Abstract
Background:
Infectious endophthalmitis is a severe ocular inflammation which can cause devastating visual loss. The aim of the study was to identify the demographic and clinical features of infectious endophthalmitis in Western China for better prophylaxis and treatment of this disease.
Methods:
A retrospective, cross-sectional study was conducted based on the medical records of inpatients having infectious endophthalmitis in a tertiary referral center in Western China between 2005 and 2016.
Results:
The common cause of infectious endophthalmitis was trauma (82.6%), endogenous (7.8%), ophthalmic surgery (6.9%), and corneal ulcer with perforation (2.7%). These four etiological groups differed in age, gender, enucleation rate, visual outcome, etc. The number of cases in the first 6 years accounted for 38.7% of the total collection, which in the second 6 years accounted for 61.3%. The etiology patterns were different between these two periods. Altogether 51.3% of patients received pars plana vitrectomy, 13.9% of patients underwent evisceration, and the remaining 34.8% received other treatments. Of the 670 cases that had culture results, 266 (39.7%) were culture positive and 177 (66.5%) were Gram-positive organisms, 64 (24.1%) were Gram-negative organisms, 11 (4.1%) had fungal infection, and 14 (5.3%) were infected by multiple pathogens.
Conclusions:
There was an upward trend of the occurrence of infectious endophthalmitis in Western China for the past decade. The demographic and clinical characteristics of infectious endophthalmitis in Western China had its own characteristics and differed from those of developed countries. Here, open globe trauma was the most common cause of endophthalmitis, most traumatic endophthalmitis patients were male, and most of the injuries were work related, implicate that we should strengthen the education and application of ocular safety regulation specifically targeting the workplace.
Keywords: Endogenous, Endophthalmitis, Etiology, Postsurgical, Posttraumatic
摘要
背景:
感染性眼内炎是一种严重危害视力的眼部感染性疾病。本研究着眼于分析中国西部一大型转诊中心12年来收治的感染 性眼内炎患者的临床特点,为预防和早期治疗提供依据。
方法:
回顾分析2005至2016年间四川大学华西医院眼科住院治疗的感染性眼内炎患者1593例的病例资料。
结果:
感染性眼内炎的常见致病因素为外伤(82.6%),内源性(7.8%),眼科手术(6.9%)和角膜溃疡穿孔(2.7%)。这 4种眼内炎在年龄、性别、眼内容物剜除率和视力等方面有所区别。前6年患者数量占38.7%,后6年占61.3%,这两个时间段 的致病因素也有所差别。51.3%的患者接受了经睫状体平坦部玻璃体切除术,13.9%的患者行眼内容物剜除术,其余34.8%的 患者接受了其他治疗。670例患者有培养结果,其中266例(24.1%)培养阳性。在这266例培养阳性患者中,177例(66.5%) 是革兰阳性菌,64例(24.1%)是革兰阴性菌,11例(4.1%)是真菌,还有14例(5.3%)是多重感染。
结论:
在过去的十年中,中国西部的感染性眼内炎发病有上升的趋势,其人口学和临床特点有别于发达国家,有其地域特色。 这里开放性眼外伤是感染性眼内炎最常见的致病因素,大部分外伤后眼内炎为男性患者且与劳动作业相关。这提示我们应该 加强工作场所的眼安全规范教育和采取眼外伤预防措施。
INTRODUCTION
Infectious endophthalmitis is a severe ocular inflammation which can cause devastating visual loss. The most common causes are open globe trauma, ocular surgery, and endogenous spread from adjacent periocular tissues or through the bloodstream.[1] The etiology and treatment settings of endophthalmitis range widely according to different geography, economy, or race.[2,3,4] Study results on etiology of endophthalmitis have been controversial. A German researcher reported that endogenous endophthalmitis accounted for 41% of the endophthalmitis patients studied, while 12% developed endophthalmitis following open ocular injury.[5] By contrast, an Indian study reported that only 16.8% of 107 endophthalmitis cases were endogenous, and up to 40.2% were posttraumatic.[6] Endophthalmitis prophylaxis and treatment have evolved tremendously over time, especially with the application of pars plana vitrectomy and systemic and/or intravitreal antibiotics. Nevertheless, even in today's world, the management of endophthalmitis still can be challenge.[7]
Western China lies in the inland region of China with multiple nations and a relatively lower economic status and there are huge population and economic disparities between urban and rural remote areas. In recent years, the upward trend for the occurrence of infectious endophthalmitis results in great economic burden for the patients and the society. As far as we know, there has been no study that analyzed the clinical features of infectious endophthalmitis in Western China. To profile the demographic and clinical features of endophthalmitis in Western China, we carried out a retrospective, cross-sectional study involving 1593 case records of endophthalmitis inpatients' records during a time span of 12 years. We also attempt to describe the time trend of the disease aiming to find better prophylaxis and treatment in this region.
METHODS
Ethical approval
The protocol of this study complied with the Declaration of Helsinki and was approved by the Institutional Review Board of West China Hospital of Sichuan University. The informed consent was exempted due to the retrospective nature of this study.
