Abstract
Background
Late-life cognitive decline, caused by progressive neuronal loss leading to brain atrophy years before symptoms are detected, is expected to double in Canada over the next two decades. Cognitive impairment in late life is attributed to vascular and lifestyle related risk factors in mid-life in a substantial proportion of cases (50%), thereby providing an opportunity for effective prevention of cognitive decline if incipient disease is detected earlier. Patients presenting with transient ischemic attack (TIA) commonly display some degree of cognitive impairment and are at a 4-fold increased risk of dementia. In the Predementia Neuroimaging of Transient Ischemic Attack (PREVENT) study, we will address what disease processes (i.e., Alzheimer’s vs. vascular disease) lead to neurodegeneration, brain atrophy, and cognitive decline, and whether imaging measurements of brain iron accumulation using quantitative susceptibility mapping predicts subsequent brain atrophy and cognitive decline.
Methods
A total of 440 subjects will be recruited for this study with 220 healthy subjects and 220 TIA patients. Early Alzheimer’s pathology will be determined by cerebrospinal fluid samples (including tau, a marker of neuronal injury, and amyloid β1–42) and by MR measurements of iron accumulation, a marker for Alzheimer’s-related neurodegeneration. Small vessel disease will be identified by changes in white matter lesion volume. Predictors of advanced rates of cerebral and hippocampal atrophy at 1 and 3 years will include in vivo Alzheimer’s disease pathology markers, and MRI measurements of brain iron accumulation and small vessel disease. Clinical and cognitive function will be assessed annually post-baseline for a period of 5-years using a clinical questionnaire and a battery of neuropsychological tests, respectively.
Discussion
The PREVENT study expects to demonstrate that TIA patients have increased early progressive rates of cerebral brain atrophy after TIA, before cognitive decline can be clinically detected. By developing and optimizing high-level machine learning models based on clinical data, image-based (quantitative susceptibility mapping, regional brain, and white matter lesion volumes) features, and cerebrospinal fluid biomarkers, PREVENT will provide a timely opportunity to identify individuals at greatest risk of late-life cognitive decline early in the course of disease, supporting future therapeutic strategies for the promotion of healthy aging.
Keywords: Magnetic resonance imaging, Neurodegeneration, Alzheimer’s disease, Vascular dementia, Small vessel disease, Cognition, Aging
Background
Cognitive impairment late in life is commonly caused by progressive neuronal loss related to two dominant diseases, Alzheimer’s disease (AD) and small vessel cerebrovascular disease (i.e. vascular dementia; VaD), both causing brain atrophy years before clinical symptoms are detected. As dementia is attributed to vascular and life-style related risk factors in mid-life in a substantial proportion of patients (50%), there is an opportunity to modify these factors if incipient disease is detected earlier [1]. Transient ischemic attack (TIA; incidence 0.37–1.1 per 1000) [2, 3], is associated with a 4-fold increase in dementia over the general population [4–6]. Therefore, TIA patients are a potential target for early modification of incipient disease processes that precede cognitive impairment guided by biologically relevant markers such as amyloid beta 1–42/ tau, whole brain and sub-structure atrophy, small vessel disease markers, and increased free iron. The Predementia Neuroimaging of Transient Ischemic Attack (PREVENT) study will focus on quantifying these biomarkers using advanced serial magnetic resonance imaging (MRI), neuropsychological assessments, APOE genotyping, and longitudinal clinical data. Specifically, PREVENT will examine what combination of pathological processes and risk factors lead to cognitive decline after TIA.
Methods
PREVENT is a prospective, single-center longitudinal study. To achieve a 30% group effect size on annualized rates of cerebral brain atrophy, N = 220 TIA patients and N = 220 healthy controls will receive clinical, neuropsychological, fluid biomarker, and MR assessments at pre-specified time points. The frequency and duration of events is summarized in Table 1.
Table 1.
Schedule of Events
| Visit Name | Baseline | Month 12 | Year 3 | Annual Review | Interim Review |
|---|---|---|---|---|---|
| Inclusion and Exclusion Criteria | • | ||||
| Medical History, Physical Exam | • | • | • | • | |
| Vital (BP, Heart rate etc.,) Signs | • | • | • | • | |
| DNA Sample Collection for APOE | • | ||||
| Neuropsychological assessment | • | • | • | ||
| Activities of Daily Living (FAQ) | • | • | • | ||
| Plasma and Serum Biomarker Collection | • | • | • | • | |
| Concomitant Medications | • | • | • | • | • |
| Adverse Events | • | • | • | • | • |
| 3 T MRI Imaging (100%) | • | • | • | ||
| CSF Collection by Lumbar Puncture | • |
Patient and control subject populations
The inclusion criteria for the TIA patient cohort are: 1) First documented TIA as defined by the following: resolved symptoms attributable to the anterior circulation (motor, speech, monocular visual loss), or posterior circulation (two or more of: ataxia, diplopia, vertigo, hemi-weakness, hemianopia) [2]; 2) no dementia as defined by National Institute of Aging-Alzheimer Association Criteria [7]; 3) clinical brain MR within 10 days of TIA symptoms to determine presence of diffusion-weighted image (DWI) lesion; 4) an age range between 45 and 75 years; and 5) fluency in English language.
