Abstract
Background
Collecting data on the localization of users is a key issue for the MASK (Mobile Airways Sentinel networK: the Allergy Diary) App. Data anonymization is a method of sanitization for privacy. The European Commission’s Article 29 Working Party stated that geolocation information is personal data.
To assess geolocation using the MASK method and to compare two anonymization methods in the MASK database to find an optimal privacy method.
Methods
Geolocation was studied for all people who used the Allergy Diary App from December 2015 to November 2017 and who reported medical outcomes. Two different anonymization methods have been evaluated: Noise addition (randomization) and k-anonymity (generalization).
Results
Ninety-three thousand one hundred and sixteen days of VAS were collected from 8535 users and 54,500 (58.5%) were geolocalized, corresponding to 5428 users. Noise addition was found to be less accurate than k-anonymity using MASK data to protect the users’ life privacy.
Discussion
k-anonymity is an acceptable method for the anonymization of MASK data and results can be used for other databases.
Keywords: Anonymization, App, MASK, Rhinitis, Asthma
Background
MASK-rhinitis (Mobile Airways Sentinel networK for allergic rhinitis) is a patient- centered ICT (Information and Communication Technology) system [1]. A mobile phone app (the Allergy Diary App), central to MASK, is available in 22 countries. It has been validated [2] and found to be an easy and effective method of assessing the symptoms of allergic rhinitis (AR) and work productivity [2–5]. MASK follows the checklist for the evaluation of Good Practices developed by the European Union Joint Action JA-CHRODIS (Joint Action on Chronic Diseases and Promoting Healthy Ageing across the Life Cycle) [6]. The major aims of MASK are to provide care pathways [7] in rhinitis and asthma multimorbidity [8] including a sentinel network using the geolocation of users [9] and to inform the App user of the pollen and/or pollution risk level in their area, by means of geolocation. Both of these functionalities are being developed.
European data protection law
The European data protection law only applies to personal data, i.e. “any information relating to an identified or identifiable natural person; an identifiable natural person is one who can be identified, directly or indirectly, in particular by reference to an identifier such as a name, an identification number, location data, an online identifier or to one or more factors specific to the physical, physiological, genetic, mental, economic, cultural or social identity of that natural person” (Art. 4 para. 1 no. 1 GDPR) [10]. Data anonymization is a method of sanitization for privacy. Anonymization renders personal data “in such a manner that the data subject is not or no longer identifiable.” (Recital 26 GDPR) [11]. As anonymous or anonymized data lack identifiability, anonymization principally enables the sharing of data in a way that preserves privacy with minimal data loss.
In 2014, lacking a clear statement within the law, the European Commission’s Article 29 Working Party (WP29) stated, with regards to the Directive 95/46/EC [12], that geolocation information is not only personal data but also to be considered as an identifier itself [13, 14]. This WP29 finding has become indisputable as the General Data Protection Regulation now clearly states within its definition of personal data (Art. 4 para. 1 no. 1 GDPR) that location data serves as an identifier.
Processing personal data legally under the European Data Protection Law first requires an assessment of the applicable law. Under the framework of Directive 95/46/EC [12], the situation was complex as the Directive may be implemented differently by the Member States of the European Union. Depending on the context of processing, compliance with additional legislation may be required.
Processing personal data by means of an app, such as the Allergy Diary App, is under the Directive 95/46/EC [12] and Directive 2002/58/EC [15] as amended by the Directive 2009/136/EC [16].
Since May 2018, the situation has become more stringent as the General European Data Protection Regulation now applies and all general national provisions on processing personal data are being overruled by European Law. Directive 2002/58/EC [15], as amended by Directive 2009/136/EC [17], is currently being revised and will also be replaced by a Regulation.
Processing personal data lawfully therefore requires (Art. 4 GDPR) either the data subject’s consent or any other legal ground being applied. Principally, such processing is unequivocally necessary for the performance of the service or contract concerned. For electronic communication services, such as apps, Directive 2002/58/EC [15] as amended by Directive 2009/136/EC [16] provides additional requirements.
Data on a subject’s smart device may only be accessed further to consent, (Article 5 para. 3 Directive 2002/58/EC [15] as amended by Directive 2009/136/EC) [16]. Such consent for technical access has to be distinguished from the potential legal ground on processing personal data [18]. Given the high sensitivity of location data, as highlighted by the clarification in Article 4 GDPR and multiple Opinions of the WP20 such as 00461/13/EN WP202 and 0829/14/EN WP216, apps should only technically access and process location data after explicit consent. The processing of personal data under data protection law may however find its legal ground in Article 6 para. 1 lit. b or lit. f GDPR and therefore does not require individual data subject’s consent in all circumstances.
Publishing and sharing location data may however require the data subject’s consent. As consent creates additional burdens, the anonymization of such data seems an appropriate option in providing a service like the Allergy Diary App. Anonymization techniques are not all considered with the same level of confidence [13, 14]. The data of the Allergy Diary App are fully anonymous except for the data related to geolocation. The two main data anonymization processes, with differing strengths and weaknesses, are randomization and generalization [19, 20]. The randomization approach includes noise addition [21] and differential confidentiality [22]. k-anonymization [23–26] and its derivative processes (l-diversity [27] and t-closeness [28]) are the most widely accepted generalization approaches and are acceptable by WP 29.
Methods
Aim and design
In order to assess whether the anonymized geolocation level of the user of the MASK Allergy Diary is sufficient for the analyses planned, a study was set up including all people who had used the App from December 1st 2015 to November 30th 2017. Noise addition and k-anonymization were evaluated.
Setting
The study included users from 22 countries who registered with the Allergy Diary App -available in 16 languages- through App stores. Geolocated data were retrieved from the users' smartphone and collected in every country where the App was available. This data retrieval was technically independent of the App.
Participants
All consecutive users who registered with the Allergy Diary were included if they agreed to be geolocated. There were no exclusion criteria. Some of the users were clinic patients who had been asked by their physician to use the App. However, due to the anonymization of data, no specific information could be gathered, as previously described in detail [3, 4]. With their consent, five users (3 from Kyomed and 2 from Peercode) were considered as “testers” for the algorithm sensitivity analysis.
Ethics
The Allergy Diary is CE1 registered [3, 4]. No ethical committee approval was needed for this study.
Allergy Diary App users agreed to be geolocated in the “terms of use” and “privacy policy” of the Allergy Diary App. Geolocation was optional, the user could allow it or not on his/her mobile phone and it could remove it at any time. Moreover, geolocation was not used in the data mining process and the phone IP was not recorded. Finally, the App functionalities were the same whether the user was geolocated or not.
Outcomes reported in the allergy diary
Users assess their daily symptom control via the touchscreen functionality on their smart phone. They were invited to click on four consecutive visual analogue scales (VAS) (global evaluation, nasal, ocular, asthma) [3, 4].
Geolocation of days reporting VAS
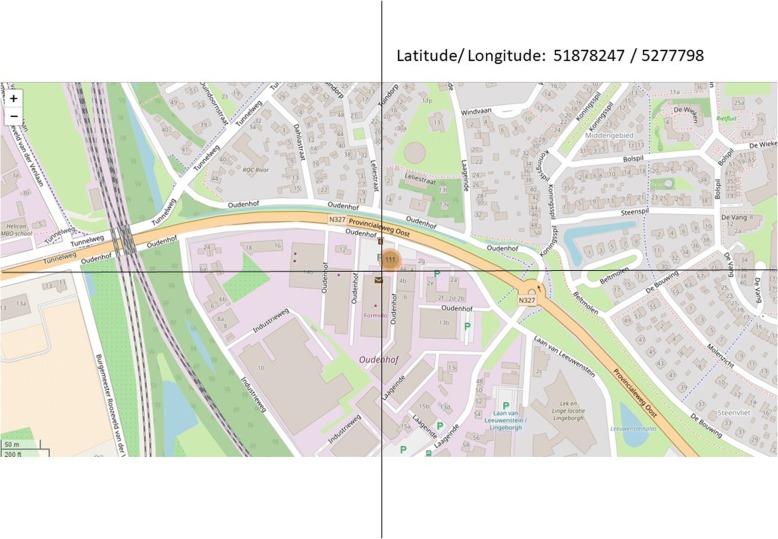
ISO/TC 211 standards are currently being used to determine precise position and location by means of coordinates or geographic identifiers. The geolocation information appears as a set of two numbers corresponding to latitude and longitude (Fig. 1).
Fig. 1.