Acquisition and extraction of data and material
It was a retrospective, cross-sectional study based on the medical records of inpatient discharge date system of West China Hospital, which is a large tertiary referral center in Western China. The time of admission was between January 1, 2005, and December 31, 2016. Demographic and clinical data of all patients with a clinical diagnosis of endophthalmitis during that period were reviewed using a retrospective chart. The diagnosis of infectious endophthalmitis was based on patient's medical history and clinical symptoms and signs. Presumptive endophthalmitis was confirmed by culture. Cultures were assessed from aqueous fluid or vitreous humor.
Patient data included case number, sex, age, living place, ethnicity, urban or rural, year of admission, month of admission, laterality, past medical condition, cause of infection (posttraumatic, postsurgical, endogenous, and corneal ulcer with perforation), classification of open globe injury (perforation, intraocular foreign body, and rupture), interval between symptom occurrence and admission, hospital stay, visual acuity on admission, visual acuity on discharge, treatment modality, and culture results (Gram-positive, Gram-negative, fungus, and culture-negative). Visual acuity tested using an international standard visual chart was converted to logMAR acuity. For statistical analyses, counting fingers (CFs), hand movement (HM), light perception (LP), and no LP were converted to 1.9 logMAR, 2.3 logMAR, 2.7 logMAR, and 3.0 logMAR, respectively.[8]
Grouping of the patients
Patients were divided into four groups according to cause of infection: posttraumatic, postsurgical, endogenous, and corneal ulcer with perforation. Clinical features and constituent ratio for the first 6 years of collection (2005–2010) versus the second 6 years of collection (2011–2016) were compared among these four groups.
Statistical analysis
Statistical analyses were performed by a professional biomedical statistician using SPSS software (version 17.0, SPSS Inc., Chicago, IL, USA). Clinical descriptive statistics were calculated as the mean ± standard deviation (SD), median, and/or n (%) depending on the circumstances. Differences in continuous outcome variables among four types of infectious endophthalmitis were sought using analysis of variance (ANOVA). Categorical data were examined using the Chi-square test. Spearman's rank correlation test was performed to evaluate the correlation between discharge and admission visual acuity. A value of P ≤ 0.05 was considered statistically significant.
RESULTS
Subject characteristics
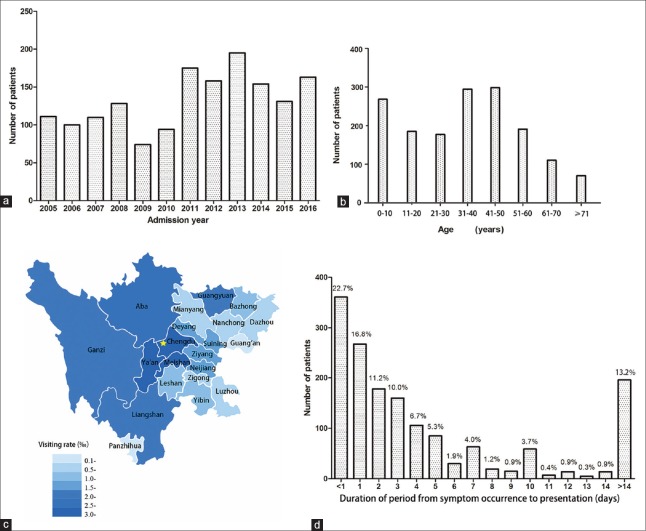
Clinical characteristics of infectious endophthalmitis enrolled in the study are shown in Figure 1. A total of 1610 eyes from 1593 patients were diagnosed as infectious endophthalmitis and the patients were hospitalized-treated in our hospital and over a 12-year period [132.8 cases per year, Figure 1a]. The mean age was 35.1 ± 20.3 years (ranging from 6 months to 88 years), and the median age was 37 years. The peak age of patients was in their 30 s and 40 s [Figure 1b]. Nearly 79.5% were male, and 20.5% were female. Almost 91.8% of patients were distributed in Sichuan Province, and 8.2% were distributed outside of Sichuan Province. The visiting rate, which is defined as the number of visiting patients during the 12 years divided by the total population of the prefecture, differed among prefectures in Sichuan Province [Figure 1c]. Nearly 90.6% of the patients were of Han-ethnicity, 5.4% were Tibetan, 3.6% were of Yi-ethnicity, and 0.4% were of other ethnicities. Furthermore, 66.1% of the patients were rural and 33.9% were urban, while 51.4% of patients had only unilateral right eye involvement, 47.5% had only unilateral left eye involvement, and 1.1% had bilateral involvement. As illustrated in Figure 1d, most patients having infectious endophthalmitis (87.6%) were admitted in the Department of Ophthalmology within 2 weeks following symptom occurrence, and the peak period was within 1 day (22.7%). The average duration of the period from symptom occurrence to admission was 9.7 ± 77.4 days (median: 2 days, range: 1 h–2920 days, with 14 cases of an unknown period). The mean hospital stay of patients was 8.45 ± 4.44 days (median: 8 days, range: 1–54 days), while the most frequent duration (80.7%) was within 10 days.
Figure 1.
