Abstract
Background:
Although fasting plasma glucose (FPG) has been highly recommended as the sole test for diabetes screening, the efficacy of FPG alone for diabetes screening is potentially limited due to its low sensitivity. The aim of this study was to improve the efficacy of FPG for diabetes screening using urinary glucose (UG).
Methods:
This study was initiated on November 12, 2015, and ended on June 28, 2016. A representative sample of individuals aged between 18 and 65 years, with no history of diabetes, from 6 cities in Jiangsu Province participated in this study. A 75-g oral glucose tolerance test was used to diagnose diabetes. All urine samples were collected within 2 h of oral glucose loading to measure UG. Partial correlation analyses were used to evaluate the associations between UG and other glycemic variables, including FPG, 2-h plasma glucose (2h-PG), and glycated hemoglobin A1c, after adjustment for age. The performance of UG was evaluated using a receiver operating characteristic (ROC) curve analysis.
Results:
Of the 7485 individuals included, 8% were newly diagnosed with diabetes and 48.7% had prediabetes. The areas under the ROC curves for UG were 0.75 for estimation of 2h-PG ≥7.8 mmol/L and 0.90 for 2h-PG ≥11.1 mmol/L, respectively. The sensitivity and specificity of UG were 52.3% and 87.8%, respectively, for 2h-PG ≥7.8 mmol/L (cutoff point ≥130 mg), and 83.5% and 87.5%, respectively, for 2h-PG ≥11.1 mmol/L (cutoff point ≥178.5 mg). The combination of FPG and UG demonstrated a significantly higher sensitivity than that of FPG alone for the identification of diabetes ([483/597] 80.9% vs. [335/597] 56.1%, χ2 = 85.0, P < 0.001) and glucose abnormalities ([2643/4242] 62.3% vs. [2365/4242] 55.8%, χ2 = 37.7, P < 0.001).
Conclusions:
The combination of UG and FPG substantially improves the efficacy of using FPG alone for diabetes screening; this combination might be a practical screening tool and is worth being recommended in the future.
Keywords: Diabetes Mellitus, Screening, Urinary glucose
摘要
背景:
空腹血糖(FPG)被推荐可单独使用进行糖尿病筛查,然而单独使用空腹血糖其灵敏度较低。本研究旨在利用尿糖(UG)提升FPG的糖尿病筛查效能。
方法:
本研究起始于2015年11月12日,并于2016年6月28日结束。抽取江苏省6个城市,年龄20至65周岁既往从未诊断过糖尿 病的城乡常住居民。所有的受试者均给予口服葡萄糖耐量试验。收集口服葡萄糖负荷2小时内所有尿液,定量检测UG。校正 年龄后,偏相关分析评估UG与其他血糖变量包括FPG,2h血糖(2h-PG)和糖化血红蛋白(HbA1c)的相关性,受试者工作 特征曲线(ROC)评价UG的筛查价值。
结果:
最终纳入分析7485名受试者,新诊断糖尿病患病率8%,糖尿病前期患病率48.7%。鉴定2h-PG≥ 7.8 mmol/L,UG的 ROC曲线下面积为0.75,鉴定2h-PG≥ 11.1 mmol/L,UG的ROC曲线下面积为0.90。UG筛查2h-PG≥ 7.8 mmol/L(最佳切点值 = 130 mg)灵敏度和特异度分别为52.3%和87.8%,筛查2h-PG≥ 11.1 mmol/L(最佳切点值≥178.5mg)灵敏度和特异度分别 为83.5%和87.5%。与单独使用FPG比较,FPG联合UG筛查糖尿病的灵敏度显著提升([483/597] 80.9% vs [335/597], 56.1%, χ2 = 85.0, p < 0.001),筛查血糖异常的灵敏度仍然显著高于单独使用FPG([2643/4242] 62.3% vs [2365/4242] 55.8%, χ2 = 37.7, p < 0.001)。
结论:
UG联合FPG能显著提升单独使用FPG的糖尿病筛查效能,这可能是一种实用的筛查工具,值得在将来被推荐。
INTRODUCTION
Diabetes has become a major public health problem in China,[1] affecting an estimated 113.9 million people in addition to the 493.4 million people with prediabetes.[2] It is often undiagnosed for many years until severe complications occur such as kidney failure, blindness, heart disease, and stroke.[3] In addition to the health burden, diabetes has imposed a large economic burden on health-care systems worldwide.[4] Early detection and diagnosis of diabetes to prevent diabetes-associated complications and to reduce the economic burden on medical care is therefore of significant importance.
