Abstract
Background:
Treatment of metastatic castration-resistant prostate cancer (mCRPC) with docetaxel often fails due to the emergence of chemoresistance. Thus, restoring chemosensitivity to docetaxel-based therapies remains a challenge in mCRPC treatment.
Methods:
microRNA (miR)-451 expression was measured in docetaxel-treated prostate cancer cells and tumor tissues by quantitative reverse-transcription polymerase chain reaction . Cell-counting kit 8 assay was performed to determine docetaxel chemoresistance. Neural-precursor-cell-expressed developmentally downregulated protein 9 (NEDD9) was identified as a novel target of miR-451 by dual-luciferase reporter system. Chromatin immunoprecipitation and co-immunoprecipitation assay were performed to confirm that histone deacetylase 3 (HDAC3)/Sp1 (a highly evolutionarily conserved transcription factor) interacted with the Sp1 binding sites in miR-451 promoter.
Results:
miR-451 was found to be silenced in docetaxel-treated prostate cancer cells and mCRPC tissues. Low miR-451 expression was closely associated with a high Gleason score, high Eastern Cooperative Oncology Group performance status score, visceral metastasis and poor prognosis. Low expression of miR-451 was significantly correlated with short progression-free survival (PFS) and overall survival (OS) according to Kaplan–Meier analysis, and miR-451 was determined to be an independent poor prognostic factor for PFS and OS in mCRPC patients by univariate and multivariate Cox regression analyses. NEDD9 was identified as a new and functional target of miR-451. Restoration of NEDD9 partially reversed the effects of miR-451 on enhancing chemosensitivity of prostate cancer cells. HDAC3 was confirmed to be involved in silencing of miR-451 expression in prostate cancer cells.
Conclusions:
The current data revealed a new HDAC3/Sp1/miR-451/NEDD9 signaling axis that regulates the chemosensitivity of prostate cancer cells and represents a novel therapeutic target for chemosensitizing mCRPC.
Keywords: chemosensitivity, HDAC3, miR-451, NEDD9, prostate cancer
Introduction
Prostate cancer is one of the most common of solid tumors. It is the third leading cause of cancer-related deaths and was estimated to be the most frequently occurring cancer in men in 2018.1 Prostate cancer frequently occurs with an occult and asymptomatic onset, and patients with clinical symptoms usually have local invasion or distant metastasis, which accounts for more than 10% of all cancer-related deaths in men.2 Despite androgen-deprivation therapy (ADT), which is regarded as the standard initial treatment for metastatic prostate cancer, the majority of patients treated with ADT eventually progress to metastatic castration-resistant prostate cancer (mCRPC), which is a lethal form of the disease, with an expected survival time of less than 2 years.3,4
Recently, taxanes have been identified as the only class of chemotherapeutic agents that prolong overall survival (OS) in patients with advanced prostate cancer, and docetaxel-based chemotherapies have been established as the first-line treatment for patients with mCRPC.5–8 While docetaxel-based treatment results in initial tumor regression, most of these patients become noncompliant to the recommended therapies due to the development of chemoresistance. Therefore, reversing resistance to docetaxel-based therapies would be of great clinical benefit in mCRPC treatment.
Microribonucleic acids (miRNAs) are endogenous, short, single-stranded, ~22-base-long, noncoding RNAs that regulate gene expression by binding to the 3′-untranslated region (UTR) of target genes. It has been recently discovered that miRNAs coordinate multiple biological processes regulating chemoresistance, such as cell proliferation, apoptosis, autophagy, cancer stem cell self-renewal, and epithelial mesenchymal transition (EMT).9–11 In addition, multiple recent efforts have collectively demonstrated that aberrant expression of miRNAs accounts for chemoresistance and the poor prognosis of various human cancers, including mCRPC.12–15
The purpose of this study was to identify novel dysregulated miRNAs responsible for the chemoresistance of mCRPC, and we established a vital role for miRNA-451 (miR-451) in the regulation of chemoresistance in mCRPC. Furthermore, neural-precursor-cell-expressed developmentally downregulated protein 9 (NEDD9) was identified as a new and direct target of miR-451, and histone deacetylase 3 (HDAC3) was confirmed to be responsible for silencing of miR-451. Finally, we revealed a new HDAC3/Sp1 (a highly evolutionarily conserved transcription factor)/miR-451/NEDD9 regulatory network that modulates the chemosensitivity of prostate cancer cells.
Methods
Cell lines, mice and chemotherapeutic reagents
The normal prostate epithelial cell line benign prostate hyperplasia (BPH)-1 and the human prostate cancer cell lines Lymph Node Carcinoma of the Prostate (LNCaP), prostatic carcinoma (PC) 3 and DU145 a hormone-insensitive prostate cell line derived from a central nervous system metastasis were obtained from the Shanghai Institute of Cell Biology (Shanghai, China). The cells were cultured in RPMI-1640 medium with 20% fetal bovine serum and penicillin/streptomycin at 37°C in a humidified atmosphere with 5% CO2.
All BALB/c athymic nude mice [male, (specific-pathogen free, SPF), 4–6 weeks] were provided by the department of Comparative Medicine of Nanjing General Hospital of Nanjing Military Command (Nanjing University, China) and maintained in laminar flow cabinets under SPF conditions. All mouse experiments and procedures were conducted with the approval of the Institutional Committee for Animal Research of Jinling Hospital (Nanjing University, China).
Docetaxel and HDAC inhibitor (Trichostatin A, TSA) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Docetaxel and TSA stock solution was prepared with dimethyl sulfoxide and diluted with phosphate-buffered saline to the required concentrations, in accordance with each experiment.
Patients
All patients with mCRPC treated with docetaxel (75 mg/m2, every 3 weeks) with 5 mg prednisone twice daily were reviewed between January 2009 and June 2013. All patients met all of the following criteria: all patients were pathologically diagnosed with prostate adenocarcinoma; all tissues from prostate biopsy specimens were collected before docetaxel treatment; all patients had received ADT; the Eastern Cooperative Oncology Group (ECOG) performance status score of all patients was ⩽2. The tissues were stored in liquid nitrogen. The tissue specimen acquisition was approved by the Review Board of Hospital Ethics Committee of Jinling Hospital (Nanjing University, China), and written informed consent was obtained from all patients before specimen collection.
To evaluate the chemotherapeutic response, the serum prostate-specific antigen (PSA) levels were measured after each chemotherapy, and a radiographic assessment (i.e. computed tomography, magnetic resonance imaging or bone scan) was conducted at every third cycle of chemotherapy. Then, the PSA progression-free survival (PFS), radiologic PFS, clinical PFS and the OS were documented, based on the assessment of the chemotherapeutic response. Only when all of the PSA, radiologic, and clinical evaluations did not show progression was the chemotherapeutic response defined as PFS. The PSA PFS, radiologic PFS and clinical PFS were defined as the time from the initial docetaxel therapy to PSA progression, radiologic progression and clinical progression, respectively. OS was calculated according to the time from the initial docetaxel therapy to death or last follow up.
PSA progression was defined as a 25% (at least 2 ng/ml) or greater increase in the PSA from the baseline that persisted for 3 or more weeks. Radiological progression was defined as two or more new lesions confirmed by computed tomography, magnetic resonance imaging or bone scan according to the recommendations of the Response Evaluation Criteria in Solid Tumor version 1.1. Clinical progression was defined as deterioration of cancer symptoms, demand for higher dose of analgesic or a new onset.
Microribonucleic acid microarray assay
Briefly, prostate cancer cells (LNCap and DU145) were treated with 10μg/l docetaxel for 48 h. Then, total RNA was isolated with TRIzol reagent (Takara, Tokyo, Japan), and miRNAs were obtained with a mirVana miRNA isolation kit (Ambion, Austin, TX, USA) according to the manufacturer’s instructions. The quality and quantity of the RNA samples were evaluated with 1% formaldehyde-agarose gel electrophoresis and spectrophotometry (Thermo Fisher Scientific, Massachusetts, USA). Fluorescein-labeled isolated miRNAs were then hybridized with an Affymetrix miRNA 4.0 (Affymetrix, Santa Clara, CA, USA). The fluorescence signal was measured with GeneChip Scanner™ 3000 7G (Thermo Fisher Scientific). The data were normalized and analyzed with Affymetrix GeneChip Command Console software. The miRNAs with a fold change of ⩾2 compared with controls were considered differentially expressed miRNAs.
