Abstract
Background
Histamine is a well-known mediator involved in skin allergic responses through up-regulation of pro-inflammatory cytokines. Antihistamines remain the mainstay of allergy treatment, but they were found limited in efficacy and associated with several common side effects. Therefore, alternative therapeutic preferences are derived from natural products in an effort to provide safe yet reliable anti-inflammatory agents. Curcumin and their derivatives are among compounds of interest in natural product research due to numerous pharmacological benefits including anti-inflammatory activities. Here, we investigate the effects of chemically synthesized curcumin derivative, 2,6-bis(2-fluorobenzylidene)cyclohexanone (MS65), in reducing cytokine production in histamine-induced HaCaT cells.
Methods
Interleukin (IL)-6 cytokine production in histamine-induced HaCaT cells were measured using enzyme-linked immunosorbent assay (ELISA) and cytotoxicity effects were determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Real-time polymerase chain reaction (RT-qPCR) was carried out to determine the inhibitory effects of MS65 on nuclear factor-kappa B (NF-κB) and mitogen activated protein kinase (MAPK) pathways.
Results
Histamine enhanced IL-6 production in HaCaT cells, with the highest production of IL-6 at 97.41 ± 2.33 pg/mL after 24 h of exposure. MS65 demonstrated a promising anti-inflammatory activity by inhibiting IL-6 production with half maximal inhibitory concentration (IC50) value of 4.91 ± 2.50 μM and median lethal concentration (LC50) value of 28.82 ± 7.56 μM. In gene expression level, we found that MS65 inhibits NF-κB and MAPK pathways through suppression of IKK/IκB/NFκB and c-Raf/MEK/ERK inflammatory cascades.
Conclusion
Taken together, our results suggest that MS65 could be used as a lead compound on developing new medicinal agent for the treatment of allergic skin diseases.
Keywords: Histamine, Interleukin-6, Curcumin derivative, Keratinocytes
Background
Allergic skin diseases generate considerable dermatologist concern due to their increases in prevalence, severity, and complexity [1]. Histamine, a biological amine, has been identified as a major mediator of inflammation and allergic response that regulates the expression of cytokine, chemokines and cell-adhesion molecules [2–4]. The release of histamine in the skin causes a variety of allergic reactions which include redness, itching as well as wheal and flare due to vasodilation and increase in vascular permeability [5]. The exposure of histamine to keratinocytes, the main cells of epidermis (outermost layer of the skin), lead to the formation of an impaired skin barrier, which initiates the expression of proinflammatory molecules that represents the starting point of primary skin inflammation [6, 7].
Histamine exerts its proinflammatory effects mainly through four different receptors (H1, H2, H3 and H4), but mediated mostly by the activation of H1 receptors (H1Rs) in allergic diseases [8]. The binding of histamine to H1R results in phosphorylation of protein kinase C (PKC) and downstream activation of NF-κB and MAPK transcription factors, which are associated with regulation of adhesion molecules, chemotaxis, antigen presentation and proinflammatory cytokine production [9, 10]. IL-6 cytokine is elevated in most inflammatory conditions and have been recognized as targets of therapeutic intervention [11]. The production of IL-6 in human epidermal keratinocytes has been widely studied due to its prominent role in various inflammatory skin diseases. In recent years, there is evidence on IL-6 production in human keratinocytes stimulated by a wide range of inducers such as IL-α, toll-like receptor (TLR) ligands and ultraviolet (UV) radiation [12, 13]. Interestingly, Matsubara et al. [10] has demonstrated the upregulation of IL-6 expression by keratinocytes in response to histamine exposure, suggesting that IL-6 is involved in the pathogenesis of skin allergies.
Considering the roles of H1R in mediating proinflammatory effects of histamine, the therapeutic intervention in allergic disorders has thus commonly focused on developing the antagonists of this receptor [14]. H1 antihistamines, also called H1 antagonists, remain as first-line medications for the treatment of allergic diseases due to their effectiveness in providing symptomatic relief [15]. The action of H1 antihistamines are associated with the suppression of cytokines, chemokines and adhesion molecules transcription [10, 16]. However, increasing evidence have shown that administration of H1 antihistamines is limited in efficacy and associated with a number of side-effects such as nausea, lightheadedness, drowsiness, headaches, agitation and dry mouth [15, 17]. Thus, the discovery of alternative anti-inflammatory agents that are more effective and safe for treatment of allergic skin disorders is of utmost important.
On natural preference of the treatment, a wide spectrum of phytochemicals and their derivatives have been identified for their potential as anti-inflammatory agents. Curcumin and its derivatives have attracted increasing interest due to numerous pharmacological benefits such as anticancer, antioxidant, antimalarial and anti-inflammatory activities [18]. The anti-inflammatory effects of curcumin are mainly mediated through regulation numerous transcription factors, cytokines, protein kinases, adhesion molecules, redox status and enzymes that have been linked to inflammation [19]. The versatility and flexibility for structural modification of natural and synthetic derivatives of curcumin have been explored extensively for designing new medicinal agents with improved potency and lesser toxicity [18]. Therefore, the aim of this work was to investigate the potential inhibitory effects of synthesized curcumin derivative (MS65) on IL-6 production in histamine-induced HaCaT cell line.
Methods
Chemicals and reagents
Curcumin derivative, 2,6-bis(2-fluorobenzylidene)cyclohexanone (MS65) was obtained from Laboratory of Natural Products, Institute of Bioscience (UPM) in a form of powder, which was dissolved in 100% dimethyl sulfoxide (DMSO) purchased from Qrec (Asia) Sdn. Bhd. (Selangor, Malaysia). Histamine and Dulbecco’s Modified Eagle’s Medium (DMEM) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Fetal bovine serum (FBS) and penicillin/streptomycin were purchased from PAA Laboratories GmbH (Austria). Levocetirizine dihydrochloride (H1 antihistamine) was purchased from Abcam (Cambridge, MA, USA) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent was purchased from Fluka Analytical (St. Louis, MO, USA). Phosphate buffered saline (PBS) tablets were purchased from Amresco Inc. (Solon, OH, USA). All the primers were obtained from First BASE Laboratories Sdn. Bhd. (Selangor, Malaysia).