The clinical characteristics of patients with infectious endophthalmitis enrolled in the study. (a) Total number of infectious endophthalmitis cases from 2005 to 2016 in Western China. (b) Ages of the 1593 cases of infectious endophthalmitis. (c) Statistical map of geographical distribution of infectious endophthalmitis. The star stands for Chengdu, the provincial capital of Sichuan Province, where the referred center is located. (d) Duration of period from symptom occurrence to presentation in all patients (n% stands for the constituent ratio).
About 10.0% of patients had past medical history including hypertension (50 cases, 31.4%), diabetes (46 cases, 28.9%), liver disease (21 cases, 13.2%), respiratory disease (11 cases, 6.9%), gastrointestinal disease (8 cases, 5%), cardiac disease (4 cases, 2.5%), cerebral disease (3 cases, 1.9%), malignant tumor (3 cases, 1.9%), immunological diseases (3 cases, 1.9%), kidney disease (2 cases, 1.3%), AIDS (2 cases, 1.3%), and others (6 cases, 3.8%). The period from symptom occurrence to admission was compared among the patients without past medical history with those having hypertension, diabetes, and other past medical histories. The results were converted to logarithm due to the skewed distribution. The difference exhibited significance (F = 7.495, P < 0.001) using ANOVA, but significant difference was only observed in the period between patients without past medical history and patients with diabetes when Dunnett T3 post hoc analysis was used to further analyze these results (P = 0.015).
Predisposing factors
As illustrated in Table 1, the most common cause of infectious endophthalmitis in this series was trauma (82.6%), followed by endogeneity (7.8%), ophthalmic surgery (6.9%), and corneal ulcer with perforation (2.7%). The injury causes of traumatic endophthalmitis were comprised of industrial tools (54.7%), living utensils (16.5%), animals and plants (8.3%), explosive materials (5.9%), medical sharp instrument (1.7%), and others (1.8%) including fall damage, fist and feet, and traffic accident. Moreover, 11.1% of the injury causes were undetermined. For postsurgical group, the mean time interval between surgery and symptom occurrence was 20.3 days, 1340.6 days, 8.0 days, 44.7 days, and 1 day in patients having endophthalmitis after cataract surgery, glaucoma filtering surgery, vitrectomy, penetrating keratoplasty, and corneal suture removal, respectively. The related infectious disease could not be determined in 71.8% of endogenous endophthalmitis, and respiratory infection (15.3%) was the most common related disease that could be determined. In the 43 cases of corneal ulcer-associated endophthalmitis, the infectious keratitis of 7 cases (16.3%) was caused by trauma such as tree branch scratch or foreign body injury, and the others had no definite causes.
Table 1.
Types of endophthalmitis in patients
| Types | Number of patients (%) |
|---|---|
| Posttraumatic | 1316 (82.6) |
| IOFB | 675 (42.4) |
| Metal | 468 (29.4) |
| Magnetic | 389 (24.4) |
| Nonmagnetic | 79 (5.0) |
| Nonmetal | 97 (6.1) |
| Metal and nonmetal IOFBs exist together | 2 (0.1) |
| Property cannot be determined | 108 (6.8) |
| Penetration | 571 (35.8) |
| Ocular rupture | 70 (4.4) |
| Postsurgical | 110 (6.9) |
| Cataract surgery | 92 (5.8) |
| Glaucoma filtering surgery | 9 (0.6) |
| Vitrectomy | 5 (0.3) |
| Penetrating keratoplasty | 3 (0.2) |
| Corneal suture removal | 1 (0.1) |
| Endogenous | 124 (7.8) |
| Cornea ulcer-associated | 43 (2.7) |
IOFB: Intraocular foreign body.
Comparison between the first half and the second half of the period
The number of cases in the first half of the period (2005–2010) accounts for 38.7% of the total collection and that in the second half of the period (2011–2016) accounts for 61.3%. The patterns of endophthalmitis with respect to the proportion of four groups for the first half of the period versus the second half of the period are illustrated in Figure 2. The proportion of posttraumatic endophthalmitis decreased from 86.2% to 80.3%, while that of postsurgical endophthalmitis increased from 3.7% to 8.9% (χ2 = 16.619, P = 0.001).
Figure 2.
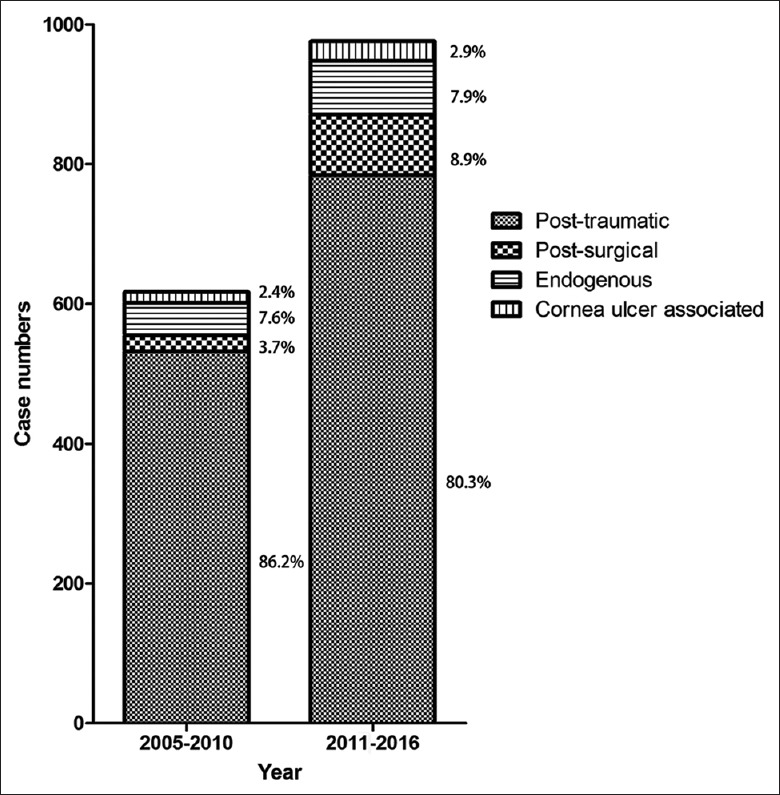
The patterns of endophthalmitis with respect to the proportion of four groups for 2005–2010 versus 2011–2016.