In vitro chemosensitivity assay
Chemosensitivity assay was measured with the cell-counting kit 8 (CCK-8) (Dojindo, Kumamoto, Japan) assay in accordance with the manufacturer’s instructions. Briefly, 3000 prostate cancer cells were plated into 96-well plates 24 h after transfection. Then, cells were treated with different doses of docetaxel and cultured for 48 h. And, CCK-8 solution was added and incubated at 37°C for 4 h. Absorbance was detected at 450 nm with a microplate reader (Bio-Rad, USA).
Xenograft transplantation
Approximately 2 × 106 DU145 cells stably transfected with plasmid complementory DNA (pcDNA)/microRNA-negative control (miR-NC), pcDNA/miR-451, control or short hairpin (sh)NEDD9#1 were suspended in 100 μl of phosphate-buffered saline and injected into the flanks of nude mice. Tumor volume was assessed with the equation V = a × b2 × 0.5 (mm3; a = largest diameter, b = perpendicular diameter). Once the tumor volume reached approximately 50 mm3, 1.0 mg/kg docetaxel (one dose per week, with four doses in total) was administered by intraperitoneal injection.
After 30 days, some of the mice were sacrificed for subsequent studies, and the other mice were maintained for further OS studies. Ki67 [1:500, monoclonal rabbit immunoglobulin G (IgG)], and proliferating cell nuclear antigen (PCNA) (1 μg/ml, monoclonal mouse IgG) staining were performed according to the manufacturer’s instructions.
Construction of plasmids and cell transfection
pcDNA/miR-451 and pcDNA/miR-NC were constructed as described in our previous study.16 The primer pairs used for sh-control, shNEDD9#1, shNEDD9#2, shNEDD9#3 and pcDNA-NEDD9 are presented in Supplementary Table 1. The siRNA-HDACs, siRNA-NC, and siRNA-Sp1 were purchased from GenePharma (Shanghai, China). All vectors were confirmed by DNA sequencing.
A luciferase reporter containing the wild-type 3′-UTR of NEDD9 (pLUC/NEDD9/3′-UTR-wt) and a mutant reporter (pluc/NEDD9/3′-UTR-mut) were constructed by Sagene Technology (Guangzhou, China). The human miR-451 promoter construct (−2370/0 miR-451) was generated from genomic DNA according to the sequence (−2370/0) of the 5′-flanking region of the human miR-451 gene. Then, the region was amplified with polymerase chain reaction (PCR) and cloned into the pGL3-basic vector (Promega, San Luis, CA, USA) at KpnI and HindIII sites. The 5′-flanking deletion promoter constructs (−826/0 miR-451, −416/0 miR-451, −278/0 miR-451, −210/0 miR-451) were generated with the −2370/0 miR-451 construct as a template and cloned similarly. Mutation of Sp1-binding site-mutant constructs was generated with a QuikChange XL site-directed mutagenesis kit from Stratagene (La Jolla, CA, USA). All primer sequences are presented in Supplementary Table 2. The vectors were confirmed by DNA sequencing.
Cells were transfected with Turbofect Transfection Reagent (Thermo Fisher Scientific) or si-RNA Mate (GenePharma, Shanghai, China) in accordance with the manufacturer’s instructions.
Ribonucleic acid extraction, real-time quantitative reverse-transcription polymerase chain reaction, flow cytometric analysis, dual luciferase reporter assay and western blotting
RNA extraction, quantitative reverse-transcription polymerase chain reaction (qRT-PCR), flow cytometric analysis, dual luciferase reporter assay and western blotting were performed as previously described.17 The primer pairs of miR-451, U6 RNA, NEDD9 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as previously described.16,18 The primary antibodies against cleaved caspase-3, caspase-3, acetyl-histone H3, Sp1, β-actin and GAPDH were used as previously described.17 HDAC3 (1:1000, monoclonal mouse IgG, immunogen affinity purified), (1:1000, polyclonal rabbit IgG, protein A purified), and NEDD9 (1:1000, monoclonal rabbit IgG, immunogen affinity purified) were purchased from Cell Signaling Technologies (Boston, USA).
Co-immunoprecipitation assay
Co-immunoprecipitation (Co-IP) assay was conducted with a Co-IP kit (Thermo Fisher Scientific) in accordance with the manufacturer’s instructions as previously described.17 Briefly, cell lysate was precleared with the control agarose resin. Next, 75 μg of affinity-purified antibody (Sp1) was coupled into the spin columns. The cell lysate was co-immunoprecipitated. The eluted sample was evaluated with western blotting.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assay was performed with Immunoprecipitation Assay Kits (Millipore, Billerica, USA) according to our previous study.17 The ChIP primers are listed in Supplementary Table 3.
Statistical analysis
SPSS software (version 17.0) (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The data are shown as means ± standard deviation of at least three independent experiments. Multiple group comparisons of quantitative data were analyzed with one-way analysis of variance, and two-group comparisons of quantitative data were performed with Student’s t test. Chi-square test was used for category data. The cumulative survival probability curves were plotted using the Kaplan–Meier method and compared with a log-rank test. A Cox proportional hazards regression model was performed to determine prognostic factors of PFS and OS. Correlation was determined by linear regression analysis. p < 0.05 was considered statistically significant.
Results
Microribonucleic acid-451 is silenced in docetaxel-treated prostate cancer cells and tumor tissues and correlated with poor prognosis of metastatic castration-resistant prostate cancer patients
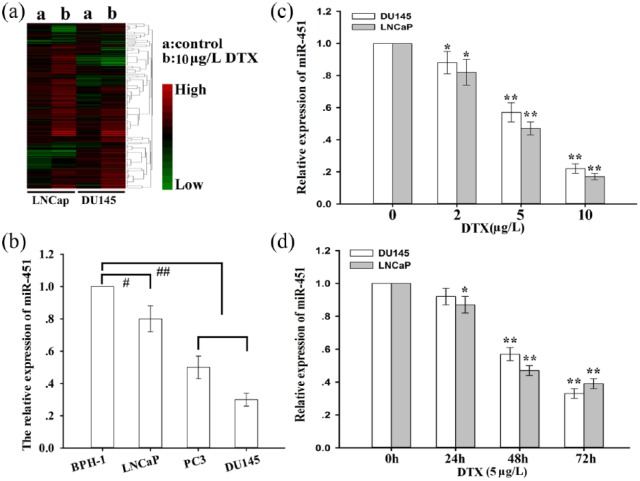
Recently, docetaxel-based chemotherapies have been established as the first-line treatment for patients with mCRPC. While the docetaxel-based treatment leads to initial tumor regression, most of these patients become noncompliant due to the emergence of chemoresistance. To understand the potential mechanisms of docetaxel chemoresistance, the miRNA expression profiles of prostate cancer cells (LNCap and DU145) treated with (or without) docetaxel were analyzed with a microRNA microarray assay [Figure 1(a)]. In total, 17 and 12 miRNAs were found to be upregulated and downregulated, respectively, in LNCap cells treated with docetaxel compared with those without docetaxel, while 12 and 14 miRNAs were upregulated and downregulated, respectively, in DU145 cells treated with docetaxel compared with those without docetaxel (fold change > 2; Supplementary Table 4). Among these miRNAs, miR-451 was identified as the significantly downregulated miRNA in docetaxel-treated LNCap and DU145 cells. Then, the expression of miR-451 in normal prostate epithelial cells (BPH-1) and several prostate cancer cells was measured, and the data indicated that miR-451 was significantly silenced in prostate cancer cells compared with that in BPH-1 cells [Figure 1(b)]. Moreover, the effects of docetaxel on miR-451 expression in prostate cancer cells were detected, and the data showed that docetaxel downregulated miR-451 expression in a dose- and time-dependent manner [Figure 1(c) and 1(d)].