Cell culture and induction
HaCaT cells obtained from AddexBio (San Diego, CA, USA) were cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin in a 5% CO2 incubator at 37 °C. HaCaT cells (2 × 104 cells/well) were then incubated in 96-well plates with various concentrations of histamine (0.1, 1, 10 and 100 μM) at different incubation hours (12, 24, 36 and 48 h). Cell culture supernatants were collected and kept in − 20 °C for determination of IL-6 production.
Cell treatments
HaCaT cells (2 × 104 cells/well) were induced with histamine and treated with MS65 (50 μM) and H1 antihistamine (20 μM) in two-fold serial dilutions for 24 h at 37 °C in fully humidified air incubator. Cell culture supernatants were then collected and analysed for IL-6 production.
Cytotoxicity assay
Cell viability was assessed using MTT assay, in which culture media of histamine-induced HaCaT cells and MS65-treated HaCaT cells were removed and replaced with 5 mg/mL MTT reagent. The cells containing MTT solution were then further incubated at 37 °C for 4 h before removing the solutions. The formazan crystals formed were dissolved in DMSO and absorbance was read at 570 nm using a microplate reader.
Measurement of IL-6 production
Enzyme-linked immunosorbent assay (ELISA) was performed using kit from eBioscience (San Diego, CA, USA). Briefly, ELISA plate (96-well) was coated with coating buffer containing capture antibody and incubated overnight at 4 °C. After washing with PBS containing 0.05% tween-20, the plate was blocked with assay diluent for 1 h at room temperature (RT). Samples and IL-6 standards were then added to the respective wells in the plate and further incubated for 2 h at RT. The plate was washed and detection antibody was added to each well for 1 h at RT. The washing step was repeated followed by addition of enzyme and incubated for 30 min at RT. The plate was then washed and substrate solution was added to each well for 15 min at RT in the dark. Stop solution was added to each well and absorbance was immediately read at 450 nm.
RNA extraction and reverse transcription
HaCaT cells were harvested and total RNA was extracted using RNeasy® Plus Mini kit from Qiagen (Hilden, Germany). The concentration and purity of RNA was determined using a NanoDrop 2000c UV-Vis Spectrophotometers from Thermo Fisher Scientific (Waltham, MA, USA). cDNA was prepared from 500 ng of total RNA using 5× iScript Reverse Transcription Supermix for RT-qPCR from Bio-Rad Laboratories (Hercules, CA, USA). The list of primers used in this study is shown in Table 1.
Table 1.
Primers sequences of target genes
| Genes | Primer sequences (5′-3′) | Accession number | |
|---|---|---|---|
| H1R | F R |
CTG GTT TCT CTC TTT TCT GTG GGT T GAT CTT GGC ATA GAA CCA GAG CAT G |
NM_001098213.1 |
| PKC | F R |
CAG GCA GAA ATT CGA GAA AGC CAA A TCC CAA CAC CAT GAG GAA ATT GAA G |
NM_002737.2 |
| IKK-β | F R |
ATC CCC GAT AAG CCT GCC A CTT GGG CTC TTG AAG GAT ACA G |
NM_001556.2 |
| IκB-α | F R |
ATT GCT GAG GCA CTT CTG GGA GCT G AGA CAC GTG TGG CCA TTG TAG TTG G |
NM_020529.2 |
| NF-κβ | F R |
GGA CCG CTG CAT CCA CAG TTT CCA G TGT CAC CTG GAA GCA GAG CCG CAC A |
NM_001145138.1 |
| c-Raf | F R |
CAG TAT CTG GGA CCC AGG AGA AAA A TAG GAT CTT TAC TGC AAC ATC TCC G |
NM_002880.3 |
| MEK | F R |
CGT ACA TCG TGG GCT TCT ATG GTG C GCA TGA TCT TGT GCT TCT CCC TCA G |
NM_002755.3 |
| ERK | F R |
CTC TAC CAG ATC CTC AGA GGG TTA A TAC CAA CGT GTG GCC ACA TAT TCT G |
NM_002745.4 |
| GAPDH | F R |
CAG CCT CAA GAT CAT CAG CA CAT CCA CAG TCT TCT GGG TG |
NM_002046.5 |
H1R histamine H1-receptor, PKC protein kinase, IKK-β inhibitor of nuclear factor kappa-B kinase subunit beta, IκB-α Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha, NF-κβ nuclear factor kappa-light-chain-enhancer of activated B cells, c-Raf RAF proto-oncogene serine/threonine-protein kinase, MEK mitogen-activated protein kinase kinase, ERK extracellular signal–regulated kinases, GAPDH glyceraldehyde 3-phosphate dehydrogenase
Real-time polymerase chain reaction (RT-qPCR)
Real-time quantitative PCR was performed on a real time PCR instrument, Bio-Rad® CFX96™. In this assay, iTaq™ Universal SYBR® Green Supermix (2×) kit from Bio-Rad Laboratories (Hercules, CA, USA) was used to determine genes expressions of H1R, PKC, IKK-β, IκB-α, NF-κB, c-Raf, MEK and ERK in histamine-induced HaCaT cells treated with MS65. Reaction setup of mastermix preparation was performed according to manufacturer’s protocol. In brief, assay mastermix was prepared by adding all required components (except cDNA template) according to the suggested volume of total 10 μL per reaction. The 9.5 μL of reaction mixtures was equally distributed into each recipient PCR tubes in triplicate prior to addition of 0.5 μL cDNA template. As for thermal cycling set up, the following PCR settings were used: an initial activation 95 °C for 3 min, followed by denaturation 95 °C for 10 s and annealing/extension at respective temperatures for 30 s (40 cycles). Then, samples were gradually heated from 70 °C to 95 °C with a ramp rate of 0.5 °C/s to obtain melting curves and fusion temperatures of the amplicons.
Kinetic analysis was measured by normalizing amplification threshold cycle (CT) values of template samples with CT values of template reference gene (GAPDH). RT-qPCR results were analyzed via relative quantification whereby expressions levels of samples were analyzed in relative amount (fold differences). In this study, induced control was used as calibrator and all samples were analyzed as increased or decreased folds in relative to calibrator by using Livak method [20] with calculation formula of 2-∆∆CT (normalized expression ratio).