Routine methods for diabetes screening include fasting plasma glucose (FPG) tests, an oral glucose tolerance test (OGTT), and glycated hemoglobin A1c (HbA1c) measurements. However, FPG alone is not sensitive enough, because isolated hyperglycemia 2 h after glucose loading is common among Chinese patients with diabetes.[5,6] Moreover, accumulating evidence shows that the efficacy of FPG alone in diabetes screening is questionable.[7,8,9,10] Although OGTT has been considered the gold standard test for the diagnosis of diabetes, it is not suitable for mass screening due to its complexity, and it is impractical to be conducted for all individuals, especially for those whose FPG is <7 mmol/L but may have diabetes.[11] HbA1c is easy to measure, and a previous study has shown that HbA1c could significantly increase the efficacy of using FPG alone in diabetes screening.[7] However, it is also not practical for mass screening due to the relatively high cost, especially in developing countries. In addition, as a diagnostic test for Chinese people, HbA1c measurement remains controversial because it has not been standardized.
Glucose will appear in the urine when plasma glucose exceeds the renal threshold for glucose resorption. This supports the assumption that urinary glucose (UG) could be an attractive alternative for assessing hyperglycemia. UG is not recommended as a routine screening test for diabetes because its sensitivity is low. However, in previous studies, urine samples were collected 1 h after the main meal of the day, before breakfast, or 1–2 h after a solid morning or evening meal, without uniform specification.[12,13,14] Moreover, quantitative detection of glycosuria allows more accurate assessment of the average elevation of glucose in the urine compared with the traditional detection with dipsticks.[15] We consider that UG measured within a specific period of time better reflects the fluctuations in blood glucose and speculate that its lack of sensitivity in diabetes screening could be overcome. Recently, a study by our group confirmed the validity of postprandial UG for diabetes screening in an urban high-risk population, but its utility in the general population is still unclear.[10] This exploratory study was aimed to examine whether the use of UG would help improve the efficacy of using FPG alone in diabetes screening.
METHODS
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Review Committee of Jiangsu Provincial Center for Disease Control and Prevention (JSJK2016-B003-03) and Zhongda Hospital, Southeast University. Each participant provided written informed consent.
Subjects
This study was initiated on November 12, 2015, and ended on June 28, 2016. We used a multistage, stratified sampling method to select a representative sample of individuals aged between 18 and 65 years in the general population from 6 cities in Jiangsu Province. Individuals without previously diagnosed diabetes were invited to take part in this study. Exclusion criteria were diabetes, pregnancy, a severe psychiatric disturbance, or an unstable health condition. A total of 8119 residents were invited and 7689 participated in this survey. The overall response rate was 94.7%. There were 7485 participants included in the final analysis after the exclusion of 204 participants whose data on HbA1c or UG were missing.
Data collection
A structured questionnaire was used to obtain the information on age, sex, education, lifestyle, family history of chronic disease, and health status. Weight, height, and blood pressure (BP) were measured according to standardized protocols. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared.
Participants were first asked to urinate and empty their bladder completely before an OGTT and were restricted from drinking water throughout the whole test. FPG, HbA1c, and 2-h plasma glucose (2h-PG) were measured. All the urine samples were collected over a 2-h period after oral glucose loading for quantitative measurement of UG. FPG and 2h-PG were measured by the glucose oxidase method using an automated chemistry analyzer (Synchron LX-20, Beckman Coulter Inc., California, USA). HbA1c was measured using high-performance liquid chromatography (HPLC, D-10, Bio-Rad Inc., California, USA). Urine glucose concentrations were determined with a quantitative urine meter (UG-201-H, Tanita Corporation, Tokyo, Japan).