Patient exclusion criteria are as follows: 1) persistence of stroke symptoms > 24 h [2]; 2) dementia as defined by the National Institute of Aging-Alzheimer Association Criteria [7]; 3) other central nervous system disease (e.g. MS), alcoholism, substance abuse, sedatives, antipsychotic medications and history of psychiatric illness; 4) contraindication to MR imaging, and other comorbidities that could significantly interfere with cognitive performance and follow-up (e.g., recent coronary artery bypass surgery (within 6 months), severe ischemic heart disease, heart failure, poorly controlled diabetes, renal or liver failure etc.).
Healthy controls will include volunteers from the community, and spouses of patients. Exclusion to participation includes prior stroke or TIA, dementia, co-morbid disease etc. as per patient exclusion criteria.
Data collection
Baseline evaluation (time point: Year 0; Y0) will include clinical review, fasting cholesterol, glucose, and renal function tests. Treatments for hypertension, diabetes, hyperlipidemia, anti-thrombotic agents, and other medications that could influence cognition such as sedatives, anxiolytics, or psychotropic medications will be determined. Apolipoprotein E (APOE) genotyping will be performed; the APOE ε4 allele is a risk factor for AD and VaD [8]. Obstructive sleep apnea may affect cognition and will be screened [9]. Blood pressure (BP) measurement will include two sitting BP measurements taken 5 minutes apart. At enrollment, patients and healthy controls will have home BP monitoring training and will perform three readings per day (waking, mid-morning, and evening) with a Bluetooth enabled, telemetric system (A&D Model UA 767BT) during the first month of recruitment [10]. All TIA patients will be managed according to current stroke prevention guidelines [11]. Cerebrospinal fluid (CSF) for AD biomarkers, amyloid β1–42 and tau, will be collected according to a standardized procedure [12, 13]. The PREVENT study consent informs the participant that all results from biological tests (i.e. APOE) will not be disclosed to the patient or to the patient’s general practitioner. Participants will be approached about brain donation.
Imaging acquisition
At study entry (Y0) patients will have MR scan as a standard of clinical care within 1–2 days of physician assessment, and then repeated at 1 (Y1) and 3 years (Y3). Healthy controls will have MR scan at the same time points (Y0, Y1, Y3). DWI will be performed to detect evidence of diffusion lesion after TIA (up to 30%) [14]. The pertinent biological imaging targets of the standard MR imaging acquisition are summarized in Table 2. The absolute brain and hippocampal volume change between Y0, Y1, and Y3 will be calculated from T1-weighted images with SIENA [15] and FIRST [16, 17] part of FSL software package [18, 19]. At baseline and follow up, FLAIR images will be registered to high-resolution T1 images using a rigid transformation and optimization of the mutual information. Quantitative Susceptibility Mapping (QSM) measurements of regional brain iron accumulation will be acquired using methodology developed and published by the Calgary Image Processing and Analysis Center (CIPAC) [20].
Table 2.
PREVENT imaging comprehensive dementia protocol < 20 min of scan time
| Imaging Sequence | Mechanistic Target | Endpoint | Geometric Parameters (2D/3D, FOV) | Acquisition Parameters (TE/TR/flip) |
|---|---|---|---|---|
| 3-plane localizer | 2D, 24 cm, 8 mm/ 8 mm, 33, 256 × 128 | 1.3 ms/ 4.8 ms/ 30° | ||
| High Resolution T1-weighted | Atrophy | Primary and secondary outcome | 3D, 24 cm2 2.0 mm/ 0 mm, 70, 256 × 256 | 3 ms/ 7 ms/ 8° (minTE, minTR) |
| 3D T2-weighted FLAIR | WML volume | Predictor of primary outcome | 3D, 24 cm, 1 mm/ 0 mm, 38, 256 × 256 | 140 ms/ 9000 ms/ 90° |
| Axial 3D QSM | Neurodegeneration Brain iron content | Predictor of primary outcome | 3D, 24 cm, 2 mm/0 mm, 192 × 192 | 29.5 ms/3 ms/8° |
| 3D Proton Density | WML volume | 3D SPGR, 1 mm/ 0 mm, 256 × 256 | ||
| Diffusion tensor DWI | Microstructure Acute cytotoxic ischemia | 2D, 24 cm, 3.5 mm/ 0 mm, 38, 144 × 144 matrix | 70 ms - 80 ms/ 13,000 ms/ 90° | |
| Axial T2 star | Microhemorrhage | 2D, 24 cm, 3.5 mm/ 0 mm, 38, 256 × 224 | 140 ms/ 9000 ms/ 90°7 |
Neuropsychological assessment
Performed at baseline (within 10 days of onset in TIA patient) and then annually by a trained research assistant (Table 3). The cognitive tests are designed to measure change over time, have been selected to minimize floor and ceiling effects, can distinguish normal aging from prodromal dementia, and will be rotated to reduce practice effects. These tests are consistent with harmonization standards for vascular cognitive impairment (VCI) [21].