The formula was derived from;
Statistical data analysis
All the results obtained were presented as mean ± standard error of mean (S.E.M) of three independent experiments unless otherwise stated. The differences between groups were determined by using one-way analysis of variance (ANOVA) followed by Dunnett test. The values of *P < 0.05, **P < 0.01 and ***P < 0.001 were considered significantly different from control group. Log IC50 calculations were performed using the built-in algorithms for dose-response curves with variable slope using Graphpad Prism software. All graphs in this study were generated by using GraphPad Prism version 7.0 (GraphPad Software, Inc.).
Results
Effect of histamine on cells viability and IL-6 production in HaCaT cells
We first evaluate the effect of histamine on HaCaT cells viability using MTT assay. Result shows that histamine demonstrated non-significant cytotoxic effect at all concentrations tested with cells viability more than 90% (Fig. 1a). Next, we examined whether histamine stimulates IL-6 production in HaCaT cells using an ELISA. As shown in Fig. 1b, histamine enhances IL-6 protein expression, with the highest production of 97.41 ± 1.65 pg/mL measured at concentration of 10 μM after 24 h incubation.
Fig. 1.
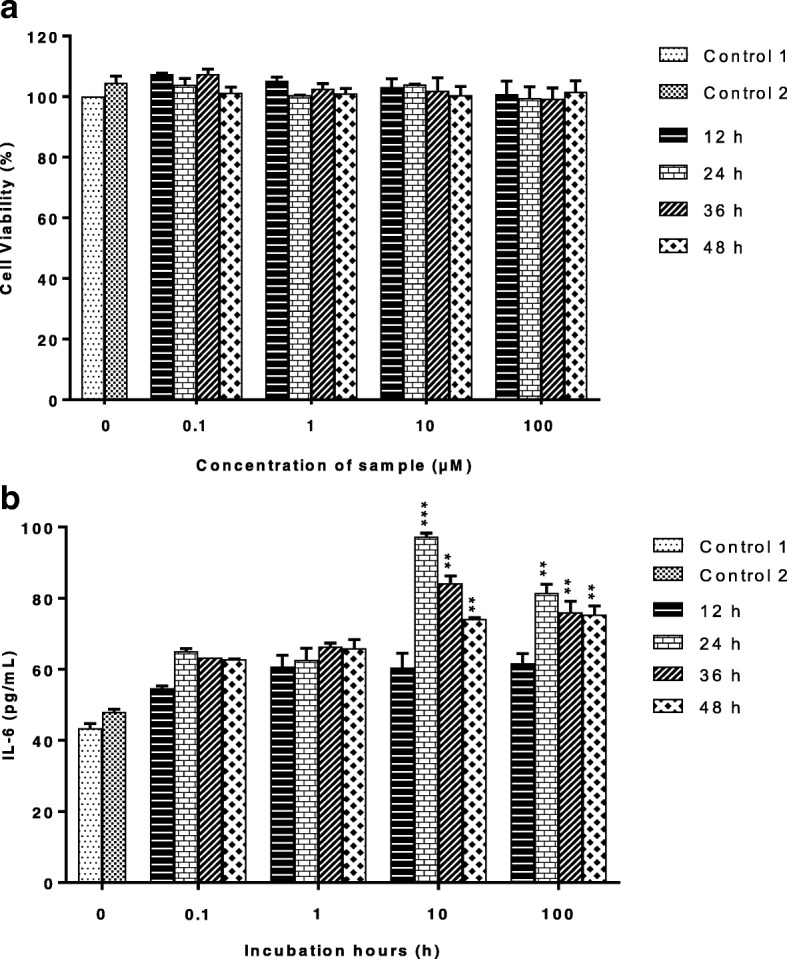
Effects of histamine on (a) cells viability and (b) IL-6 production in HaCaT cells. Cells were tested in the presence or absence of histamine. Cells were seeded for 24 h before inducing with different concentrations of histamine (0.1-100 μM). Cells were then further incubated for 0-48 h. C1; uninduced cells in DMEM only. C2; uninduced cells with 0.1% DMSO was set as negative control. All values are the mean ± S.E.M. of three independent experiments. Values of **P < 0.01 and ***P < 0.001 were considered significantly different to uninduced control group (C2)
MS65 inhibits IL-6 production in histamine-induced HaCaT cells
In order to evaluate the anti-inflammatory activity of MS65, we examined the effect of MS65 on IL-6 production and HaCaT cells viability in two-fold serial dilutions. As shown in Fig. 2, IL-6 production was dose-dependently down-regulated by MS65, in which MS65 showed inhibition at all concentrations tested with IC50 value of 4.91 ± 2.50 μM. The cytotoxicity assay shows that MS65 demonstrated non-significant cytotoxicity effect at low concentrations tested (0.78, 1.56 and 6.25 μM); however, HaCaT cell viability started to decrease at concentration of 12.5 μM (69.20 ± 5.06%), followed by 25 μM (56.09 ± 3.95%) and 50 μM (45.42 ± 6.93%), suggesting that MS65 at concentration of 6.25 μM and below were safe to be used on HaCaT cells due to high cell viability (more than 80%).
Fig. 2.