Definitions
Diabetes was diagnosed as FPG ≥7.0 mmol/L and/or 2h-PG ≥11.1 mmol/L and/or HbA1c ≥6.5% on the basis of the 2012 American Diabetes Association diagnostic criteria. Prediabetes was defined as FPG ≥5.6 mmol/L and ≤6.9 mmol/L, 2h-PG ≥7.8 mmol/L and <11.0 mmol/L, or 5.7%≤ HbA1c ≤6.4%.
Statistical analysis
Continuous variables are presented as the means (95% confidence intervals), and categorical variables are presented as percentages. Student's t-test and Kappa test were used to compare the characteristics of participants in different groups for continuous variables, and Chi-square tests were used to compare categorical variables. Partial correlation analyses were used to evaluate the associations between UG and other glycemic variables, including FPG, 2h-PG, and HbA1c, after adjustment for age. The receiver operating characteristic (ROC) curves were constructed, and the area under the ROC curve (AUC) was used to evaluate the performance of using UG to detect 2h-PG ≥7.8 mmol/L and 2h-PG ≥11.1 mmol/L. The optimal cutoff points of UG were determined using the maximum of Youden's index. A value of P < 0.05 was considered statistically significant. All statistical analyses were conducted using SPSS 22.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
General characteristics of study participants
According to the predefined criteria, 597 (8.0%) individuals were newly diagnosed with diabetes mellitus, 3645 (48.7%) had prediabetes, and 3243 (43.3%) had normal glucose tolerance. The general characteristics of the study population are presented in Table 1. Among them, 3298 (44.1%) were males and 4187 (55.9%) were females. BP, FPG, cholesterol, triglycerides, HDL-C, LDL-C, BMI, and UG were significantly higher in males than those in females. No significant differences in age, 2h-PG, and HbA1c were observed between the two groups.
Table 1.
Characteristics of study participants according to gender
| Characteristics | Male (n = 3298) | Female (n = 4187) | Statistics | P |
|---|---|---|---|---|
| Age (years) | 43.6 (43.2–44.1) | 44.0 (43.7–44.4) | −1.512* | 0.131 |
| HR (beats/min) | 75.9 (75.5–76.3) | 78.9 (78.5–79.4) | −9.894* | <0.001 |
| Blood pressure (mmHg) | ||||
| Systolic | 131.2 (130.6–131.8) | 126.0 (125.4–126.6) | 11.904* | <0.001 |
| Diastolic | 82.0 (81.6–82.4) | 77.0 (76.5–77.5) | 14.930* | <0.001 |
| Plasma glucose (mmol/L) | ||||
| FPG | 5.6 (5.5–5.6) | 5.4 (5.4–5.4) | 8.150* | <0.001 |
| 2h-PG | 6.6 (6.5–6.7) | 6.6 (6.5–6.7) | 0.292* | 0.770 |
| HbA1c (%) | 5.7 (5.6–5.7) | 5.7 (5.6–5.7) | −0.115* | 0.909 |
| Cholesterol (mmol/L) | 4.7 (4.7–4.7) | 4.6 (4.6–4.7) | 3.269* | 0.001 |
| Triglycerides (mmol/L) | 1.9 (1.8–2.0) | 1.4 (1.4–1.4) | −14.179† | <0.001 |
| HDL-C (mmol/L) | 1.3 (1.3–1.3) | 1.4 (1.4–1.4) | −14.332* | <0.001 |
| LDL-C (mmol/L) | 2.7 (2.6–2.7) | 2.6 (2.5–2.6) | 4.703* | <0.001 |
| BMI (kg/m2) | 25.5 (25.3–25.6) | 24.9 (24.8–25.0) | 5.929* | <0.001 |
| UG (mg) | 299.6 (273.3–325.9) | 130.7 (116.4–144.9) | −15.869† | <0.001 |
Data are means (95% CI) as indicated. 1 mmHg = 0.133 kPa. *t value; †Z value. HR: Heart rate; HbA1c: Glycated hemoglobin; FPG: Fasting plasma glucose; 2h-PG: 2-h plasma glucose; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; BMI: Body mass index; UG: Urinary glucose; CI: Confidence interval.
