Abstract
Electroporation is a well-known phenomenon that occurs at the cell membrane when cells are exposed to high-intensity electric pulses. Depending on electric pulse amplitude and number of pulses, applied electroporation can be reversible with membrane permeability recovery or irreversible. Reversible electroporation is used to introduce drugs or genetic material into the cell without affecting cell viability. Electrochemotherapy refers to a combined treatment: electroporation and drug injection to enhance its cytotoxic effect up to 1000-fold for bleomycin. Since several years, electrochemotherapy is gaining popularity as minimally invasive oncologic treatment. The adoption of electrochemotherapy procedure in interventional oncology poses several unsolved questions, since suitable tumor histology and size as well as therapeutic efficacy still needs to be deepen. Electrochemotherapy is usually applied in palliative settings for the treatment of patients with unresectable tumors to relieve pain and ameliorate quality of life. In most cases, it is used in the treatment of advanced stages of neoplasia when radical surgical treatment is not possible (eg, due to lesion location, size, and/or number). Further, electrochemotherapy allows treating tumor nodules in the proximity of important structures like vessels and nerves as the treatment does not involve tissue heating. Overall, the safety profile of electrochemotherapy is favorable. Most of the observed adverse events are local and transient, moderate local pain, erythema, edema, and muscle contractions during electroporation. The aim of this article is to review the recent published clinical experiences of electrochemotherapy use in deep-seated tumors with particular focus on liver cases. The principle of electrochemotherapy as well as the application to cutaneous metastases is briefly described. A short insight in the treatment of bone metastases, unresectable pancreas cancer, and soft tissue sarcoma will be given. Preclinical and clinical studies on treatment efficacy with electrochemotherapy of hepatic lesions and safety of the procedure adopted are discussed.
Keywords: electrochemotherapy, ECT, reversible electroporation, percutaneous, liver, hepatic, metastases, interventional oncology, bleomycin, hepatocellular carcinoma
Introduction
Electrochemotherapy (ECT) is a locally enhanced chemotherapy that combines the administration of chemotherapeutic drugs with well-dosed electric pulses for cell membrane electroporation (EP). In 1957, scientists discovered the influence of electric fields on axons and cell membranes.1–3 The exposure of biological membranes to a sufficiently high external electric field can lead to a rapid and large increase in electric conductivity and permeability, called membrane EP. While applying an electric field on cell membranes, their surface tension will be destabilized and nonpermanent molecules can diffuse into the cytosol. According to the theory of aqueous pore formation, pores are able to form spontaneously, when the bilayer is exposed to an electrical field (>50 V).4,5 It is assumed that the induced voltage causes lipid molecule rearrangement on the bilayer that supports pore formation.4,6,7 Even with modern molecular dynamic simulation software, the pore formation of electric stimulated membranes is not completely understood. Thus, the presumed pore formation caused by EP has not yet been observed directly, possibly due to the microscale dimension and fragile structure of pores and cells.
When electric pulses are applied to cells, 2 different phenomena are observed: reversible EP and irreversible electroporation (IRE), both used in clinical practice. Reversible EP will increase cell membrane permeability and open an access route for molecules that are too big to cross the cell membrane (DNA, RNA) or facilitates cell enter by hydrophilic molecules (bleomycin [BLM], cisplatin).8–10 These molecules once crossed the cell membrane exert their effect in the resealing and intact cells. In contrast, IRE (>600 V/cm) is used as nonthermal form of soft tissue ablation in clinical routine.11 The IRE of cell membranes leads to a disturbance of the cell homeostasis and thus ultimately to apoptosis in the treated tissue. This method of tissue ablation has found widespread use to destroy malignant tissue.12–14
As the application of ECT in the case of cutaneous tumors is widespread adapted in clinical routine, only a brief outline will be given. The main focus of this review is set on the treatment of deep-seated tumors, especially hepatic metastases, as this approach offers new and interesting treatment options in the case of liver cancer. Moreover, research in animal models as well as current human studies is presented and possible use in clinical practice is discussed.
Principle of ECT
The concept of ECT is based on the aforementioned properties of reversible EP combined with therapeutic efficacy of chemotherapeutic agents. Due to the increased permeability of the cell membrane, chemotherapeutic agents can pass into cells and induce cell death mitosis in the targeted tissue. In general, a patient receiving ECT treatment will first get an intravenous or intratumoral administration of an anticancer drug (eg, BLM, cisplatin) so it can evenly distribute over the vascular system and extracellular space of the tissue. Electrodes located around or inside the tumor will deliver defined electric pulses which enables diffusion of otherwise membrane nonpermeant anticancer drug into the target cells (Figure 1). After a short time, a few seconds to several minutes after exposure to the electric field, the membrane permeability will return to its initial state and the specific chemotherapeutics will cause multiple DNA breaks (BLM) or intra- and inter-strand DNA bonds (cisplatin) in the abnormal tumor cells. Benefits arising from this combination therapy include a low dose of cytostatic drug due to locally increased cytotoxic effect (BLM, 1000-fold and cisplatin, 80-fold increase of toxicity),15–17 as only a few molecules per cell are needed to induce cell death during a single treatment session. Another effect caused by the treatment is the release of intact tumor antigens due to substance shedding by the damaged cells. Released tumor antigens are exposed to the immune system of patients and activate tumor antigen-directed immune response. Since ECT is a local treatment, distant metastases should not be affected.8,18 Nevertheless, the treatment of tumor nodules with ECT recruiting components of the immune system may elicit a systemic immune response against distant metastases: abscopal effect.19,20 Furthermore, a vascular disruption and hypoperfusion caused by EP (vascular lock) can be observed during intervention. This effect has been attributed to electrical-induced reflexive constriction of arterioles and interstitial edema of endothelial structures.21 A decreased blood flow can be observed in the treated areal which increases drug retaining time as well as prevents bleeding of treated well-vascularized organs.22 The synergistic effects of immune checkpoint inhibitory therapy can achieve a further stimulation of the antitumor immune response.23
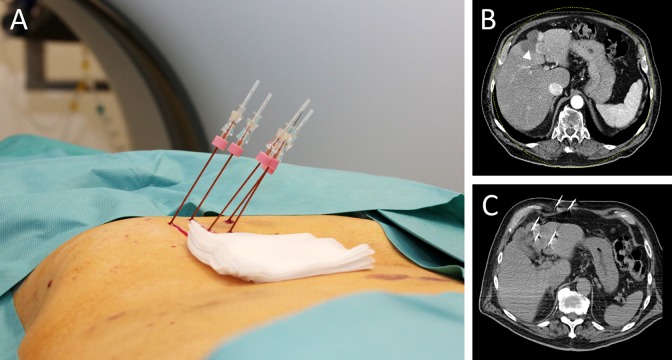
Figure 1.
An 88-year-old male patient receiving hepatic ECT treatment. A, Percutaneous placement of electrode needles for hepatic lesion treatment. B, Preinterventional CT imaging of hepatic metastasis (white arrowhead; IV liver segment). C, Control CT imaging during electrode needle placement. CT indicates computed tomography; ECT indicates electrochemotherapy.