Table 3.
PREVENT Neuropsychological Battery
| Domain | Test | Description |
|---|---|---|
| Attention | Trail Making A [52] | Measure of attention, speed and mental flexibility through connecting numbers from 1 to 25 in ascending order. |
| Processing Speed | Digit Symbol Coding Test (WAIS-IV) [53] | Assessment consisting of 9 digit-symbol pairs followed by a list of digits. Subject instructed to fill in the corresponding symbol within 120 s. |
| Verbal Memory | WHO/UCLA’s Auditory Verbal Learning Test [54] | Measure of verbal learning and memory. Subject asked to repeat a list of words (List A) for 5 consecutive trials. An interference trial is introduced (List B) followed by immediate recall and a 25-min delayed recall. |
| Visual Memory | Brief Visuospatial Memory Test – Revised (BVMT) [55] | Measure of visual learning and memory. Target figures presented to subject for 10 s for 3 consecutive drawing trials. After a 25-min delay subject is asked to redraw target figures. Recognition trial is almost administered using 6 novel and 6 target figures. |
| Executive Function | Stroop Color Word Test [56] | Measure of executive function to assess cognitive control. Subjects instructed to read colors out loud while ignoring the printed words in 45-s. |
| Trail Making B [52] | Measure of mental flexibility. Subject asked to connect alternating numbers (1–13) and letters (A-L). | |
| Clox-1: Executive Clock Drawing [57] | Measure of visual spatial construction and executive abilities; also screens for dementia. The subject is instructed to draw a clock with hands and numbers and a specific time. | |
| Working Memory | Digit Span Forward [58] | Test of working memory. Subject is asked to repeat back a list of numbers verbatim as they increase in difficulty. |
| Digit Span Backwards [58] | Test of working memory. Subject is asked to repeat the list of words in backwards order as they increase in difficulty. | |
| Language | Boston Naming Test (Short form) [59] | Assessment of visual naming ability. Subject is asked to name 15 objects after being presented with an illustration. |
| Complex Ideation Material [60] | Assessment of error detection that may be attributed to dementia. Subject is asked yes/no questions about areas that are considered general knowledge. Subject is then read two stories and asked yes/no questions post-comprehension. | |
| Premorbid Intelligence | North American Adult Reading Test (NAART) [61] | Measure of premorbid intelligence. The subject is asked to read a list of 61 words out loud. |
| Mood | Center for Epidemiological Studies - Depression Scale (CES-D) [62] | Self-reported questionnaire. The subject is asked to fill out a questionnaire that pertains to their mood and well-being. |
| Cognitive Screening Tests | Montreal Cognitive Assessment (MoCA) [63] | Assessment of multiple cognitive domains; sensitive to mild cognitive impairment and dementia. |
| Addenbrooke’s Cognitive Assessment – Revised (ACE-R) [64] | Assessment of multiple cognitive domains; sensitive to MCI and dementia. The test has a built in mini mental state examination (MMSE). |
Power analyses
The PREVENT study will recruit 220 TIA patients to participate in this study and compare them to 220 controls, which is inclusive of 20% attrition rate in the whole sample, informed by our preliminary data and by other well documented longitudinal studies [22–24]. The study is powered based on multiple linear regressions of the association between the primary outcome of interest, first year cerebral atrophy rate (R1), and group effect, after adjusting for other socio-demographic, clinical, and vascular risk factors, and other potential confounders. With a sample size of 440, the study will have at least 85% power to detect a 30% group effect size (i.e. TIA versus control) following baseline measurement and follow up measurement taken at Y1, informed by similar rates of whole brain atrophy in published reports of prodromal dementia [25, 26], and our pilot data [27, 28]. This sample size will provide at least 90% power to detect a standardized difference of 0.35 between TIA and control groups for change over time on the WHO/UCLA AVLT (verbal memory) and Trails A and B tests (processing speed/executive function).
Statistical analyses
Statistical analysis will include descriptive statistics to compare the mean, median, standard deviations, and frequency distribution s of all variables.
A mixed repeated measure regression model will be used to model the association between overall cognitive domain specific and composite z-score variable measures at baseline, 1 year, and 3 years and patient group (TIA versus healthy control), after adjusting for demographic characteristics, baseline cognitive function, vascular risk factors mentioned before, in vivo biomarkers, and patient interactions as fixed effects covariates. The size of the standardized regression coefficients associated with each predictor will be used to quantify its relative importance in predicting rate of cognitive decline in patients versus controls. A random intercept will be included to account for subject-specific variation in cognitive scores. The 95% confidence intervals and the corresponding p-values for adjusted group effect on overall cognitive outcomes will be estimated.