Geolocation using the ISO/TC211 standards (example: Peercode tester position)
Data analysis
We initially mapped the data in order to validate the fact that the geolocation data of the App users is an identifying process (https://folium.readthedocs.io/en/latest/). Experiments have been conducted by using the Folium Python Library and Leaflet maps (http://leafletjs.com/). Folium builds on the data wrangling strengths of the Python ecosystem and on the mapping strengths of the Leaflet. Folium visualizes data on an interactive Leaflet map. It enables the binding of data to a map for choropleth visualizations and Vincent/Vega visualizations as markers on the map. Clustering image pixels is an important image segmentation technique. We used the algorithm of Hou et al. [29] who combined DSets (dominant sets) and DBSCAN (Density-Based Spatial Clustering of Applications with Noise) to generate the clusters of arbitrary shapes without any parameter input.
We then assessed geolocation methods. A first experiment was to apply a random anonymization technique to the data set. We used noise addition by replacing the last two digits of the geolocation data by a zero value, which corresponds to blur geolocation data in a 10 by 10 km area [14].
A second set of experiments used a k-anonymization [26] method. k-anonymity allows the tolerable disclosure risk to be selected at the outset. For k-anonymity, the risk of identity disclosure is upper-bounded by 1/k. ε-Differential privacy can ensure a very low identity and disclosure (especially for small ε), but at the expense of an important utility loss. However, k-anonymity does not protect against attribute disclosure in general (e.g. if the values of a confidential attribute are very similar in a group of k records sharing quasi-identifier values). A common method for complying to the k-anonymity criterion is to generalize values in the quasi-identifiers by reducing their precision [30]. A release of data has the k-anonymity property if the information for each person contained in the release cannot be distinguished from at least k-1 individuals whose information also appears in the release. In our context, k stands for the minimal distinct days of symptoms. Obviously, the number of users must be greater than one, failing which it is still possible to identify this person. After a quantitative exploratory research, we gathered users at least by 2 and data at least by 5, which is a method accepted by the EU directive [8, 31].
For k-anonymity, we tested several values of ε on our data set. We tested data aggregation to get 5 minimum points from at least 2 users in a circle of 1 km of radius (ε = 1), 2.5 km (ε = 2.5 km), and 5 km (ε = 5 km). The haversine formula was used for the calculation of distances [32] as it determines the great-circle distance between two points on a sphere, given their longitude and latitude. This is the method recommended for calculating short distances by NASA’s Jet Propulsion Laboratory (https://www.jpl.nasa.gov).
Random anonymization techniques and k-anonymity were tested first of all on the five “testers” (with their consent) who used the App for over 200 days. The two techniques were then tested for confirmation on 518 users who declared more than 30 days of symptoms. The users declaring 7 to 15 days of VAS were given special focus, as they represent the targeted App users. Seven to 15 days of VAS allowed a sufficient number of events and appeared to be clinically relevant as most AR patients suffer from 7 to 15 days during the pollen season [33]. We did not study periods of between 15 and 29 days since the analyses of the database showed that there was a low number of users in this category (< 15%) and that the data were very heterogeneous (unpublished data). Finally, the two methods were tested on the users having declared only one day of VAS.
Results
Participants
From December 1st 2015 to November 30th 2017, 93,116 days of VAS were collected from 8535 users. 5428 (60.1%) users in 22 countries were geolocated, corresponding to 54,500 (58.5%) days (Tables 1 and 2). There was no major difference in the users’ geolocation rates between countries.
Table 1.
World-wide repartition of geolocated days and users
| Country | Nb of geolocalized data | Nb of geolocalized users |
|---|---|---|
| AT | 1323 | 200 |
| AU | 354 | 45 |
| BE | 398 | 63 |
| BR | 2553 | 489 |
| CA | 66 | 11 |
| CH | 661 | 238 |
| CZ | 101 | 5 |
| DE | 4416 | 309 |
| DK | 485 | 54 |
| ES | 4043 | 283 |
| FI | 1305 | 167 |
| FR | 2206 | 316 |
| GB | 3168 | 278 |
| GR | 1583 | 89 |
| IT | 8500 | 706 |
| LT | 4073 | 211 |
| MX | 9707 | 496 |
| NL | 1304 | 218 |
| PL | 2300 | 300 |
| PT | 4819 | 810 |
| SE | 639 | 62 |
| TR | 496 | 78 |
| Total | 54,500 | 5428 |
Table 2.
Online: repartition of VAS geolocated days and users included in the evaluation
| VAS days of reporting | 1 | 2–6 | 7–15 | 16–30 | > 30 |
|---|---|---|---|---|---|
| Number of users | 2273 | 2311 | 234 | 92 | 518 |
Geolocation of users
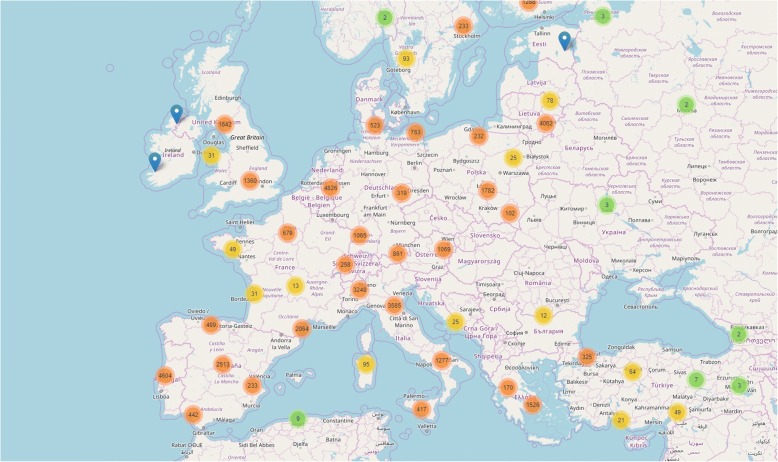
The geolocation of VAS days collected in Europe is reported in Fig. 2. The plot refers to days of symptoms. The color code is linked to the number of days reported. When zooming, we can associate days of symptoms to specific users (as described in Fig. 3), confirming that geolocation is an identifying process which is usable worldwide.
Fig. 2.
Geolocation of VAS days collected in Europe
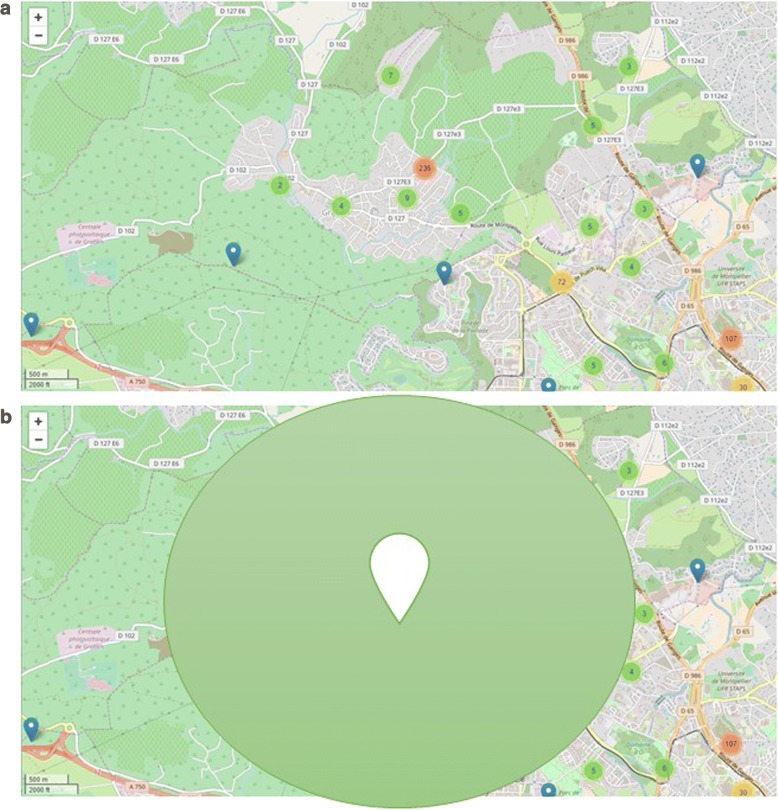
Fig. 3.
a VAS data of a single “tester”. b Geolocation data treatment with random method blurs, here only one user’s data
Random anonymity
By including a zero value for the last two digits of the localization data, we could blur the location zone. When distinct users were close to each other (as in an urban zone), this process enabled the merging of different VAS data (of distinct users) in a single location zone. But in areas where only one user was using the App, miles away from the other closest user, the reported VAS data was linked only to this individual user at that location (Fig. 3). In these circumstances, the random method by noise addition did not enable the dissociation of the VAS data days from their owner. Figure 3a and b show data collected on one of the five “testers”.
