | Yes | 61 | 37.7 | .0891 | − | 9 | 31.1 | .0773 | − | 52 | 38.8 | .256 | − |
| No | 89 | 48.9 | 19 | 59 | 70 | 46.1 | |||||||
| Previous treatment (RFA/PEI) | Yes | 41 | 37.5 | .1796 | − | 5 | 33.3 | .2962 | − | 35 | 38.1 | .2956 | − |
| No | 109 | 46.6 | 23 | 54.4 | 87 | 44.6 | |||||||
| Adverse effects | <Grade 3 | 122 | 46.4 | .6421 | − | 23 | 48.8 | .43 | − | 99 | 45.6 | .3302 | − |
| ≧Grade 3 | 28 | 34.8 | 5 | 53.3 | 23 | 30.4 | |||||||
| Combination with TACE | Yes | 28 | 49 | .1889 | − | ||||||||
| No | 122 | 42.9 | |||||||||||
Abbreviations: BCLC; Barcelona Clinic Liver Cancer; CTP; Child-Turcotte-Pugh; HCV; hepatitis C virus; MVA, multivariate analyses; OS, overall survival; PFS, progression-free survival; RFA/PEI; radiofrequency ablation/percutaneous ethanol injection; SBRT, stereotactic body radiotherapy; TACE, transcatheter arterial chemoembolization; UVA, univariate analyses.
Table 7.
Prognostic Factors of 2-Year Local Progression-Free Survival; Univariate Analysis and Multivariate Analysis.
| Prognostic Factor | All Patients (n = 150) | SBRT Alone (n = 28) | SBRT + TACE (n = 122) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | 2-Year OS (%) | UVA, P Value | MVA, P Value | Number | 2-Year OS (%) | UVA, P Value | MVA, P Value | Number | 2-Year OS (%) | UVA, P Value | MVA, P Value | ||
| Age, years | ≧75 | 73 | 79.1 | .3252 | − | 16 | 71.4 | .4439 | − | 57 | 79.6 | .4054 | − |
| <75 | 77 | 79.8 | 12 | 71.4 | 65 | 80.9 | |||||||
| Gender | Male | 99 | 77.4 | .6955 | − | 17 | 55.6 | .0762 | − | 82 | 80.3 | .2481 | − |
| Female | 51 | 83.5 | 11 | 100 | 40 | 80.9 | |||||||
| Performance status | 0 | 128 | 78.4 | .6636 | − | 21 | 66.7 | .7207 | − | 107 | 79.8 | .5429 | − |
| 1 or 2 | 22 | 87.8 | 7 | 100 | 15 | 84.6 | |||||||
| CTP class | A | 127 | 80.7 | .4293 | − | 23 | 66.7 | .3522 | − | 104 | 82.5 | .2362 | − |
| B | 23 | 72 | 5 | 100 | 18 | 68 | |||||||
| Viral infection | HCV | 109 | 80.3 | .472 | − | 18 | 75 | .9252 | − | 91 | 80.5 | .4731 | − |
| Non-HCV | 41 | 77 | 10 | 60.7 | 31 | 79.1 | |||||||
| BCLC stage | 0 | 80 | 82.4 | .1257 | − | 17 | 85.7 | .468 | − | 63 | 84.9 | .0537 | − |
| A | 70 | 70.9 | 11 | 71.4 | 59 | 76.4 | |||||||
| T stage | T1 | 113 | 81 | .064 | − | 24 | 70 | .6682 | − | 89 | 83.7 | .0324 | .3312 |
| T2 | 37 | 78.5 | 4 | 75 | 33 | 70.4 | |||||||
| Greatest tumor dimensions | ≧20 mm | 63 | 81 | .124 | − | 12 | 71.4 | .6502 | − | 47 | 84.6 | .1121 | − |
| <20 mm | 87 | 78.5 | 12 | 83.3 | 75 | 77.9 | |||||||
| Tumor location | Central | 13 | 77.9 | .915 | − | 4 | 72.7 | .9167 | − | 9 | 85.7 | .7481 | − |
| Periferal | 137 | 79.5 | 24 | 66.7 | 113 | 80.1 | |||||||
| Diagnosis history | Initial | 36 | 81.5 | .5717 | − | 13 | 85.7 | .1775 | − | 23 | 79.6 | .9232 | − |
| Recurrence | 114 | 78.9 | 15 | 57.1 | 99 | 80.5 | |||||||
| Previous treatment (surgery) | Yes | 61 | 79.2 | .6117 | − | 9 | 50 | .2259 | − | 52 | 81.5 | .9263 | − |
| No | 89 | 79.6 | 19 | 80 | 70 | 79.3 | |||||||
| Previous treatment (RFA/PEI) | Yes | 41 | 81.6 | .5635 | − | 5 | 66.7 | .6748 | − | 35 | 83.4 | .6284 | − |
| No | 109 | 78.7 | 23 | 72.7 | 87 | 79.3 | |||||||
| Adverse effects | <Grade 3 | 122 | 81.3 | .0766 | − | 23 | 70 | .42 | − | 99 | 82.5 | .0277 | .1012 |
| ≧Grade 3 | 28 | 72.5 | 5 | 75 | 23 | 71.7 | |||||||
| Combination with TACE | Yes | 28 | 71.4 | .9661 | − | ||||||||