Finally, we will develop novel prediction models based on machine learning and generalized estimating equation models to identify individual TIA patients with a high likelihood to develop cognitive decline. The basic idea of this is to predict occurrence of cognitive decline using prediction models developed and trained on the longitudinal data with known clinical outcome (cognitive decline yes / no), which can then be used to predict cognitive impairment in new patients based on the same features used for training of the high-level machine learning model. More precisely, we will develop prediction models based on random forest [29], support vector machines [30], deep neural networks [31], and quadratic inference function classifiers for predicting the occurrence of dementia using available baseline information such as cognitive test results, blood and CSF parameters, as well as quantitative image-based biomarkers such as regional QSM values, volumetric brain and hippocampal values, and white matter lesion (WML) load. The evaluation of the prediction models based on the TIA and control cohorts will be conducted using well-established cross validation techniques. This means that we will, for example, exclude one patient with known dementia outcome from the training set. After this, the prediction model trained based on the remaining patients can be used to predict dementia outcome for the one patient not part of the training set and the predicted outcome can be compared to the real outcome. By iteratively repeating this so-called leave-one-out cross validation for all patients available, the accuracy of the prediction model can be determined and used to identify the optimal high-level machine learning method for this purpose.
Discussion
In light of social and economic burdens, early identification of high-risk patients for dementia is the most important step in prevention and postponement [32, 33]. With no cure and recent clinical trial failures, it is becoming increasingly important that trials must be redesigned with a focus on high-risk populations and standardized biomarkers, which include clinical, demographic, imaging and neuropsychological considerations. The PREVENT study proposes the early identification of people at risk for late-life cognitive decline as the single most important approach for trial designs. To date, studies have established a link between vascular risk and stroke, and late life cognitive impairment but overlooked fundamental challenges such as the efficacy of vascular reduction treatment (antihypertensive treatments [34–38], diabetes management, and cholesterol lowering therapy [39]) on slowing cognitive decline, thus leading to inconclusive results. Special consideration needs to be given to identifying patients at the highest risk of dementia (i.e. TIA patients) since these patients are most likely to benefit from earlier intervention [40] before symptoms manifest, and from therapies that focus on vascular risk reduction [34]. Intervention trials such as the Finnish Geriatric Interventions to Prevent Cognitive Impairment (FINGER) study and the Prevention of Dementia by Intensive Vascular care (preDIVA) trial, show that focusing on diet, exercise, cognitive training, and vascular risk management as a multi-domain treatment approach improves or maintains cognitive function in at risk elderly when compared to healthy controls [24, 41]. The potential mechanisms of disease progression (AD, vascular disease or both), response of these multi-domain therapeutic interventions, and their long-term benefits remain unknown [42]. However, epidemiological studies show that the incidence or age specific prevalence of dementia has declined in the past 20 years [43–45], implying that the risk of dementia is modifiable through vascular risk reduction therapies. The early identification of patients at greatest risk of cognitive decline, by measuring change in cognition, is an important issue to be addressed by the PREVENT study.
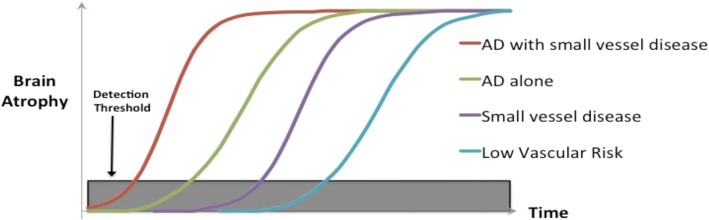
The PREVENT study is expected to support the concept that rate of cerebral atrophy is a meaningful measure of disease progression that precedes cognitive decline and provide justification for the use of rates of cerebral atrophy as an outcome in pre-clinical dementia prevention trials. The PREVENT study in its design will stratify dementia risk according to biological markers of neurodegeneration, and small vessel cerebrovascular disease. We anticipate that the PREVENT study will demonstrate the temporal order of biomarker (in vivo AD and small vessel disease features) change as shown in the figure below with more advanced decline of brain volume occurring in order as follows: Alzheimer’s pathology plus cerebrovascular disease, Alzheimer’s disease in isolation, small vessel cerebrovascular disease and low vascular risk group without identifiable in vivo markers of either vascular disease or AD, all starting before clinical symptoms manifest (Fig. 1).
Fig. 1.
PREVENT stratification of dementia risk by biomarker profile for future dementia prevention clinical trials
We anticipate that recruitment will be the most important challenge to the feasibility of the PREVENT Study. Clinical evaluations, cognitive tests, and the MRI protocol have been designed to minimize patient fatigue, and will be only performed yearly (inclusive of year 5). Recruitment is ahead of schedule providing powerful evidence that enrollment of TIA patients and healthy controls will be complete by the end of 2018. We are actively recruiting from a repository of healthy community volunteers (Calgary Normative Study). We anticipate that by the end of 2019 we will have completed our primary outcome measure of early rates of brain atrophy (R1). By 2021, secondary outcome measures of rates of brain atrophy will have been completed 3 years (R2) and biomarkers of Alzheimer’s disease in WML will have been analyzed. All patients will have completed 4 years of serial neuropsychological assessments.