Figure 3b shows the data collected from one of the five “testers” when the random method by noise addition has been considered. It shows that it does not enable the dissociation of the VAS data days from their owner. Even if it is not possible to determine the precise location of the user, it is possible to infer his/her main location. The point is now located at the barycentre of all the previous locations. This method was tested on the three data subsets that were analyzed. We observed that 70% of the users declared symptoms within a circle of 1 to 9 km. This method is therefore not a de-identification method in our data set.
Generalization approach using k-anonymity
The k-anonymity algorithm was tested on users according to the number of VAS they reported (Table 2).
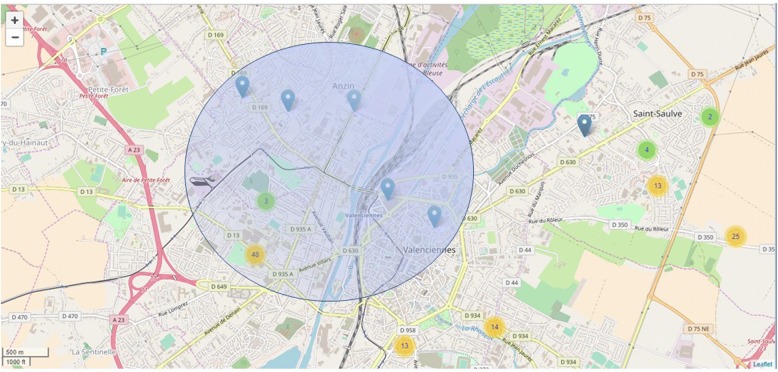
The k-anonymity property was tested with several ε parameters and users’ anonymity was respected if (i) the geolocation data were aggregated by at least 5 by 5 for two distinct users and (ii) the designated perimeter was a circle of 5 km in diameter (Fig. 4) for urban zones. The circle perimeter would be automatically adjusted as needed by the algorithm to fit the first condition (aggregate at least 5 distinct data corresponding to at least 2 different users). If we reconsider the example of the “testers”, the algorithm could merge the data of another user to create a location zone big enough to merge the data of two distinct users. We used the same process with users having declared more than 30 VAS days or between 7 to 15 VAS days and showed that anonymization was found for all users. For users having declared only 1 day of VAS, it is mandatory to merge their geolocation data to at least one other user in order to de-identify their information. The algorithm could merge the one VAS day- user’s location with up to 5 users if they had all declared only 1 day of VAS. But even if the users declare only 1 day of VAS, the k-anonymity method allows the de-identification of the related results since all the results are aggregated to get a virtual position as the barycentre of the circle.
Fig. 4.
k-anonymity applied to users in Valenciennes (France)
This method does not alter the initial quality of the VAS data but creates a location zone big enough to respect users’ privacy. When more users are identified in this area, the algorithm will be automatically adjusted to create a sharper location zone to fit the above condition.
Below is an example of k-anonymity applied to the users of Valenciennes (France). The circle is calculated to gather 5 data of at least 2 distinct users. This creates a circle of 2.5 km of radius that provides an artificial location at the centre of the circle for each data.
Discussion
The present study in 5428 users from 22 countries showed that the precision of the geolocation data transferred by the smart phone is useful and reliable. The privacy of geolocation was evaluated by two methods, first on the five “testers” then on the 518 users declaring more than 30 days of VAS, and also in a sample of 234 users reporting 7 to 15 days of VAS. Special attention was also paid to users declaring VAS data only once. k-anonymity appeared to be relevant for data privacy of the Allergy Diary.
Discussion of methods
The General Data Protection Regulation (GDPR) still recognizes quantification and gradation of anonymization methods.
For the Allergy Diary App, pseudonymizing cannot be considered as an anonymization technique because linking information data sets (such as pollen exposure) cannot guarantee that the initial sensitive data will not be recovered [23].
For random approaches (i) Permutation of data was not considered, as it would alter the quality of the database (DB); (ii) Differential confidentiality would imply the calculation of an aggregation estimator on sensitive data. We did not investigate this option since the DB could no longer be used to fit the MASK project objectives; (iii) Noise addition was tested. Using a zero value for the two last digits of the geolocation data, we were able to blur geolocation data in a 10 by 10 km area. Nevertheless, in our data set, some isolated users were still identifiable (Fig. 3).
Using k-anonymity, we tested several values of ε on our data set, and especially on the data collected for users registering 7 to 15 VAS days, these being our expected App user profiles. Users’ anonymization could always be obtained for a circle of 5 km in diameter. Interestingly, a 5 km circle would blur the localization data which is better than deleting the last two digits of the corresponding data in the noise addition approach (for example in Valenciennes as in Fig. 4). More generally, the algorithm can automatically adjust the radius of the circle when needed in order to fit the appropriate conditions (the k number of users and data).
We did not study any other generalization approaches. For instance, l-diversity [13] is an extension of the k-anonymity method but would imply the consideration of l distinct values, which is not possible in our data set. t-proximity [13] is even more stringent than the k-anonymity and l-diversity methods but we would need to know the general distribution of the sensitive data. This method would also imply the segregation of the data to obtain homogenous distribution classes. These data treatments would be too restrictive, and the overall DB quality would be affected.
The general strengths and weaknesses of the tools should be compared in terms of the three basic requirements proposed by WP29 [13] (Table 3).
Table 3.
Strengths and weaknesses of anonymization tools (adapted from [13])
| Is singling out still a risk? | Is linkability still a risk? | Is inference still a risk? | Spoiling DB | |
|---|---|---|---|---|
| Pseudonymisation | Yes | Yes | Yes | Yes |
| Noise addition | Yes | Yes | May not | May |
| k-anonymity(general) | No | May not | May not | No |
| k-anonymity on MASK-DB | No | No | No | No |
k-anonymity applied to the MASK DB is sufficient to guarantee the users’ anonymity, not only on the current medical data set but also considering the integration of environmental data sets (e.g. pollen counts and pollution risks) yet to be gathered. No other DB containing personal data will be merged to our current data set in the future for the allergic rhinitis worldwide survey.
We therefore recommend the k-anonymization method (with our selected conditions/parameters) to anonymize this kind of geolocated medical data since this method does not interfere with the overall DB quality. This post treatment of sensitive data is an irreversible way of de-identifying the data collected through the App. The individualization of data is therefore respected, since even with k = 2, the probability of getting 5 days of identical VAS values is extremely low and, so far, has never been observed in our dataset. Considering the other data collected in our DB (such as the impact of allergic symptoms on daily activities), no correlation is possible with other data sets. Even if we integrate pollen counts and pollution risks, no personal data will be added to our database that could question the anonymization of our data set. Finally, interference (induction of sensitive information on any user) is impossible.
Perspectives
Privacy of information is an increasing concern with the availability of large amounts of data from many individuals. In the Allergy Diary App, the mandatory data retrieved to use the app only include age, sex and country of living. This information is essential for adjusting the list of treatments available in the country of living. This is why the privacy concern has to focus only on geolocation data. In the future, we plan to apply our de-identification method, allowing us to merge our database with other sources of information that include precise geolocation data (for example: pollen and pollution exposition), while respecting users’ life privacy. These results are applicable to other DBs using geolocated data for any field of medicine.
The DB anonymization of “trajectories” (i.e. time and position information) will be considered as the number of users increases as well as the duration of the reporting. We will then consider anonymizing the data at the export phase (for analysis) with clusters of trajectories [34].
Guidelines are based on the assumption that patients regularly use their treatment and that recommendations are not tested with real-life data. Moreover, for many questions, recommendations are uncertain. Next-generation guidelines will need to use anonymized real-life data optimally retrieved using mobile technology to fill the current gaps. The results of this paper will then be used for guideline development.
Conclusions
k-anonymity is an acceptable method for the anonymization of MASK data. It can also be used in other medical app-collected DBs in any fields of medicine. The remaining risk of identification is quite acceptable when considering the “reasonable means” [8, 31] used for re-identification with regards to the Recital 26 GDPR [11]. This k-anonymization method will be used for all data collected through the MASK project and this process will be written in the users’ legal document (“Terms of Use”/“Privacy Policy”). The post treatment of personal data is therefore considered to be compatible with the information given to the users when installing the App on their personal phone.