Comparison among four groups
Demographic and clinical features were compared among the four groups of endophthalmitis and are shown in Table 2. There were significantly more males in the posttraumatic group (84.3% males, χ2 = 109.635, P < 0.001) compared with the other three groups. The endophthalmitis following corneal ulcer with perforation tended to affect females more (46.5% females, P < 0.001). Age displayed a significant difference among the four groups by ANOVA (F = 73.33, P < 0.001), and Dunnett T3 post hoc analysis revealed that the posttraumatic group (32.1 ± 18.1 years) was younger than the other three groups, while the postsurgical group (57.3 ± 22.8 years) had the oldest age. There was no significant difference in ethnic distribution among the four groups (Fisher's exact test: P =0.052). There was a significant difference in rural/urban distribution among the four groups, and the posttraumatic group and the following corneal ulcer with perforation group tended to have more rural patients (69.5% and 72.1%, respectively, P < 0.001), while the postsurgical and endogenous group tended to have more urban patients (52.7% and 55.6%, respectively, P < 0.001). The laterality had no significant difference among the groups (χ2 = 2.797, P = 0.424). Patients having endogenous endophthalmitis (40.3%) were more likely to have past medical history compared to those having other types of endophthalmitis (χ2 = 271.990, P < 0.001). The rates of positive cultures had no significant difference among the four groups (χ2 = 0.638, P = 0.888). Most of endogenous endophthalmitis tended to be caused by Gram-positive organisms (92.0%, Fisher's exact test: P =0.001). Among the four groups, endogenous endophthalmitis had the best discharge visual acuity (1.73 ± 0.91, F = 26.30, P < 0.001) and corneal ulcer-associated endophthalmitis had the highest enucleation rate (74.4%, χ2 = 139.300, P < 0.001). Almost 76.5% of patients (625 eyes) who underwent vitrectomy presented with admission acuity of HM or worse and included 525 eyes following trauma, 48 eyes following ophthalmic surgery, 51 eyes from the endogenous group, and 1 eye following corneal ulcer with perforation. Meanwhile, 23.5% of patients who underwent vitrectomy presented with better than HM admission acuity and included 164 eyes following trauma, 15 eyes following ophthalmic surgery, and 13 eyes from the endogenous group. Furthermore, endogenous group had the longest hospitalization stay (11.35 ± 8.66 days, F = 21.46, P < 0.001). Finally, the delayed time to diagnosis of endogenous and corneal ulcer-associated endophthalmitis (31.57 ± 58.13 days and 82.15 ± 448.79 days, respectively) was significantly longer than the other two types of endophthalmitis (5.57 ± 19.09 days and 8.07 ± 15.30, respectively, F = 17.13, P < 0.001).
Table 2.