Our sample size calculations for the PREVENT study are inclusive of an estimated attrition rate of 20%, which is similar to drop out rates in other longitudinal studies of cerebrovascular disease and cognition [46]. Potential bias of our results through the withdrawal or death of subjects at highest risk of dementia will be mitigated by the age inclusion criteria (45–75). Moreover, unlike stroke patients, TIA patients will be able to physically and cognitively comply with serial cognitive and MR imaging. All study participants will be under regular surveillance during clinical reassessments for the development of cardiovascular events and death. Confirmed cases of recurrent stroke will be excluded from our primary outcome analysis; recurrence is anticipated to be low (< 2%) [47].
A proportion of PREVENT subjects may have diffusion-weighted lesions (possibly up to 30%) [14], but previous studies have shown that presence of such small silent infarcts may not explain the cognitive profile [48], nor the progressive cognitive decline [49], perhaps implicating neurodegenerative and inflammatory processes [50, 51]. The presence of DWI lesions may be another predictor of progressive brain atrophy. Exploring disease mechanisms implicated in the recently reported interactions between incident stroke and persistent progressive cognitive decline will be important to furthering our understanding of the disease interaction of neurodegenerative and cerebrovascular processes on cognitive decline [49].
We have set rigorous standards for maintaining the highest quality MR images, including the application of similar benchmark standards used by the Alzheimer’s Disease Neuroimaging group (adni.loni.usc.edu). Objective quality assurance to detect image distortion will be diligently followed to measure properties that include grading calibration, image contrast, and signal to noise ratio. Any scans that fail quality control will be repeated within 4 weeks.
The PREVENT study will be the first critical step for identifying individuals at increased risk of late life cognitive decline. Future steps will target individuals at the highest risk of dementia before symptoms develop, thereby identifying an enriched disease group to test preventative and disease modifying strategies in clinical trials that aim to reduce the microscopic brain tissue loss over a relatively short period of time, thereby optimizing the opportunity for preventing dementia in the future.
Acknowledgements
We thank the participants and the research staff at the Calgary Stroke Program, Calgary Image Processing and Analysis Centre, Calgary Normative Study and Seaman Family MR Research Center for their cooperation and support.
Funding
The present study is supported by Heart and Stroke Foundation of Canada, operating grant (G-16-00012595). The Calgary Normative data used as part of the normal controls portion of this study was funded by the Canadian Institutes for Health Research (220064). We thank Linda Andersen, Cheryl McCreary, and Marina Salluzzi for enabling access to these data.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AD
Alzheimer’s disease
- APOE
Apolipoprotein E
- BP
Blood pressure
- CSF
Cerebrospinal fluid
- DWI
Diffusion weighted imaging
- FINGER
Finnish Geriatric Interventions to Prevent Cognitive Impairment and Disability study
- MRI
Magnetic resonance imaging
- MS
Multiple sclerosis
- PREVENT
Predementia Neuroimaging of Transient Ischemic Attack study
- QSM
Quantitative susceptibility mapping
- TIA
Transient ischemic attack
- VaD
Vascular Dementia
- VCI
Vascular cognitive impairment
- WHO/UCLA AVLT
World Health Organization/University of California Los Angeles auditory verbal learning test
- WML
White matter lesion
Authors’ contributions
TTS, EES, RSL, RF, SBC, NDF, PAB have made substantial contributions to conception and design of this study. ST and PAB wrote the draft of the manuscript and all authors were involved in revising the manuscript critically for important intellectual content. ST was involved in coordinating the study, recruitment, and administering neuropsychological tests. TTS provided significant statistical guidance for the project. RSL conceptualized the neuropsychological test battery and oversaw research assistant (RA) training for administration. RF and NDF provided guidance related to imaging modalities and the conceptual framework for analysis. All authors have given full approval of the version to be published.
Ethics approval and consent to participate
The study has been approved by the Conjoint Health and Research Ethics Board (CHREB; 13–0240). Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sana Tariq, Email: stariq@ucalgary.ca.
Christopher D. d’Esterre, Email: cdesterr@gmail.com
Tolulope T. Sajobi, Email: ttsajobi@ucalgary.ca
Eric E. Smith, Email: eric.smith@albertahealthservices.ca
Richard Stewart Longman, Email: stewart.longman@albertahealthservices.ca.
Richard Frayne, Email: rfrayne@ucalgary.ca.