Application Area of ECT
The efficacy of ECT treatment is well demonstrated for cutaneous and subcutaneous melanomas, and the technique can be applied in a variety of malignant lesions.24,25 Also, patients with diabetes as comorbidity or pretreated by surgery, radiofrequency ablation (RFA), or transarterial chemoembolization (TACE) can be treated by ECT.26 The range of applications can be divided into 3 groups: (1) treatment of cutaneous and subcutaneous metastases located in head or neck, melanoma, nonmelanoma skin cancer, or breast cancer metastases to the skin8,24,27–30; (2) treatment of noncutaneous metastases located in bone, liver, or soft tissue sarcoma (STS)31–33; and (3) clinical trials for the treatment of primary tumors, such as ovary or colon cancer.34,35
Although there seems to be no restrictions in the application by various malignant lesions, some limitations still occur. For individual tissues, variable specific treatment protocols are available. Also, different lesions need special shaped electrodes or numbers of electrodes as well as individual pulse generators.36 The successful ECT performance is also depended on tumor size and location. The drug perfusion of large metastases may be insufficient and lead to decreased drug concentration in the inner portion and impede the drug cytotoxic effect.37 Also, large metastases will show low concentration of the drug at the time of EP due to delayed drug perfusion.38 In the case of big metastases, correct needle placement in order to ensure an effective and homogeneous coverage with the electric field is mandatory.39,40 As there are many variables that individually need to be taken into account for each tumor, a general protocol can be hardly designed. Also, the time of response can vary depending on the size and location,41 which impedes a consistent follow-up evaluation schedule. Repetitive ECT intervention showed to influence the treatment result in a positive way.30 Nevertheless, patients treated with ECT show fewer side effects than patients treated with systemic chemotherapy. In addition, ECT can be applied as adjuvant therapy.
Factors affecting ECT effectiveness include tumor size, previous treatments, location, and histotype. Cutaneous metastases smaller than 3 cm, not preirradiated, show the best objective response rate. Moreover, electrode geometry, number, and separation will affect the electric field in the tumor as well as the treatment outcome. However, cell homeostasis can be disturbed permanently if certain thresholds are exceeded. Critical factors are among others especially amplitude and pulse duration. If duration or amplitude of electric pulses is increased, irreversible disruption of cell homeostasis will happen (reversible EP: eg, 1.3 kV/cm, 99 µs; IRE: eg, 1.5 kV/cm, 300 µs).12,42
Assisting and Supporting Tools During ECT
Correct spatial individual long-needle positioning needs skills and the support of intraoperative imaging: X-ray, ultrasound, or computed tomography (CT) imaging. Specific instruments have been developed to guide needle insertion (navigation systems) and positioning and to guarantee the complete and homogenous coverage of the tumor volume by the applied electric field. Moreover, treatment needs to be carefully planned with software that indicates where to position electrodes and the required electric field conditions.43 Dedicated EP preoperative planning tools were developed. To help the surgeon, the software called PULSAR (PULSUR ver. 1.0) was realized by with the functionality to calculate an optimized placement of the electrodes within or around a predefined area segmented by the user. The software estimates the coverage of the electric field and minimizes the number of electrodes required. The tool provides an estimation of the electric field in the region of interest selected by expert operators by means of approximate calculations giving indication of electrodes configuration, voltage, and distance for each couple of electrodes.
Another software proposed in literature43 was a web-based tool that automatically builds a 3-D model of the target tissue from the medical images uploaded by the user and then uses this 3-D model to optimize treatment parameters. In addition, this web-based tool is intended to facilitate the treatment planning process and reduce the time needed for it. It is crucial for facilitating expansion of EP-based treatments in clinical practice and ensuring reliable treatment for the patients.
Also keeping a safety margin while needle placement helps to treat the tumor and the tumor margin to prevent possible tumor cell infiltrating normal tissue. This can be ensured by navigated needle placement and confirmed by ultrasound or CT imaging. As helpful tool, navigation systems can be applied additionally. Navigation systems can be separated into 2 groups, “robotic”-assisted needle placement and electrode tracking systems.44–46 During intervention, the integration of these systems decreases the probability of needle repositioning and increases the probability to accurately fit the needle position according to the treatment plan. Nevertheless, control imaging complements this, as the needle position can be visually tracked and compared to the treatment plan. So the integration of software tools for simulation of local electric field distribution, image guidance (CT, ultrasound) during electrode positioning, and navigation systems for needle insertion will increase accuracy, decrease intervention times, and ensure probe positioning.44,47
Electrochemotherapy in Clinical Practice
Electrochemotherapy of Cutaneous and Subcutaneous Metastases
Electrochemotherapy treatment of superficial (ie, skin and subcutaneous) neoplastic lesions regardless of their histology is well established.30,48–54
Electrochemotherapy has been adopted for the treatment of skin metastases of squamous cell carcinoma, melanoma, basal cell carcinoma, adenocarcinomas (of the breast, salivary glands, and kidneys), Kaposi sarcoma, Merkel cell carcinoma, transitional cell carcinoma of the urinary bladder, and head and neck cancer. It can be considered when there is no possibility of surgical intervention, when surgery is contraindicated or would be too harming, and when addressing lesions resistant to chemotherapy and radiotherapy. A thorough discussion of these modalities can be found elsewhere in the literature.25,36,55 In a 10-year audit, the effect of ECT in the case of cutaneous breast cancer was analyzed. The authors proposed to apply ECT as an early treatment modality, as the treatment response seems to be influenced by the lesion size and a good response can be obtained (objective response 79.7%).56 Several clinical studies as well as the European Standard Operating Procedures for Electrochemotherapy and Electrogenetherapy (ESOPE) project have set up defined standard operating procedures in order to ensure patient safety and to achieve optimal treatment of cutaneous and subcutaneous metastases. These recommendations include chemotherapeutic drugs, drug delivery route, electrode shape, and pulse parameters as well as tumor properties (measurement, location, and number).41 Reports from clinical experiences adopting the operating procedures of the ESOPE study were consistent with those of controlled clinical trials. The meta-analysis of Mali et al,25 which included 44 studies involving 1894 tumors, reported an objective response rate to ECT of 84%. When ECT procedures are performed according to these recommendations, an objective response rate of 68% to 86% and complete response rate of 33% to 60% of treated malignant melanoma nodules were observed. Dependent on tumor size, the therapy outcome varies whereas tumors smaller than 3 cm show the highest response rates.38 Due to the treatment, temporary flushing, fluid accumulation, and swelling may occur as side effects.24,57,58 In their study on ECT (BLM followed by EP) in 85 patients, Campana et al 59 showed that tumor size and number of lesions are significant predictive factors for response. An increasing number of electrode applications and ECT cycles were predictors of local control. There were no predictors of toxicity.
According to Bertino et al,58 in a recent clinical trial on skin cancer of the head and neck, better responses are obtained with small lesions (≤3 cm), primary tumors, and naive tumors. Previous surgery least affected the outcome compared to (chemo)radiotherapy or multiple treatments of recurrent tumor nodules. They reported that no ECT-related serious adverse events were observed and the most common adverse events were postoperative pain (92%) and skin reactions (18%) that decreased over time.
Recently, Kunte et al,60 in a prospective cohort study by InsPECT, experienced an objective response of 74% in 151 patients with metastatic melanoma, and 229 of 394 treated lesions showed a complete response of 58%. One-year overall survival was 67%, while melanoma-specific survival was 74%. Caraco et al 61 reported that 44.8% of complete responders experienced a long-lasting response after 1 ECT session and were disease-free after a mean duration of follow-up of 27.5 months.