Figure 1.
Microribonucleic acid-451 is significantly downregulated in docetaxel-treated prostate cancer cells.
(a) A heatmap of differentially expressed miRNAs in DTX-treated prostate cancer cells (LNCap and DU145) compared with normal control, which indicated that miR-451 was significantly downregulated in DTX-treated LNCap cells; blue: low expression, red: high expression; (b) the relative expression of miR-451 in BPH-1 and prostate cancer cells (LNCap, PC3 and DU145); miR-451 expression was determined with qRT-PCR; U6 was used as an internal control; (c) miR-451 expression was determined by qRT-PCR in prostate cancer cells treated with different concentrations of DTX for 48 h; U6 was used as an internal control; (d) qRT-PCR detection of miR-451 expression in prostate cancer cells treated with DTX (5μg/l) at different time points; U6 was used as an internal control; the data are representative of at least three independent experiments (means ± standard deviation), #p < 0.05; ##p < 0.01; *p < 0.05; **p < 0.01 compared with the control group.
DTX, docetaxel; miR-451, microribonucleic acid-451; qRT-PCR, quantitative reverse-transcription polymerase chain reaction; BPH-1, prostate epithelial cells.
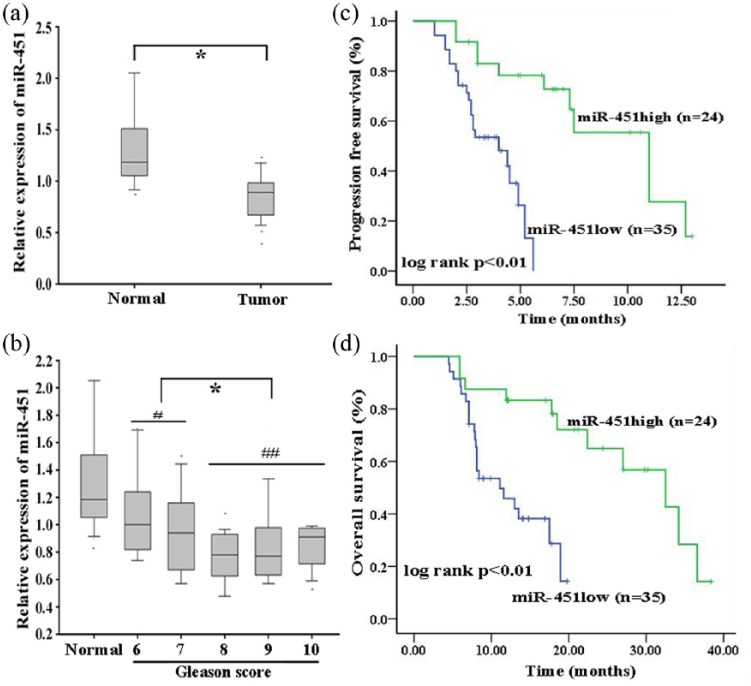
To further understand the clinical significance of miR-451 in docetaxel chemoresistance, qRT-PCR detection of miR-451 expression was performed in normal prostate and tumor tissues [Figure 2(a)]. The data revealed that miR-451 was significantly downregulated in tumor tissues compared with normal prostate tissues [Figure 2(a)]. Furthermore, low miR-451 expression was closely associated with high Gleason score, high ECOG performance status score, visceral metastasis and poor prognosis [Figure 2(b); Supplementary Table 5]. A Kaplan–Meier analysis indicated that low expression of miR-451 was significantly correlated with shorter PFS and OS [Figure 2(c) and 2(d)]. In addition, low expression of miR-451 was identified as an independent poor prognostic factor for PFS and OS in mCRPC patients by univariate and multivariate Cox regression analysis (Supplementary Tables 6 and 7).
Figure 2.
Microribonucleic acid-451 is silenced in tumor tissues and correlated with poor prognosis of patients with metastatic castration-resistant prostate cancer.
(a) miR-451 expression detected by qRT-PCR in normal prostate tissues (n = 18) and tumor tissues (n = 59); the cut-off value (0.895) of tumor tissues was determined by an ROC curve; (b) miR-451 expression levels in normal prostate tissues and prostate cancer tissues of varying Gleason scores; (c) Kaplan–Meier analysis of the correlation between miR-451 expression and PFS of mCRPC patients; (d) Kaplan–Meier analysis of the association between miR-451 expression and OS of mCRPC patients.
*p < 0.05; #p < 0.05, ##p < 0.01.
mCRPC, metastatic castration-resistant prostate cancer patients; miR-451, microribonucleic acid-451; OS, overall survival; PFS, progression-free survival; qRT-PCR, quantitative reverse-transcription polymerase chain reaction; ROC, receiver operating characteristic.
Restoration of microribonucleic acid-451 increases chemosensitivity of prostate cancer cells to docetaxel both in vitro and in vivo
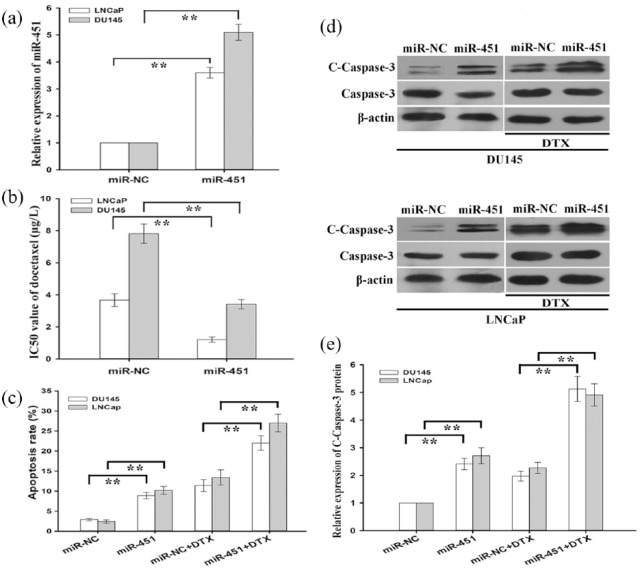
To further explore the functional role of miR-451 in promoting chemoresistance of prostate cancer cells (LNCap and DU145) to docetaxel, prostate cancer cells were transfected with pcDNA/miR-NC or pcDNA/miR-451 plasmid. miR-451 expression was significantly upregulated in LNCap and DU145 cells transfected with pcDNA/miR-451 compared with those transfected with pcDNA/miR-NC [Figure 3(a)]. Then, the half maximal inhibitory concentration (IC50) values for docetaxel in pcDNA/miR-NC (or pcDNA/miR-451)-transfected LNCap and DU145 cells were determined with a CCK-8 assay. The data showed that the IC50 values of docetaxel in pcDNA/miR-451-transfected LNCap and DU145 cells (1.21 ± 0.17 μg/l and 3.41 ± 0.29 μg/l) were significantly lower than those in pcDNA/miR-NC-transfected LNCap and DU145 cells (3.67 ± 0.40 μg/l and 7.82 ± 0.61 μg/l) [Figure 3(b)]. Then, a flow cytometric analysis was performed to detect early apoptosis, and the data indicated that upregulation of miR-451 increased early apoptosis in pcDNA/miR-451-transfected LNCap and DU145 cells compared with that in pcDNA/miR-NC-transfected LNCap and DU145 cells [Figure 3(c)]. Western blotting detection of cleaved caspase-3 was conducted in pcDNA/miR-NC (or pcDNA/miR-451)-transfected LNCap and DU145 cells, and the data indicated that the cleaved-caspase-3 protein level was significantly upregulated in pcDNA/miR-451-transfected LNCap and DU145 cells compared with that in pcDNA/miR-NC-transfected LNCap and DU145 cells [Figure 3(d) and 3(e)].
