Abstract
Introduction
Psychosocial stress contributes to onset/exacerbation of mood episodes and alcohol use, suggesting dysregulated diurnal cortisol rhythms underlie episodic exacerbations in Bipolar Disorder (BD). However, mechanisms underlying dysregulated HPA rhythms in BD and alcohol use disorders (AUD) are understudied. Knowledge of associated variance factors have great clinical translational potential by facilitating development of strategies to reduce stress-related relapse in BD and AUD. Evidence suggests structural changes to mitochondrial translocator protein (TSPO) (a regulator of steroid synthesis) due to the single nucleotide polymorphism rs6971, may explain much of this variance. However, whether rs6971 is associated with abnormal HPA rhythms and clinical exacerbation in humans is unknown.
Methods
To show this common TSPO polymorphism impacts HPA rhythms in BD, we tested whether rs6971 (dichotomized: presence/absence of polymorphism) predicted variance in diurnal cortisol rhythm (saliva: morning and evening for 3 days) in 107 BD (50 with and 57 without AUD) and 28 healthy volunteers of similar age and ethno-demographic distribution.
Results
Repeated measures ANOVA confirmed effects BD (F5,525 = 3.0, p = 0.010) and AUD (F5,525 = 2.9, p = 0.012), but not TSPO polymorphism (p > 0.05). Interactions were confirmed for TSPO × BD (F5,525 = 3.9, p = 0.002) and for TSPO × AUD (F5,525 = 2.8, p = 0.017).
Discussion
We identified differences in diurnal cortisol rhythm depending on presence/absence of common TSPO polymorphism in BD volunteers with or without AUD and healthy volunteers. These results have wide ranging implications but further validation is needed prior to optimal clinical translation.
Keywords: bipolar disorder, biomarker, stress, cortisol, immune, genetics, TSPO, rs6971, alcohol use disorder, variance factor, diurnal rhythm, HPA axis, precision medicine
Introduction
Bipolar disorder (BD) is a common (Merikangas et al., 2007), debilitating, life-threatening illness associated with substantial psychosocial and interpersonal dysfunction (Baldessarini et al., 2010). The impact of BD is substantial (Chang et al., 2016), owing in part to the cumulative effects of relapsing and remitting episodic mood changes, often exacerbated in the context of elevated psychosocial stress (Altman et al., 2006). While knowledge of mechanisms underlying stress-related behavioral (and biological) changes has greatly improved over the past few decades, factors underlying inter-individual variation in susceptibility remain unclear. This lack of biological clarity may mask mechanisms contributing to high rate of stress-related relapse in BD patients, potential familial and clinical linkages to higher rate of alcohol use disorder (AUD) (Saunders et al., 2012), and increased switching of mood states and rapid cycling in patients with co-morbid stress-associated illness like AUD (Ostacher et al., 2010). Determining stress mechanisms related to clinical variation in BD will be well pioised to facilitate development of novel, patient tailored treatment and prevention strategies in this debilitating, life threatening illness.
The impact of environmental stress on Hypothalamus Pituitary Adrenal (HPA)-axis activity is well established (Kirschbaum et al., 1993; Tanaka et al., 2013). Decline of cortisol concentration from morning to evening represents a diurnal pattern often referred to as diurnal cortisol rhythm or diurnal cortisol cycle (Mormont and Levi, 1997; Sephton et al., 2013). Variance in cortisol’s diurnal rhythm has been attributed to various factors including sex (Larsson et al., 2009), age (Amir et al., 2015; Larsson et al., 2009), and stress exposure (Dahlgren et al., 2005) and significant differences have been identified in stress-associated illnesses including BD (Belvederi Murri et al., 2016; Tanaka et al., 2013) and AUD (Spencer and Hutchison, 1999). Offspring of BD patients appear to exhibit different diurnal cortisol rhythms when compared with offspring of parents with no mental disorder (Ellenbogen et al., 2006). Taken together, this evidence suggests the presence of heritable pathophysiology underlying dysregulated stress processing in BD. Specifically how mechanisms underlying dysregulated HPA rhythms relate to interindividual clinical variability in BD is unclear.