Multiple clinical experiences report preliminary data on the safety and the benefit of the ECT and target therapy combinations.62–66 In clinical practice, ECT is successfully used for the treatment of cutaneous and subcutaneous tumors and receives satisfying results treating melanoma. Current research aims now to identify the best treatment conditions and guidelines in order to treat deep-seated tumors.32,67
Electrochemotherapy of Noncutaneous Deep-Seated Tumors
As stated by Miklavcic et al,39 efforts to translate the application of ECT from easily accessible lesions (eg, cutaneous metastasis of different malignancies) to the treatment of nonsuperficial tumors require addressing the critical importance of achieving complete EP of the target lesion(s). The preliminary results of the published studies on these new clinical indications show that the treatment of metastases of deep-seated tumors is feasible and safe. Electrochemotherapy is effective in resolution or regression of tumor nodules and quality of life of patients is improved.68
Locally advanced pancreatic cancer
Preclinical experiences
Electrochemotherapy in pancreatic cancer has been investigated by Jaroszeski et al 69 in a preclinical trial using a hamster animal model. The combination of EP with intratumoral BLM was able to induce a 25% response rate in hamster in which tumoral cells were injected directly into pancreas. In vivo effects of EP were evaluated by Girelli et al 70 in normal pancreas of the rabbits. The EP has shown to be a valid alternative for the local control of nonresectable pancreatic cancer because the procedure did not affect pancreatic parenchyma.
Clinical experience
A prospective clinical phase I/II study to evaluate feasibility, safety, and efficacy of ECT by means of VGP02 Cliniporator model was published by Granata et al 71 and reported encouraging results. No acute (intraoperative) and/or postoperative serious adverse events related to ECT were observed; no clinically significant electrocardiographic, hemodynamic, or serum biologic changes were noted. No clinically relevant elevation in amylase or lipase levels was observed in any patient and no bleeding or damage to surrounding viscera occurs. Functional imaging based on magnetic resonance imaging (MRI) and positron emission tomography scan demonstrated to be more suitable to evaluate ECT response in patients with locally advanced pancreatic adenocarcinoma than CT imaging. According to the authors, ECT of locally advanced pancreatic adenocarcinoma is feasible, safe, and effective treatment modality.71,72 Clinical study is currently in progress, and ECT using variable geometry technology will be used to treat all patients who will be enrolled starting from this time.
Bone metastasis
Bone metastases are common disabling complications in patients with cancer that impair general health status, quality of life, and survival. Pain relief is one of the most challenging and significant problems in the management of these patients. Many treatments are available for the palliative management of patients with metastatic bone disease, including radiotherapy, thermal and cryoablation, high-intensity-focused ultrasound, resection, and the application of bone cement to fill osteolytic areas. When the surgical removal or ablation of metastases is not indicated (unresectable bones such as pelvis, vertebral bodies, or in case of disseminated disease), the use of narcotics for pain relief and of local ablative techniques such as RFA or selective embolization can be chosen even if these treatments are not always effective.
Electrochemotherapy is a local treatment modality characterized by minimal invasiveness, is completed in short time and absence of severe adverse events compared to other palliative surgical procedures, and characterized by repeated irradiation, hyperthermia, or isolated limb perfusion or infusion. Minimally invasive approach to bone metastases treatment with ECT, using variable geometry and Cliniporator VITAE (IGEA S.p.A., Carpi, Italy) and BLM, was performed for the first time in Bologna.
Preclinical studies with ECT
A preclinical study on 10.5-month-old New Zealand White rabbits was performed by Fini et al.31 The study was designed to prove that EP was effective in cell ablation also in the presence of a mineralized matrix. An appropriate combination of voltage and electric pulses induced ablation of bone cells without affecting bone mechanical competence and the recovery of osteogenic activity that was completely recovered by 30 days after EP. Electrochemotherapy was used to control bone osteolysis induced in rat tibia by injection of syngeneic breast cancer cells. Bone resorption and tibia fracture were prevented in all animals by one single treatment with ECT.31 Furthermore, in animal treated with ECT, trabeculae maintained a normal structure and morphology. Overall, preclinical investigation provides the rational bases to investigate ECT in the treatment of human bone metastasis.
Clinical experience
After preclinical studies that demonstrated bone osteogenic activity and bone hardness to be preserved after ECT, a phase I–II clinical trial has been conducted at Instituto Ortopedico Rizzoli (Bologna, Italy) in order to assess safety and feasibility of ECT on bone metastases. No complications were reported, and fast recovery was observed in patients with impending or actual fracture of the treated bone.73,74
After primary staging (bone scan, CT/MRI, etc), 10 patients affected by bone metastases of the appendicular skeleton were treated with ECT according to ESOPE method. Nine more patients with pelvic or sacral metastases were treated under CT guidance “out of protocol.” The procedure was performed in general anesthesia, and the patient was discharged the day after the treatment. The ECT response was assessed by either MRI or CT imaging 4 and 8 weeks after ECT. Early results show pain relief in 56% of the patients with quality of life improvement. In few patients, radiographic response was also observed with necrosis of the metastatic lesion. Although these early clinical results are promising, a longer follow-up and larger cohort of patients are warranted.
A phase II clinical study with ECT was conducted on 29 patients affected by painful bone metastases. The appendicular skeleton was affected in 15 patients, while in 14 patients, other sites were involved. All patients well tolerated the procedure, and no intraoperative or postoperative complications were observed. At a mean follow-up of 7 months, 24 patients were available for evaluation. Patients affected by bone metastases obtained an improvement of pain (≥50%) in 84% of the cases after ECT. Results reported in this study demonstrated ECT to be safe and feasible in the treatment of painful bone metastases even when other previous treatments were ineffective.74
Case Report
A case report of a spine metastasis treated during open surgery was reported.75 Mini-open surgery with a left L5 laminectomy was performed under general anesthesia to introduce the electrodes. The assessed follow-up period was 48 months after the ECT procedure. Neither serious ECT-related adverse events nor BLM toxicity were reported. The patient obtained an excellent improvement in pain intensity. The experience gathered in the treatment of bone metastases allowed to prepare specific guidelines adopted by Società Italiana Ortopedia e Traumatologia and Associazione Italiana Oncologia Medica.76
Nonsuperficial large soft tissue tumors
Preliminary results of “Phase I/II study of ECT using variable geometry poration (VGP) and bleomycin for the treatment of deep and large soft tissue tumors” have been presented at the European Society for Medical Oncology (ESMO) Conference 2014.
An ex vivo study on surgical specimen was performed to explore the difference in tissue electrical properties of STS. These results may be used to verify whether tuning electric field intensity according to the specific STS histotype could improve tissue EP and ultimately treatment efficacy.77,78
Liver metastases
Since the liver is an organ of particular current research interests in ECT, results of preclinical studies and clinical experiences will be given in more detail. Besides established ablation modalities like microwave ablation79 or RFA80 and IRE,81 there is need for a controlled nonthermal ablation modality that enhances the efficacy of chemotherapeutic agents. The benefit of nonthermal ablation, like IRE, is the ability to treat lesions without thermonecrosis. This enables the ability to ablate near-sensitive structures like vessels and nerves, as no heat will be produced which spread around the treated area. An already established locoregional method is TACE. During TACE, embolizing agents and chemotherapeutics will be administrated via catheter to obtain tissue-specific necrosis. In cases of hepatocellular carcinoma (HCC), a complete response of 23% and objective response of 66% as well as an improvement in survival can be observed after TACE.82,83 When treating malignant liver metastases, a partial response rate up to 56% in the case of liver metastasis with neuroendocrine tumors (stable disease 40%),84 partial response up to 11.5% in the case of liver metastases from pancreatic cancer (stable disease 78%),85 and partial response up to 80% in the case of colorectal liver metastases (stable disease up to 48%)86 were reported. As both therapy concepts are based on locoregional application of cytostatic in hepatocellular parenchyma, a similar response rate of ECT by liver lesions can be possible. Similar to TACE, ECT allows for a controlled locoregional additional chemotherapy without marked systemic side effects. As the chemotherapeutics applied during ECT are membrane impermeant, even better results might be possible. The establishment and expansion of ECT in deep-seated tumors (eg, liver, bone metastases) will open up new opportunities for minimally invasive treatment of metastases and carcinomas.