Figure 3.
Microribonucleic acid-451 sensitizes prostate cancer cells to DTX in vitro.
(a) qRT-PCR detection of miR-451 expression in prostate cancer cells transfected with pcDNA/miR-NC (miR-NC) or pcDNA/miR-451 (miR-451); U6 was used as an internal control; (b) a CCK-8 assay was performed to detect the IC50 values of DTX in pcDNA/miR-NC (or pcDNA/miR-451)-transfected prostate cancer cells; (c) flow cytometric analysis of early apoptosis rate of pcDNA/miR-NC (or pcDNA/miR-451)-transfected prostate cancer cells treated without (or with) DTX (5μg/l); (d) and (e) western blotting was used to detect cleaved caspase-3 (C-caspase-3) and caspase-3 in pcDNA/miR-NC (or pcDNA/miR-451)-transfected prostate cancer cells treated without (or with) DTX (5μg/l); β-actin was used as an internal control; the data represent the average of three independent experiments (means ± standard deviation), **p < 0.01.
DTX, docetaxel; miR-451, microribonucleic acid-451; pcDNA, plasmid complementory DNA; miR-NC, microRNA-negative control.
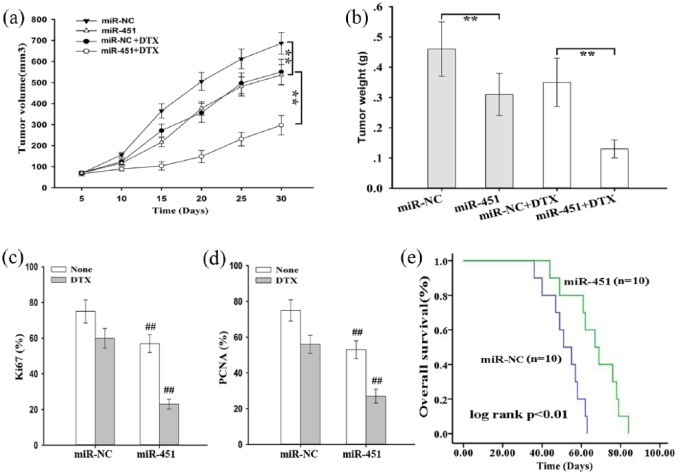
Next, we investigated the effects of miR-451 on chemosensitivity of prostate cancer cells to docetaxel in vivo. First, 2 × 106 DU145 cells stably transfected with pcDNA/miR-NC or pcDNA/miR-451 were subcutaneously inoculated into nude mice. Once palpable tumors formed, docetaxel was administered, and tumor volume was measured. As shown in Figure 4(a) and 4(b) tumor volume and weight in the pcDNA/miR-451 group were significantly lower than those in the pcDNA/miR-NC group. Then, Ki67 and PCNA expression were examined via immunohistochemistry staining, and the cells with positive expression were counted. The data indicated that upregulation of miR-451 significantly decreased the rate of positive Ki67 and PCNA staining [Figure 4(c) and 4(d)]. Last, a Kaplan–Meier analysis was conducted to measure OS, and the data indicated that high expression of miR-451 was significantly associated with longer OS of mice [Figure 4(e)].
Figure 4.
Microribonucleic acid-451 enhances in vivo chemosensitivity of prostate cancer cells to docetaxel.
(a) Tumor growth was measured by tumor volume in nude mice subcutaneously transplanted with pcDNA/miR-NC (or pcDNA/miR-451)-transfected DU145 cells combined with DTX treatment; the data are displayed as the means ± standard deviation, **p < 0.01; (b) tumor weight of xenograft tumors at the end of the treatment period; the data are presented as the means ± standard deviation, **p < 0.01; (c) and (d) the positive rate of Ki67 and PCNA expression in tumors developed from pcDNA/miR-NC (or pcDNA/miR-451)-transfected DU145 cells combined with DTX treatment; the data are presented as the means ± standard deviation, **p < 0.01; (e) Kaplan–Meier analysis of OS of nude mice subcutaneously transplanted with pcDNA/miR-NC (or pcDNA/miR-451)-transfected DU145 cells combined with DTX treatment.
pcDNA, ; DTX, docetaxel; miR-NC, microribonucleic acid-451; miR-451, ; PCNA, proliferating cell nuclear antigen; OS, overall survival.
Neural-precursor-cell-expressed developmentally downregulated protein 9 is a new and direct target of microribonucleic acid-451
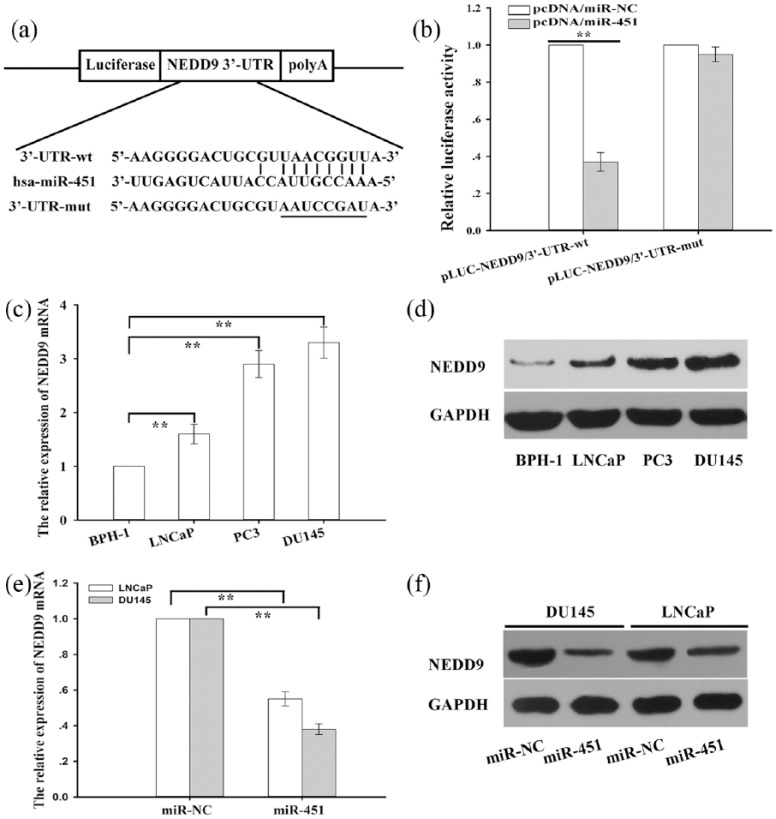
To unravel the underlying mechanisms by which miR-451 participates in regulation of chemosensitivity in prostate cells, candidate target genes were searched with two open access software systems (TargetScan http://www.targetscan.org/vert_72/ and microRNA.org http://www.microrna.org/ This was used to predict candidate target genes of miR-451), and the results indicated that NEDD9 might be a putative target of miR-451. NEDD9, a scaffolding protein, promotes tumor growth and EMT and is correlated with poor prognosis in lung cancer, hepatocellular carcinoma, and prostate cancer. Thus, NEDD9 might play an important role in regulation of chemoresistance. Therefore, we then focused on proving this hypothesis. First, as shown in Figure 5(a) a schematic representation of the 3′-UTR of NEDD9 is depicted with the complementary binding site for miR-451. Then, a NEDD9 3′-UTR fragment containing the wild (or mutant)-type miR-451-binding sequence was cloned into downstream of the luciferase reporter gene in a pLUC-luc vector (pLUC-NEDD9/3′-UTR-wt and pLUC-NEDD9/3′-UTR-mut). Next, pcDNA/miR-NC or pcDNA/miR-451 was cotransfected into DU145 cells transfected with wild-type (or mutant) 3′-UTR reporter plasmids, and the data indicated that luciferase activity in pcDNA/miR-451-transfected DU145 cells cotransfected with the wild-type 3′-UTR reporter plasmid was significantly inhibited compared with that in the pcDNA/miR-NC group [Figure 5(b)]. However, there was no difference in the mut-type 3′-UTR reporter plasmid-transfected DU145 cells [Figure 5(b)]. Then, qRT-PCR and western blotting detection of NEDD9 messenger ribonucleic acid (mRNA) and protein expression were performed in a normal prostate epithelial cell line (BPH-1) and prostate cancer cells, and the results indicated that NEDD9 mRNA and protein expression were significantly upregulated in prostate cancer cells compared with that in BPH-1 cells [Figure 5(c) and 5(d)]. Finally, qRT-PCR and western blotting detection of NEDD9 mRNA and protein expression were performed in prostate cancer cells transfected with pcDNA/miR-NC (miR-NC) or pcDNA/miR-451 (miR-451), and the data indicated that NEDD9 mRNA and protein expression were significantly suppressed in prostate cancer cells transfected with pcDNA/miR-451compared with those transfected with pcDNA/miR-NC [Figure 5(e) and 5(f)].