Acknowledgements
The MASK group was listed as an author. It should be searchable through individual Pubmed records.
MASK Study group: J Bousquet1,2, I Agache3, R Almeida4, R Angles 5, I Annesi-Maesano6, JM Anto7-10, S Arnavielhe11, E Asayag12, E Bacci13, C Bachert14, I Baiardini15, I Baroni16, BA Barreto17, X Basagana7, A Bedbrook 1,M Bedolla-Barajas18, KC Bergmann19, L Bertorello13, M Bewick20, S Bialek21, T Bieber22, C Bindslev-Jensen23, L Bjermer24, A Blua25, M Bochenska Marciniak26, I Bogus-Buczynska26, S Bosnic-Anticevich27, I Bosse28, J Bouchard29, R Bourret30, V Briedis31, C Bucca32, R Buonaiuto33, MT Burguete Cabanas34, D Caiazza35, D Caillot36, D Caimmi37, P Camargos38, G Canfora39, V Cardona40, AM Carriazo41, C Cartier42, A Carla Carvalho Coelho43, G Castellano44, L Cecchi45, NH Chavannes46, MM Ciaravolo47, C Cingi48, A Ciceran49 L Colas50, E Colgan51, J Coll52, D Conforti53, J Correia da Sousa54, RM Cortés-Grimaldo55, F Corti56, DJ Costa57, MC Costa Dominguez58, AL Courbis59, AA Cruz60, A Custovic61, W Czarlewski62, C Dario63, J da Silva64, Y Dauvilliers65, G De Carlo66, F De Blay67, T Dedeu68, M de Fátima Emerson69, G De Feo70, M H Garcia Cruz71, B De Martino72, P Demoly37, N de Paula Motta Rubini73, P Devillier74, G De Vries75, S Di Capua Ercolano76, N Di Carluccio33, G Dray59, R Dubakiene77, E Eller23, R Emuzyte77, JM Espinoza-Contreras78, A Estrada-Cardona79, J Farrell51, A Farsi45, J Ferreira de Mello Jr.80, J Ferrero81, WJ Fokkens82, J Fonseca4, JF Fontaine83, S Forti53, J Garcia-Aymerich7, JL Gálvez-Romero84, CI García-Cobas85, B Gemicioğlu86, R Gerth van Wijk87, M Guidacci88, J Gómez-Vera89, NA Guldemond90, Z Gutter91, T Haahtela92, J Hajjam93, PW Hellings94, L Hernández95, M Illario96, JC Ivancevich97, E Jares98, G Joos99, J Just100, O Kalayci101, AF Kalyoncu102, J Karjalainen103, T Keil104, N Khaltaev105, L Klimek106, ML Kowalski107, I Kull108, P Kuna26, V Kvedariene109, V Kolek110, E Krzych-Fałta111, M Kupczyk26, P Lacwik26, D Larenas-Linnemann112, D Laune11, D Lauri113, J Lavrut114, MA Lessa115, G Levato116, L Lewis117, I Lieten118, A Lipiec111, R Louis119, JA Luna-Pech120, K Maciej26, A Magnan50, J Malva121,119, JF Maspero120, E Mathieu-Dupas11, AL Matos122, O Mayora53, MA Medina-Ávalos123, E Melen124, E Menditto125, J Millot-Keurinck126, MA Mizani127, G Moda128, M Morais-Almeida129, FF Morato-Castro130, P Moura Santo131, R Mösges132, A Mota-Pinto133, J Mullol134, A Murraro135, R Murray136, M Nalin16, M Noguès126, E Novellino137, L Napoli138, H Neffen139, RE O’Hehir140, GL Onorato1, S Palkonen66, NG Papadopoulos141, G Passalacqua142, JL Pépin143, AM Pereira144, M Persico145, O Pfaar146,147, R Picard148, P Poncelet149, F Portejoie1, AC Pozzi150, D Price151, EP Prokopakis152, R Puy140, B Pugin153, M Przemecka-Green26, F Raciborski111, R Rajabian-Soderlund152, S Reitsma82, I Ribeirinho153, J Rimmer154, JA Rizzo155, MC Rizzo156, C Robalo-Cordeiro154, X Rodo155, S Rodrigues Valle158, M Rodríguez-González156, G Rolla160, RE Roller-Wirnsberger161, A Romano157, M Romano16, N Rosario163, D Ryan164, J Salimäki165, B Samolinski111, D, Samreth11, S Shamai132, A Sheikh127, M Sierra52, FER Simons158, D Solé159, M Sorlini160, O Spranger161, C Stellato A70, R Stelmach162, J Strozek111, R Stukas163, M Sutherland164, A Szylling111, JN Tebyriçá165, M Thibaudon166, V Tibaldi167, A Todo-Bom168, S Toppila-Salmi92, P Tomazic169, U Trama170, M Triggiani70, M Urrutia-Pereira171, A Valero172, E Valovirta173, A Valiulis174, O Vandenplas175, M van Eerd75, T Vasankari176, A Vatrella70, MT Ventura177, MT Verissimo121, F Viart42, S Williams178, M Wagenmann179, M Westman15, M Wickman191, P Wroczynski180, A Yorgancioglu181, E Zernotti182, T Zurbierber19, C Zubrinich140, A Zurkuhlen183.
1 MACVIA-France, Fondation partenariale FMC VIA-LR, Montpellier, France.
2 INSERM U 1168, VIMA: Ageing and chronic diseases Epidemiological and public health approaches, Villejuif, Université Versailles St-Quentin-en-Yvelines, UMR-S 1168, Montigny le Bretonneux, France and Euforea, Brussels, Belgium.
3 Faculty of Medicine, Transylvania University, Brasov, Romania.
4 Center for Health Technology and Services Research- CINTESIS, Faculdade de Medicina, Universidade do Porto; and Medina, Lda Porto, Portugal.
5 Innovación y nuevas tecnologías, Salud Sector sanitario de Barbastro, Barbastro, Spain.
6 Epidemiology of Allergic and Respiratory Diseases, Department Institute Pierre Louis of Epidemiology and Public Health, INSERM and Sorbonne Université, Medical School Saint Antoine, Paris, France.
7 ISGlobAL, Centre for Research in Environmental Epidemiology (CREAL), Barcelona, Spain.
8 IMIM (Hospital del Mar Research Institute), Barcelona, Spain.
9 CIBER Epidemiología y Salud Pública (CIBERESP), Barcelona, Spain.
10 Universitat Pompeu Fabra (UPF), Barcelona, Spain.
11 Kyomed, Montpellier, France.
12 Argentine Society of Allergy and Immunopathology, Buenos Aires, Argentina.
13 Regione Liguria, Genoa, Italy.
14 Upper Airways Research Laboratory, ENT Dept, Ghent University Hospital, Ghent, Belgium.
15 Department of Medicine Solna, Immunology and Allergy Unit, Karolinska Institutet and Department of ENT diseases, Karolinska University Hospital, Stockholm, Sweden.
16 Telbios SRL, Milan, Italy.
17 Universidade do Estado do Pará, Belem, Brazil.
18 Hospital Civil de Guadalajara Dr Juan I Menchaca, Guadalarara, Mexico.
19 Comprehensive Allergy-Centre-Charité, Department of Dermatology and Allergy, Charité - Universitätsmedizin Berlin; Global Allergy and Asthma European Network (GA2LEN), Berlin, Germany.
20 iQ4U Consultants Ltd, London, UK.
21 Dept of Biochemistry and Clinical Chemistry-Dpt of Postgraduate Education, Faculty of Pharmacy with the Laboratory Medicine Division, Medical University of Warsaw, Poland.
22 Department of Dermatology and Allergy, Rheinische Friedrich-Wilhelms-University Bonn, Bonn, Germany.
23 Department of Dermatology and Allergy Centre, Odense University Hospital, Odense Research Center for Anaphylaxis (ORCA), Odense, Denmark.
24 Department of Respiratory Medicine and Allergology, University Hospital, Lund, Sweden.
25 Argentine Association of Respiratory Medicine, Buenos Aires, Argentina.
26 Division of Internal Medicine, Asthma and Allergy, Barlicki University Hospital, Medical University of Lodz, Poland.
27 Woolcock Institute of Medical Research, University of Sydney and Sydney Local Health District, Glebe, NSW, Australia.
28 Allergist, La Rochelle, France.
29 Associate professor of clinical medecine, Laval's University, Quebec city, Head of medecine department, Hôpital de la Malbaie, Quebec , Canada.