Shelagh B. Coutts, Email: scoutts@ucalgary.ca
Nils D. Forkert, Email: nils.forkert@ucalgary.ca
Philip A. Barber, Phone: 403-210-3881, Email: pabarber@ucalgary.ca
References
- 1.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet. Neurol. [Internet] 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 2.Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardio. Stroke. [Internet]. 2009;40:2276–2293. Available from: papers3://publication/doi/10.1161/STROKEAHA.108.192218 [DOI] [PubMed]
- 3.Li L, Yiin GS, Geraghty OC, Schulz UG, Kuker W, Mehta Z, et al. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: A population-based study. Lancet Neurol. [Internet]. Li et al. Open Access article distributed under the terms of CC BY-NC-ND; 2015;14:903–13. Available from: 10.1016/S1474-4422(15)00132-5. [DOI] [PMC free article] [PubMed]
- 4.Pendlebury S, Chen P-J, Mehta Z, Rothwell PM, Study O. Long-term risk of dementia after TIA and stroke: current estimates from a large population-based study. Cerebrovasc. Dis. [Internet] 2013;35:187. doi: 10.1159/000345491. [DOI] [Google Scholar]
- 5.Li J-Q, Tan L, Wang H-F, Tan M-S, Tan L, Xu W, et al. Risk factors for predicting progression from mild cognitive impairment to Alzheimer’s disease: a systematic review and meta-analysis of cohort studies. J. Neurol. Neurosurg. Psychiatry [Internet]. 2016:1–9. Available from: http://jnnp.bmj.com/cgi/doi/10.1136/jnnp-2014-310095 [DOI] [PubMed]
- 6.Heath CA, Mercer SW, Guthrie B. Vascular comorbidities in younger people with dementia: a cross-sectional population-based study of 616 245 middle-aged people in Scotland. J Neurol Neurosurg Psychiatry [Internet]. 2014;jnnp-2014-309033-. Available from: http://jnnp.bmj.com/content/early/2014/11/18/jnnp-2014-309033 [DOI] [PubMed]
- 7.GM MK, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers. Dement. [Internet] 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Yu J-T, Wang H-F, Han P-R, Tan C-C, Wang C, et al. APOE genotype and neuroimaging markers of Alzheimer’s disease: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry [Internet]. 2015;86:127–134. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24838911 [DOI] [PMC free article] [PubMed]
- 9.Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest [Internet] 1993;103:30–36. doi: 10.1378/chest.103.1.30. [DOI] [PubMed] [Google Scholar]
- 10.Webb AJS, Rothwell PM. Physiological correlates of beat-to-beat, ambulatory, and day-to-day home blood pressure variability after transient ischemic attack or minor stroke. Stroke [Internet] 2014;45:533–538. doi: 10.1161/STROKEAHA.113.003321. [DOI] [PubMed] [Google Scholar]
- 11.Coutts SB, Wein TH, Lindsay MP, Buck B, Cote R, Ellis P, et al. Canadian Stroke Best Practice Recommendations: secondary prevention of stroke guidelines, update 2014. Int. J. Stroke [Internet] 2015;10:282–291. doi: 10.1111/ijs.12439. [DOI] [PubMed] [Google Scholar]
- 12.Lista S, Garaci FG, Ewers M, Teipel S, Zetterberg H, Blennow K, et al. CSF Aβ1–42 combined with neuroimaging biomarkers in the early detection, diagnosis and prediction of Alzheimer’s disease. Alzheimers. Dement. [Internet]. Elsevier Ltd; 2014;10:381–392. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23850330 [DOI] [PubMed]
- 13.Mattsson N, Insel P, Nosheny R, Zetterberg H, Trojanowski JQ, Shaw LM, et al. CSF protein biomarkers predicting longitudinal reduction of CSF β-amyloid42 in cognitively healthy elders. Transl. Psychiatry [Internet] 2013;3:e293. doi: 10.1038/tp.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brazzelli M, Chappell FM, Miranda H, Shuler K, Dennis M, PAG S, et al. Diffusion-weighted imaging and diagnosis of transient ischemic attack. Ann. Neurol. [Internet] 2014;75:67–76. doi: 10.1002/ana.24026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage [Internet] 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 16.Nugent AC, Luckenbaugh DA, Wood SE, Bogers W, Zarate CA, Drevets WC. Automated subcortical segmentation using FIRST: test-retest reliability, interscanner reliability, and comparison to manual segmentation. Hum Brain Mapp [Internet]. 2013;34:2313–2329. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22815187 [DOI] [PMC free article] [PubMed]
- 17.Thomann PA, Wüstenberg T, Nolte HM, Menzel PB, Wolf RC, Essig M, et al. Hippocampal and entorhinal cortex volume decline in cognitively intact elderly. Psychiatry Res. [Internet] 2013;211:31–36. doi: 10.1016/j.pscychresns.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, TEJ B, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage [Internet] 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 19.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage [Internet]. 2012;62:782–790. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21979382 [DOI] [PubMed]
- 20.Lauzon ML, McCreary CR, McLean DA, Salluzzi M, Frayne R. Quantitative susceptibility mapping at 3 T: comparison of acquisition methodologies. NMR Biomed [Internet]. 2017;30. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26887659 [DOI] [PubMed]
- 21.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National Institute of Neurological Disorders and Stroke-Canadian stroke network vascular cognitive impairment harmonization standards. Stroke [Internet]. 2006;37:2220–2241. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16917086 [DOI] [PubMed]
- 22.Gouw AA, van der Flier WM, Fazekas F, van Straaten ECW, Pantoni L, Poggesi A, et al. Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: the Leukoaraiosis and disability study. Stroke [Internet]. 2008;39:1414–1420. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18323505 [DOI] [PubMed]
- 23.Madureira S, Verdelho A, Ferro J, Basile A-M, Chabriat H, Erkinjuntti T, et al. Development of a neuropsychological battery for the Leukoaraiosis and disability in the elderly study (LADIS): experience and baseline data. Neuroepidemiology [Internet]. 2006;27:101–116. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16943684 [DOI] [PubMed]
- 24.Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet (London, England) [Internet] 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 25.Evans MC, Barnes J, Nielsen C, Kim LG, Clegg SL, Blair M, et al. Volume changes in Alzheimer’s disease and mild cognitive impairment: cognitive associations. Eur Radiol [Internet]. 2010;20:674–682. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19760240 [DOI] [PubMed]
- 26.Ahmed RM, Paterson RW, Warren JD, Zetterberg H, O’Brien JT, Fox NC, et al. Biomarkers in dementia: clinical utility and new directions. J Neurol Neurosurg Psychiatry. 2014;85:1426–34. Available from: https://www.ncbi.nlm.nih.gov/pubmed/25261571. [DOI] [PMC free article] [PubMed]
- 27.Munir MA, Zamboni G, Griffanti L, Zhang Y, Tariq S, Smith EE, et al. TIA and Minor Stroke Patients Experience Higher Brain Atrophy During Preclinical Stages. Tokyo. 2015; Available from: http://www2.convention.co.jp/vas-cog-world2015/program/Program_Poster.pdf.