Figure 5.
Neural-precursor-cell-expressed developmentally downregulated protein 9 is identified as a direct target of microribonucleic acid-451 .
(a) Schematic representation of the 3′-UTR of NEDD9 with a complementary binding site for miR-451; a NEDD9 3′-UTR fragment containing the wild (or mutant)-type miR-451-binding sequence was cloned the downstream of the luciferase reporter gene in a pLUC-luc vector; (b) determination of relative luciferase activity after co-transfection with pcDNA/miR-NC or pcDNA/miR-451 into DU145 cells transfected with wild-type (or mutant) 3′-UTR reporter plasmids; the histogram represents the means ± standard deviation of the normalized luciferase activity from three independent experiments performed in triplicate; (c) the relative expression of NEDD9 mRNA in BPH-1 and prostate cancer cells (LNCap, PC3 and DU145); NEDD9 mRNA expression was determined with qRT-PCR; GAPDH was used as an internal control; the data are presented as the means ± standard deviation of three independent experiments performed in triplicate; (d) western blotting was used to detect NEDD9 protein expression in BPH-1 and prostate cancer cells (LNCap, PC3 and DU145); (e) qRT-PCR detection of NEDD9 mRNA expression in prostate cancer cells transfected with pcDNA/miR-NC (miR-NC) or pcDNA/miR-451 (miR-451); GAPDH mRNA was used as an internal control; the data are presented as the means ± standard deviation of three independent experiments performed in triplicate; (f) western blotting analysis of the NEDD9 protein level in prostate cancer cells transfected with pcDNA/miR-NC (miR-NC) or pcDNA/miR-451 (miR-451); **p < 0.01.
hsa, homo sapiens; NEDD9, neural-precursor-cell-expressed developmentally downregulated protein 9; mRNA, messenger ribonucleic acid; BPH-1, normal prostate epithelial cell line; qRT-PCR, quantitative reverse-transcription polymerase chain reaction; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; pcDNA, ; miR-NC, ; miR-451, microribonucleic acid-451; polyA, polyadenylic acid.
Inhibition of neural-precursor-cell-expressed developmentally downregulated protein 9 phenocopies the biological functions of microribonucleic acid-451 in prostate cancer cells
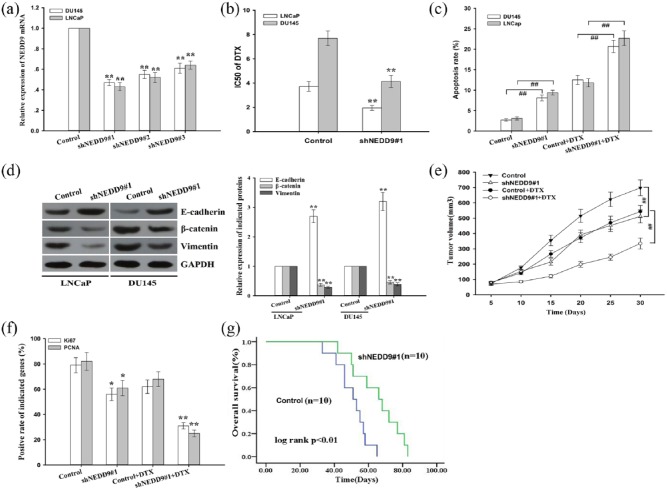
To further define whether NEDD9 is a functional target of miR-451 in prostate cancer cells, prostate cancer cells were transfected with shRNA(sh)NEDD9#1, shNEDD9#2, shNEDD9#3 or control, and the qRT-PCR data indicated that shNEDD9#1 was the most efficient shRNA against NEDD9 [Figure 6(a)]. Next, a CCK-8 assay was conducted to detect the IC50 values for docetaxel in shNEDD9#1 (or control)-transfected LNCap and DU145 cells. The data showed that the IC50 value for docetaxel in shNEDD9#1-transfected LNCap and DU145 cells was significantly lower than that in the control group [Figure 6(b)]. Then, a flow cytometric analysis was performed to detect early apoptosis, and the data indicated that inhibition of NEDD9 significantly increased early apoptosis in LNCap and DU145 cells [Figure 6(c)]. Furthermore, NEDD9 suppression reversed EMT to mesenchymal epithelial transition (MET) in prostate cancer cells [Figure 6(d)].
Figure 6.
Knockdown of neural-precursor-cell-expressed developmentally downregulated protein 9 expression enhances chemosensitivity of prostate cancer cells to docetaxel both in vitro and in vivo.
(a) qRT-PCR detection of NEDD9 in prostate cancer cells transfected with shNEDD9#1, shNEDD9#2, shNEDD9#3 or control; the data are presented as the means ± standard deviation of three independent experiments performed in triplicate; GAPDH was used as an internal control; (b) CCK-8 assay analysis of IC50 values of DTX in shNEDD9#1 (or control)-transfected prostate cancer cells; the data are displayed as the means ± standard deviation of three independent experiments performed in triplicate; (c) a flow cytometric analysis was performed to detect the early apoptosis rate of shNEDD9#1 (or control)-transfected prostate cancer cells treated without (or with) DTX (5 μg/l). The data are presented as the means ± standard deviation of three independent experiments performed in triplicate; (d) western blotting was performed to detect the protein expression of epithelial marker (E-cadherin) and mesenchymal markers (β-catenin and vimentin) in prostate cancer cells treated with control or shNEDD9#1 vectors. GAPDH was used as an internal control; (e) tumor growth was measured by tumor volume in nude mice subcutaneously transplanted with shNEDD9#1 (or control)-transfected DU145 cells combined with (or without) DTX treatment; the data are displayed as the means ± standard deviation, **p < 0.01; (f) the positive rate of Ki67 and PCNA expression in tumors developed from shNEDD9#1 (or control)-transfected DU145 cells combined with (or without) DTX treatment; the data are presented as the means ± standard deviation; (g) Kaplan–Meier analysis of the OS of nude mice subcutaneously transplanted with shNEDD9#1 (or control)-transfected DU145 cells combined with DTX treatment; **p < 0.01, compared with control group, ##p < 0.01.
DTX, docetaxel; mRNA, messenger ribonucleic acid; qRT-PCR, quantitative reverse-transcription polymerase chain reaction; NEDD9, neural-precursor-cell-expressed developmentally downregulated protein 9; sh, short hairpin; GAPDH, ; PCNA, proliferating cell nuclear antigen; CCK-8, cell-counting kit 8.
Then, we focused on exploring the effects of NEDD9 on chemoresistance of prostate cancer cells to docetaxel in vivo. Briefly, 2 × 106 DU145 cells stably transfected with control or shNEDD9#1 were subcutaneously inoculated into nude mice. When palpable tumors had formed, docetaxel was administered, and tumor volume was measured. Tumor volume in the shNEDD9#1 group increased more slowly in comparison with that in the control group [Figure 6(e)]. Then, Ki67 and PCNA expression were examined with immunohistochemistry staining, and the results indicated that suppression of NEDD9 significantly decreased the rate of Ki67- and PCNA-positive cells [Figure 6(f)]. Finally, a Kaplan–Meier analysis was performed to detect OS, which indicated that NEDD9 inhibition was significantly associated with longer OS of mice [Figure 6(g)]. Therefore, downregulation of NEDD9 mimics the biological functions of miR-451 in prostate cancer cells.