Heritable factors underlying HPA dysregulation in BD may involve mechanisms regulating steroid production. A key outer mitochondrial membrane protein, the translocator protein (18 kDa; TSPO), formerly known as the peripheral-type benzodiazepine receptor (i.e. PBR) (Braestrup and Squires, 1977; Papadopoulos et al., 2006), is a rate limiting factor in steroid synthesis (Anholt et al., 1985; Lacapere and Papadopoulos, 2003; Lucki et al., 2012). The TSPO provides a binding site for cytoplasmic cholesterol on the interface between the fifth transmembrane domain and the cytosol facing C-terminus. Under normal circumstances, binding to the TSPO facilitates cholesterol transport through the outer and inner mitochondrial membranes via formation of functional supramolecular complexes and contact sites (Li and Papadopoulos, 1998). Through these complexes, cholesterol is made available to cytochrome P450 side chain cleavage via CYP11A1 on the matrix side of the inner mitochondrial membrane, yielding pregnenolone. Pregenolone, through a series of reactions within both mitochondria and endoplasmic reticula, is metabolized to final tissue-specific steroid products (i.e. cortisol in the adrenal). Genes regulating TSPO expression appear to have been conserved, regulating specialized functions underlying oxygen mediated metabolic processes (Fan et al., 2012), suggesting the TSPO is crucial to mammalian adaptation, regulating production of stress-related steroid hormones that ultimately impact learning, memory and recursively, behavior.
Structural changes to TSPO’s cholesterol binding domain reduces cholesterol binding (Lacapere and Papadopoulos, 2003), disrupting steroid synthesis and dysregulating cortisol rhythms. A specific, common, nonsynomnymous, missense single nucleotide polymorphism (snp) (rs6971) results in a base substitution from alanine to threonine at position 147 in TSPO’s fifth transmembrane domain (Owen et al., 2012). This functional polymorphism, associated with the A allele, alters mitochondrial TSPO protein structure, reducing cholesterol transport into the mitochondria and subsequently, reducing production of pregnenolone as compared to that occurring in the presence of the ancestral, G allele (Costa et al., 2009). The rs6971 polymorphism directly impacts TSPO binding by specific radiotracer ligands (e.g. [11C]PBR-28). Individuals homozygous for the ancestral, G-allele, express a high-affinity binding (HAB) site on their TSPO proteins, while individuals heterozygous (AG) or homozygous (AA) for the polymorphism will express moderate (MAB) or low-affinity binding (LAB) sites for [11C]PBR-28 respectively (Li et al., 2016; Yoder et al., 2013). Recent evidence suggests this common TSPO polymorphism is associated with dysregulated steroid production in humans (Owen et al., 2017).
Emerging evidence suggests this TSPO functional polymorphism is associated with a diagnosis of BD, possibly by reducing both cholesterol binding/transport and subsequent cortisol production, enhancing susceptibility to environmental stress, and ultimately, facilitating development of stress related psychiatric disorders including BD (Colasanti et al., 2013). The presence of a polymorphism associated with reduced homeostatic HPA-reactivity is relevant to BD patients, particularly those with a history of dysfunctional alcohol self-medication during periods of heightened psychosocial stress. However, whether this TSPO functional polymorphism is associated with dysregulated cortisol rhythms and consequent clinical exacerbations in BD with or without co-morbid AUD is unknown. Identifying variance factors associated with altered diurnal cortisol rhythm would reveal novel treatment targets to facilitate personalization and enhance medical precision in this debilitating illness.
To show that a common TSPO functional polymorphism is associated with HPA dysregulation in individuals with stress-related clinical vulnerability, we tested whether the presence/absence of the TSPO functional polymorphism (rs6971) predicted differences in cortisol’s diurnal rhythm in healthy control volunteers as well as in BD volunteers with and without co-morbid AUD. Building on existing evidence, we predicted strong interactions between diagnosis (BD, co-morbid AUD), TSPO variant, and diurnal cortisol rhythm in study volunteers. We believe that knowledge of biological factors accounting for inter-individual variation in the human diurnal cortisol cycle would have immense clinical utility, facilitating development of novel personalized treatment and prevention strategies, particularly in BD patients at highest risk of stress-related clinical relapse.
Methods
This study was approved by the institutional review board of the University of Michigan. All volunteers provided written, informed consent. Initiated in 2004, the Prechter Bipolar Longitudinal Study (Prechter) runs within the University of Michigan Comprehensive Depression Center, collecting longitudinal data on individuals diagnosed with BD in an effort to enhance understanding of its pathophysiology. Upon entry into the Prechter Longitudinal Study, diagnoses of psychiatric illness (e.g. bipolar disorder type I (BD-I), bipolar disorder type II, etc.) or lack of psychiatric illness (e.g. healthy control) were determined using the Diagnostic Instrument for Genetic Studies (DIGS), a structured diagnostic tool often used in psychiatric studies (Nurnberger et al., 1994). Adhering to DSM-IV diagnostic criteria, diagnoses are obtained from the DIGS and are confirmed (and re-confirmed annually) via consensus review by a panel of clinicians resulting in the consensus “best estimate” diagnosis. AUD was defined as meeting DSM-IV diagnostic criteria of either Alcohol Dependence or Alcohol Abuse disorder. Additional information collected from the DIGS used in subsequent analyses includes anthropometric and socio-demographic measures, age of onset (AAO: reported in years) of either first manic or first depressive episode and functional severity of a volunteer’s most clinically impairing manic or depressive episodes. Functional severity was determined using a likert scale varying from 0 (no impairment), 1 (mild impairment but still functioning in primary role), 2 (severe impairment to function in primary role), to 3 (incapacitated: unable to function in primary role). Patients also provided an estimate of the number of lifetime episodes of mania or depression, represented in our data as an ordinal number. More information on DIGS interviews in the Prechter Bipolar Longitudinal Study are outlined elsewhere (Kamali et al., 2012).