Electrochemotherapy of hepatic tumors
Electrochemotherapy in animal trial
First trials of ECT by hepatic tumors were conducted in animal models (Table 1). Electrochemotherapy was shown to be effective to reduce the volume of hepatic metastases of colorectal cancer in the rats.88 The first study of the effects of ECT on tumors transplanted to livers in rabbits was conducted by Ramirez et al.90 Almost complete tumor necrosis was observed after ECT, resulting from both BLM direct cytotoxic effects on electropermeabilized tumor cells and indirect effects on the tumor vessels. A large reduction in tumor growth rate and significantly longer survival times were scored in comparison with control rabbits. Moreover, ECT of liver tumors was well tolerated and devoid of systemic side effects.
Table 1.
Preclinical and Phase I/II Studies of ECT on Damaged and Healthy Hepatic Tissue.
| Hepatic Disease | Chemotherapeutic; Injection; Dose | Number of Electrodes | Number of Electrical Pulses; Frequency | Duration (µs) | Electric Field Intensity (V/cm) or Vo Electric Field Intensity (V/cm) or Voltage Value (V) | Time Between Injection and EP | Effect | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Rat | N1S1-induced tumor | BLM; IT; 0.5 U | 6-needle electrodes | 6; 1 Hz | 99 | 1000 V/cm | 90 seconds | 69% (CR); 15% (PR) | 87 |
| Rat | DHD/K12/Prob hepatic metastases | BLM; IT; 0.45 mg | 6-needle electrodes | 6; NA | 99 | 650 V | 3 minutes | 22% (CR); 88% (PR) | 88 |
| Rat | Healthy | BLM; IT; 0.5-2.5 U | 6-needle electrodes | 6; 1 Hz | 100 | 500-2250 V/cm | 2 minutes | Minimal level of necrosis; field strengths between 500 and 1200 V/cm showed minimal necrosis at day 14. The amount of necrosis (20%-30%) increased for the >1500 V/cm groups | 89 |
| Rabbit | VX2 tumor nodules | BLM; IV; 0.5-1 mg/kg | 2-plate electrode or 2- to 7-needle electrodes | 8; 1-8 Hz | 100 | 850 V/cm | 4 minutes | 50% (CR) and 30% disease-free long-term survivors | 90 |
| Human | Single case; colorectal liver metastasis | BLM; IV; 15 000 IU/m2 | 6-needle electrodes; open surgery | 8; heart beat adapted | 100 | 1700-2100 V | 8 minutes | Complete necrosis | 32 |
| Human | 16 patients; colorectal liver metastasis | BLM; IV; 15 000 IU/m2 | 7-needle electrodes; open surgery | 8; heart beat adapted | 100 | 1000 V/cm | 8-28 minutes | 85% (CR); 15% (PR) | 91 |
| Human | 8 patients; colorectal liver metastasis | BLM; IV; 15 000 IU/m2 | 5- to 7-needle electrode; open surgery | 8; heart beat adapted | 100 | 713-2810 V | 8 minutes | ECT minor effects on heart function | 92 |
| Human | 6 patients; PVTT from HCC | BLM; IV; 15 000 IU/m2 | 4- to 6-needle electrodes; percutaneously | 8; heart beat adapted | 100 | NA- | 8 minutes | 83% complete necrosis of Vp3-VP4 PVTT | 26 |
| Human | 7 patients; colorectal liver metastases | BLM; IV; 15 000 IU/m2 | 6-needle electrodes; open surgery | 8; heat beat adapted | 100 | 450-900 V/cm | 8 minutes | Necrosis of the treated area; preservation of vessels and biliary structures | 93 |
| Human | 10 patients; 17 HCC | BLM; IV; 15 000 IU/m2 | 1- to 7-needle electrodes; open surgery | 8-24; heart beat adapted | 100 | 1000 V/cm | 8-28 minutes | 80%-88% (CR); 12% (PR) | 94 |
Abbreviations: BLM, bleomycin; CR, complete response; ECT, electrochemotherapy; EP, electroporation; HCC, hepatocellular carcinoma; IT, intratumoral; IV, intravenous; NA, not applicable; PR, partial response; PVTT, portal vein tumor thrombus.
Jaroszeski et al reported first results of ECT in rats with induced hepatic tumor growth by injection of N1S1 rat hepatoma cells. Animals treated with cytostatic and electrical pulses showed a significantly better response (69% complete response; 15% partial response) to the treatment than the control groups (EP only; BLM only; no treatment).87 In a following study,89 the effect of ECT on normal liver tissue was analyzed in a rat model. Treatment with electric pulses higher than 1500 V/cm resulted in slightly elevated levels of necrosis, whereas lower amplitudes showed no remarkable effects. The combined application of BLM (0.5 U) and electric pulses showed, in cases treated with 1500 to 2250 V/cm, 1.5% and 20% to 30% necrosis of the treated area, whereas lower amplitudes caused almost no necrosis. Low levels or no necrosis were observable 56 days after ECT.
As the duration and intensity of the electrical pulse determines whether EP is reversible or irreversible, measuring of the passive electrical properties of electroporated tissues may provide a quality assurance and real-time feedback on the ECT process. However, tests revealed that the overall long-term pattern of change in conductivity in hepatic cells after EP is complex and differs between reversible EP and IRE.95
Electrochemotherapy in Human Clinical Trial
Electrochemotherapy of solid organs has been evaluated in phase I and II trials in humans. Here, ECT was conducted intraoperatively by individually inserted long-needle electrodes and intravenous BLM administration.32,91 A significant reduction of viable tumor tissue in ECT-treated metastases was observed. Infarct-like necrosis occurred presumably caused by the cytotoxic and vascular-disrupting effect on tumor cells and small tumor blood vessels.22,91,96 Histopathological analysis of colorectal liver metastases after ECT treatment revealed necrotic and fibrotic changes of tumor and normal tissue in the treated area, whereas 3 months later, a regeneration was observable. The analysis also revealed that after ECT treatment, most vessels (>5 mm) and biliary structures were preserved.93 Due to close anatomical relation of the liver to the heart, cardiac arrhythmia can occur. Synchronized application of electrical pulses is therefore recommended to prevent this unintended effect.32 Internal solid organs and tissues lack the protective barrier of the skin and possess a relatively large electrical conductivity. This has to be taken into account while treatment planning, as the electrical current delivered during ECT can propagate through a larger volume of tissue surrounding the treated region and might interfere with the patient’s heart function.32,97 With synchronized application of EP, minor arrhythmias can occur (2.2%), so patients with epilepsy or previous myocardial infarction should be treated with caution.26,98,99
In a small clinical trial, patients received an open approach of ECT for the treatment of unresectable colorectal liver metastases. The obtained response rates 4 weeks after ECT were 55% as complete response and 45% stable disease.100 Additionally, an overall and progression-free survival at 6 months of 80% to 100% was achieved by the treatment. Another study evaluated the treatment response of HCC and concomitant Vp3-Vp4 portal vein tumor thrombus (PVTT) treated by percutaneous ECT. In 5 of 6 patients, complete necrosis of the PVTT could be observed, as well as cases with complete tumor necrosis by associated HCC nodules.26 These first results show the potential of percutaneous ECT in case of Vp3-Vp4 PVTT treatment. A recently published study94 tested, in a prospective pilot study on HCC, the effectiveness of ECT. They obtained a complete response rate of 88% per treated lesion (lesion size: 8-41 mm) and confirmed feasibility and safety, as no treatment or postoperative adverse events were recorded, even in cases with lesions located near the major hepatic vessels.