Restoration of neural-precursor-cell-expressed developmentally downregulated protein 9 partially abrogates the effects of microribonucleic acid-451 on enhancing chemosensitivity of prostate cancer cells
To further confirm the role of NEDD9 in miR-451 signaling, pcDNA/control or pcDNA/NEDD9 was synthesized and transfected into prostate cancer cells. As shown in Supplementary Figure 1(a), pcDNA/NEDD9 significantly upregulated NEDD9 mRNA expression in prostate cancer cells transfected with pcDNA/NEDD9 in comparison with that in the control group. Intriguingly, the IC50 values of DTX in pcDNA/miR-451-transfected prostate cancer cells were partially rescued by transfection with pcDNA/NEDD9 [Supplementary Figure 1(b)]. In addition, early apoptosis and cleaved caspase-3 expression in the pcDNA/miR-451-transfected prostate cancer cells were partially reversed by transfection with pcDNA/NEDD9 [Supplementary Figure 1(c) and (d)].
Neural-precursor-cell-expressed developmentally downregulated protein 9 is inversely correlated with microribonucleic acid-451 and upregulated in metastatic castration-resistant prostate cancer tissues
To further interrogate the clinicopathological significance of NEDD9 and the correlation between NEDD9 and the miR-451 level, we first detected the NEDD9 mRNA expression in normal prostate and tumor tissues, and the data indicated that NEDD9 mRNA expression was significantly upregulated in tumor tissues compared with that in normal prostate tissues [Supplementary Figure 2(a)]. Interestingly, the Kaplan–Meier analysis indicated that high expression of NEDD9 was significantly correlated with shorter PFS and OS [Supplementary Figure 2(b) and (c)]. Additionally, high expression of NEDD9 was identified as an independent poor prognostic factor for PFS and OS in mCRPC patients by univariate and multivariate Cox regression analysis (Supplementary Tables 6 and 7). Next, a statistically significant inverse correlation was verified between NEDD9 and miR-451 by linear regression analysis [r = −0.592, p < 0.01; Supplementary Figure 2(d)].
Histone deacetylase 3 is involved in silencing of microribonucleic acid-451 expression in prostate cancer cells
HDACs have been regarded as one of the most important epigenetic mechanisms governing gene expression. To further ascertain whether HDACs are responsible for silencing of miR-451 in prostate cancer cells, HDAC inhibitor TSA was administered to prostate cancer cells. qRT-PCR indicated that treatment with TSA significantly upregulated miR-451 expression, which indicated that HDACs might be a potential epigenetic factor involved in silencing of miR-451 expression in prostate cancer cells [Supplementary Figure 3(a)]. Next, to further define the specific HDACs responsible for regulation of miR-451 expression, small interfering RNAs (siRNAs) against eleven isoforms of HDACs were transfected into prostate cancer cells, and qRT-PCR demonstrated that siRNA against HDAC3 (siRNA-HDAC3) significantly upregulated miR-451 expression [Supplementary Figure 3(b)]. Next, western blotting revealed that HDAC3 protein expression was upregulated in prostate cancer cells compared with that in BPH-1 cells [Supplementary Figure 3(c)].
HDACs are unable to bind to DNA directly due to lack of a DNA binding domain, and they interact with DNA through specific proteins. Sp1 participates in the transcriptional regulation of genes associated with chemoresistance. First, we focused on elucidating whether HDAC3 could interact with Sp1, and co-immunoprecipitation (Co-IP) data revealed that HDAC3 co-immunoprecipitated with Sp1 in vivo in prostate cancer cells [Supplementary Figure 3(d)]. Next, the results of a qRT-PCR analysis indicated that HDAC3 mediated miR-451 expression in an Sp1-dependent manner [Supplementary Figure 3(e)].
To further define HDAC3/Sp1 signaling-mediated regulation of miR-451 expression, the promoter region of the miR-451 gene was analyzed using open online databases (Consite and PROMO), and four putative complementary Sp1 binding sites were found and are depicted in the schematic of the human miR-451 promoter [Supplementary Figure 3(f)]. Then, serially truncated and site-mutated regions of the miR-451 promoter were constructed and cloned into a pGL3-basic vector [Supplementary Figure 3(f)]. Next, the above-luciferase reporter vectors were then cotransfected into DU145 cells with Renilla luciferase and siRNA-HDAC3 (or siRNA-NC) [Supplementary Figure 3(f)]. Then, the relative luciferase activity was measured with a dual-luciferase reporter assay, and the results demonstrated that the second and fourth Sp1 binding sites were responsible for HDAC3-mediated miR-451 silencing [Supplementary Figure 3(f)]. To further confirm that Sp1 interacted with the second and fourth Sp1 binding sites in vivo, a chromatin immunoprecipitation (ChIP) assay was performed in DU145 cells with antibodies directly against Sp1, and the data indicated that Sp1 bound to the second and fourth Sp1 binding sites in vivo [Supplementary Figure 3(g)]. Next, a ChIP assay further demonstrated that inhibition of HDAC3 increased the histone-H3 acetylation level of the miR-451 promoter at Sp1-binding sites [Supplementary Figure 3(h)]. Intriguingly, IC50 values indicated that HDAC3 mediated chemosensitivity in an miR-451-dependent manner [Supplementary Figure 3(i)].
In summary, the current data revealed a new HDAC3/Sp1/miR-451/NEDD9 signaling axis that regulates the chemosensitivity of prostate cancer cells (Supplementary Figure 4). HDAC3-mediated silencing of miR-451 decreases chemosensitivity of patients with metastatic castration-resistant prostate cancer by targeting NEDD9. This new signaling axis suggests novel therapeutic targets for chemosensitizing prostate cancer.
Discussion
Recently, miRNAs have been extensively studied in various types of cancers and aberrant expression of miRNAs is critically involved in the chemoresistance and progression of cancers, including mCRPC.15,19,20 Nevertheless, the underlying mechanisms contributing to docetaxel resistance in prostate cancer remain largely unknown and need to be further elucidated. To address this issue, miRNA expression profiles in prostate cancer cells treated with (or without) docetaxel were analyzed with a microRNA microarray assay and 29 miRNAs were dysregulated in prostate cancer cells treated with docetaxel compared with those without docetaxel treatment. Among these miRNAs, miR-451 was identified as the most downregulated miRNA in docetaxel-treated prostate cancer cells. Importantly, prior studies have shown that downregulation of miR-451 contributes to a malignant phenotype in several types of cancers. For instance, it has been shown that miR-451 was downregulated in colorectal cancer stem cells, and upregulation of miR-451 induced a decrease in tumorigenicity, self-renewal, and chemoresistance to irinotecan in colorectal cancer stem cells.21 Another study revealed that miR-451 was downregulated in hepatoma cell lines, and restoration of miR-451 decreased cell viability and accelerated cell apoptosis in a caspase-3-dependent manner.22 In addition, miR-451 has also been reported to be significantly downregulated in docetaxel-resistant lung adenocarcinoma cells, and restoration of miR-451 inhibited invasion and metastasis of docetaxel-resistant lung adenocarcinoma cells both in vitro and in vivo.23
In this manuscript, miR-451 was found to be downregulated in docetaxel-treated prostate cancer cells and mCRPC tissues. Furthermore, low miR-451 expression was closely associated with high Gleason score, high ECOG performance status score, visceral metastasis and poor prognosis. Low expression of miR-451 was significantly correlated with shorter PFS and OS based on a Kaplan–Meier analysis. Moreover, low expression of miR-451 was identified as an independent poor prognostic factor for PFS and OS in mCRPC patients by univariate and multivariate Cox regression analysis. Intriguingly, restoration of miR-451 increased the chemosensitivity of prostate cancer cells to docetaxel both in vitro and in vivo.