30 Centre Hospitalier Valenciennes, France.
31 Head of Department of Clinical Pharmacy of Lithuanian University of Clinical Sciences, Kaunas, Lithuania.
32 Chief of the University Pneumology Unit- AOU Molinette, Hospital City of Health and Science of Torino, Italy.
33 Pharmacist, Municipality Pharmacy, Sarno, Italy.
34 Nuove Leon, Monterey, Mexico.
35 Pharmacist, Municipality Pharmacy, Mercato Sam Severino, Italy.
36 Service de pneumologie, CHU et université d'Auvergne, Clermont-Ferrand, France.
37 Department of Respiratory Diseases, Montpellier University Hospital, France.
38 Federal University of Minas Gerais, Medical School, Department of Pediatrics, Belo Horizonte, Brazil.
39 Mayor of Sarno and President of Salerno Province, Director, Anesthesiology Service, Sarno "Martiri del Villa Malta" Hospital, Italy.
40 S Allergologia, S Medicina Interna, Hospital Vall d'Hebron, Barcelona, Spain.
41 Regional Ministry of Health of Andalusia, Seville, Spain.
42 ASA - Advanced Solutions Accelerator, Clapiers, France.
43 Universidade Federal da Bahia, Salvador, Brazil.
44 Celentano pharmacy, Massa Lubrense, Italy.
45 SOS Allergology and Clinical Immunology, USL Toscana Centro, Prato, Italy.
46 Department of Public Health and Primary Care, Leiden University Medical Center, Leiden, The Netherlands.
47 Specialist social worker, Sorrento, Italy.
48 Eskisehir Osmangazi University, Medical Faculty, ENT Department, Eskisehir,Turkey.
49 Argentine Federation of Otorhinolaryngology Societies, Buenos Aires, Argentina.
50 University of Nantes, Service de Pneumologie, UMR INSERM, UMR 1087 and CNR 6291, l'institut du thorax, Nantes, France.
51 LANUA International Healthcare Consultancy, Northern Ireland.
52 Innovación y nuevas tecnologías, Salud Sector sanitario de Barbastro, Barbastro, Spain.
53 Fondazione Bruno Kessler (FBK), Trento, Italy.
54 Life and Health Sciences Research Institute (ICVS), School of Medicine, University of Minho, Braga, Portugal; ICVS/3B's, PT Government Associate Laboratory, Braga/Guimarães, Portugal.
55 Guadalarara, Mexico.
56 FIMMG (Federazione Italiana Medici di Medicina Generale), Milan, Italy.
57 General Practice, Nîmes, France.
58 Dominguez C. Ciutad Mexico, Mexico.
59 IMT Mines-Alès, Univ. Montpellier, Alès, France.
60 ProAR – Nucleo de Excelencia em Asma, Federal University of Bahia, Brasil and WHO GARD Planning Group, Brazil.
61 Department of Pediatric, Imperial College London, UK.
62 Medical Consulting Czarlewski, Levallois, France.
63 Azienda Provinciale per i Servizi Sanitari di Trento (APSS-Trento), Italy.
64 Department of Internal Medicine and Allergy Clinic of Professor Polydoro Ernani de São Thiago University Hospital, Federal University of Santa Catarina (UFSC), Florianopolis-SC, Brazil.
65 Sleep Unit, Department of Neurology, Hôpital Gui-de-Chauliac Montpellier, Inserm U1061, France.
66 EFA European Federation of Allergy and Airways Diseases Patients’ Associations, Brussels, Belgium.
67 Allergy Division, Chest Disease Department, University Hospital of Strasbourg, Strasbourg, France.
68 AQuAS, Barcelna, Spain & EUREGHA, European Regional and Local Health Association, Brussels, Belgium.
69 Policlínica Geral do Rio de Janeiro, Rio de Janeiro, Brazil.
70 Department of Medicine, Surgery and Dentistry "Scuola Medica Salernitana", University of Salerno, Salerno, Italy.
71 Allergy Clinic, National Institute of Respiratory Diseases, Mexico City, Mexico.
72 Social workers oordinator, Sorrento, Italy.
73 Universidade Federal do Estado do Rio de Janeiro, Rio de Janeiro.
74 UPRES EA220, Pôle des Maladies des Voies Respiratoires, Hôpital Foch, Université Paris-Saclay, Suresnes, France.
75 Peercode BV, Geldermalsen,The Netherlands.
76 Farmacie Dei Golfi Group, Massa Lubrense, Italy.
77 Clinic of Children's Diseases, Faculty of Medicine, Vilnius University, Vilnius, Lithuania.
78 University of Aguascalientes, Chihuaha, Mexico.
79 Playa del Carmen, Mexico.
80 JUniversidade de São Paulo, São Paulo, Brazil.
81 Andalusian Agency for Healthcare Quality, Seville, Spain.
82 Department of Otorhinolaryngology, Academic Medical Centre, Amsterdam, the Netherlands.
83 Allergist, Reims, France.
84 Regional hospital of ISSSTE, Puebla, Mexico.
85 Guadalarara, Mexico.
86 Department of Pulmonary Diseases, Istanbul University, Cerrahpasa Faculty of Medicine, Turkey.
87 Department of Internal Medicine, section of Allergology, Erasmus MC, Rotterdam, the Netherlands.
88 Hospital de Base de Brasilia, Brazil.
89 Allergy Clinic, Hospital Regional del ISSSTE ‘Lic. López Mateos’, Mexico City, Mexico.
90 Institute of Health Policy and Management iBMG, Erasmus University, Rotterdam, The Netherlands.
91 University Hospital Olomouc – National eHealth Centre, Czech Republic.
92 Skin and Allergy Hospital, Helsinki University Hospital, Helsinki, Finland.
93 Centich : centre d’expertise national des technologies de l’information et de la communication pour l’autonomie, Gérontopôle autonomie longévité des Pays de la Loire, Conseil régional des Pays de la Loire, Centre d’expertise Partenariat Européen d’Innovation pour un vieillissement actif et en bonne santé, Nantes, France.
94 Dept of Otorhinolaryngology, Univ Hospitals Leuven, Belgium, and Academic Medical Center, Univ of Amsterdam, The Netherlands and Euforea, Brussels, Belgium.
95 Ensenada, Mexico.
96 Division for Health Innovation, Campania Region and Federico II University Hospital Naples (R&D and DISMET) Naples, Italy.
97 Servicio de Alergia e Immunologia, Clinica Santa Isabel, Buenos Aires, Argentina.
98 President, Libra Foundation, Buenos Aires, Argentina.
99 Dept of Respiratory Medicine, Ghent University Hospital, Ghent, Belgium.
100 Allergology department, Centre de l'Asthme et des Allergies Hôpital d'Enfants Armand-Trousseau (APHP); Sorbonne Université, UPMC Univ Paris 06, UMR_S 1136, Institut Pierre Louis d'Epidémiologie et de Santé Publique, Equipe EPAR, Paris, France.
101 Pediatric Allergy and Asthma Unit, Hacettepe University School of Medicine, Ankara, Turkey.
102 Hacettepe University, School of Medicine, Department of Chest Diseases, Immunology and Allergy Division, Ankara, Turkey.
103 Allergy Centre, Tampere University Hospital, Tampere, Finland.
104 Institute of Social Medicine, Epidemiology and Health Economics, Charité - Universitätsmedizin Berlin, Berlin, and Institute for Clinical Epidemiology and Biometry, University of Wuerzburg, Germany.
105 GARD Chairman, Geneva, Switzerland.
106 Center for Rhinology and Allergology, Wiesbaden, Germany.
107 Department of Immunology, Rheumatology and Allergy, Medical University of Lodz, and HARC, Poland.
108 Department of Clinical Science and Education, Södersjukhuset, Karolinska Institutet, Stockholm, Sweden.
109 Faculty of Medicine, Vilnius University, Vilnius, Lithuania.
110 Department of Respiratory Medicine, Faculty of Medicine and Dentistry, Palacky University Olomouc and University Hospital Olomouc, Czech Republic.
111 Department of Prevention of Envinronmental Hazards and Allergology, Medical University of Warsaw, Poland.
112 Center of Excellence in Asthma and Allergy, Hospital Médica Sur, México City, Mexico.
113 Presidente CMMC, Milano, Italy.
114 Head of the Allergy Department of Pedro de Elizalde Children's Hospital, Buenos Aires, Argentina.
115 Faculdade de Medicina da Universidade Federal da Bahia, Salvador de Bahia, Brazil.
116 Sifmed, Milano, Italy.
117 Promotor B3 Action GRoup EIP on AHA and Senior Fellow, International Foundation for Integreted Care, Aberystwyth, UK.