Clinical features of the four groups of patients with endophthalmitis
| Variables | Posttraumatic (n = 1316) | Postsurgical (n = 110) | Endogenous (n = 124) | Corneal ulcer-associated (n = 43) | Statistics | P |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 1110 (84.3) | 67 (60.9) | 67 (54.0) | 23 (53.5) | χ2 = 109.635 | <0.001 |
| Female | 206 (15.7) | 43 (39.1) | 57 (46.0) | 20 (46.5) | ||
| Age (years) | 32.14 ± 18.05 | 57.33 ± 22.80 | 41.75 ± 23.37 | 49.30 ± 24.76 | F = 73.33 | <0.001 |
| Ethnic groups | ||||||
| Han | 1186 (90.1) | 107 (97.3) | 112 (90.3) | 38 (88.4) | – | 0.052** |
| Minority | 130 (9.9) | 3 (2.7) | 12 (9.7) | 5 (11.6) | ||
| Area | ||||||
| Rural | 915 (69.5) | 52 (47.3) | 55 (44.4) | 31 (72.1) | χ2 = 51.163 | <0.001 |
| Urban | 401 (30.5) | 58 (52.7) | 69 (55.6) | 12 (27.9) | ||
| Laterality* | ||||||
| Right | 681 (51.7) | 49 (44.5) | 66 (53.2) | 23 (53.5) | χ2 = 2.797 | 0.424 |
| Left | 631 (47.9) | 57 (51.8) | 49 (39.5) | 20 (46.5) | ||
| Associated systemic diseases | ||||||
| No | 1259 (95.7) | 73 (66.4) | 74 (59.7) | 28 (65.1) | χ2 = 271.990 | <0.001 |
| Yes | 57 (4.3) | 37 (33.6) | 50 (40.3) | 15 (34.9) | ||
| Culture results† | ||||||
| Positive | 207 (40.3) | 25 (35.7) | 29 (40.3) | 5 (35.7) | χ2 = 0.638 | 0.888 |
| Negative | 307 (59.7) | 45 (46.3) | 43 (59.7) | 9 (64.3) | ||
| Causative organisms‡ | ||||||
| Gram-positive | 138 (66.7) | 23 (92.0) | 13 (44.8) | 3 (60.0) | – | 0.001** |
| Gram-negative | 52 (25.1) | 2 (8.0) | 9 (31.0) | 1 (20.0) | ||
| Fungus | 5 (2.4) | 0 (0.0) | 6 (20.7) | 0 (0.0) | ||
| Multiple pathogens | 12 (5.8) | 0 (0.0) | 1 (3.4) | 1 (20.0) | ||
| Discharge VA§ | 2.19 ± 0.69 | 1.73 ± 0.91 | 2.34 ± 0.72 | 2.80 ± 0.72 | F = 26.30 | <0.001 |
| Enucleation | ||||||
| No | 1161 (88.2) | 98 (89.1) | 101 (81.5) | 11 (25.6) | χ2 = 139.300 | <0.001 |
| Yes | 155 (11.8) | 12 (10.9) | 23 (18.5) | 32 (74.4) | ||
| Vitrectomy|| | ||||||
| Total | 689 | 63 | 64 | 1 | – | – |
| VA ≤ HM | 525 (76.5) | 48 (76.2) | 51 (79.7) | 1 (100) | ||
| VA > HM | 164 (23.5) | 15 (23.8) | 13 (20.3) | 0 (0.0) | ||
| Duration of hospitalization (days) | 8.21 ± 3.67 | 8.66 ± 5.23 | 11.35 ± 8.66 | 6.95 ± 2.40 | F = 21.46 | <0.001 |
| Delay to diagnosis (days)¶ | 5.57 ± 19.09 | 8.07 ± 15.30 | 31.57 ± 58.13 | 82.15 ± 448.79 | F = 17.13 | <0.001 |
Values are n (%), n, or mean ± standard deviation. *17 cases of bilateral involvement were excluded in the statistical analysis; †670 cases which had cultures results were analyzed statistically; ‡266 cases of positive cultures were included in the statistical analysis; §17 cases of bilateral involvement, 151 cases of missing acuity and 1 case of impatient death were excluded in the statistical analysis; ||817 cases of patients who received vitrectomy were included in the descriptive analysis and VA standed for admission VA here; ¶14 cases of an unknown period were excluded; **Fisher’s exact test was used here. HM: Hand movement; VA: Visual acuity.
Treatment
Almost 51.3% of patients received pars plana vitrectomy combined with systemic intravenous infusion of antibiotics, 13.9% of patients underwent evisceration, and the remaining 34.8% received other treatments including intravitreal injection of antimicrobials, systemic intravenous infusion of antibiotics, and anterior chamber irrigation.
Culture results
Overall 670 of 1593 cases had culture results. In these 670 cases, 266 (39.7%) were culture positive, while 404 (60.3%) were culture negative. In the 266 culture-positive cases, 177 (66.5%) were Gram-positive organisms, 64 (24.1%) were Gram-negative organisms, 11 (4.1%) had fungal infection, and 14 (5.3%) were infected by multiple pathogens. Staphylococcus epidermidis was the most common pathogen (69 cases, 25.9%).
Primary outcomes
Some patients were too young to be tested for visual acuity and others were in pain, thus unable to cooperate and resulting in 151 cases of missing acuity. With regard to visual acuity, 17 cases of bilateral involvement, 151 cases of missing acuity, and 1 case of impatient death were excluded from the study. The mean admission LogMAR visual acuity was 2.26 ± 0.68, and the mean discharge LogMAR visual acuity was 2.18 ± 0.72 [Figure 3]. Almost 34.4% of the cases showed improvement in their presenting acuity. However, visual acuity deteriorated in 23.4% of the patients at discharge, and the remaining 42.2% had no change in acuity. The discharge visual acuity was significantly correlated with admission visual acuity (rs= 0.691, P < 0.001).
Figure 3.