In the present study, 135 volunteers (including only individuals with a BD-I diagnosis and Healthy Controls of similar age and sex) were enrolled from the Prechter study on a first come, first serve basis, provided the required data (TSPO genetic information, salivary cortisol) was available. Individuals within the BD-I group were included with or without co-morbid AUD. Specific whole blood samples (genetics), and saliva samples (cortisol) were obtained from study volunteers on intake (or on follow-up assessment for those previously enrolled in the longitudinal study). Following completion of the DIGS (or on follow-up assessment for prior study enrollees), subjects completed the Hamilton Depression Rating Scale (HAM-D) (Hamilton, 1960) and the Young Mania Rating Scale (YMRS) (Young et al., 1978). Additional clinical information on each episode (whether depressive or manic) was obtained from the DIGS (or on follow-up assessment). This information included age of onset of first episode (AAO), number of lifetime episodes (NOE), and functional severity of most severe episode. Functional severity provides a rough estimate of a mood episode’s clinical impact with results ranging on a scale from 0 to 4 (0 – no symptoms, 1 - no impairment; 2 – impairment in secondary role; 3 – impairment in primary role; 4 – incapacitation). Medication information was dichotomized as the following categorical variables: anti-depressants, mood stabilizers, anti-psychotics, sedative-hypnotics, and thyroid hormones. None of the study volunteers was in receipt of treatment with cortisol, hydrocortisone, or other cortisol medications at the time of the study.
Saliva samples
A group of individuals provided saliva samples upon request, following either the initial diagnostic interview or the follow-up interview, whichever was most recent. Of the individuals who provided saliva samples, only those for whom we previously obtained genetic information on a common functional polymorphism of the TSPO gene (snp rs6971) were planned for inclusion in the current study (n = 135). Saliva samples were collected by volunteers at their home 15 min after wake-up and 15 min before bedtime on each of 3 consecutive days, using a Salivette synthetic swab (Sarstedt, Nümbrecht, Germany). Volunteers were provided with detailed instructions pertaining to the process of sample collection. Participants were informed to be sure not to engage in eating, drinking (except water), smoking, or teeth brushing for 30 min prior to sample collection. Saliva samples were stored in volunteers’ home freezer until sample set completion. Samples were shipped to the study PI in provided mailers and and immediately stored at −20 °C until ready to assay. Using a Siemen Centaur automated analyzer with chemi-luminescent technology, cortisol competitive immunoassays were completed on each saliva sample in a University of Alan R. Prossin et al. Michigan core lab. Inter- and intra-assay coefficients of variation at 0.7 µg/dl were 12.4% and 3.6% respectively. Within a small subset (n=21), 1 or 2 saliva samples had insufficient volume to complete cortisol assays. Rather than attempt to impute any missing data, only those volunteers who provided complete sample sets (3 wake-up samples and 3 bedtime samples) were included in analyses (BD-I without AUD: n=44; BD-I with AUD: n=44; Healthy Control: n=26). While some overlap exists between the current sample and that of another study (Kamali et al., 2012), the sample in the current study is distinct from (and incompletely overlapping with) the sample included in the study of Kamali et al (n=185). Furthermore, in the current study, raw cortisol concentrations were log10 normalized for statistical analyses while in the study by Kamali et al., non-parametric analyses were completed using raw (non-normalized) cortisol concentrations. As such, the numerical data used in the current study is distinct from that used in the study by Kamali et al.
Genetic Testing
Existing evidence associates allelic variation in a common functional polymorphism of the TSPO (snp rs6971) with both altered [11C]PBR-28 binding affinity in PET studies (Owen et al., 2012) and differential efficacy of TSPO selective ligands that bind to the mitochondrial TSPO (e.g. XBD173) (Owen et al., 2011). At study intake (prior to obtaining saliva samples) all subjects provided whole blood samples for genetic testing of specific single nucleotide polymorphisms. Methods pertaining to genetic testing are described in detail elsewhere (Cross-Disorder Group of the Psychiatric Genomics et al., 2013). For purposes of the current study, genetic factors focused on the TSPO rs6971 snp, dichotomized based on the presence/absence of the GG homozygosity, indicating the presence/absence of the high affinity binding (HAB) phenotype. Given the substantial reductions in radiotracer binding profiles observed in both AG (MAB) and AA (LAB) individuals in comparison to those in GG (HAB) individuals, MAB and LAB volunteers were combined to represent the group without the HAB phenotype. For purposes of rmANOVA analyses in the current study, TSPOHAB (a dichotomized variable) represents either presence of TSPOHAB (GG: G-allele homozygosity) or absence of TSPOHAB (AA or AG) as a main factor of interest.