Correlation of urinary glucose with glycemic variables
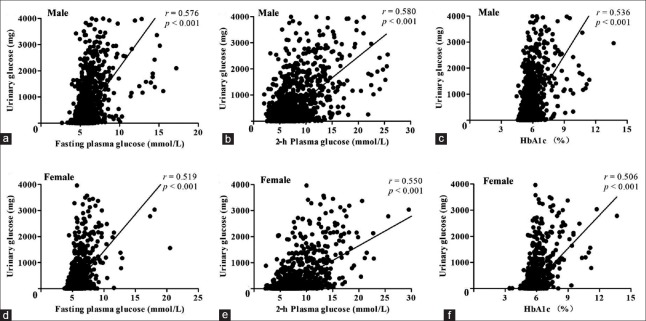
As shown in Figure 1, UG was significantly correlated with FPG (r = 0.576, P < 0.001), 2h-PG (r = 0.580, P < 0.001), and HbA1c (r = 0.536, P < 0.001) in males after adjustment for age. Similar correlations were observed in females. In addition, the correlation between 2h-PG and UG was slightly stronger than the correlation between FPG and UG.
Figure 1.
Correlations between UG and glycemic variables including FPG, 2h-PG, and HbA1c after adjustment for age. UG was significantly correlated with FPG, 2h-PG, and HbA1c in males (r = 0.576, 0.580, and 0.536, respectively, all P < 0.001; a-c, n = 3298). Significant positive correlations were also observed in females (r = 0.519, 0.550, and 0.506, respectively, all P < 0.001; d-f, n = 4187). UG: Urinary glucose; FPG: Fasting plasma glucose; 2h-PG: 2-h plasma glucose; HbA1c: Glycated hemoglobin A1c.
Performance of urinary glucose in the prediction of 2-h plasma glucose
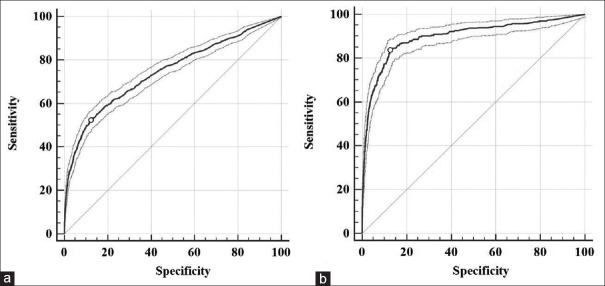
The ROC curve was employed to evaluate the validity of UG in the prediction of 2h-PG. The AUC for UG was 0.75 for the prediction 2h-PG ≥7.8 mmol/L and 0.90 for the prediction of 2h-PG ≥11.1 mmol/L [Figure 2]. Furthermore, UG exhibited a sensitivity of 52.3% and a specificity of 87.8% for the detection of 2h-PG ≥7.8 mmol/L at a corresponding optimal cutoff point of 130 mg and a high sensitivity of 83.5% and a specificity of 87.5% at an optimal cutoff point of 178.5 mg for the prediction 2h-PG ≥11.1 mmol/L [Table 2].
Figure 2.
ROC curves for UG for identifying 2h-PG ≥7.8 mmol/L (a) and 2h-PG ≥11.1 mmol/L (b). The areas under the ROC curves were 0.75 (95% CI: 0.74–0.75) and 0.90 (95% CI: 0.89–0.91). ROC: Receiver operating characteristic; UG: Urinary glucose; 2h-PG: 2-h plasma glucose; CI: Confidence interval.
Table 2.