A recent study by Gasljevic et al 93 demonstrates regressive changes in the whole ECT-treated area of the liver. The detailed histopathological evaluation of the resected tumors in a specific group of patients who underwent 2-stage operation was shown. Regressive changes in ECT-treated liver metastases were evaluated after the second operation (in 8 ± 10 weeks) in 7 patients and 13 metastases when the treated metastases were resected. The ECT was able to induce coagulation necrosis in the treated area encompassing both tumor and a narrow band of normal tissue. The functionality of vessel larger than 5 mm was preserved at the time of observation, with evidence of disruption of vessels less than 5 mm. Regenerative changes in the peripheral parts of the treated area were seen 8 ± 10 weeks after ECT. These findings are important because it confirmed that ECT can be indicated for the therapy of metastases near major blood vessels in the liver to provide a safe approach with good antitumor efficacy.
Djokic et al 94 reported the results of a prospective study conducted in patients with HCC. Electrochemotherapy with BLM was performed on 17 HCCs in 10 patients. The complete response rate at 3 to 6 months was 80% per patient and 88% per treated lesion. Electrochemotherapy of HCC proved to be a feasible and safe treatment in all 10 patients. The results at average of 20.5 months after ECT show that in 15 out of 17 lesions complete response was obtained.
The obtained response rates by ECT treatment of hepatic lesions vary a lot. This might be caused by different sample sizes among studies, as well as individual treatment planning. As each lesion has a special size and structure, specific amplitude calculations and probe positioning need to be planned. Therefore, the results of the different groups can be hardly compared, as each intervention is biased by the involved operators. Also, needle placement differs in accuracy, even with imaging control, as some interventions are free handed and other based on assisting systems. Another restriction is the individual vascular structure of each hepatic lesion, which influences the distribution of the drugs in the tumor vascular system and extracellular space. Therefore, it is possible that big lesions lack in a sufficient concentration of the drug, as the distribution is insufficient or delayed at the time point of EP. An improvement in response rates can be obtained by accurate treatment simulations, which help to obtain correct amplitudes, as well as real-time feedback of transmembrane voltage, which prevents IRE or insufficient EP. Another point of improvement is the inclusion of navigation systems to minimize manual errors. A sufficient concentration of cytostatic drug can be obtained by a combination of intratumoral and intravenous injection. The successful establishment of ECT as hepatic lesion treatment can offer an additional minimally invasive treatment in the case of malignant lesions with decreased systemic side effects.
Conclusion
Electrochemotherapy for cutaneous and subcutaneous malignancies is already well established. Even if only few experiences have been published for colorectal liver metastases treated by ECT, considering the proven safety and the promising results, this treatment option deserves further attention. In the case of malignant hepatic lesions, different therapy options exist. A tumor resection leads to immediate absence of tumor tissue, in contrast (thermo)ablative procedures will induce necrotic damages whereas chemoablative procedures induce cell apoptosis. As patients with cancer suffer from multiple stress factors (psychical, illness, oncological treatment), a minimally invasive procedure can relieve associated symptoms, while decreasing interventional caused complaints. In contrast to chemoembolization techniques, ECT relies on membrane nonpermeant chemotherapeutics, which need EP for cell uptake. Improvements need to be done to achieve homogeneous EP in the treated area, as well as steady concentrations of cytostatic drug in big lesions. If this can be accomplished, it is possible that response rates achieved with ECT can exceed those achieved with TACE. For sure, ECT can induce tumor volume shrinkage, suggesting an implementation in clinical routine as neoadjuvant treatment to enhance future tumor resection.
Electrochemotherapy is usually applied in palliative settings for patients with unresectable tumors, resulting in amelioration of quality of life. In most cases, it is used in treatment of advanced neoplastic lesions in which radical surgical treatment is not possible (eg, due to lesion location, size, and/or number). Electrochemotherapy allows treating tumor nodules in the proximity of important structures like vessels and nerves. The safety profile of ECT is favorable. Most of the observed adverse events are local and transient, including moderate local pain, erythema, edema, and muscle contractions during EP. No serious adverse events or deaths related to ECT have been reported in studies conducted according to ESOPE SOPs. Electrochemotherapy is well accepted by patients, as indicated by the high percentage of those who would accept retreatment, if necessary.
Treatment of nonsuperficial nodules with ECT is at its early stages, either during laparotomy procedures or by percutaneous introduction of the electrodes. However, from an operational procedure, placement of multiple parallel electrodes can be difficult.
Limitations of ECT are the need for interventional individual electric pulse generating systems, individual electrode needles, and complex preinterventional planning. As the success of difficult interventions in deep-seated tumors will rely on accurate needle placement, robotic navigated systems as well as image guidance improve successful ECT treatment and minimize reintervention.
Percutaneous ECT of solid organs is a novel, potentially very effective treatment option in minimally invasive oncologic treatments. Further trials have to be conducted to establish best treatment algorithms (ie, strength and length of EPs, dosage, and type of chemotherapeutic agents) as well as to prove therapeutic efficacy for different tumor entities.