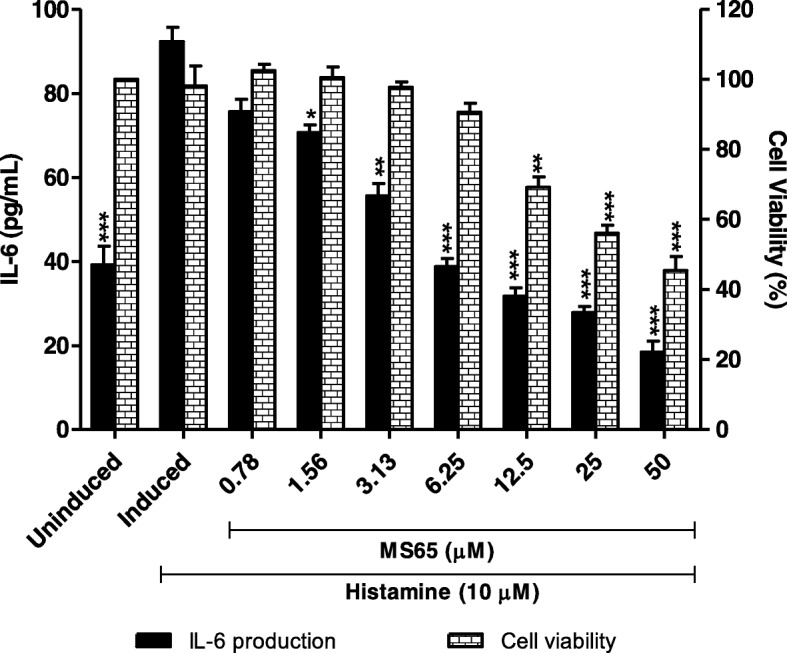
Effects of curcumin derivative, MS65 on IL-6 production and cells viability in histamine-induced HaCaT cells. Cells were seeded for 24 h before inducing with 10 μM of histamine. MS65 was tested in two-fold serial dilution with the highest concentration of 50 μM. Cells were then further incubated for 24 h. All values are the mean ± S.E.M. of three independent experiments. Values of *P < 0.05, **P < 0.01 and ***P < 0.001 were considered significantly different to histamine-induced control group
H1 antihistamine inhibits IL-6 production with high cytotoxicity effects in histamine-induced HaCaT cells
In this study, the effect of H1 antihistamine on IL-6 production and HaCaT cells viability were evaluated as a comparison with MS65. As shown in Fig. 3, IL-6 production was dose-dependently down-regulated by H1 antihistamine at all concentrations tested with IC50 value of 1.91 ± 1.80 μM. In cytotoxicity assay, result shows that H1 antihistamine demonstrated significant cytotoxicity effect at concentration of 2.5 μM and above. Therefore, it can be suggested that H1 antihistamine demonstrated lower IL-6 inhibition and higher cytotoxicity effects (LC50 value of 2.90 ± 1.50 μM) compared to MS65 (LC50 value of 28.82 ± 7.56 μM).
Fig. 3.
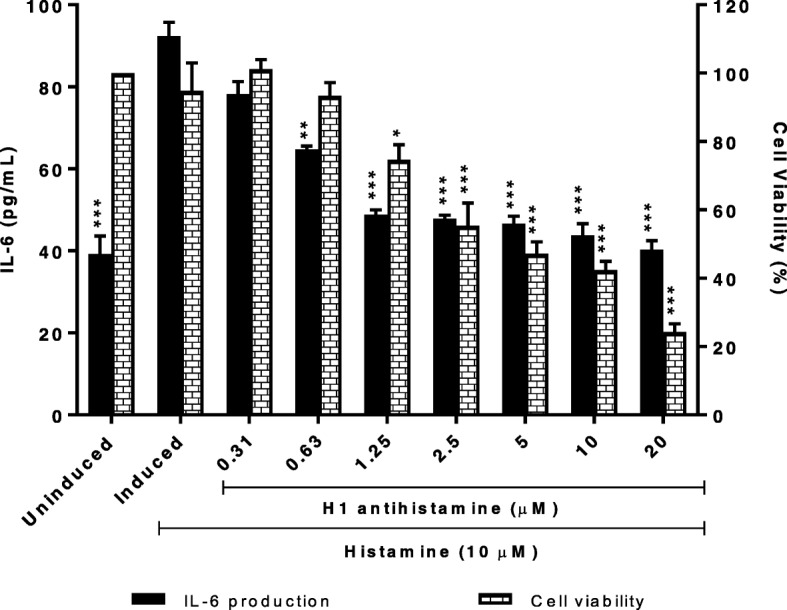
Effects of H1 antihistamine on IL-6 production and cells viability of histamine-induced HaCaT cells. Cells were seeded for 24 h before inducing with 10 μM of histamine. H1 antihistamine was tested in two-fold serial dilution with the highest concentration of 20 μM. Cells were then futher incubated for 24 h. All values are the mean ± S.E.M. of three independent experiments. Values of *P < 0.05, **P < 0.01 and ***P < 0.001 were considered significantly different to histamine-induced control group
MS65 suppresses gene expression of H1R, PKC, IKK-β, IκB-α, NF-κβ, c-Raf, MEK and ERK
In order to determine the effects of MS65 on H1R-dependent NF-κβ and MAPK pathway, we evaluated the gene expression of H1R, PKC, IKK-β, IκB-α, NF-κβ, c-Raf, MEK and ERK in MS65-treated histamine-induced HaCaT cells in five-fold serial dilutions (0.25, 1.25 and 6.25 μM) (Fig. 4a and b). In this study, histamine-induced HaCaT cells treated with MS65 showed decrement on all the genes tested with highest decrement at concentration of 6.25 μM. H1R gene demonstrated highest reduction of gene expression at 6.25 μM (0.23 ± 0.02 fold expression), followed by NF-ĸB (0.41 ± 0.06 fold expression), IKK-β (0.48 ± 0.04 fold expression), ERK (0.49 ± 0.09 fold expression), IκB-α (0.57 ± 0.05 fold expression), MEK (0.64 ± 0.06 fold expression), c-Raf (0.77 ± 0.14 fold expression) and PKC (0.77 ± 0.09 fold expression).
Fig. 4.