| No | 122 | 80.8 | |||||||||||
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; CTP, Child-Turcotte-Pugh; HCV, hepatitis C virus; LPFS, local progression-free survival; MVA, multivariate analyses; OS, overall survival; RFA/PEI; radiofrequency ablation/percutaneous ethanol injection; SBRT, stereotactic body radiotherapy; TACE, transcatheter arterial chemoembolization; UVA, univariate analyses.
Discussion
This retrospective study demonstrated that there were no significant differences in treatment results, including OS, PFS, LPFS, LC, and adverse effects, between the groups. In addition, the combination with TACE was not a significant prognostic factor for OS, PFS, and LPFS. However, we lack strong evidence based on prospective randomized studies regarding the superiority of the combination with TACE + SBRT, and the role of TACE combined with local therapies such as SBRT or RFA remains unclear.
Several authors evaluated SBRT with or without TACE in patients with HCC. Sapir et al compared SBRT with TACE using an inverse probability of treatment weighting technique to adjust for potential treatment assignment imbalances in 209 patients with HCC (TACE, n = 84; SBRT, n = 125).13 The 2-year LC was significantly better in the SBRT-alone group (91%) than that in the TACE-alone group (23%; hazard ratio 66.5, P <.001). This finding was similar to our results in the SBRT-alone group (95.4%). There was no difference in OS between patients treated with TACE or SBRT (2-year OS; 54.9% and 34.9%, respectively). The authors concluded that SBRT is a safe alternative to TACE for 1 to 2 tumors and provides better LC, with no observed difference in OS. On the other hand, Takayasu et al reported excellent 1- and 3-year OS of 93% and 72% among 836 patients with T1N0M0 and 90% and 60% of 2070 patients with T2N0M0 who underwent TACE alone as an initial treatment, respectively.14 However, this study may have been influenced by selection bias because these patients were selected from the following specific criteria applied to 60 773 patients who underwent TACE from 2000 to 2005 in Japan.
Kang et al reported an excellent LC (2-year local control, 94.6%) with SBRT for inoperable HCC as a local salvage treatment after incomplete TACE, concluding that SBRT + TACE was a promising treatment.7 This finding was similar to our results in the SBRT + TACE group (98.5%). Huo and Eslick reported the results of a meta-analysis of TACE with radiotherapy including SBRT versus TACE alone.15 The survival benefit of TACE + radiotherapy progressively increased for 5-year survival (odds ratio, 3.98 [95% CI, 1.86-8.51]). They concluded that TACE + radiotherapy including SBRT was more therapeutically beneficial compared to TACE alone for the treatment of HCC and should be recommended for suitable patients with unresectable HCC. Su et al compared long-term survival of SBRT alone (n = 50) with that of SBRT + transcatheter arterial embolization (TAE)/TACE (n = 77) in patients with large HCC (>5 cm, median tumor size, 8.5 cm).16 The 5-year OS was significantly higher in the SBRT + TAE/TACE group (46.9%) than that in the SBRT alone group (32.9%; P = .049). The PFS and LPFS did not differ significantly between the 2 groups. The authors suggested that SBRT combined with TAE/TACE may be an effective complementary treatment approach for HCC >5 cm.