Abbreviations
- BLM
bleomycin
- CT
computed tomography
- ECT
electrochemotherapy
- EP
electroporation
- ESOPE
European Standard Operating Procedures for Electrochemotherapy and Electrogenetherapy
- HCC
hepatocellular carcinoma
- IRE
irreversible electroporation
- MRI
magnetic resonance imaging
- PVTT
portal vein tumor thrombus
- RFA
radiofrequency ablation
- STS
soft tissue sarcoma
- TACE
transarterial chemoembolization.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Irene Fuhrmann  http://orcid.org/0000-0001-9532-9973
http://orcid.org/0000-0001-9532-9973
References
- 1. Staempfli R, Willi M. Membrane potential of a Ranvier node measured after electrical destruction of its membrane. Experientia. 1957;13(7):297–298. [DOI] [PubMed] [Google Scholar]
- 2. Sale A, Hamilton W. Effects of high electric fields on microorganisms I. Killing of bacteria and yeasts. Biochim Biophys Acta. 1967;148(3):781–788. [Google Scholar]
- 3. Neumann E, Rosenheck K. Permeability changes induced by electric impulses in vesicular membranes. J Membr Biol. 1972;10(3):279–290. [DOI] [PubMed] [Google Scholar]
- 4. Weaver J, Chizmadzhev Y. Theory of electroporation: a review. Bioelectrochem Bioenerg. 1996;41(2):135–160. [Google Scholar]
- 5. Pliquett U, Langer R, Weaver J. Changes in the passive electrical properties of human stratum corneum due to electroporation. Biochim Biophys Acta. 1995;1239(2):111–121. [DOI] [PubMed] [Google Scholar]
- 6. Glaser R, Leikin S, Chernomordik L, Pastushenko V, Sokirko A. Reversible electrical breakdown of lipid bilayers: formation and evolution of pores. Biochim Biophys Acta. 1988;940(2):275–287. [DOI] [PubMed] [Google Scholar]
- 7. Kotnik T, Kramar P, Pucihar G, Miklavcic D, Tarek M. Cell membrane electroporation—part 1: the phenomenon. IEEE Electric Insul Mag. 2012;28(5):14–23. [Google Scholar]
- 8. Belehradek M, Domenge C, Luboinski B, Orlowski S, Belehradek J, Mir LM. Electrochemotherapy, a new antitumor treatment. First clinical phase I–II trial. Cancer. 1993;72(12):3694–3700. [DOI] [PubMed] [Google Scholar]
- 9. Calvet C, Andre F, Mir L. Dual therapeutic benefit of electroporation-mediated DNA vaccination in vivo. Oncoimmunology. 2014;3(4):e28540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ivanov MA, Lamrihi B, Szyf M, Scherman D, Bigey P. Enhanced antitumor activity of a combination of MBD2-antisense electrotransfer gene therapy and bleomycin electrochemotherapy. J Gene Med. 2003;5(10):893–899. [DOI] [PubMed] [Google Scholar]
- 11. Onik G, Mikus P, Rubinsky B. Irreversible electroporation: implications for prostate ablation. Technol Cancer Res Treat. 2007;6(4):295–300. [DOI] [PubMed] [Google Scholar]
- 12. Miller L, Leor J, Rubinsky B. Cancer cells ablation with irreversible electroporation. Technol Cancer Res Treat. 2005;4(6):699–705. [DOI] [PubMed] [Google Scholar]
- 13. Wiggermann P, Bruenn K, Baeumler W. . Irreversible electroporation (IRE): a minimally invasive therapeutic option in prostate cancer [in German]. Radiologe. 2017;57(8):637–640. [DOI] [PubMed] [Google Scholar]
- 14. Niessen C, Thumann S, Beyer L. et al. percutaneous irreversible electroporation: long-term survival analysis of 71 patients with inoperable malignant hepatic tumors. Sci Rep. 2017;7:43687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Orlowski S, Belehradek J, Jr, Paoletti C, Mir LM. Transient electropermeabilization of cells in culture. Increase of the cytotoxicity of anticancer drugs. Biochem Pharmacol. 1988;37(24):4727–4733. [DOI] [PubMed] [Google Scholar]
- 16. Gehl J, Skovsgaard T, Mir LM. Enhancement of cytotoxicity by electropermeabilization: an improved method for screening drugs. Anticancer Drugs. 1998;9(4):319–325. [DOI] [PubMed] [Google Scholar]
- 17. Jaroszeski M, Dang V, Pottinger C, Hickey J, Gilbert R, Heller R. Toxicity of anticancer agents mediated by electroporation in vitro. Anticancer Drugs. 2000;11(3):201–208. [DOI] [PubMed] [Google Scholar]
- 18. Mir LM, Orlowski S, Belehradek J, Paoletti C. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur J Cancer Clin Oncol. 1991;27(1):68–72. [DOI] [PubMed] [Google Scholar]
- 19. Postow M, Callahan M, Barker C, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366(10):925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grimaldi A, Simeone E, Giannarelli D, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology. 2014;3: e28780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gehl J. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand. 2003;177(4):437–447. [DOI] [PubMed] [Google Scholar]
- 22. Jarm T, Cemazar M, Miklavcic D, Sersa G. Antivascular effects of electrochemotherapy: implications in treatment of bleeding metastases. Expert Rev Anticancer Ther. 2010;10(5):729–746. [DOI] [PubMed] [Google Scholar]
- 23. Stam A, de Gruijl T. From local to systemic treatment: leveraging antitumor immunity following irreversible electroporation In: Meijerink MR, Scheffer HJ, Narayanan G, eds. Irreversible Electroporation in Clinical Practice. Cham, Switzerland: Springer International Publishing; 2018:249–270. [Google Scholar]
- 24. Marty M, Sersa G, Garbay J, et al. Electrochemotherapy—an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. EJC Suppl. 2006;4(11):3–13. [Google Scholar]
- 25. Mali B, Jarm T, Snoj M, Sersa G, Miklavcic D. Antitumor effectiveness of electrochemotherapy: a systematic review and meta-analysis. Eur J Surg Oncol. 2013;39(1):4–16. [DOI] [PubMed] [Google Scholar]
- 26. Tarantino L, Busto G, Nasto A, et al. Percutaneous electrochemotherapy in the treatment of portal vein tumor thrombosis at hepatic hilum in patients with hepatocellular carcinoma in cirrhosis: a feasibility study. World J Gastroenterol. 2017;23(5):906–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Quaglino P, Mortera C, Osella-Abate S, et al. Electrochemotherapy with intravenous bleomycin in the local treatment of skin melanoma metastases. Ann Surg Oncol. 2008;15(8):2215–2222. [DOI] [PubMed] [Google Scholar]
- 28. Eggermont A. Treatment of melanoma in-transit metastases confined to the limb. Cancer Surv. 1996;26:335–349. [PubMed] [Google Scholar]
- 29. Sersa G, Cufer T, Cemazar M, Rebersek M, Zvonimir R. Electrochemotherapy with bleomycin in the treatment of hypernephroma metastasis: case report and literature review. Tumori. 2000;86(2):163–165. [DOI] [PubMed] [Google Scholar]
- 30. Campana L, Mocellin S, Basso M, et al. Bleomycin-based electrochemotherapy: clinical outcome from a single institution’s experience with 52 patients. Ann Surg Oncol. 2009;16(1):191–199. [DOI] [PubMed] [Google Scholar]
- 31. Fini M, Salamanna F, Parrilli A, et al. Electrochemotherapy is effective in the treatment of rat bone metastases. Clin Exp Metas. 2013;30(8):1033–1045. [DOI] [PubMed] [Google Scholar]
- 32. Edhemovic I, Gadzijev E, Brecelj E, et al. Electrochemotherapy: a new technological approach in treatment of metastases in the liver. Technol Cancer Res Treat. 2011;10(5):475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Linnert M, Iversen H, Gehl J. Multiple brain metastases—current management and perspectives for treatment with electrochemotherapy. Radiol Oncol. 2012;46(4):271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perrone A, Cima S, Pozzati F, et al. Palliative electro-chemotherapy in elderly patients with vulvar cancer: a phase II trial. J Surg Oncol. 2015;112(5):529–532. [DOI] [PubMed] [Google Scholar]
- 35. Pellegrino A, Damiani G, Mangioni C, et al. Outcomes of bleomycin-based electrochemotherapy in patients with repeated loco-regional recurrences of vulvar cancer. Acta Oncol. 2016;55(5):619–624. [DOI] [PubMed] [Google Scholar]
- 36. Cadossi R, Ronchetti M, Cadossi M. Locally enhanced chemotherapy by electroporation: clinical experiences and perspective of use of electrochemotherapy. Future Oncol. 2014;10(5):877–890. [DOI] [PubMed] [Google Scholar]
- 37. Sersa G, Cemazar M, Miklavcic D. Tumor blood flow modifying effects of electrochemotherapy: a potential vascular targeted mechanism. Radiol Oncol. 2003;37(1):43–48. [Google Scholar]
- 38. Mali B, Miklavcic D, Campana L, et al. Tumor size and effectiveness of electrochemotherapy. Radiol Oncol. 2013;47(1):32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miklavcic D, Snoj M, Zupanic A, et al. Towards treatment planning and treatment of deep-seated solid tumors by electrochemotherapy. Biomed Eng Online. 2010;9:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pavliha D, Kos B, Zupanic A, Marcan M, Sersa G, Miklavcic D. Patient-specific treatment planning of electrochemotherapy: procedure design and possible pitfalls. Bioelectrochemistry. 2012;87:265–273. [DOI] [PubMed] [Google Scholar]