It is well known that miRNAs exert their biological effects by regulating their specific targeting genes. Accordingly, by in silico analysis with TargetScan and miRanda algorithms, NEDD9 was identified as a new target of miR-451. Overexpression of the NEDD9 scaffolding protein promotes tumor EMT and metastasis and is correlated with poor prognosis in several types of cancers, including lung cancer, hepatocellular carcinoma, and breast cancer. NEDD9 promoted lung cancer cell migration and invasion via induction of EMT and significantly inhibited metastasis of lung cancer in vivo.24 Furthermore, NEDD9 was upregulated in liver tumor models and was associated with worse prognosis in human hepatocellular carcinoma.25 Additionally, re-expression of NEDD9 was able to restore matrix metalloproteinase-14 activity and promoted invasion of breast cancer cells, while NEDD9 inhibition decreased primary tumor growth and suppressed metastasis in xenograft models of breast cancer.26 Here, NEDD9 was shown to be inversely correlated with miR-451 and significantly upregulated in prostate cancer cells and mCRPC tissues. Moreover, NEDD9 suppression phenocopied the biological functions of miR-451 and reversed EMT to MET in prostate cancer cells, which might be a potential mechanism for regulation of chemoresistance in prostate cancer cells. As expected, restoration of NEDD9 partially reversed the effects of miR-451 on enhancing chemosensitivity of prostate cancer cells.
Despite recent advancements, the mechanisms responsible for dysregulation of miRNAs remain largely unclear. Histone acetylation, modulated by HDACs that remove acetyl functional groups from lysine residues around gene promoters, represents one of the critical epigenetic mechanisms that transcriptionally regulate gene expression during tumor development.27,28 Recently, HDACs have been shown to be one of the most important epigenetic factors governing gene expression, and they regulate various pathological processes, including chemoresistance, through transcriptional regulation of genes.29–32 Accordingly, treatment with an HDAC inhibitor efficiently suppressed cell growth of prostate cancer lines through post-transcriptional upregulation of miR-320a that was correlated with prostate cancer prognosis.33 Furthermore, miR-320a inhibition significantly abrogated the growth suppression induced by HDAC inhibitor treatment.33 Intriguingly, our manuscript indicated that the HDAC inhibitor TSA significantly upregulated miR-451 expression. Moreover, HDAC3 was specifically identified as being involved in silencing of miR-451 expression in prostate cancer cells.
Due to lack of a DNA binding domain, HDAC3 is unable to bind to DNA directly and interacts with DNA through specific proteins. Sp1 is a highly evolutionarily conserved transcription factor that has been generally thought to participate in transcriptional regulation of genes related to cell proliferation, apoptosis, EMT and chemoresistance.34–37 Intriguingly, HDAC1 interacts with Sp1 within the miR-29b regulatory sequence, and HDAC1 inhibition results in higher miR-29b expression in acute myeloid leukemia.38 Moreover, Sp1 recruits HDAC4 to the miR-200a promoter, leading to miR-200a inhibition that promotes proliferation and migration of hepatocellular carcinoma cells.39 In this study, by analysis of the promoter region of miR-451 with two open online databases (Consite and PROMO) and a series of molecular biology experiments, two complementary Sp1 binding sites were identified in the miR-451 promoter. Furthermore, HDAC3 was found to interact with Sp1 in vivo, and inhibition of HDAC3 increased the histone-H3 acetylation level in the miR-451 promoter at Sp1-binding sites and decreased the IC50 value in an miR-451-dependent manner. Thus, our study revealed that Sp1 could recruit HDAC3 to the promoter region of miR-451, resulting in miR-451 inhibition and promoting chemoresistance of prostate cancer cells.
Altogether, to our knowledge, this is the first study to reveal a novel HDAC3/Sp1/miR-451/NEDD9 signaling axis that regulates chemosensitivity of prostate cancer cells. miR-451 increased chemosensitivity by targeting NEDD9 in prostate cancer cells. Moreover, HDAC3 mediated silencing of miR-451 in an Sp1-dependent manner. Therefore, this new signaling axis represents a novel therapeutic target for chemosensitizing prostate cancer.
Supplementary Material
Acknowledgments
The authors thank Jiangsu Overseas Research and Training Program for University Prominent Young and Middle-aged Teachers and Presidents, the talents program of Jiangsu Cancer Hospital, Key health talent program of Suzhou and the young talents program of Jiangsu Cancer Hospital. The authors thank Dr Jin Chen and Kai Zhang for technical assistance. Ji-feng Feng and Hong Zhu were Co-corresponding authors. Dong-qin Chen, Chen Yu and Xue-feng Zhang contributed equally to this article.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This study was supported by the National Science Foundation of China (no. 81672928), Financial Grant from the China Postdoctoral Science Foundation (no. 2018T110466 and 2017M621678) and the National Science Foundation of China (nos. 81572345 and 81503528). The Jiangsu Province Office of Education is very much appreciated for its project that sponsors the outstanding young and middle-aged teachers to go overseas as visiting scholars.
Contributor Information
Dong-qin Chen, Department of Medical Oncology, Jiangsu Cancer Hospital & Jiangsu Institute of Cancer Research & the Affiliated Cancer Hospital of Nanjing Medical University, Nanjing, China; Department of Medical Oncology, the First Affiliated Hospital of Soochow University, Suzhou, China; Department of Medical Oncology, Nanjing University, Nanjing, China.
Chen Yu, Department of Medical Oncology, Jiangsu Cancer Hospital & Jiangsu Institute of Cancer Research & the Affiliated Cancer Hospital of Nanjing Medical University, Nanjing, China.
Xue-feng Zhang, Wake Forest Institute for Regenerative Medicine, Wake Forest School of Medicine, Winston-Salem, USA; Department of Urology, the First Affiliated Hospital of Soochow University, Suzhou, China.
Zhong-fang Liu, Department of Medical Oncology, the First Affiliated Hospital of Soochow University, Suzhou, China.
Rui Wang, Department of Medical Oncology, Nanjing University, Nanjing, China.
Min Jiang, Department of Medical Oncology, the First Affiliated Hospital of Soochow University, Suzhou, China.
Hao Chen, Department of Urology, the First Hospital of Jiaxing, Jiaxing, China.
Feng Yan, Department of Medical Oncology, Jiangsu Cancer Hospital & Jiangsu Institute of Cancer Research & the Affiliated Cancer Hospital of Nanjing Medical University, Nanjing, China.
Min Tao, Department of Medical Oncology, the First Affiliated Hospital of Soochow University, Suzhou, China.
Long-bang Chen, Department of Medical Oncology, Nanjing University, Nanjing, China.
Hong Zhu, The First Affiliated Hospital of Soochow University, 899 Pinghai Road, Suzhou 215006, China.