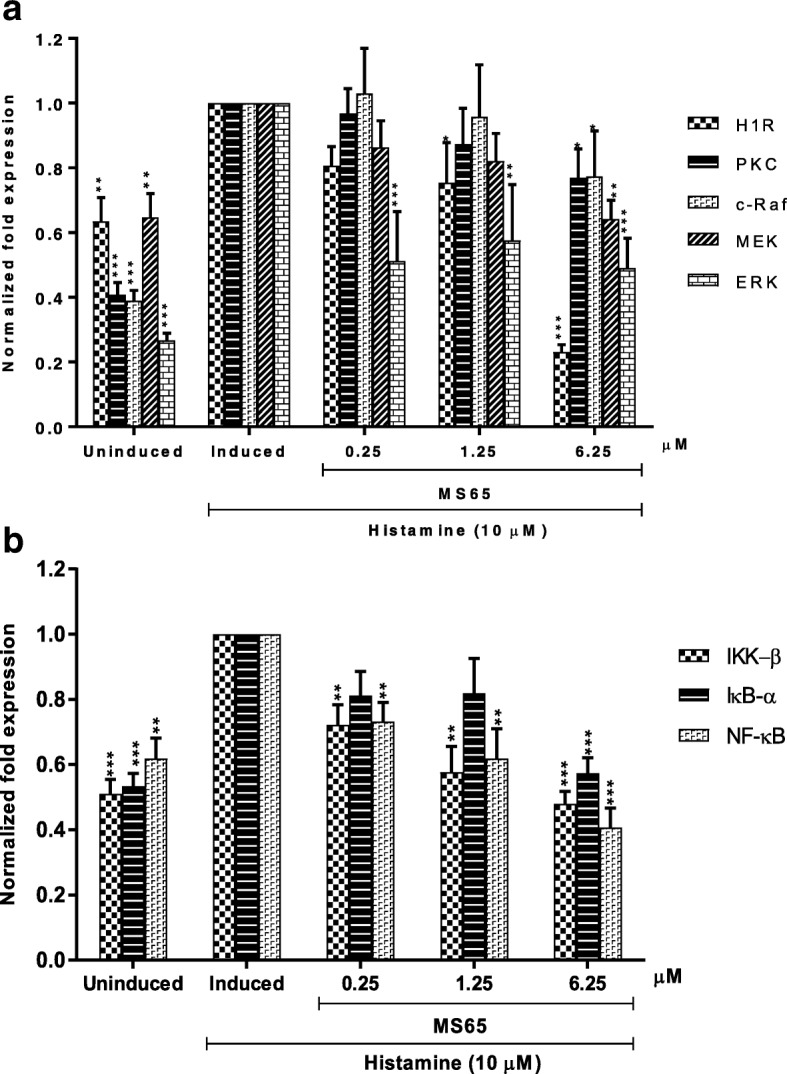
Effects of MS65 on genes expression of (a) H1R, PKC, c-Raf, MEK, ERK and (b) IKK-β, IκB-α, NF-κβ. Cells were treated with 10 μM of histamine for 24 h in presence or absence of MS65. Total mRNA was extracted, and gene expression was analyzed by RT-qPCR. The fold expressions were normalized by GAPDH expression levels. Data are expressed as the mean ± S.E.M. of three separate experiments. Values of *P < 0.05, **P < 0.01 and ***P < 0.001 were considered significantly different to histamine-induced control group
Discussion
Keratinocytes, which comprise 95% of epidermis, are highly active immunological cells with a major control over acute and chronic phases of skin inflammation. During allergies, keratinocytes sense protein allergens and initiate the expression of inflammatory molecules, which represents the starting point of primary skin inflammation. Upon stimulation, keratinocytes are able to secrete a wide spectrum of cytokines, chemokines and accessory molecules, which potently amplifies the innate and adaptive immune responses in the skin [7]. IL-6 cytokine, one of the cytokine secreted by keratinocytes, has been reported to be involved in many inflammatory skin conditions including autoinflammatory and allergic disorders such as psoriasis [21], atopic dermatitis [22] and urticaria [23].
In the present study, we evaluated the effect of histamine on IL-6 production in cultured HaCaT cells. Our study found that histamine enhanced the production of IL-6, adding further evidence about the capacity of this amine to induce cytokine release from keratinocytes. The present result is in agreement with previous studies showing that histamine induces IL-6 production in epidermal keratinocytes [10], lung macrophages [24] and nasal fibroblast [25], indicating that histamine activates a common pathway leading to IL-6 expression in these cells. The enhanced production of IL-6 has been reported to cause wheal formation and rash development, confirming the role of IL-6 in modulating allergic inflammation in skin [13].
In recent years, curcumin and its derivatives have demonstrated promising anti-inflammatory activities by inhibiting several important inflammatory mediators such as tumor necrosis factor (TNF)-α, IL-6, IL-1β, nitric oxide (NO) and nitric oxide synthase (iNOS) [26, 27]. The therapeutic potentials of curcumin are attributed mainly to its chemical structure, in which the presence of various substituents such as methoxy, hydroxyl, alkyl, halogens, amino, nitro, nitril, acetamido, carboxyl, benzene, heterocyclic and condensed rings in their structure may influence their specific biological activities to a certain degree [28, 29]. In pharmacological evaluation, the halogen substitution has been found to enhance anti-inflammatory potency of the compound. Vairappan et al. [30] has demonstrated the anti-inflammatory activity of halogenated secondary metabolites through inhibition of TNF-α, IL-1β, IL-6, and NO production. Several studies also showed that synthesized products with halogens on their aromatic ring favor anti-inflammatory activities [31, 32]. These reports were consistent with our study, whereby halogen-containing curcumin derivative MS65 showed promising anti-inflammatory activity by significantly inhibited IL-6 production in HaCaT cells.
Recent study has shown that structural modification of curcumin enhanced its anti-inflammatory activity by improving the stability in aqueous solutions at physiological pH [33]. The incorporation of halogen atoms causes an increase in thermal and oxidative stability of the compound, resulting in a lack of responsiveness towards oxidation by the liver P450 detoxification. Halogen-containing compounds are also more lipophilic and less water soluble, thus providing better penetration of lipid membranes [34]. Most of halogenated drugs are fluorine drugs, followed by chlorine, while bromine is rare and the only iodine drug is the thyroid hormone thyroxine [35]. In relation to our study, MS65 contains fluorine atoms, which are believed to play a remarkable role in improving its bioavailability. The small and highly electronegativity fluorine atom can modify electron distribution in the molecule, affecting its absorption, distribution and metabolism [36]. Therefore, the fluorinated compounds could have improved metabolic stability, binding affinity and physicochemical properties, leading to better bioavailability [37, 38].