118 Tech Life Valley, Diepenbeek, Belgium.
119 Department of Pulmonary Medicine, CHU Sart-Tilman, University of Liege, GIGA I3 research group, Liege, Belgique.
120 University of Guadalajara, Guadalara, Mexico.
121 Institute of Biomedical Imaging and Life Sciences (IBILI), Faculty of Medicine, University of Coimbra, Portugal; Ageing@Coimbra EIP-AHA Reference Site, Portugal.
122 University of Southeast Bahia, Brazil.
123 Veracruz, Mexico.
124 Sachs’ Children and Youth Hospital, Södersjukhuset, Stockholm and Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden.
125 CIRFF, Federico II University, Naples, Italy.
126 Caisse d'assurance retraite et de la santé au travail du Languedoc-Roussillon (CARSAT-LR), Montpellier, France.
127 Centre of Medical Informatics, Usher Institute of Population Health Sciences and Informatics, The University of Edinburgh, Edinburgh, UK.
128 Regione Piemonte, Torino, Italy.
129 Allergy Center, CUF Descobertas Hospital, Lisbon, Portugal.
130 Universidade de São Paulo, São Paulo, Brazil.
131 Federal University of Bahia, Brazil.
132 Institute of Medical Statistics, and Computational Biology, Medical Faculty, University of Cologne, Germany and CRI-Clinical Research International-Ltd., Hamburg, Germany.
133 General Pathology Institute, Faculty of Medicine, University of Coimbra, Portugal; Institute of Biomedical Imaging and Life Sciences (IBILI), Faculty of Medicine, University of Coimbra, Portugal; Ageing@Coimbra EIP-AHA Reference Site, Portugal.
134 Rhinology Unit & Smell Clinic, ENT Department, Hospital Clínic; Clinical & Experimental Respiratory Immunoallergy, IDIBAPS, CIBERES, University of Barcelona, Spain.
135 Food Allergy Referral Centre Veneto Region, Department of Women and Child Health, Padua General University Hospital, Padua, Italy.
136 Director, Medical Communications Consultant, MedScript Ltd, Dundalk, Co Louth, Ireland.
137 Director of Department of Pharmacy of University of Naples Federico II, Naples, Italy.
138 Director, Consortium of Pharmacies and Services COSAFER, Salerno, Italy.
139 Director of Center of Allergy, Immunology and Respiratory Diseases, Argentina.
140 Department of Allergy, Immunology and Respiratory Medicine, Alfred Hospital and Central Clinical School, Monash University, Melbourne, Victoria, Australia; Department of Immunology, Monash University, Melbourne, Victoria, Australia.
141 Center for Pediatrics and Child Health, Institute of Human Development, Royal Manchester Children's Hospital, University of Manchester, Manchester M13 9WL, UK Allergy Department, 2nd Pediatric Clinic, Athens General Children's Hospital "P&A Kyriakou," University of Athens, Athens, Greece.
142 Allergy and Respiratory Diseases, Ospedale Policlino San Martino -University of Genoa, Italy.
143 Université Grenoble Alpes, Laboratoire HP2, Grenoble, INSERM, U1042 and CHU de Grenoble, France.
144 Allergy Unit, CUF-Porto Hospital and Institute; Center for Research in Health Technologies and information systems CINTESIS, Universidade do Porto, Portugal.
145 Sociologist, municipality area n33, Sorrento, Italy.
146 Center for Rhinology and Allergology, Wiesbaden, Germany.
147 Department of Otorhinolaryngology, Head and Neck Surgery, Universitätsmedizin Mannheim, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany.
148 Conseil Général de l'Economie Ministère de l'Economie, de l'Industrie et du Numérique, Paris, France.
149 LIRMM, Montpellier, France.
150 Vice-Presidente of IML, Milano, Italy.
151 Observational and Pragmatic Research Institute, Singapore, Optimum Patient Care, Cambridge, UK, and Academic Centre of Primary Care, University of Aberdeen, UK.
152 Department of Otorhinolaryngology University of Crete School of Medicine, Heraklion, Greece.
153 European Forum for Research and Education in Allergy and Airway Diseases (EUFOREA), Brussels, Belgium.
154 Centre of Pneumology, Coimbra University Hospital, Portugal.
155 Climate and Health Program, ISGlobal and ICREA, Barcelona, Spain.
156 Pediatric Allergy and Clinical Immunology, Hospital Angeles Pedregal, Mexico City Mexico.
157 Allergy Unit, Presidio Columbus, Rome, Catholic University of Sacred Heart, Rome and IRCCS Oasi Maria SS, Troina, Italy.
158 Department of Pediatrics & Child Health, Department of Immunology, Faculty of Medicine, University of Manitoba, Winnipeg, Manitoba, Canada.
159 Universidade Federal de São Paulo, São Paulo, Brazil.
160 Presidente, IML (Lombardy Medical Initiative), Bergamo, Italy.
161 Global Allergy and Asthma Platform GAAPP, Altgasse 8-10, 1130 Vienna, Austria.
162 Pulmonary Division, Heart Institute (InCor), Hospital da Clinicas da Faculdade de Medicina da Universidade de Sao Paulo, Sao Paulo, Brazil.
163 Public Health Institute of Vilnius University, Vilnius, Lithuania.
164 Alfred Hospital, Melbourne, Victoria, Australia.
165 Universidade Federal do Estado do Rio de Janeiro, Rio de Janeiro - Brazil.
166 RNSA (Réseau National de Surveillance Aérobiologique), Brussieu, France.
167 Regione Piemonte, Torino, Italy.
168 Imunoalergologia, Centro Hospitalar Universitário de Coimbra and Faculty of Medicine, University of Coimbra, Portugal.
169 Department of ENT, Medical University of Graz, Austria.
170 Campania Region, Division on Pharmacy and devices policy, Naples, Italy.
171 Universidade Federal dos Pampas, Uruguaiana, Brazil.
172 Pneumology and Allergy Department CIBERES and Clinical & Experimental Respiratory Immunoallergy, IDIBAPS, University of Barcelona, Spain.
173 Department of Lung Diseases and Clinical Immunology, University of Turku and Terveystalo allergy clinic, Turku, Finland.
174 Vilnius University Institute of Clinical Medicine, Clinic of Children's Diseases, and Institute of Health Sciences, Department of Public Health, Vilnius, Lithuania; European Academy of Paediatrics (EAP/UEMS-SP), Brussels, Belgium.
175 Department of Chest Medicine, Centre Hospitalier Universitaire UCL Namur, Université Catholique de Louvain, Yvoir, Belgium.
176 FILHA, Finnish Lung Association, Helsinki, Finland.
177 University of Bari Medical School, Unit of Geriatric Immunoallergology, Bari, Italy.
178 International Primary Care Respiratory Group IPCRG, Aberdeen, Scotland.
179 Dept of Otorhinolaryngology, HNO-Klinik, Universitätsklinikum Düsseldorf, Germany.
180 Department of Physical Pharmacy and Bioanalysis, Faculty of Pharmacy with the Laboratory Medicine Division, Medical University of Warsaw, Warsaw, Poland.
181 Department of Pulmonary Diseases, Celal Bayar University, Faculty of Medicine, Manisa, Turkey and GARD Executive Committee, Turkey.
182 Universidad Católica de Córdoba, Córdoba, Argentina.
183 Gesundheitsregion KölnBonn - HRCB Projekt GmbH, Kohln, Germany.
Funding
Partly funded by POLLAR (Horizon 2020, EIT health).
Availability of data and materials
The database is available under the GDPR regulations and agreements obtained from Kyomed.
Abbreviations
- AR
Allergic rhinitis
- ARIA
Allergic Rhinitis and its Impact on Asthma
- DB
Data base
- EU
European Union
- GDPR
General data protection regulation
- ICT
Information and communication technology
- MACVIA
Contre les MAladies Chroniques pour un VIeillissement Actif et en bonne santé
- MASK
MACVIA-ARIA Sentinel networK; Mobile Airways Sentinel networK
- VAS
Visual analogue scale
Authors’ contributions
DS (1) performed the study, SA (2) and PP (64) designed and analyzed the study and wrote the paper, JB (2,63) leads MASK and participated in the design and analysis of the study and writing of the paper, AB (2) and GO are the operating managers of MASK (2), RM (3) participated in the analysis of the study and writing of the paper, RA (4) and MM (5) made specific comments to the paper, GdV and MvE (62) are the developers of the App. All other authors included patients for the study. All authors have read, discussed and approved the paper.