Performance of UG for predicting 2h-PG ≥7.8 mmol/L and ≥11.1 mmol/L
| Parameters | AUC | Optimal cutoff point (mg) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| 2h-PG ≥7.8 mmol/L | 0.75 (0.74–0.75) | 130.0 | 52.3 (49.6–55.0) | 87.8 (87.0–88.6) |
| 2h-PG ≥11.1 mmol/L | 0.90 (0.89–0.91) | 178.5 | 83.5 (79.5–87.1) | 87.5 (86.7–88.2) |
| Parameters | PPV (%) | NPV (%) | +LR | −LR |
| 2h-PG ≥7.8 mmol/L | 49.2 (46.6–51.8) | 89.1 (88.3–89.9) | 4.3 (3.9–4.7) | 0.5 (0.5–0.6) |
| 2h-PG ≥11.1 mmol/L | 27.1 (24.6–29.7) | 99.0 (98.7–99.2) | 6.7 (6.2–7.2) | 0.2 (0.2–0.2) |
Data are means (95% CI). 2h-PG: 2-h plasma glucose; UG: Urinary glucose; AUC: The area under the ROC curves; PPV: Positive predictive value; NPV: Negative predictive value; +LR: Positive likelihood ratio; −LR: Negative likelihood ratio; CI: Confidence interval; ROC: Receiver operating characteristic.
Evaluation of fasting plasma glucose combined with urinary glucose in diabetes screening
Using FPG ≥7 mmol/L alone to identify diabetes had an excellent specificity of 100%, but a low sensitivity of only 56.1% (335/597), whereas the combined utilization of FPG and UG showed a significantly higher sensitivity ([483/597] 80.9% vs. 56.1%, χ2 = 85.0, P < 0.001) for detecting diabetes. Further analysis showed that combined utilization of FPG and UG had an absolute sensitivity advantage of 44.2% over FPG alone. Moreover, the sensitivity of using FPG alone (≥5.6 mmol/L) to identify glucose abnormalities was 55.8% (2365/4242), while the sensitivity of combined utilization of FPG and UG was 62.3% (2643/4242), showing an absolute sensitivity advantage of 11.6% over FPG alone.
DISCUSSION
Although FPG has been recommended for routine screening for diabetes, its use is limited because of its low sensitivity.[6] In support of this, the results of the study showed that using FPG alone does not have sufficient sensitivity to screen for newly diagnosed diabetes (56.1%) in a general population. This also means that nearly half of newly diagnosed diabetes cases could not be detected using FPG alone. In the present study, the sensitivity of UG for the estimation of 2h-PG ≥11.1 mmol/L was 83.5%, suggesting that this test might be useful in detecting individuals with 2h-PG ≥11.1 mmol/L. In addition, the combined utilization of UG and FPG improved the sensitivity to 80.9% for identifying diabetes and to 62.3% for identifying glucose abnormalities. Since FPG is easy to access and UG is noninvasive and available, the combined utilization of UG and FPG is a practical and sufficient test model for diabetes screening, especially in large populations.
Diabetes is a major risk factor for morbidity and mortality, especially in low- and middle-income countries where the diabetes prevalence grows increasingly higher.[16] Early diagnosis and timely treatments or interventions have been shown to reduce diabetic complications and to prevent or delay the onset of diabetes.[9] Therefore, screening for diabetes is warranted. Because early-stage type 2 diabetes is often asymptomatic and FPG alone is not sensitive enough for people with only mildly elevated postprandial blood glucose,[1,7,17] a large proportion of patients with prediabetes or diabetes often have delayed diagnosis until a severe complication occurs. The sensitivity of using FPG alone for detecting diabetes was 56.1%, which is consistent with several other cross-sectional studies.[18,19] However, the efficacy of FPG for diabetes screening has been questioned. Therefore, some studies have attempted to improve the efficacy of FPG in diabetes screening by introducing new strategies. A study by Wang et al.[7] showed that FPG combined with HbA1c could significantly improve the sensitivity to 88%. In this study, we also investigated the sensitivity of FPG in combination with HbA1c, and a sensitivity of 84.6% was observed (data are not shown). The HbA1c test can be performed at any time of a day and does not require an overnight fast. However, HbA1c may not be suitable for mass screening of large populations because it is relatively expensive. Our previous study demonstrated that the use of serum advanced glycation end products-peptides substantially improved the efficacy of FPG in diabetes screening among high-risk Chinese individuals,[19] for which a sensitivity of 91.2% was found by combined utilization of advanced glycation end products-peptides and FPG. Nevertheless, the efficacy of advanced glycation end products-peptides in the general population for diabetes screening is unknown.