Collectively, the results of these studies suggest the potential advantage of SBRT + TACE compared to SBRT or TACE alone. Stereotactic body radiation therapy combined with TACE has several potential theoretical advantages such as tumor shrinkage, the use of remaining lipiodol as a target for image-guided radiotherapy, and enhanced sensitivity to irradiation.7,17 In addition, Kawahara et al estimated the dose enhancement in lipiodol’s proximity using Monte Carlo calculation, which indicated a larger and more accurate dose increase in lipiodol compared to the algorithms used in commercially available treatment planning systems.18 They concluded that the observed dose enhancement in the tumor area could be clinically significant. The embolization using TACE has a disadvantage which prevents a sufficient supply with oxygen and nutrition. However, the retention of chemotherapeutic agents and lipiodol from embolization could enhance radiation sensitivity and increasing dose around the tumor area during SBRT. There were theoretical advantages over disadvantages of embolization as several positive reports suggested. In contrast, Takeda et al reported the results of phase II study of SBRT with or without TACE for HCC.9 They used TACE before SBRT in 58 of 90 patients and observed no significant difference in 3-year OS and cause-specific survival (P = .58 and .44, respectively). Our analysis was similar to these findings which did not observe a superiority for the combination with TACE. One reason for this finding might be a smaller tumor size (median <4 cm in Takeda study vs almost 2 cm in our study).
We discussed the comparison of TACE alone and SBRT with or without TACE as mentioned above. Selective internal radiotherapy (SIRT) with yttrium-90 microspheres is also effective treatment option for patients who were ineligible for resection or ablation therapies. According to NCCN guidelines, SIRT is recommended one of the locoregional therapies,19 but it has not been common because it is not covered by Japanese national insurance. Considering these results, SBRT alone might be sufficient for patients with small HCC who are ineligible for resection or ablation therapies. Further prospective randomized trials comparing SBRT alone and SBRT + TACE are warranted.
The present study has several limitations, including its retrospective nature and relatively short follow-up periods. Moreover, although patient characteristics were well balanced between the groups in many aspects due to the same eligibility criteria, characteristics such as naive patients and follow-up periods favored the SBRT-alone group, while age relatively favored the SBRT + TACE group. Most of our patients previously underwent these therapies which could have influenced the SBRT treatment results.
Conclusions
There were no significant differences in the survival and adverse effects in patients with small HCCs who underwent SBRT with or without TACE. Stereotactic body radiotherapy alone could be a good treatment option for patients with small HCC who are ineligible for resection or ablation therapies.
Abbreviations
- 3D-CRT
3-dimensional conformal radiation therapy
- CI
confidence interval
- CT
computed tomography
- D95%
the dose covering 95% of the PTV
- GTV
gross tumor volume
- HCC
hepatocellular carcinoma
- mRECIST
modified Response Evaluation Criteria in Solid Tumors
- LC
local control rates
- LPFS
local progression-free survival
- MRI
magnetic resonance imaging
- MVA
multivariate analyses
- NCCN
National Comprehensive Cancer Network
- OS
overall survival
- PFS
progression-free survival
- PS
performance status
- PTV
planning target volume
- RFA
radiofrequency ablation
- SBRT
stereotactic body radiation therapy
- SIRT
selective internal radiotherapy
- TACE
transarterial chemoembolization
- UVA
univariate analyses
- V10-30 Gy
percentages of uninvolved liver volume exceeding 10 Gy, 20 Gy, and 30 Gy
- VMAT
volumetric modulated arc therapy
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant no. 17K10478).