Ethics approval and consent to participate
No ethical committee was required for the study.
Consent for publication
Not needed.
Competing interests
JB reports personal fees and other from Chiesi, Cipla, Hikma, Menarini, Mundipharma, Mylan, Novartis, Sanofi-Aventis, Takeda, Teva, Uriach, other from Kyomed, outside the submitted work.
RA reports grants from North Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL2020 Partnership Agreement and through the European Regional Development Fund (ERDF), during the conduct of the study.
AC reports grants and personal fees from GlaxoSmithKline, personal fees from Boehringer Ingelheim, MEDA Pharma, CHIESI, AstraZeneca, om Merck, Sharp & Dohme, Novartis EUROFARMA outside the submitted work.
-
H reports personal fees from Munidpharma, Novartis, OrionPharma, outside the submitted work.
PK reports personal fees from Adamed, Boehringer Ingelheim, AstraZeneca, Chiesi, FAES, Berlin Chemie, Novartis, Polpharma, Allergopharma, outside the submitted work.
V.K has received payment for consultancy from GSK and for lectures from StallergensGreer, Berlin-CHemie outsaide the submitted work.
LL reports personal fees from MSD, Pfizer, GSK, Astrazeneca, MEDA, Boehringer Ingelheim, Novartis, Grunenthal, UCB, TEVA, Amstrong, Siegfried, DBV Technologies, grants from Sanofi, Pfizer, Astrazeneca, Novartis, UCB, outside the submitted work.
RM reports personal fees from ALK, allergopharma, Allergy Therapeutics, Bayer, Friulchem, FAES, GSK, Hexal, Servier, Klosterfrau, MSD, Johnson&Johnson, Meda, Stada, UCB, Nuvo; grants and personal fees from Bencard, Stallergenes; grants from Leti, Optima, ASIT biotech, BitopAG, Hulka, Ursapharm; grants, personal fees and non-financial support from Lofarma; non-financial support from Atmos, Roxall, Bionorica, Otonomy, Ferrero; personal fees and non-financial support from Novartis; outside the submitted work.
NGP reports personal fees from Abbvie Novartis, Faes Farma, BIOMAY, HAL, Nutricia Research, Menarini, Novartis, MEDA, MSD, Omega Pharma, Danone, grants from Menarini, outside the submitted work.
0P reports grants and personal fees from ALK-Abelló, Allergopharma, Stallergenes Greer, HAL Allergy Holding B.V./HAL Allergie GmbH, Bencard Allergie GmbH/Allergy Therapeutics, Lofarma, Biotech Tools S.A., Laboratorios LETI/LETI Pharma, Anergis S.A., grants from Biomay, Nuvo, Circassia, Glaxo Smith Kline, personal fees from Novartis Pharma, MEDA Pharma, Mobile Chamber Experts (a GA2LEN Partner), Pohl-Boskamp, Indoor Biotechnologies, grants from, outside the submitted work.
RS reports grants from São Paulo Research Foundation, MSD, grants and personal fees from Novartis, grants, personal fees and non-financial support from AstraZeneca, Chiesi, personal fees and non-financial support from Boheringer Ingelheim, outside the submitted work.
TBAM reports grants and personal fees from Novartis, Boehringer Ingelheim, Mundipharma, GSK (GlaxoSmithKline), personal fees from Teva Pharma, AstraZeneca, grants from Leti, outside the submitted work.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
J. Bousquet, Phone: +33 611 42 88 47, Email: jean.bousquet@orange.fr
the MASK study group:
J. Bousquet, I. Agache, R. Almeida, R. Angles, I. Annesi-Maesano, J. M. Anto, S. Arnavielhe, E. Asayag, E. Bacci, C. Bachert, I. Baiardini, I. Baroni, B. A. Barreto, X. Basagana, A. Bedbrook, M. Bedolla-Barajas, K. C. Bergmann, L. Bertorello, M. Bewick, S. Bialek, T. Bieber, C. Bindslev-Jensen, L. Bjermer, A. Blua, M. Bochenska Marciniak, I. Bogus-Buczynska, S. Bosnic-Anticevich, I. Bosse, J. Bouchard, R. Bourret, V. Briedis, C. Bucca, R. Buonaiuto, M. T. Burguete Cabanas, D. Caiazza, D. Caillot, D. Caimmi, P. Camargos, G. Canfora, V. Cardona, A. M. Carriazo, C. Cartier, A. Carla Carvalho Coelho, G. Castellano, L. Cecchi, N. H. Chavannes, M. M. Ciaravolo, C. Cingi, A. Ciceran, L. Colas, E. Colgan, J. Coll, D. Conforti, J. Correia da Sousa, R. M. Cortés-Grimaldo, F. Corti, D. J. Costa, M. C. Costa Dominguez, A. L. Courbis, A. A. Cruz, A. Custovic, W. Czarlewski, C. Dario, J. da Silva, Y. Dauvilliers, G. De Carlo, F. De Blay, T. Dedeu, M. de Fátima Emerson, G. De Feo, M. H. Garcia Cruz, B. De Martino, P. Demoly, N. de Paula Motta Rubini, P. Devillier, G. De Vries, S. Di Capua Ercolano, N. Di Carluccio, G. Dray, R. Dubakiene, E. Eller, R. Emuzyte, J. M. Espinoza-Contreras, A. Estrada-Cardona, J. Farrell, A. Farsi, J. Ferreira de Mello, Jr, J. Ferrero, W. J. Fokkens, J. Fonseca, J. F. Fontaine, S. Forti, J. Garcia-Aymerich, J. L. Gálvez-Romero, C. I. García-Cobas, B. Gemicioğlu, R. Gerth van Wijk, M. Guidacci, J. Gómez-Vera, N. A. Guldemond, Z. Gutter, T. Haahtela, J. Hajjam, P. W. Hellings, L. Hernández, M. Illario, J. C. Ivancevich, E. Jares, G. Joos, J. Just, O. Kalayci, A. F. Kalyoncu, J. Karjalainen, T. Keil, N. Khaltaev, L. Klimek, M. L. Kowalski, I. Kull, P. Kuna, V. Kvedariene, V. Kolek, E. Krzych-Fałta, M. Kupczyk, P. Lacwik, D. Larenas-Linnemann, D. Laune, D. Lauri, J. Lavrut, M. A. Lessa, G. Levato, L. Lewis, I. Lieten, A. Lipiec, R. Louis, J. A. Luna-Pech, K. Maciej, A. Magnan, J. Malva, J. F. Maspero, E. Mathieu-Dupas, A. L. Matos, O. Mayora, M. A. Medina-Ávalos, E. Melen, E. Menditto, J. Millot-Keurinck, M. A. Mizani, G. Moda, M. Morais-Almeida, F. F. Morato-Castro, P. Moura Santo, R. Mösges, A. Mota-Pinto, J. Mullol, A. Murraro, R. Murray, M. Nalin, M. Noguès, E. Novellino, L. Napoli, H. Neffen, R. E. O’Hehir, G. L. Onorato, S. Palkonen, N. G. Papadopoulos, G. Passalacqua, J. L. Pépin, A. M. Pereira, M. Persico, O. Pfaar, R. Picard, P. Poncelet, F. Portejoie, A. C. Pozzi, D. Price, E. P. Prokopakis, R. Puy, B. Pugin, M. Przemecka-Green, F. Raciborski, R. Rajabian-Soderlund, S. Reitsma, I. Ribeirinho, J. Rimmer, J. A. Rizzo, M. C. Rizzo, C. Robalo-Cordeiro, X. Rodo, S. Rodrigues Valle, M. Rodríguez-González, G. Rolla, R. E. Roller-Wirnsberger, A. Romano, M. Romano, N. Rosario, D. Ryan, J. Salimäki, B. Samolinski, D. Samreth, S. Shamai, A. Sheikh, M. Sierra, F. E. R. Simons, D. Solé, M. Sorlini, O. Spranger, C. Stellato A, R. Stelmach, J. Strozek, R. Stukas, M. Sutherland, A. Szylling, J. N. Tebyriçá, M. Thibaudon, V. Tibaldi, A. Todo-Bom, S. Toppila-Salmi, P. Tomazic, U. Trama, M. Triggiani, M. Urrutia-Pereira, A. Valero, E. Valovirta, A. Valiulis, O. Vandenplas, M. van Eerd, T. Vasankari, A. Vatrella, M. T. Ventura, M. T. Verissimo, F. Viart, S. Williams, M. Wagenmann, M. Westman, M. Wickman, P. Wroczynski, A. Yorgancioglu, E. Zernotti, T. Zurbierber, C. Zubrinich, and A. Zurkuhlen
References
- 1.Bousquet J, Hellings PW, Agache I, et al. ARIA 2016: care pathways implementing emerging technologies for predictive medicine in rhinitis and asthma across the life cycle. Clin Transl Allergy. 2016;6:47. doi: 10.1186/s13601-016-0137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caimmi D, Baiz N, Tanno LK, et al. Validation of the MASK-rhinitis visual analogue scale on smartphone screens to assess allergic rhinitis control. Clin Exp Allergy. 2017. [DOI] [PubMed]
- 3.Bousquet J, Bewick M, Arnavielhe S, et al. Work productivity in rhinitis using cell phones: the MASK pilot study. Allergy. 2017;72:1475–1484. doi: 10.1111/all.13177. [DOI] [PubMed] [Google Scholar]