Levocetirizine dihydrochloride is a third generation H1 antihistamines used for the treatment of various allergies including atopic dermatitis and urticarial [39]. In this study, levocetirizine was used as a comparison with MS65. Levocetirizine contains different halogen atoms, which is chlorine, therefore it may have different efficiency than MS65. Different from fluorine, chlorine is a moderate halogen bond acceptor, besides being larger in size than fluorine. However, the C-Cl bond is enough stable, allowing its insertion on diverse heterocyclics of pharmacological value [35]. In this study, we found that histamine-induced IL-6 production was dose-dependently down-regulated by H1 antihistamine and MS65, with the highest inhibition measured at the highest concentrations tested, 20 μM and 50 μM respectively. However, the cytotoxicity assay shows that MS65 is functional at non-cytotoxic levels even though its effective dose range appears to be higher than H1 antihistamine (1.56-50 μM versus 0.63-1.25 μM), suggesting that MS65 is more effective as well as safe and well tolerated to be used than levocetirizine. These findings also raise the intriguing possibility that levocetirizine is unsuitable for a long-term treatment and drug overdose could result in serious health consequences. This assumption is supported by previous study which has reported the first case of levocetirizine-induced hepatotoxicty in a patient with chronic urticarial [40]. Additionally, recent studies have documented few cases of fixed drug eruption (FDE) due to levocetirizine that was given to the patient for allergic rhinitis and scabies [41, 42].
Further investigation is prompted in order to investigate the effect of MS65 on gene expression levels. Reviews of literature have demonstrated the regulation of H1 receptor on cytokine production via downstream activation of NF-κB and MAPK pathways [10, 25]. Matsubara et al. [10] has proposed the underlying mechanism of H1R-dependent NF-κB and MAPK pathways in human epidermal keratinocytes, which involves the phosphorylation of PKC. The coupling of PKC with H1R signalling has been reported in epidermal keratinocytes [10], chinese hamster ovary (CHO) cells [43] and HeLa cells [44]. Briefly, the phosphorylation of PKC by histamine activates IKK to phosphorylate IκB, allowing active NF-κB dimer to translocate into nucleus. The phosphorylated PKC was also involved in the phosphorylation of c-Raf, which further phosphorylates MEK, a MAPKK upstream of ERK/MAPK pathway [10]. In the present study, the effects of histamine and MS65-treated histamine-induced cells were evaluated on gene expression of H1R, PKC, IKK-β, IκB-α, NF-κβ, c-Raf, MEK and ERK in HaCaT cells. As previously reported by Matsubara et al. [10], the stimulation of H1R, PKC, IKK/IκB/NFκB and c-Raf/MEK/ERK inflammatory cascades by histamine confirmed the involvement of NF-κB and MAPK pathways in histamine-induced physiological responses. The treatment of histamine-induced keratinocytes with MS65 demonstrated gene suppression in dose-dependent manner for all the genes tested, with the highest inhibition on H1R gene at the highest concentration of 6.25 μM. H1R expression levels are closely related with the severity of allergic symptoms, therefore, compounds that suppress the up-regulation of H1R gene may found effective in alleviating the allergic symptoms [45].
Conclusion
Our present study demonstrated that MS65 interacts with H1R gene, thereby blocking the phosphorylation of PKC, allowing the suppression of IKK/IκB/NFκB and c-Raf/MEK/ERK inflammatory cascades as well as preventing their translocation into the nucleus. Therefore, it is attractive to speculate that down-regulation of NF-kB and MAPK pathways may represent a possible mechanism for MS65 to inhibit the IL-6 production in HaCaT cells. Hence, this study may provide a starting point for understanding the potentials of MS65 as an add-on drug in allergic-mediated anti-inflammatory therapies.
Acknowledgements
This study is financially supported by Universiti Putra Malaysia (UPM) under Research University Grant Scheme (RUGS) (05-02-12-2137RU).
Funding
This research was financially supported by Universiti Putra Malaysia (UPM) under Research University Grant Scheme (RUGS) (05-02-12-2137RU).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ANOVA
Analysis of variance
- cDNA
Complementary deoxyribonucleic acid
- c-Raf
RAF proto-oncogene serine/threonine-protein kinase
- DMEM
Dulbecco’s modified eagle medium
- DMSO
Dimethyl sulfoxide
- ELISA
Enzyme-linked immunosorbent assay
- ERK
Extracellular signal–regulated kinase
- FBS
Fetal bovine serum
- FDE
Fixed drug eruption
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- H1R
Histamine H1 receptor
- HaCaT
Human adult low calcium high temperature
- IC50
Half-maximal inhibitory concentration
- IKK
Inhibitor of nuclear factor kappa-B kinase
- IL
Interleukin
- iNOS
Nitric oxide synthase
- IκB
Inhibitor of nuclear factor kappa-B
- LC50
Half-maximal lethal concentration
- MAPK
Mitogen activated protein kinase
- MEK/MAPKK
Mitogen-activated protein kinase kinase
- MS65
2,6-bis(2-fluorobenzylidene)cyclohexanone
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NO
Nitric oxide
- PBS
Phosphate buffered saline
- PKC
Protein kinase C
- RNA
Ribonucleic acid
- RT
Room temperature
- RT-qPCR
Quantitative real-time polymerase chain reaction
- S.E.M
Standard error of mean
- TNF
Tumor necrosis factor
- TLR
Toll-like receptor
- UV
Ultraviolet
Authors’ contributions
NAR is the Master student who conducted the current project and was a major contributor in writing the manuscript. NAN was another master student who also involved in some part of the project. LKS and FA were co-researchers of the project and SA was the project leader. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable
Competing interests
The authors declare that they have no competing interests in this section.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nurul Atika Razali, Email: atika.razali@hotmail.com.
Nur Amiza Nazarudin, Email: amiza.nazarudin@gmail.com.
Kok Song Lai, Email: laikoksong@upm.edu.my.
Faridah Abas, Email: faridah_abas@upm.edu.my.
Syahida Ahmad, Phone: +603-89466699, Email: syahida@upm.edu.my.