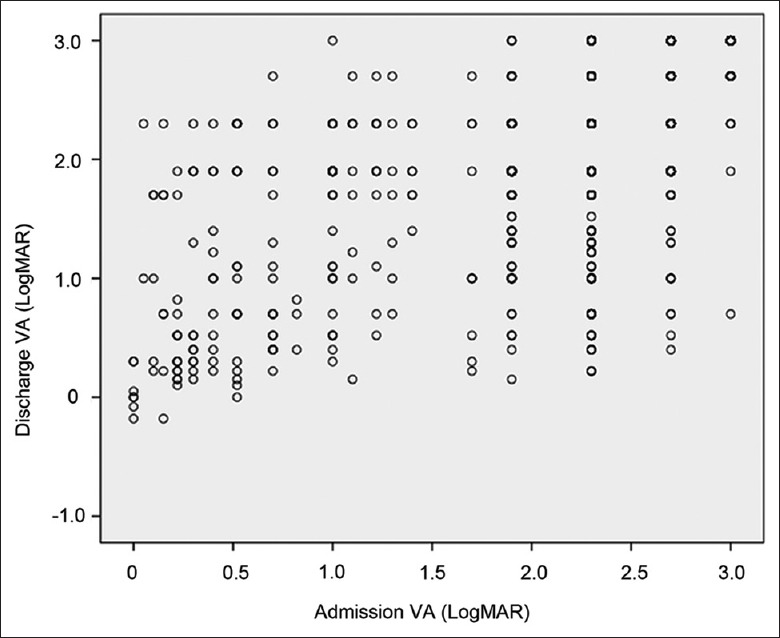
Admission VA and discharge VA of all participants. VA: Visual acuity.
One case of infectious endophthalmitis ended in mortality. A 54-year-old female patient had endogenous endophthalmitis transferred from pulmonary infection and died of infectious shock. However, her culture result was negative.
DISCUSSION
This study revealed the clinical characteristics of infectious endophthalmitis in Western China, which enrolled 1593 cases.
In developed countries, ophthalmic surgery, especially cataract surgery, was the most common cause of infectious endophthalmitis, while trauma-related endophthalmitis accounted for only 12–25%.[2,4,5] In developing countries, however, trauma was a very common cause of endophthalmitis. In an Indian study, posttraumatic endophthalmitis accounted for 40.2% of the patients, which is a lower prevalence than that of postoperative endophthalmitis (43.0%).[6] Trauma was the most common cause (43.1%) in a Thailand study.[9] In our study, 82.6% of all endophthalmitis cases were caused by trauma, which was a higher rate than that of previous studies. Moreover, the use of industrial tools (54.7%) was the most common cause of trauma, suggesting the importance of avoiding industrial injury to reduce the prevalence of traumatic endophthalmitis.
In contrast to previous studies of developed countries that exhibited a balanced distribution between sexes,[2,10,11] our study showed that male patients accounted for most of the infectious endophthalmitis cases (79.5%). This finding may largely be attributed to the fact that Western China is located in China's inland regions with a relatively lower economic status, where trauma was the most common cause of endophthalmitis (82.6%). Because most traumatic endophthalmitis patients were male (84.3%), male patients accounted for a large proportion of the total population. Similarly, there were more male patients with infectious endophthalmitis (66%) in India,[7] which is also a developing country. We also found that most of the injuries were work related. As a matter of fact, regulations regarding ocular safety have been in practice in China for years. Nevertheless, publicizing of and education on ocular safety has been far from sufficient in reality. Specific injury prevention strategies, such as the use of safety goggles, need to target the workplace to reduce the incidence and severity of ocular trauma.
We compared the patterns of endophthalmitis with respect to the proportion of posttraumatic versus postsurgical versus endogenous for the first 6 years of collection versus the second 6 years of collection. We found that the number of endophthalmitis cases presented an increasing pattern (38.7% in the first half of the period and 61.3% in the second half of the period). This increasing trend is probably consistent with the development of economy and increasing industrial activities for the past decade in Western China. The proportion of posttraumatic endophthalmitis decreased from 86.2% to 80.3%, while that of postsurgical endophthalmitis increased from 3.7% to 8.9%, which may be due to the propagation of ocular safety and increasing number of ocular operations over the years with the economic growth in Western China.
As indicated by previous studies, advanced age is a risk factor of postsurgical endophthalmitis.[10,11] In our study, patients of postsurgical endophthalmitis were also older than those of traumatic and endogenous endophthalmitis.
The duration of the period from symptom onset to admission in our study (9.7 ± 77.4 days) was distinctively longer than studies conducted in Korea[2] (3.9 ± 11.2 days) and New Zealand[4] (4.2 ± 7.9 days). Twenty-one (1.3%) patients came to our hospital more than 60 days after symptom occurrence. Low socioeconomic status and unawareness of the harm of endophthalmitis may lead to delayed hospital visit.
Most of the past medical histories were hypertension (31.4%) and diabetes (28.9%), which was in line with previous studies.[2,12,13] Patients with endogenous endophthalmitis were more likely to have past medical history than those with other types of endophthalmitis.
Given the large number of cases reviewed and period of collection, it is hard to collect the final visual outcome after a certain period of follow-up. Hence, we used the discharge visual acuity in the medical records instead for the present study. It is reasonable that the visual outcome in the present study was poorer than that of reported studies:[2,12,14,15] 11.4% of the discharge visual acuity was beyond 20/200 and 74.6% of the patients had a discharge visual acuity of CF or worse. In the Korean study, 33.3% of patients had a final visual outcome (the vision after a follow-up period) over 20/100.[2] Moreover, 74% and 68.8% of patients achieved 20/100 or better vision in the Endophthalmitis Vitrectomy Study[12] and the Netherlands[15] studies, respectively. To evaluate the treatments, we should analyze the final visual outcome instead of discharge visual acuity in subsequent studies.