Ji-feng Feng, Jiangsu Cancer Hospital & Jiangsu Institute of Cancer Research & the Affiliated Cancer Hospital of Nanjing Medical University, 42 Baiziting Road, Nanjing 210009, China.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Paranjape AN, Soundararajan R, Werden SJ, et al. Inhibition of FOXC2 restores epithelial phenotype and drug sensitivity in prostate cancer cells with stem-cell properties. Oncogene 2016; 35: 5963–5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galletti G, Leach BI, Lam L, et al. Mechanisms of resistance to systemic therapy in metastatic castration-resistant prostate cancer. Cancer Treat Rev 2017; 57: 16–27. [DOI] [PubMed] [Google Scholar]
- 4. Rathkopf DE, Smith MR, Ryan CJ, et al. Androgen receptor mutations in patients with castration-resistant prostate cancer treated with apalutamide. Ann Oncol 2017; 28: 2264–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tannock IF, De Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–1512. [DOI] [PubMed] [Google Scholar]
- 6. Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol 2008; 26: 242–245. [DOI] [PubMed] [Google Scholar]
- 7. Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004; 351: 1513–1520. [DOI] [PubMed] [Google Scholar]
- 8. Antonarakis ES, Tagawa ST, Galletti G, et al. Randomized, noncomparative, phase II trial of early switch from docetaxel to cabazitaxel or vice versa, with integrated biomarker analysis, in men with chemotherapy-naive, metastatic, castration-resistant prostate cancer. J Clin Oncol 2017; 35: 3181–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fischer KR, Durrans A, Lee S, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015; 527: 472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weng H, Huang H, Dong B, et al. Inhibition of miR-17 and miR-20a by oridonin triggers apoptosis and reverses chemoresistance by derepressing BIM-S. Cancer Res 2014; 74: 4409–4419. [DOI] [PubMed] [Google Scholar]
- 11. Xiong H, Ni Z, He J, et al. LncRNA HULC triggers autophagy via stabilizing Sirt1 and attenuates the chemosensitivity of HCC cells. Oncogene 2017; 36: 3528–3540. [DOI] [PubMed] [Google Scholar]
- 12. Ramalho-Carvalho J, Martins JB, Cekaite L, et al. Epigenetic disruption of miR-130a promotes prostate cancer by targeting SEC23B and DEPDC1. Cancer Lett 2017; 385: 150–159. [DOI] [PubMed] [Google Scholar]
- 13. Ye FG, Song CG, Cao ZG, et al. Cytidine deaminase axis modulated by miR-484 differentially regulates cell proliferation and chemoresistance in breast cancer. Cancer Res 2015; 75: 1504–1515. [DOI] [PubMed] [Google Scholar]
- 14. Cai J, Fang L, Huang Y, et al. Simultaneous overactivation of Wnt/beta-catenin and TGF beta signalling by miR-128–3p confers chemoresistance-associated metastasis in NSCLC. Nat Commun 2017; 8: 15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wen D, Peng Y, Lin F, et al. Micellar delivery of miR-34a modulator rubone and paclitaxel in resistant prostate cancer. Cancer Res 2017; 77: 3244–3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang R, Wang ZX, Yang JS, et al. MicroRNA-451 functions as a tumor suppressor in human non-small cell lung cancer by targeting ras-related protein 14 (RAB14). Oncogene 2011; 30: 2644–2658. [DOI] [PubMed] [Google Scholar]
- 17. Chen DQ, Pan BZ, Huang JY, et al. HDAC 1/4-mediated silencing of microRNA-200b promotes chemoresistance in human lung adenocarcinoma cells. Oncotarget 2014; 5: 3333–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xia L, Huang W, Tian D, et al. Overexpression of forkhead box C1 promotes tumor metastasis and indicates poor prognosis in hepatocellular carcinoma. Hepatology 2013; 57: 610–624. [DOI] [PubMed] [Google Scholar]
- 19. Chen MW, Yang ST, Chien MH, et al. The STAT3-miRNA-92-Wnt signaling pathway regulates spheroid formation and malignant progression in ovarian cancer. Cancer Res 2017; 77: 1955–1967. [DOI] [PubMed] [Google Scholar]
- 20. Wang Y, Lieberman R, Pan J, et al. miR-375 induces docetaxel resistance in prostate cancer by targeting SEC23A and YAP1. Mol Cancer 2016; 15: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bitarte N, Bandres E, Boni V, et al. MicroRNA-451 is involved in the self-renewal, tumorigenicity, and chemoresistance of colorectal cancer stem cells. Stem Cells 2011; 29: 1661–1671. [DOI] [PubMed] [Google Scholar]
- 22. Liu X, Zhang X, Xiang J, et al. miR-451: potential role as tumor suppressor of human hepatoma cell growth and invasion. Int J Oncol 2014; 45: 739–745. [DOI] [PubMed] [Google Scholar]
- 23. Chen D, Huang J, Zhang K, et al. MicroRNA-451 induces epithelial-mesenchymal transition in docetaxel-resistant lung adenocarcinoma cells by targeting proto-oncogene c-Myc. Eur J Cancer 2014; 50: 3050–3067. [DOI] [PubMed] [Google Scholar]
- 24. Jin Y, Li F, Zheng C, et al. NEDD9 promotes lung cancer metastasis through epithelial-mesenchymal transition. Int J Cancer 2014; 134: 2294–2304. [DOI] [PubMed] [Google Scholar]
- 25. Matter MS, Marquardt JU, Andersen JB, et al. Oncogenic driver genes and the inflammatory microenvironment dictate liver tumor phenotype. Hepatology 2016; 63: 1888–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loskutov YV, Kozyulina PY, Kozyreva VK, et al. NEDD9/Arf6-dependent endocytic trafficking of matrix metalloproteinase 14: a novel mechanism for blocking mesenchymal cell invasion and metastasis of breast cancer. Oncogene 2015; 34: 3662–3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ciesla M, Marona P, Kozakowska M, et al. Heme oxygenase-1 controls an HDAC4-miR-206 pathway of oxidative stress in rhabdomyosarcoma. Cancer Res 2016; 76: 5707–5718. [DOI] [PubMed] [Google Scholar]
- 28. Napoli M, Venkatanarayan A, Raulji P, et al. DeltaNp63/DGCR8-dependent microRNAs mediate therapeutic efficacy of HDAC inhibitors in cancer. Cancer Cell 2016; 29: 874–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hentati-Kallel M, Le Jan S, Bernard P, et al. Histone deacetylases meet microRNA-associated MMP-9 expression regulation in glucocorticoid-sensitive and -resistant cell lines. Int J Oncol 2017; 50: 717–726. [DOI] [PubMed] [Google Scholar]
- 30. Lai TH, Ewald B, Zecevic A, et al. HDAC inhibition induces microRNA-182, which targets RAD51 and impairs HR repair to sensitize cells to sapacitabine in acute myelogenous leukemia. Clin Cancer Res 2016; 22: 3537–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Amodio N, Stamato MA, Gullà AM, et al. Therapeutic targeting of miR-29b/HDAC4 epigenetic loop in multiple myeloma. Mol Cancer Ther 2016; 15: 1364–1375. [DOI] [PubMed] [Google Scholar]
- 32. Groselj B, Ruan JL, Scott H, et al. Radiosensitisation in vivo by histone deacetylase inhibition with no increase in early normal tissue radiation toxicity. Mol Cancer Ther. Epub ahead of print 24 August 2017. DOI: 10.1158/1535-7163.MCT-17-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sato S, Katsushima K, Shinjo K, et al. Histone deacetylase inhibition in prostate cancer triggers miR-320-mediated suppression of the androgen receptor. Cancer Res 2016; 76: 4192–4204. [DOI] [PubMed] [Google Scholar]
- 34. Qian Y, Yao W, Yang T, et al. aPKC-iota/P-Sp1/Snail signaling induces epithelial-mesenchymal transition and immunosuppression in cholangiocarcinoma. Hepatology 2017; 66: 1165–1182. [DOI] [PubMed] [Google Scholar]
- 35. Kaushik AK, Shojaie A, Panzitt K, et al. Inhibition of the hexosamine biosynthetic pathway promotes castration-resistant prostate cancer. Nat Commun 2016; 7: 11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu X, Fan Z, Liang C, et al. A signature motif in LIM proteins mediates binding to checkpoint proteins and increases tumour radiosensitivity. Nat Commun 2017; 8: 14059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kasagi Y, Harada Y, Morodomi Y, et al. Peritoneal dissemination requires an Sp1-dependent CXCR4/CXCL12 signaling axis and extracellular matrix-directed spheroid formation. Cancer Res 2016; 76: 347–357. [DOI] [PubMed] [Google Scholar]
- 38. Liu S, Wu LC, Pang J, et al. Sp1/NFkappaB/HDAC/miR-29b regulatory network in KIT-driven myeloid leukemia. Cancer Cell 2010; 17: 333–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yuan JH, Yang F, Chen BF, et al. The histone deacetylase 4/SP1/microRNA-200a regulatory network contributes to aberrant histone acetylation in hepatocellular carcinoma. Hepatology 2011; 54: 2025–2035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.