ORCID iD: Tomoki Kimura, MD, PhD  http://orcid.org/0000-0002-7779-0536
http://orcid.org/0000-0002-7779-0536
References
- 1. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Hepatobiliary Cancers 2017 ver. 4. [internet] Fort Washington, PA: National Comprehensive Cancer Network; ®; http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed January 16, 2018. [Google Scholar]
- 2. Bruix J, Reig M, Sherman M. Reviews in basic and clinical gastroenterology and hepatology. Gastroenterology. 2016;150(4):835–853. [DOI] [PubMed] [Google Scholar]
- 3. Miyayama S, Matsui O, Yamashiro M, et al. Ultraselective transcatheter arterial chemoembolization with a 2-f tip microcatheter for small hepatocellular carcinomas: relationship between local tumor reccurence and visualization of portal vein with iodized oil. J Vasc Interv Radiol. 2007;18(3):365–376. [DOI] [PubMed] [Google Scholar]
- 4. Bujold A, Massey CA, Kim JJ, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31(13):1631–1639. doi:10.1200/JCO.2012.44.1659 [DOI] [PubMed] [Google Scholar]
- 5. Andolino DL, Johnson CS, Maluccio M, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81(4):e447–e453. [DOI] [PubMed] [Google Scholar]
- 6. Kwon JH, Bae SH, Kim JY, et al. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer. 2010;10:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kang JK, Kim MS, Cho CK, et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer. 2012;118(21):5424–5431. [DOI] [PubMed] [Google Scholar]
- 8. Sanuki N, Takeda A, Oku Y, et al. Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol. 2014;53(3):399–404. [DOI] [PubMed] [Google Scholar]
- 9. Takeda A, Sanuki N, Tsurugai Y, et al. Phase 2 study of stereotactic body radiotherapy and optional transarterial chemoembolization for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer. 2016;122(13):2041–2049. [DOI] [PubMed] [Google Scholar]
- 10. Kubo M, Kimura T, Aikata H, et al. Long-term outcome of stereotactic body radiotherapy for patients with small hepatocellular carcinoma [published online January 14, 2018] Hepatol Res. 2018. [DOI] [PubMed] [Google Scholar]
- 11. Kimura T, Aikata H, Takahashi S, et al. Stereotactic body radiotherapy for patients with small hepatocellular carcinoma ineligible for resection or ablation therapies. Hepatol Res. 2015;45(4):378–386. [DOI] [PubMed] [Google Scholar]
- 12. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sapir E, Tao Y, Schipper MJ, et al. Stereotactic body radiation therapy as an alternative to transarterial chemoembolization for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2018;100(1):122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takayasu K, Arii S, Kudo M, et al. Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guidelines. J Hepatol. 2012;56(4):886–892. [DOI] [PubMed] [Google Scholar]
- 15. Huo YR, Eslick GD. Transcatheter arterial chemoembolization plus radiotherapy compared with chemoembolization alone for hepatocellular carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2015;1(6):756–765. [DOI] [PubMed] [Google Scholar]
- 16. Su TS, Lu HZ, Cheng T, et al. Long-term survival analysis in combined transarterial embolization and stereotactic body radiation therapy versus stereotactic body radiation monotherapy for unresectable hepatocellular carcinoma >5 cm. BMC Cancer. 2016;16(1):834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murray LJ, Dawson LA. Advances in stereotactic body radiation therapy for hepatocellular carcinoma. Semin Radiat Oncol. 2017;27(3):247–255. [DOI] [PubMed] [Google Scholar]
- 18. Kawahara D, Ozawa S, Saito A, et al. Dosimetric impact of lipiodol in stereotactic body radiation therapy on liver after transarterial chemoembolization. Med Phys. 2017;44(1):342–348. [DOI] [PubMed] [Google Scholar]
- 19. Benson AB III, D’Angelica MI, Abbott D, et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Hepatobiliary Cancers ver. 1. 2018. Fort Washington, PA: National Comprehensive Cancer Network; ® updated 2018 Feb 14 http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed April 25, 2018. [Google Scholar]