Based on the findings that UG reflects the prevailing plasma glucose level,[20,21] studies demonstrated that a postprandial urine test for glucose is an effective approach for diabetes self-monitoring and self-management.[22,23] A study by our group also showed that quantitative monitoring of urine glucose has comparable efficacy on glycemic control.[24] However, UG as a screening test for diabetes has been underestimated. Because of poor stability and low sensitivity,[12,13] traditional urine testing with dipsticks is not recommended for diabetes screening. With the development of a urine glucose meter, quantitative measurement of UG has exhibited a wider measurement range of 0–2 g/L and showed a rapid response within 6 s. Furthermore, it is stable and is not likely to be influenced by various interferents such as ascorbic acid and acetaminophen.[15] In contrast to traditional urine dipsticks, this quantitative measurement is more sensitive, objective, and accurate. In addition, urine samples were collected without uniform specification in previous studies. The data showed that determining UG over a specific period of time is useful for diabetes screening. Due to its noninvasiveness and availability to pregnant women, UG was also confirmed as the strongest predictor of gestational diabetes.[25] In addition, our previous study has indicated that UG is a valid tool for detecting both undiagnosed diabetes and prediabetes in high-risk populations.[10] As a result, UG testing should not be completely given up, especially in low-income regions.
The present study was conducted in a large sample of representative Chinese adults without a history of diagnosed diabetes. The large number of individuals provided our study with high statistical power for data analyses. However, the limitations of this study should be recognized. First, we did not collect multiple urine samples (30 min, 60 min, and 90 min) after the OGTT test. To sufficiently evaluate the use of UG as a screening test and guarantee scientific rigor, participants were required to empty their bladders before the OGTT and were restricted from drinking water during the whole study. Furthermore, all the urine samples were collected over a 2-h period after oral glucose loading. Future studies are required to investigate whether 2-h postprandial UG is the best indicator of diabetes and whether a “spot” urine test after a solid meal is sufficiently effective for diabetes screening. Second, the excretion of urine glucose is mainly mediated by the kidneys, and filtered glucose is reabsorbed by the proximal tubules. This alternative and efficient screening approach might therefore be inappropriate for those with renal function impairment or proximal tubule disorders. Third, it should be mentioned that the present study only involved individuals of Chinese Han ethnicity from the same province. It remains largely unknown whether involving different ethnicities might contribute to differences in sensitivities. More studies are required to confirm our findings.
In conclusion, UG substantially improves the efficacy of using FPG alone in diabetes screening in a general population. Due to its non-invasiveness and effectiveness, the combined measurement of FPG and UG might be a novel approach for mass screening in the general population, and further study is warranted to evaluate whether 2-h postprandial UG is the best indicator of diabetes and whether a “spot” urine test after a solid meal is sufficiently effective for diabetes screening.