- 4.Bousquet J, Caimmi DP, Bedbrook A, et al. Pilot study of mobile phone technology in allergic rhinitis in European countries: the MASK-rhinitis study. Allergy. 2017;72:857–865. doi: 10.1111/all.13125. [DOI] [PubMed] [Google Scholar]
- 5.Bousquet J, Arnavielhe S, Bedbrook A, et al. The ARIA score of allergic rhinitis using mobile technology correlates with quality-of-life: the MASK study. Allergy. 2018;73(2):505-10. [DOI] [PubMed]
- 6.Bousquet J, Onorato GL, Bachert C, et al. CHRODIS criteria applied to the MASK (MACVIA-ARIA sentinel NetworK) good practice in allergic rhinitis: a SUNFRAIL report. Clin Transl Allergy. 2017;7:37. doi: 10.1186/s13601-017-0173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellings PW, Borrelli D, Pietikainen S, et al. European summit on the prevention and self-Management of Chronic Respiratory Diseases: report of the European Union Parliament summit (29 march 2017) Clin Transl Allergy. 2017;7:49. doi: 10.1186/s13601-017-0186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cingi C, Gevaert P, Mosges R, et al. Multi-morbidities of allergic rhinitis in adults: European academy of allergy and clinical immunology task force report. Clin Transl Allergy. 2017;7:17. doi: 10.1186/s13601-017-0153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bousquet J, Schunemann HJ, Fonseca J, et al. MACVIA-ARIA sentinel NetworK for allergic rhinitis (MASK-rhinitis): the new generation guideline implementation. Allergy. 2015;70:1372–1392. doi: 10.1111/all.12686. [DOI] [PubMed] [Google Scholar]
- 10.Article 4 EU GDPR. « Definitions ». EU general data protection regulation 2016/679 (GDPR). http://www.privacy-regulation.eu/en/article-4-definitions-GDPR.htm.
- 11.Recital 26-EU GDPR. EU general data protection regulation 2016/679. http://www.privacy-regulation.eu/en/recital-26-GDPR.htm.
- 12.Directive 95/46/EC of the European Parliament and of the Council 24 October 1995 on the protection of individuals with regard to the processing of personal data and on the free movement of such data. Off J Eur Communities. 1995;L281(31):23. [Google Scholar]
- 13.Protection of personal data. Article 29 data protection working party. Opinion 05/2014 on Anonymisation Techniques. Eur Comm Justice Data Prot 2014;0829/14/EN WP216:http://ec.europa.eu/justice/data-protection/index_en.htm.
- 14.REGULATION (EU) 2016/679 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL. 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation): Official Organ of the European Union; 2016. https://publications.europa.eu/en/publication-detail/-/publication/3e485e15-11bd-11e6-ba9a-01aa75ed71a1/language-en.
- 15.Directive 2002/58/EC of the European Parliament and of the Council 12 July 2002 concerning the processing of personal data and the protection of privacy in the electronic communications sector (Directive on privacy and electronic communications) Off J Eur Comm. 2002;L201:37. [Google Scholar]
- 16.Directive 2009/136/EC of The European Parliament and of the Council 25 November 2009 amending Directive 2002/22/EC on universal service and users’ rights relating to electronic communications networks and services, Directive 2002/58/EC concerning the processing of personal data and the protection of privacy in the electronic communications sector and Regulation (EC) No 2006/2004 on cooperation between national authorities responsible for the enforcement of consumer protection laws. Off J Eur Union. 2009;L337:11. [Google Scholar]
- 17.Knoppers B. Broaden human-rights focus for health data under GDPR. Nature. 2018;558(7709):189. [DOI] [PubMed]
- 18.Article 29. Data protection working party. 00461/13/EN WP 202. Opinion 02/2013 on apps on smart devices. http://ec.europa.eu/justice/data-protection/index_enhtm 2013.
- 19.Lane J, Schur C. Balancing access to health data and privacy: a review of the issues and approaches for the future. Health Serv Res. 2010;45:1456–1467. doi: 10.1111/j.1475-6773.2010.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Li XB, Motiwalla L, Li W, Zheng H, Franklin PD. Preserving patient privacy when sharing same-disease data. ACM J Data Inf Qual. 2016;7(4). [DOI] [PMC free article] [PubMed]
- 21.Adam N, Wortmann J. Security control methods for statistical databases: a comparative study. AMC Comput Surv. 1999;21:515–556. doi: 10.1145/76894.76895. [DOI] [Google Scholar]
- 22.Dwork C, Pottenger R. Toward practicing privacy. J Am Med Inform Assoc. 2013;20:102–108. doi: 10.1136/amiajnl-2012-001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sweeney L. K-anonymity: a model for protecting privacy. Int J Uncertain Fuz Knowl Syst. 2002;10:557–570. doi: 10.1142/S0218488502001648. [DOI] [Google Scholar]
- 24.Aristodimou A, Antoniades A, Pattichis CS. Privacy preserving data publishing of categorical data through k-anonymity and feature selection. Healthc Technol Lett. 2016;3:16–21. doi: 10.1049/htl.2015.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Emam K, Dankar FK. Protecting privacy using k-anonymity. J Am Med Inform Assoc. 2008;15:627–637. doi: 10.1197/jamia.M2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El Emam K, Dankar FK, Issa R, et al. A globally optimal k-anonymity method for the de-identification of health data. J Am Med Inform Assoc. 2009;16:670–682. doi: 10.1197/jamia.M3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Machanavajjhala A, Kifer D, Gehrke J. Venkitasubramiam L. l-diversity: privacy beyond k-anonymity. ACM Trans Knowl Discov Data. 2007;1:52. doi: 10.1145/1217299.1217302. [DOI] [Google Scholar]
- 28.Kohlmayer F, Prasser F, Eckert C, Kuhn KA. A flexible approach to distributed data anonymization. J Biomed Inform. 2014;50:62–76. doi: 10.1016/j.jbi.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Hou J, Gao H, Li X. DSets-DBSCAN: a parameter-free clustering algorithm. IEEE Trans Image Process. 2016;25:3182–3193. doi: 10.1109/TIP.2016.2559803. [DOI] [PubMed] [Google Scholar]
- 30.Ciriani V, De-Capitani-di-Vimercati S, Samarati P. k-anonymity secure data Management in Decentralized Systems, MJ Atallah, M Blanton eds, Algorithms and theory of computation handbook 2, Special topics and techniques. London: Chapman & Hall/CRC; 2010. p. 18.
- 31.Article 28 EU General Data Protection Regulation (EU-GDPR). 2018. https://www.eugdpr.org/.
- 32.Inman J. The haversine formula determines the great-circle distance between two points on a sphere given their longitudes and latitudes. London: R & J Rivington; 1849. [Google Scholar]
- 33.Price D, Scadding G, Ryan D, et al. The hidden burden of adult allergic rhinitis: UK healthcare resource utilisation survey. Clin Transl Allergy. 2015;5:39. doi: 10.1186/s13601-015-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nergiz M, Atzori M, Saygin Y. Towards Trajectory Anonymization: A Generalization-Based Approach. Comput Sci Tech Rep Paper. 2008;1702 https://docs.lib.purdue.edu/cstech/1702.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The database is available under the GDPR regulations and agreements obtained from Kyomed.