References
- 1.Warner JO, Kaliner MA, Crisci CD, Del Giacco S, Frew AJ, Liu GH, et al. Allergy practice worldwide: a report by the world allergy organization specialty and training council. Int Arch Allergy Immunol. 2006;139(2):166–174. doi: 10.1159/000090502. [DOI] [PubMed] [Google Scholar]
- 2.Canonica GW, Blaiss M. Antihistaminic, anti-inflammatory, and antiallergic properties of the nonsedating second-generation antihistamine desloratadine: a review of the evidence. World Allergy Organ J. 2011;4(2):47–53. doi: 10.1097/WOX.0b013e3182093e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bäumer W, Glatzer F, Roßbach K, Ohtsu H, Seike M, Mommert S, et al. Histamine in atopic disorders: atopic dermatitis and pruritus. In: Stark H, et al., editors. Histamine H4 receptor: a novel drug target in immunoregulatory and inflammatory diseases. London: Man; 2013. pp. 173–200. [Google Scholar]
- 4.Fitzsimons R, van der Poel LA, Thornhill W, du Toit G, Shah N, Brough HA. Antihistamine use in children. Arch Dis Child Educ Pract Ed. 2015;100:122–131. doi: 10.1136/archdischild-2013-304446. [DOI] [PubMed] [Google Scholar]
- 5.Thurmond RL, Gelfand EW, Dunford PJ. The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nat Rev Drug Discov. 2008;7:41–53. doi: 10.1038/nrd2465. [DOI] [PubMed] [Google Scholar]
- 6.Gschwandtner M, Mildner M, Mlitz V, Gruber F, Eckhart L, Werfel T, et al. Histamine suppresses epidermal keratinocyte differentiation and impairs skin barrier function in a human skin model. Allergy. 2013;68:37–47. doi: 10.1111/all.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albanesi C. Keratinocytes in allergic skin diseases. Curr Opin Allergy Clin Immunol. 2010;10(5):452–456. doi: 10.1097/ACI.0b013e32833e08ae. [DOI] [PubMed] [Google Scholar]
- 8.Marone G, Granata F, Spadaro G. The histamine-cytokine network in allergic inflammation. J Allergy Clin Immunol. 2003;112(4):83–88. doi: 10.1016/S0091-6749(03)01881-5. [DOI] [PubMed] [Google Scholar]
- 9.Dávila I, Sastre J, Bartra J, Cuvillo A, Jáuregui I, Montoro J, et al. Effect of H1 antihistamines upon the cardiovascular system. J Investig Allergol Clin Immunol. 2006;16(1):13–23. [PubMed] [Google Scholar]
- 10.Matsubara M, Tamura T, Ohmori K, Hasegawa K. Histamine H1 receptor antagonist blocks histamine-induced proinflammatory cytokine production through inhibition of Ca2 +-dependent protein kinase C, Raf/MEK/ERK and IKK/IκB/NF-κB signal cascades. Biochem Pharmacol. 2005;69:433–449. doi: 10.1016/j.bcp.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 12.Sugawara T, Gallucci RM, Simeonova PP, Luster MI. Regulation and role of interleukin 6 in wounded human epithelial keratinocytes. Cytokine. 2001;15(6):328–336. doi: 10.1006/cyto.2001.0946. [DOI] [PubMed] [Google Scholar]
- 13.Stannard J, Myers E, Reed TJ, Lowe L, Kahlenberg JM. Keratinocyte-associated IL-6 is elevated in cutaneous lupus rashes and production of IL-6 by keratinocytes is enhanced in non-involved lupus skin [abstract] Arthritis Rheumatol. 2015;67(10):1072–1073. [Google Scholar]
- 14.Zappia CD, Granja-galeano G, Fernández N, Shayo C, Davio C, Fitzsimons CP, et al. Effects of histamine H1 receptor signalling on glucocorticoid receptor activity. Role of canonical and non-canonical pathways. Sci Rep. 2015;5:17476. doi: 10.1038/srep17476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motala C. H1 antihistamines in allergic disease. Curr Allergy Clin Im. 2009;22(2):71–74. [Google Scholar]
- 16.Leurs R, Church MK, Taglialatela M. Review article H1-antihistamines: inverse agonism, anti-inflammatory actions and cardiac effects. Clin Exp Allergy. 2002;32:489–498. doi: 10.1046/j.0954-7894.2002.01314.x. [DOI] [PubMed] [Google Scholar]
- 17.Gutzmer R, Gschwandtner M, Rossbach K, Mommert S, Werfel T, Kietzmann M, et al. Pathogenetic and therapeutic implications of the histamine H4 receptor in inflammatory skin diseases and pruritus. Front Biosci (Schol Ed) 2011;3:985–994. doi: 10.2741/s203. [DOI] [PubMed] [Google Scholar]
- 18.Kumar D, Kumar M, Kumar A, Singh SK. Chalcone and curcumin derivatives: a way ahead for malarial treatment. Mini Rev Med Chem. 2013;13(14):2116–2133. doi: 10.2174/13895575113136660101. [DOI] [PubMed] [Google Scholar]
- 19.Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41(1):40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Grossman RM, Krueger J, Yourish D, Granelli-piperno A, Murphy DP, May LT, et al. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci U S A. 1989;86:6367–6371. doi: 10.1073/pnas.86.16.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navarini AA, French LE, Hofbauer GFL. Interrupting IL-6-receptor signalling improves atopic dermatitis but associates with bacterial superinfection. J Allergy Clin Immunol. 2011;128(5):1128–1130. doi: 10.1016/j.jaci.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Kasperska-Zajac A, Grzanka A, Damasiewicz-Bodzek A. IL-6 transsignalling in patients with chronic spontaneous urticaria. PLoS One. 2015;10(12):e0145751. doi: 10.1371/journal.pone.0145751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Triggiani M, Gentile M, Secondo A, Granata F, Oriente A, Taglialatela M, et al. Histamine induces exocytosis and IL-6 production from human lung macrophages through interaction with H1 receptors. J Immunol. 2014;166(6):4083–4091. doi: 10.4049/jimmunol.166.6.4083. [DOI] [PubMed] [Google Scholar]
- 25.Park I, Um J, Cho J, Lee SH, Lee SH, Lee H. Histamine promotes the release of interleukin-6 via the H1R/p38 and NF-κB pathways in nasal fibroblasts. Allergy Asthma Immunol Res. 2014;6(6):567–572. doi: 10.4168/aair.2014.6.6.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu W, Li Y, Yue Y, Zhang K, Chen Q, Wang H, et al. Bioorganic & medicinal chemistry letters synthesis and biological evaluation of curcumin derivatives containing NSAIDs for their anti-inflammatory activity. Bioorg Med Chem Lett. 2015;25(15):3044–3051. doi: 10.1016/j.bmcl.2015.04.077. [DOI] [PubMed] [Google Scholar]
- 27.Song MY, Yim JY, Yim JM, Kang IJ, Rho HW, Kim HS, et al. Use of curcumin to decrease nitric oxide production during the induction of antitumor responses by IL-2. J Immunother. 2011;34(2):149–164. doi: 10.1097/CJI.0b013e3182056ec4. [DOI] [PubMed] [Google Scholar]
- 28.Priyadarsini KI. Chemical and structural features influencing the biological activity of curcumin. Curr Pharm Des. 2013;19(11):2093–2100. doi: 10.2174/138161213805289228. [DOI] [PubMed] [Google Scholar]
- 29.Malik P, Mukherjee TK. Review article: structure-function elucidation of antioxidative and prooxidative activities of the polyphenolic compound curcumin. Chin J Biol. 2014;396708:1–8.