Financial support and sponsorship
This work was supported by grants from the National Key Technology R&D Program of China (No. 2016YFC1305700) and the key Program of Jiangsu Natural Science Foundation (No. BL2014079).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–101. doi: 10.1056/NEJMoa0908292. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–59. doi: 10.1001/jama.2013.168118. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 3.Laiteerapong N, Cifu AS. Screening for prediabetes and type 2 diabetes mellitus. JAMA. 2016;315:697–8. doi: 10.1001/jama.2015.17545. doi: 10.1001/jama.2015.17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.da Rocha Fernandes J, Ogurtsova K, Linnenkamp U, Guariguata L, Seuring T, Zhang P, et al. IDF diabetes atlas estimates of 2014 global health expenditures on diabetes. Diabetes Res Clin Pract. 2016;117:48–54. doi: 10.1016/j.diabres.2016.04.016. doi: 10.1016/j.diabres.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Qiao Q, Nakagami T, Tuomilehto J, Borch-Johnsen K, Balkau B, Iwamoto Y, et al. Comparison of the fasting and the 2-h glucose criteria for diabetes in different Asian cohorts. Diabetologia. 2000;43:1470–5. doi: 10.1007/s001250051557. doi: 10.1007/s001250051557. [DOI] [PubMed] [Google Scholar]
- 6.Jia WP, Pang C, Chen L, Bao YQ, Lu JX, Lu HJ, et al. Epidemiological characteristics of diabetes mellitus and impaired glucose regulation in a Chinese adult population: The Shanghai Diabetes Studies, a cross-sectional 3-year follow-up study in Shanghai urban communities. Diabetologia. 2007;50:286–92. doi: 10.1007/s00125-006-0503-1. doi: 10.1007/s00125-006-0503-1. [DOI] [PubMed] [Google Scholar]
- 7.Wang W, Lee ET, Fabsitz R, Welty TK, Howard BV. Using HbA(1c) to improve efficacy of the American Diabetes Association fasting plasma glucose criterion in screening for new type 2 diabetes in American Indians: The strong heart study. Diabetes Care. 2002;25:1365–70. doi: 10.2337/diacare.25.8.1365. doi: 10.2337/diacare.25.8.1365. [DOI] [PubMed] [Google Scholar]
- 8.Deepa R, Shanthi Rani S, Premalatha G, Mohan V. Comparison of ADA 1997 and WHO 1985 criteria for diabetes in South Indians – The Chennai urban population study. American Diabetes Association. Diabet Med. 2000;17:872–4. doi: 10.1046/j.1464-5491.2000.00385.x. doi: 10.1046/j.1464-5491.2000.00385.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhou X, Pang Z, Gao W, Wang S, Zhang L, Ning F, et al. Performance of an A1C and fasting capillary blood glucose test for screening newly diagnosed diabetes and pre-diabetes defined by an oral glucose tolerance test in Qingdao, China. Diabetes Care. 2010;33:545–50. doi: 10.2337/dc09-1410. doi: 10.2337/dc09-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang BQ, Lu Y, He JJ, Wu TZ, Xie ZL, Lei CH, et al. Performance of fasting plasma glucose and postprandial urine glucose in screening for diabetes in Chinese high-risk population. Chin Med J. 2015;128:3270–5. doi: 10.4103/0366-6999.171353. doi: 10.4103/0366-6999.171353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Hu G, Zhang L, Mayo R, Chen L. A novel testing model for opportunistic screening of pre-diabetes and diabetes among US adults. PLoS One. 2015;10:e0120382. doi: 10.1371/journal.pone.0120382. doi: 10.1371/journal.pone.0120382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friderichsen B, Maunsbach M. Glycosuric tests should not be employed in population screenings for NIDDM. J Public Health Med. 1997;19:55–60. doi: 10.1093/oxfordjournals.pubmed.a024588. doi: 10.1093/oxfordjournals.pubmed.a024588. [DOI] [PubMed] [Google Scholar]
- 13.Davies MJ, Williams DR, Metcalfe J, Day JL. Community screening for non-insulin-dependent diabetes mellitus: Self-testing for post-prandial glycosuria. Q J Med. 1993;86:677–84. doi: 10.1093/qjmed/86.10.677. doi: 10.1093/qjmed/86.10.677. [DOI] [PubMed] [Google Scholar]