- 28.Munir MA, Zamboni G, Griffanti L, Zhang Y, Tariq S, Smith EE, et al. Cognitive decline at 3 years in minor stroke and TIA patients. Tokyo. 2015; Available from: http://www2.convention.co.jp/vas-cog-world2015/program/Program_Poster.pdf
- 29.Lebedev A V, Westman E, Van Westen GJP, Kramberger MG, Lundervold A, Aarsland D, et al. Random Forest ensembles for detection and prediction of Alzheimer’s disease with a good between-cohort robustness. NeuroImage. Clin. [Internet]. 2014;6:115–125. Available from: https://www.ncbi.nlm.nih.gov/pubmed/25379423. [DOI] [PMC free article] [PubMed]
- 30.Ferreira LK, Rondina JM, Kubo R, Ono CR, Leite CC, Smid J, et al. Support vector machine-based classification of neuroimages in Alzheimer’s disease: direct comparison of FDG-PET, rCBF-SPECT and MRI data acquired from the same individuals. Rev Bras Psiquiatr [Internet]. 2017; Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1516-44462017005017101&lng=en&tlng=en. [DOI] [PMC free article] [PubMed]
- 31.Hosseini-Asl E, Gimel’farb G, El-Baz A. Alzheimer’s disease diagnostics by a deeply supervised adaptable 3D convolutional network. 2016. [DOI] [PubMed] [Google Scholar]
- 32.Prince M, Albanese E, Guerchet M, Prina M. World Alzheimer report 2014 dementia and risk reduction: an analysis of protective and modifiable factors. London Alzheimer’s Dis. Int. [Internet]. 2014:104. Available from: https://www.alz.co.uk/research/WorldAlzheimerReport2014.pdf
- 33.Haan MN, Wallace R. Can dementia be prevented? Brain aging in a population-based context. Annu Rev Public Health [Internet]. 2004;25:1–24. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15015910 [DOI] [PubMed]
- 34.Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA [Internet]. 1991;265:3255–3264. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2046107 [PubMed]
- 35.Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhäger WH, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators Lancet (London, England) [Internet] 1997;350:757–764. doi: 10.1016/s0140-6736(97)05381-6. [DOI] [PubMed] [Google Scholar]
- 36.Tzourio C, Anderson C, Chapman N, Woodward M, Neal B, MacMahon S, et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch. Intern. Med. [Internet] 2003;163:1069–1075. doi: 10.1001/archinte.163.9.1069. [DOI] [PubMed] [Google Scholar]
- 37.Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, et al. The study on cognition and prognosis in the elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens [Internet]. 2003;21:875–886. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12714861 [DOI] [PubMed]
- 38.Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet. Neurol. [Internet] 2008;7:683–689. doi: 10.1016/S1474-4422(08)70143-1. [DOI] [PubMed] [Google Scholar]
- 39.Mielke MM, Zandi PP, Sjögren M, Gustafson D, Ostling S, Steen B, et al. High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology [Internet]. 2005;64:1689–1695. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15911792 [DOI] [PubMed]
- 40.Schneider LS. Reduce vascular risk to prevent dementia? Lancet [Internet] 2016;388:738–740. doi: 10.1016/S0140-6736(16)31129-1. [DOI] [PubMed] [Google Scholar]
- 41.van Charante EPM, Richard E, Eurelings LS, van Dalen J-W, Ligthart SA, van Bussel EF, et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet [Internet]. Elsevier Ltd; 2016;388:797–805. Available from: 10.1016/S0140-6736(16)30950-3. [DOI] [PubMed]
- 42.Tariq S, Barber PA. Dementia risk and prevention by targeting modifiable vascular risk factors. J. Neurochem. [Internet]. 2017 [cited 2017 Sep 9]; Available from: http://doi.wiley.com/10.1111/jnc.14132 [DOI] [PubMed]
- 43.Prince M, Ali G-C, Guerchet M, Prina AM, Albanese E, Wu Y-T, et al. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers. Res. Ther. [Internet]. Alzheimer’s Research & Therapy. 2016;8:23. doi: 10.1186/s13195-016-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin. Neurosci. [internet], Available from. 2009;11:111–28. http://www.ncbi.nlm.nih.gov/pubmed/19585947 [DOI] [PMC free article] [PubMed]
- 45.Langa KM. Is the risk of Alzheimer’s disease and dementia declining? Alzheimer’s Res. {&} Ther. [Internet]. 2015;7:34. Available from: 10.1186/s13195-015-0118-1%5Cnhttp://alzres.com/content/7/1/34%5Cnhttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4374373&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed]