- 30.Vairappan CS, Kamada T, Lee WW, Jeon YJ. Anti-inflammatory activity of halogenated secondary metabolites of Laurencia snackeyi (weber-van Bosse) Masuda in LPS-stimulated RAW264.7 macrophages. J Appl Phycol. 2013;25:1805–1813. doi: 10.1007/s10811-013-0023-6. [DOI] [Google Scholar]
- 31.Rani P, Pal D, Hegde RR, Hashim SR. Anticancer, anti-inflammatory, and analgesic activities of synthesized 2-(substituted phenoxy) acetamide derivatives. Biomed Res Int. 2014;386473:1–9. doi: 10.1155/2014/386473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joshi H, Singh BK, Saxena G, Singh V, Singh RP, Arya E. Novel halogens substituted coumarin-aldehyde as an anti-inflammatory agent. World J Pharm Sci. 2013;2(3):1–15. [Google Scholar]
- 33.Somchit N, Kimseng R, Dhar R, Hiransai P, Changtam C, Suksamrarn A, et al. Curcumin pyrazole blocks lipopolysaccharide-induced inflammation via suppression of JNK activation in RAW 264.7 macrophages. Asian Pac J of Allergy Immunol. 2017; 10.12932/AP-130417-0073. [DOI] [PubMed]
- 34.Herrera-Rodriguez LN, Khan F, Robins KT, Meyer HP. Perspectives on biotechnological halogenation. Part I: halogenated products and enzymatic halogenation. Chim Oggi-Chem Today. 2011;29:31–33. [Google Scholar]
- 35.Hernandes MZ, Cavalcanti SMT, Moreira DRM, de Azevedo Jr WF, Leite ACL. Halogen atoms in the modern medicinal chemistry: hints for the drug design. Curr Drug Targets. 2010;11:1–12. doi: 10.2174/138945010790711996. [DOI] [PubMed] [Google Scholar]
- 36.Strunecká A, Patočka J, Connett P. Fluorine in medicine. J Appl Biomed. 2004;2:141–150. [Google Scholar]
- 37.Wang B-C, Wang L-J, Jiang B, Wang S-Y, Wu N, Li X-Q, et al. Application of fluorine in drug design during 2010-2015 years: a mini-review. Mini-Rev Med Chem. 2017;17:683–692. doi: 10.2174/1389557515666151016124957. [DOI] [PubMed] [Google Scholar]
- 38.Shah P, Westwell AD. The role of fluorine in medicinal chemistry. J Enzyme Inhib Med Chem. 2007;22(5):527–540. doi: 10.1080/14756360701425014. [DOI] [PubMed] [Google Scholar]
- 39.Goindi S, Kumar G, Kaur A. Novel flexible vesicles based topical formulation of levocetirizine: in vivo evaluation using oxazolone-induced atopic dermatitis in murine model. J Liposome Res. 2014;24(3):249–257. doi: 10.3109/08982104.2014.899365. [DOI] [PubMed] [Google Scholar]
- 40.Ekiz F, Yuksel I, Ekiz O, Coban S, Basar O, Yuksel O. Levocetirizine induced hepatotoxicity in a patient with chronic urticarial. Ann Hepatol. 2011;10(2):237–238. [PubMed] [Google Scholar]
- 41.Kataria G, Saxena A, Sharma S. Levocetirizine induced fixed drug eruption: a rare case report. Int J Sci Stud. 2014;2(7):228–229. [Google Scholar]
- 42.Jhaj R, Asati DP, Chaudhary D. Case report fixed drug eruption due to levocetirizine. J Pharmacol Pharmacother. 2016;7:109–111. doi: 10.4103/0976-500X.184778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Megson AC, Walker EM, Hill SJ. Role of protein kinase Cα in signalling from the histamine H1 receptor to the nucleus. Mol Pharmacol. 2001;69:1012–1021. doi: 10.1124/mol.59.5.1012. [DOI] [PubMed] [Google Scholar]
- 44.Das AK, Yoshimura S, Mishima R, Fujimoto K, Mizuguchi H, Fukui H. Full paper stimulation of histamine H1 receptor up-regulates histamine H1 receptor itself through activation of receptor gene transcription. J Pharm Sci. 2007;103(4):374–382. doi: 10.1254/jphs.FP0061411. [DOI] [PubMed] [Google Scholar]
- 45.Mizuguchi H, Terao T, Kitai M, Ikeda M, Yoshimura Y, Das AK, et al. Involvement of protein kinase cδ/extracellular signal-regulated kinase/poly(ADP-ribose) polymerase-1 (PARP-1) signalling pathway in histamine-induced up-regulation of histamine H1 receptor gene expression in Hela cells. J Biol Chem. 2011;286(35):30542–30551. doi: 10.1074/jbc.M111.253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