There are different therapeutic strategies for infectious endophthalmitis according to the severity. Intravitreal antimicrobial and small-gauge pars plana vitrectomy are most commonly used, sometimes combined with systemic antibiotics for severe cases. In our study, the rate of vitrectomy was 51.3% (817/1593). The rate of vitrectomy as an intervention of endophthalmitis differed between studies. In the Netherlands study, 10% of the cases underwent vitrectomy.[15] However, the rate of vitrectomy in another Korean endophthalmitis study was 76.3%,[2] which was higher than our current findings. Tsai and Tseng found that evisceration or enucleation was performed in 23.2% of the patients.[16] In our study, only 13.9% of patients underwent evisceration. A high incidence of enucleation or evisceration was observed among patients with corneal ulcer-associated endophthalmitis. It was reported that 21.4–62.2% of patients with infectious keratitis-related endophthalmitis underwent enucleation or evisceration.[17,18,19] In our study, 32 of 43 cases (74.4%) with corneal ulcer-associated endophthalmitis underwent enucleation, which was substantially higher than in previous reports. Low socioeconomic status, residence in remote places, poor accessibility to high-quality eye care services, unawareness of the harm of keratitis, and poor medication compliance may result in delayed treatment and failure to save the eyes of these patients.
In a retrospective study reporting the etiology of endophthalmitis, cataract surgery (70%) and trabeculectomy (16%) were the first and second causes of postsurgical endophthalmitis, respectively.[20] Our results confirmed this finding. Our study also showed that the time interval between filter surgery and endophthalmitis occurrence could be as long as several years because of the connection between the outer and inner eye, which was a result also found in previous studies.[21]
The positive culture rate was 39.7% in our study and is relatively lower compared with other reports.[4,22,23,24] Thus, further investigation of causes is required. Gram-positive bacteria were the predominant causative microorganisms, which was also similarly found in previous studies.[4,23]
In conclusion, since 2011, there was a significant increase in the number of infectious endophthalmitis patients in this dataset. It might imply that more measures of prevention and control need to be taken for the upward trend of the occurrence of infectious endophthalmitis in Western China. The demographic and clinical characteristics of infectious endophthalmitis in Western China had its own characteristics and were quite different from those in developed countries. Here, trauma was the most common cause of endophthalmitis, most traumatic endophthalmitis patients were male, and most of the injuries were work related, implicate that we should strengthen the education and application of ocular safety regulation. Specific injury prevention strategies, such as the use of protective eyewear, need to target the workplace to reduce the incidence of ocular trauma and traumatic endophthalmitis. Infectious keratitis should be aware and effectively treated since corneal ulcer-associated endophthalmitis had a high incidence of enucleation.
Financial support and sponsorship
This work was supported by a grant from the National Major Scientific Equipment Program (No. 2012YQ12008005).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
REFERENCES
- 1.Connell PP, O'Neill EC, Fabinyi D, Islam FM, Buttery R, McCombe M, et al. Endogenous endophthalmitis: 10-year experience at a tertiary referral centre. Eye (Lond) 2011;25:66–72. doi: 10.1038/eye.2010.145. doi: 10.1038/eye.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheu SJ. Endophthalmitis. Korean J Ophthalmol. 2017;31:283–9. doi: 10.3341/kjo.2017.0036. doi: 10.3341/kjo.2017.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shrader SK, Band JD, Lauter CB, Murphy P. The clinical spectrum of endophthalmitis: Incidence, predisposing factors, and features influencing outcome. J Infect Dis. 1990;162:115–20. doi: 10.1093/infdis/162.1.115. doi: 10.1093/infdis/162.1.115. [DOI] [PubMed] [Google Scholar]
- 4.Fan JC, Niederer RL, von Lany H, Polkinghorne PJ. Infectious endophthalmitis: Clinical features, management and visual outcomes. Clin Exp Ophthalmol. 2008;36:631–6. doi: 10.1111/j.1442-9071.2008.01813.x. doi: 10.1111/j.1442-9071.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- 5.Krause L, Bechrakis NE, Heimann H, Kildal D, Foerster MH. Incidence and outcome of endophthalmitis over a 13-year period. Can J Ophthalmol. 2009;44:88–94. doi: 10.3129/i08-160. doi: 10.3129/i08-160. [DOI] [PubMed] [Google Scholar]