- 41. Mir LM, Gehl J, Sersa G, et al. Standard operating procedures of the electrochemotherapy: instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the Cliniporator (TM) by means of invasive or non-invasive electrodes. Eur J Cancer Suppl. 2006;4:14–25. [Google Scholar]
- 42. Heller R, Jaroszeski M, Glass L, et al. Phase I/II trial for the treatment of cutaneous and subcutaneous tumors using electrochemotherapy. Cancer. 1996;77(5):964–971. [DOI] [PubMed] [Google Scholar]
- 43. Marcan M, Kos B, Miklavcic D. Effect of blood vessel segmentation on the outcome of electrochemotherapy of liver tumors—preliminary theoretical study In: Lacković I, Vasic D, eds. 6th European Conference of the International Federation for Medical and Biological Engineering: MBEC 2014, 7–11 September 2014, Dubrovnik, Croatia. Cham, Switzerland: Springer International Publishing; 2015:825–828. [Google Scholar]
- 44. Beyer L, Wiggermann P. Treatment planning, needle insertion, image guidance, and endpoint assessment In: Meijerink M, Scheffer H, Narayanan G, eds. Irreversible Electroporation in Clinical Practice. Cham, Switzerland: Springer International Publishing; 2018:115–120. [Google Scholar]
- 45. Beyer L, Wiggermann P. Planning and guidance: new tools to enhance the human skills in interventional oncology. Diagn Interv Imaging. 2017;98(9):583–588. [DOI] [PubMed] [Google Scholar]
- 46. Wood B, Kruecker J, Abi-Jaoudeh N, et al. Navigation systems for ablation. J Vasc Interv Radiol. 2010;21(suppl 8):S257–S263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Groselj A, Kos B, Cemazar M, et al. Coupling treatment planning with navigation system: a new technological approach in treatment of head and neck tumors by electrochemotherapy. Biomed Eng Online. 2015;14(suppl 3):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reinhold U. Electrochemotherapy of skin tumors [in German]. Hautarzt. 2011;62(7):549–558; quiz 559. [DOI] [PubMed] [Google Scholar]
- 49. Skarlatos I, Kyrgias G, Mosa E, et al. Electrochemotherapy in cancer patients: first clinical trial in Greece. In vivo. 2011;25(2):265–274. [PubMed] [Google Scholar]
- 50. Munoz M, Ortega P. Electrochemotherapy for treatment of skin and soft tissue tumours. Update and definition of its role in multimodal therapy. Clin Transl Oncol. 2011;13(1):18–24. [DOI] [PubMed] [Google Scholar]
- 51. Testori A, Tosti G, Martinoli C, et al. Electrochemotherapy for cutaneous and subcutaneous tumor lesions: a novel therapeutic approach. Dermatol Ther. 2010;23(6):651–661. [DOI] [PubMed] [Google Scholar]
- 52. Carrera C, Bennassar A, Ishioka P, et al. Desmoplastic melanoma on the nose: electrochemotherapy as an alternative treatment to local advanced disease. J Eur Acad Dermatol Venereol. 2014;28(4):424–432. [DOI] [PubMed] [Google Scholar]
- 53. Wiater K, Zdzienicki M, Morysinski T, et al. Effective treatment of recurrent, advanced dermatofibrosarcoma protuberans by electrochemotherapy. Eur J Dermatol. 2013;23(2):260–261. [DOI] [PubMed] [Google Scholar]
- 54. Curatolo P, Rotunno R, Miraglia E, Mancini M, Calvieri S, Giustini S. Complete remission of Merkel cell carcinoma treated with electrochemotherapy and etoposide. G Ital Dermatol Venereol. 2013;148(3):310–311. [PubMed] [Google Scholar]
- 55. Bakonyi M, Berko S, Eros G, et al. A review of electroporation-based antitumor skin therapies and investigation of betulinic acid-loaded ointment [published online ahead of print November 13, 2017]. Anticancer Agents Med Chem. 2017. [DOI] [PubMed] [Google Scholar]
- 56. Bourke M, Salwa S, Sadadcharam M, et al. Effective treatment of intractable cutaneous metastases of breast cancer with electrochemotherapy: ten-year audit of single centre experience. Breast Cancer Res Treat. 2017;161(2):289–297. [DOI] [PubMed] [Google Scholar]
- 57. Plaschke C, Bertino G, McCaul J, et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) Project: results from the treatment of mucosal cancers. Eur J Cancer. 2017;87:172–181. [DOI] [PubMed] [Google Scholar]
- 58. Bertino G, Sersa G, De Terlizzi F, et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) Project: results of the treatment of skin cancer. Eur J Cancer. 2016;63:41–52. [DOI] [PubMed] [Google Scholar]
- 59. Campana L, Valpione S, Mocellin S, et al. Electrochemotherapy for disseminated superficial metastases from malignant melanoma. Br J Surg. 2012;99(6):821–830. [DOI] [PubMed] [Google Scholar]
- 60. Kunte C, Letule V, Gehl J, et al. Electrochemotherapy in the treatment of metastatic malignant melanoma: a prospective cohort study by InspECT. Br J Dermatol. 2017;176(6):1475–1485. [DOI] [PubMed] [Google Scholar]
- 61. Caraco C, Mozzillo N, Marone U, et al. Long-lasting response to electrochemotherapy in melanoma patients with cutaneous metastasis. BMC Cancer. 2013;13:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gerlini G, Di Gennaro P, Borgognoni L. Enhancing anti-melanoma immunity by electrochemotherapy and in vivo dendritic-cell activation. Oncoimmunology. 2012;1(9):1655–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dolinsek T, Prosen L, Cemazar M, Potocnik T, Sersa G. Electrochemotherapy with bleomycin is effective in BRAF mutated melanoma cells and interacts with BRAF inhibitors. Radiol Oncol. 2016;50(3):274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Valpione S, Campana L, Pigozzo J, Chiarion-Sileni V. Consolidation electrochemotherapy with bleomycin in metastatic melanoma during treatment with dabrafenib. Radiol Oncol. 2015;49(1):71–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Heppt M, Eigentler T, Kahler K, et al. Immune checkpoint blockade with concurrent electrochemotherapy in advanced melanoma: a retrospective multicenter analysis. Cancer Immunol Immunother. 2016;65(8):951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Theurich S, Rothschild S, Hoffmann M, et al. Local tumor treatment in combination with systemic ipilimumab immunotherapy prolongs overall survival in patients with advanced malignant melanoma. Cancer Immunol Res. 2016;4(9):744–754. [DOI] [PubMed] [Google Scholar]
- 67. Zupanic A, Kos B, Miklavcic D. Treatment planning of electroporation-based medical interventions: electrochemotherapy, gene electrotransfer and irreversible electroporation. Phys Med Biol. 2012;57(17):5425–5440. [DOI] [PubMed] [Google Scholar]
- 68. Cadossi R, Valeria D, Roberta F, Ronchetti M. Minimally invasive treatment by electroporation: remaining challenges In: Miklavcic D, ed. Handbook of Electroporation. Cham, Switzerland: Springer; 2016. [Google Scholar]
- 69. Jaroszeski M, Illingworth P, Pottinger C, Hyacinthe M, Heller R. Electrically mediated drug delivery for treating subcutaneous and orthotopic pancreatic adenocarcinoma in a hamster model. Anticancer Res. 1999;19(2a):989–994. [PubMed] [Google Scholar]
- 70. Girelli R, Prejano S, Cataldo I, et al. Feasibility and safety of electrochemotherapy (ECT) in the pancreas: a pre-clinical investigation. Radiol Oncol. 2015;49(2):147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Granata V, Fusco R, Setola S, et al. Early radiological assessment of locally advanced pancreatic cancer treated with electrochemotherapy. World J Gastroenterol. 2017;23(26):4767–4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tafuto S, von Arx C, De Divitiis C, et al. Electrochemotherapy as a new approach on pancreatic cancer and on liver metastases. Int J Surg. 2015;21(suppl 1): S78–S82. [DOI] [PubMed] [Google Scholar]
- 73. Bianchi G, Campanacci L, Rimondi E, Ruggieri P. Palliative treatments: electrochemotherapy and thermoablation [published ahead of print March 21, 2012]. Adv Bone Metastasis Manag. doi: 10.2217/ebo.11.362. [Google Scholar]
- 74. Bianchi G, Campanacci L, Ronchetti M, Donati D. Electrochemotherapy in the treatment of bone metastases: a phase II trial. World J Surg. 2016;40(12):3088–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gasbarrini A, Campos W, Campanacci L, Boriani S. Electrochemotherapy to metastatic spinal melanoma: a novel treatment of spinal metastasis? Spine (Phila Pa 1976). 2015;40(24): E1340–E1346. [DOI] [PubMed] [Google Scholar]
- 76. Guideline SIOT 2016. http://www.siot.it/pagine/soci/linee-guida.html. Accessed March 14, 2018.