- 14.Hanson RL, Nelson RG, McCance DR, Beart JA, Charles MA, Pettitt DJ, et al. Comparison of screening tests for non-insulin-dependent diabetes mellitus. Arch Intern Med. 1993;153:2133–40. doi: 10.1001/archinte.1993.00410180083010. [PubMed] [Google Scholar]
- 15.Miyashita M, Ito N, Ikeda S, Murayama T, Oguma K, Kimura J, et al. Development of urine glucose meter based on micro-planer amperometric biosensor and its clinical application for self-monitoring of urine glucose. Biosens Bioelectron. 2009;24:1336–40. doi: 10.1016/j.bios.2008.07.072. doi: 10.1016/j.bios.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 16.Roglic G, Unwin N. Mortality attributable to diabetes: Estimates for the year 2010. Diabetes Res Clin Pract. 2010;87:15–9. doi: 10.1016/j.diabres.2009.10.006. doi: 10.1016/j.diabres.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Beagley J, Guariguata L, Weil C, Motala AA. Global estimates of undiagnosed diabetes in adults. Diabetes Res Clin Pract. 2014;103:150–60. doi: 10.1016/j.diabres.2013.11.001. doi: 10.1016/j.diabres.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Bao Y, Ma X, Li H, Zhou M, Hu C, Wu H, et al. Glycated haemoglobin A1c for diagnosing diabetes in Chinese population: Cross sectional epidemiological survey. BMJ. 2010;340:c2249. doi: 10.1136/bmj.c2249. doi: 10.1136/bmj. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Z, He J, Qiu S, Lei C, Zhou Y, Xie Z, et al. Using serum advanced glycation end products-peptides to improve the efficacy of World Health Organization fasting plasma glucose criterion in screening for diabetes in high-risk Chinese subjects. PLoS One. 2015;10:e0137756. doi: 10.1371/journal.pone.0137756. doi: 10.1371/journal.pone.0137756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruhnau B, Faber OK, Borch-Johnsen K, Thorsteinsson B. Renal threshold for glucose in non-insulin-dependent diabetic patients. Diabetes Res Clin Pract. 1997;36:27–33. doi: 10.1016/s0168-8227(97)01389-2. doi: 10.1016/S0168-8227(97)01389-2. [DOI] [PubMed] [Google Scholar]
- 21.Rave K, Nosek L, Posner J, Heise T, Roggen K, van Hoogdalem EJ, et al. Renal glucose excretion as a function of blood glucose concentration in subjects with type 2 diabetes – Results of a hyperglycaemic glucose clamp study. Nephrol Dial Transplant. 2006;21:2166–71. doi: 10.1093/ndt/gfl175. doi: 10.1093/ndt/gfl175. [DOI] [PubMed] [Google Scholar]
- 22.Dallosso HM, Bodicoat DH, Campbell M, Carey ME, Davies MJ, Eborall HC, et al. Self-monitoring of blood glucose versus self-monitoring of urine glucose in adults with newly diagnosed type 2 diabetes receiving structured education: A cluster randomized controlled trial. Diabet Med. 2015;32:414–22. doi: 10.1111/dme.12598. doi: 10.1111/dme.12598. [DOI] [PubMed] [Google Scholar]
- 23.Müller N, Kämmer K, Kloos C, Wolf G, Müller UA. Postprandial self-monitoring of urine glucose reflects glycaemic control in people with relatively well controlled type 2 diabetes mellitus not treated with insulin: A retrospective cohort study. Diabet Med. 2015;32:958–62. doi: 10.1111/dme.12718. doi: 10.1111/dme.12718. [DOI] [PubMed] [Google Scholar]
- 24.Lu J, Bu RF, Sun ZL, Lu QS, Jin H, Wang Y, et al. Comparable efficacy of self-monitoring of quantitative urine glucose with self-monitoring of blood glucose on glycaemic control in non-insulin-treated type 2 diabetes. Diabetes Res Clin Pract. 2011;93:179–86. doi: 10.1016/j.diabres.2011.04.012. doi: 10.1016/j.diabres.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Lawlor DA, Fraser A, Lindsay RS, Ness A, Dabelea D, Catalano P, et al. Association of existing diabetes, gestational diabetes and glycosuria in pregnancy with macrosomia and offspring body mass index, waist and fat mass in later childhood: Findings from a prospective pregnancy cohort. Diabetologia. 2010;53:89–97. doi: 10.1007/s00125-009-1560-z. doi: 10.1007/s00125-009-1560-z. [DOI] [PubMed] [Google Scholar]