- 6.Sharma S, Padhi TR, Basu S, Kar S, Roy A, Das T, et al. Endophthalmitis patients seen in a tertiary eye care centre in Odisha: A clinico-microbiological analysis. Indian J Med Res. 2014;139:91–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Relhan N, Forster RK, Flynn HW., Jr Endophthalmitis: Then and now. Am J Ophthalmol. 2018;187:xx–xxvii. doi: 10.1016/j.ajo.2017.11.021. doi: 10.1016/j.ajo.2017.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities “hand motion” and “counting fingers” can be quantified with the freiburg visual acuity test. Invest Ophthalmol Vis Sci. 2006;47:1236–40. doi: 10.1167/iovs.05-0981. doi: 10.1167/iovs.05-0981. [DOI] [PubMed] [Google Scholar]
- 9.Bhoomibunchoo C, Ratanapakorn T, Sinawat S, Sanguansak T, Moontawee K, Yospaiboon Y, et al. Infectious endophthalmitis: Review of 420 cases. Clin Ophthalmol. 2013;7:247–52. doi: 10.2147/OPTH.S39934. doi: 10.2147/OPTH.S39934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friling E, Lundström M, Stenevi U, Montan P. Six-year incidence of endophthalmitis after cataract surgery: Swedish national study. J Cataract Refract Surg. 2013;39:15–21. doi: 10.1016/j.jcrs.2012.10.037. doi: 10.1016/j.jcrs.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 11.Hatch WV, Cernat G, Wong D, Devenyi R, Bell CM. Risk factors for acute endophthalmitis after cataract surgery: A population-based study. Ophthalmology. 2009;116:425–30. doi: 10.1016/j.ophtha.2008.09.039. doi: 10.1016/j.ophtha.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 12.Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Endophthalmitis Vitrectomy Study Group. Arch Ophthalmol. 1995;113:1479–96. doi: 10.1001/archopht.1995.01100120009001. [PubMed] [Google Scholar]
- 13.Kang KT, Kim KS, Kim YC. Factors affecting final visual acuity in infectious endophthalmitis following cataract surgery. J Korean Ophthalmol Soc. 2013;54:1025–31. doi: 10.3341/jkos.2013.54.7.1025. [Google Scholar]
- 14.Wong TY, Chee SP. The epidemiology of acute endophthalmitis after cataract surgery in an Asian population. Ophthalmology. 2004;111:699–705. doi: 10.1016/j.ophtha.2003.07.014. doi: 10.1016/j.ophtha.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Pijl BJ, Theelen T, Tilanus MA, Rentenaar R, Crama N. Acute endophthalmitis after cataract surgery: 250 consecutive cases treated at a tertiary referral center in the Netherlands. Am J Ophthalmol. 2010;149:482–70. doi: 10.1016/j.ajo.2009.09.021. doi: 10.1016/j.ajo.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 16.Tsai YY, Tseng SH. Risk factors in endophthalmitis leading to evisceration or enucleation. Ophthalmic Surg Lasers. 2001;32:208–12. doi: 10.1038/srep28100. [PubMed] [Google Scholar]
- 17.Henry CR, Flynn HW, Jr, Miller D, Forster RK, Alfonso EC. Infectious keratitis progressing to endophthalmitis: A 15-year study of microbiology, associated factors, and clinical outcomes. Ophthalmology. 2012;119:2443–9. doi: 10.1016/j.ophtha.2012.06.030. doi: 10.1016/j.ophtha.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott IU, Flynn HW, Jr, Feuer W, Pflugfelder SC, Alfonso EC, Forster RK. Endophthalmitis associated with microbial keratitis. Ophthalmology. 1996;103:1864–70. doi: 10.1016/s0161-6420(96)30415-6. doi: 10.1016/S0161-6420(96)30415-6. [DOI] [PubMed] [Google Scholar]
- 19.O'Neill EC, Yeoh J, Fabinyi DC, Cassidy D, Vajpayee RB, Allen P, et al. Risk factors, microbial profiles and prognosis of microbial keratitis-associated endophthalmitis in high-risk eyes. Graefes Arch Clin Exp Ophthalmol. 2014;252:1457–62. doi: 10.1007/s00417-014-2732-1. doi: 10.1007/s00417-014-2732-1. [DOI] [PubMed] [Google Scholar]
- 20.Karacal H, Kymes SM, Apte RS. Retrospective analysis of etiopathogenesis of all cases of endophthalmitis at a large tertiary referral center. Int Ophthalmol. 2007;27:251–9. doi: 10.1007/s10792-007-9068-3. doi: 10.1007/s10792-007-9068-3. [DOI] [PubMed] [Google Scholar]
- 21.Ye H, Sun X, Gan D, Yu X, Zhou W, Xu G, et al. Bleb-associated endophthalmitis in a Chinese population (2003-2010): Clinical characteristics and visual outcome. Eur J Ophthalmol. 2012;22:719–25. doi: 10.5301/ejo.5000110. doi: 10.5301/ejo.5000110. [DOI] [PubMed] [Google Scholar]
- 22.Janknecht P, Zühlke S, Hansen LL. Did the spectrum of endophthalmitis change? A study comprising 13 years experience with operative therapy of endophthalmitis. Eur J Ophthalmol. 2006;16:300–5. doi: 10.1177/112067210601600217. doi: 10.1177/112067210601600217. [DOI] [PubMed] [Google Scholar]
- 23.Han DP, Wisniewski SR, Wilson LA, Barza M, Vine AK, Doft BH, et al. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study. Am J Ophthalmol. 1996;122:1–7. doi: 10.1016/s0002-9394(14)71959-2. doi: 10.1016/S0002-9394(14)71959-2. [DOI] [PubMed] [Google Scholar]
- 24.Okhravi N, Towler HM, Hykin P, Matheson M, Lightman S. Assessment of a standard treatment protocol on visual outcome following presumed bacterial endophthalmitis. Br J Ophthalmol. 1997;81:719–25. doi: 10.1136/bjo.81.9.719. doi: 10.1136/bjo.81.9.719. [DOI] [PMC free article] [PubMed] [Google Scholar]