- 77. Valpione S, Campana LG, Rastrelli M, et al. Phase I/II study of electrochemotherapy with intravenous bleomycin and variable geometry electric fields for the treatment of deep and large soft tissue tumors. Ann Oncol. 2014;25(suppl 4):iv510–iv510. [Google Scholar]
- 78. Campana L, Cesari M, Dughiero F, et al. Electrical resistance of human soft tissue sarcomas: an ex vivo study on surgical specimens. Med Biol Eng Comput. 2016;54(5):773–787. [DOI] [PubMed] [Google Scholar]
- 79. Simon C, Dupuy D, Mayo-Smith W. Microwave ablation: principles and applications. Radiographics. 2005;25(suppl 1):S69–S83. [DOI] [PubMed] [Google Scholar]
- 80. Gazelle G, Goldberg S, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology. 2000;217(3):633–646. [DOI] [PubMed] [Google Scholar]
- 81. Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality—clinical implications. Technol Cancer Res Treat. 2007;6(1):37–48. [DOI] [PubMed] [Google Scholar]
- 82. Kamel Abdel Aal A, Moawad S, Jackson B, et al. Tumor response and survival outcomes of very small drug-eluting beads used in trans-arterial chemoembolization for unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2017;28(2): S124–S125. [DOI] [PubMed] [Google Scholar]
- 83. Camma C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224(1):47–54. [DOI] [PubMed] [Google Scholar]
- 84. Okuyama H, Ikeda M, Takahashi H, et al. Transarterial (chemo)embolization for liver metastases in patients with neuroendocrine tumors. Oncology. 2017;92(6):353–359. [DOI] [PubMed] [Google Scholar]
- 85. Vogl T, Mohamed S, Albrecht M, et al. Transarterial chemoembolization in pancreatic adenocarcinoma with liver metastases: MR-based tumor response evaluation, apparent diffusion coefficient (ADC) patterns, and survival rates. Pancreatology. 2018;18(1):94–99. [DOI] [PubMed] [Google Scholar]
- 86. Massmann A, Rodt T, Marquardt S, et al. Transarterial chemoembolization (TACE) for colorectal liver metastases—current status and critical review. Langenbecks Arch Surg. 2015;400(6):641–659. [DOI] [PubMed] [Google Scholar]
- 87. Jaroszeski M, Gilbert R, Heller R. In vivo antitumor effects of electrochemotherapy in a hepatoma model. Biochim Biophys Acta. 1997;1334(1):15–18. [DOI] [PubMed] [Google Scholar]
- 88. Chazal M, Benchimol D, Baque P, Pierrefite V, Milano G, Bourgeon A. Treatment of hepatic metastases of colorectal cancer by electrochemotherapy: an experimental study in the rat. Surgery. 1998;124(3):536–540. [PubMed] [Google Scholar]
- 89. Jaroszeski M, Coppola D, Nesmith G, et al. Effects of electrochemotherapy with bleomycin on normal liver tissue in a rat model. Eur J Cancer. 2001;37(3):414–421. [DOI] [PubMed] [Google Scholar]
- 90. Ramirez L, Orlowski S, An D, et al. Electrochemotherapy on liver tumours in rabbits. Br J Cancer. 1998;77(12):2104–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Edhemovic I, Brecelj E, Gasljevic G, et al. Intraoperative electrochemotherapy of colorectal liver metastases. J Surg Oncol. 2014;110(3):320–327. [DOI] [PubMed] [Google Scholar]
- 92. Mali B, Gorjup V, Edhemovic I, et al. Electrochemotherapy of colorectal liver metastases—an observational study of its effects on the electrocardiogram. Biomed Eng Online. 2015;14(suppl 3):S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gasljevic G, Edhemovic I, Cemazar M, et al. Histopathological findings in colorectal liver metastases after electrochemotherapy. PLos One. 2017;12(7):e0180709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Djokic M, Cemazar M, Popovic P, et al. Electrochemotherapy as treatment option for hepatocellular carcinoma, a prospective pilot study. Eur J Surg Oncol. 2018;44(5):651–657. [DOI] [PubMed] [Google Scholar]
- 95. Ivorra A, Rubinsky B. In vivo electrical impedance measurements during and after electroporation of rat liver. Bioelectrochemistry. 2007;70(2):287–295. [DOI] [PubMed] [Google Scholar]
- 96. Sersa G, Jarm T, Kotnik T, et al. Vascular disrupting action of electroporation and electrochemotherapy with bleomycin in murine sarcoma. Br J Cancer. 2008;98(2):388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Deodhar A, Dickfeld T, Single G, et al. Irreversible electroporation near the heart: ventricular arrhythmias can be prevented with ECG synchronization. Am J Roentgenol. 2011;196(3): W330–W335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nielsen K, Scheffer H, van den Tol M, Nilsson A. Irreversible electroporation of liver tumors In: Meijerink MR, Scheffer HJ, Narayanan G, eds. Irreversible Electroporation in Clinical Practice. Cham, Switzerland: Springer International Publishing; 2018:139–166. [Google Scholar]
- 99. Scheffer H, Nielsen K, de Jong M, et al. Irreversible electroporation for nonthermal tumor ablation in the clinical setting: a systematic review of safety and efficacy. J Vasc Interv Radiol. 2014;25(7):997–1011. [DOI] [PubMed] [Google Scholar]
- 100. Coletti L, Battaglia V, De Simone P, Turturici L, Bartolozzi C, Filipponi F. Safety and feasibility of electrochemotherapy in patients with unresectable colorectal liver metastases: a pilot study. Int J Surg. 2017;44:26–32. [DOI] [PubMed] [Google Scholar]