- 46.Mandzia JL, Smith EE, Horton M, Hanly P, Barber PA, Godzwon C, et al. Imaging and baseline predictors of cognitive performance in minor ischemic stroke and patients with transient ischemic attack at 90 days. Stroke. 2016;47:726–731. doi: 10.1161/STROKEAHA.115.011507. [DOI] [PubMed] [Google Scholar]
- 47.Coutts SB, Simon JE, Eliasziw M, Sohn C-H, Hill MD, Barber PA, et al. Triaging transient ischemic attack and minor stroke patients using acute magnetic resonance imaging. Ann Neurol [Internet]. 2005;57:848–854. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15929051 [DOI] [PubMed]
- 48.van Rooij FG, Schaapsmeerders P, Maaijwee NAM, van Duijnhoven DAHJ, de Leeuw F-E, Kessels RPC, et al. Persistent cognitive impairment after transient ischemic attack. Stroke [Internet]. 2014;45:2270–2274. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25070959 [DOI] [PubMed]
- 49.Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, et al. Trajectory of cognitive decline after incident stroke. JAMA [Internet]. 2015;314:41–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26151265 [DOI] [PMC free article] [PubMed]
- 50.Whitehead SN, Cheng G, Hachinski VC, Cechetto DF. Progressive increase in infarct size, neuroinflammation, and cognitive deficits in the presence of high levels of amyloid. Stroke [Internet]. 2007;38:3245–3250. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17962591 [DOI] [PubMed]
- 51.Amtul Z, Nikolova S, Gao L, Keeley RJ, Bechberger JF, Fisher AL, et al. Comorbid Aβ toxicity and stroke: hippocampal atrophy, pathology, and cognitive deficit. Neurobiol. Aging [Internet] 2014;35:1605–1614. doi: 10.1016/j.neurobiolaging.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Arnett JA, Labovitz SS. Effect of physical layout in performance of the trail making test. Psychol Assess [Internet] 1995;7:220–221. Available from: http://doi.apa.org/getdoi.cfm?doi=10.1037/1040-3590.7.2.220
- 53.Strauss E. A compendium of neuropsychological tests: Administration, norms, and commentary. Third. Sherman EMS, Spreen O, editors. Oxford University Press; 2006.
- 54.Maj M, D’Elia L, Satz P, Janssen R, Zaudig M, Uchiyama C, et al. Evaluation of two new neuropsychological tests designed to minimize cultural bias in the assessment of HIV-1 seropositive persons: a WHO study. Arch. Clin. Neuropsychol. [Internet] 1993;8:123–135. doi: 10.1093/arclin/8.2.123. [DOI] [PubMed] [Google Scholar]
- 55.Benedict RHB. Brief visuospatial memory test-revised. 2012. [Google Scholar]
- 56.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol [Internet] 1935;18:643–662. doi: 10.1037/h0054651. [DOI] [Google Scholar]
- 57.Royall D, Cordes J, Polk M. CLOX: an executive clock drawing task. J Neurol Neurosurg Psychiatry [Internet] 1998;64:588–594. doi: 10.1136/jnnp.64.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaufman AS, Lichtenberger EO. Wiley. 2006. Assessing adolescent and adult intelligence. 3rd ed. [Google Scholar]
- 59.Lansing AE, Ivnik RJ, Cullum CM, Randolph C. An empirically derived short form of the Boston naming test. Arch. Clin. Neuropsychol. [Internet] 1999;14:481–487. doi: 10.1093/arclin/14.6.481. [DOI] [PubMed] [Google Scholar]
- 60.Erdodi LA, Tyson BT, Abeare CA, Lichtenstein JD, Pelletier CL, Rai JK, et al. The BDAE complex ideational material???A measure of receptive language or performance validity? Psychol. Inj. Law [Internet]. Psychological Injury and Law; 2016;9:112–120. Available from: 10.1007/s12207-016-9254-6
- 61.Blair JR, Spreen O. Predicting premorbid IQ: a revision of the national adult reading test. Clin Neuropsychol [Internet] 1989;3:129–136. Available from: http://www.tandfonline.com/doi/abs/10.1080/13854048908403285
- 62.Radloff LS. The CES-D scale: A self report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- 63.Nasreddine Z, Phillips N, Bédirian V, Charbonneau S, Whitehead V, Colllin I, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc [Internet] 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 64.Larner AJ. Dementia in clinical practice: a neurological perspective: studies in the dementia clinic: Springer; 2012.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.