Statistical Plan
Initial testing determined whether group differences (BD-I vs. Control; TSPO genotype, etc.) existed amongst anthropomorphic and clinical variables within our sample. T-Testing detected diagnostic differences in real number variables (subject age, BMI) and chi-squared testing detected differences in categorical variables (sex, TSPO variant). Due to non-normal distributions and/or ordinal nature of certain variables, Mann Whitney U testing Alan R. Prossin et al. examined whether the functional TSPO polymorphism impacted age of onset (AAO), functional severity, and number of episodes (NOE) of either mania or depression in BD-I volunteers. Subsequent analyses involved using repeated measures analysis of variance (rmANOVA) to understand whether specific factors of interest impacted the diurnal cortisol rhythm (also described as the diurnal cortisol cycle) consistent with similar analyses described elsewhere (Abelson et al., 2014). More specifically, testing with rmANOVA confirmed effects of independent factors of interest (BD-I, AUD, TSPO HAB; all dichotomous) on the dependent variable, diurnal salivary cortisol rhythm/cycle (repeated measure: salivary cortisol obtained in the morning and evening on each of three consecutive days). Repeated measures analyses were completed twice, once on the entire sample (n = 114) and once within the BD-I group (n = 88), the latter while controlling for categorical medication effects using the variables described above. Analyses were completed using SPSS Statistics (version 24) software (IBM, IL). Threshold for significance was set at p < 0.05 to control for a type I error.
Results
In total, we identified 107 BD-I volunteers (50 with and 57 without AUD), and 28 healthy control volunteers of similar age and demographic background. Within subjects who provided a full set of saliva samples (n=114), distribution of demographic, genetic, and anthropometric information is outlined in Table 1. No significant differences in ratio of males to females, age, distribution of the TSPO polymorphism, and BMI were found when comparing BD-I and Healthy Control groups (p > 0.05 for each). In Table 2 we outline the distribution of behavioral, genetic and anthropometric information in BD-I volunteers, separated by presence or absence of AUD, including additional information on depressive symptoms (HAM-D) and manic-type symptoms (YMRS).
Table 1.
Diagnostic distribution of subjects’ demographic, genetic, and anthropometric information reported across groups of BD-I and Healthy Control volunteers.
| Bipolar Disorder Type I | Healthy Controls | Stats | |||||
|---|---|---|---|---|---|---|---|
| Mean Age (years +/− SD) | 41.9+/− 12.1 | 40.6 +/− 16.6 | T = 0.37, p = 0.71 | ||||
| Sex | F (n = 56) | M (n = 32) | F (n = 18) | M (n = 8) | Χ2 = 0.28, p = 0.60 | ||
| Body Mass Index | 27.7 +/− 6.3 | 22.6 +/− 2.0 | T = 1.8, p = 0.09 | ||||
| TSPO variant | AA/AG (n = 47) | GG (n = 41) | AA/AG (n = 14) | GG (n = 12) | Χ2 < 0.01, p = 0.97 | ||
| Allele | A1: A | A2: G | A1: A | A2: G | |||
| frequency (%) | 29.0% | 71.0% | 30.8% | 69.2% | |||
| Genotype | A1: AA | A1/A2: AG | A2:GG | A1: AA | A1/A2: AG | A2:GG | |
| counts | 4 | 43 | 41 | 2 | 12 | 12 | |
| frequency (%) | 4.5% | 48.9% | 46.6% | 7.7% | 46.2% | 46.2% | |
Table 2.
Here we dspict the distribution of genetic, and other subject information within groups of BD-I subjects based on presence or absence of comorbid AUD. AAO refers to age (in years) at onset of first episode. Functional Severity provides a rough estimate of the episode’s clinical impact with results ranging on a scale from 0 to 4 (0 – no symptoms, 1 - no impairment; 2 – impairment in secondary role; 3 – impairment in primary role; 4 – incapacitation). Number of Lifetime Episodes refers to patient report of the total number of respective mood episodes (i.e. mania or depression) in their lifetime.
| Bipolar Disorder Type I | Stats | ||||||
|---|---|---|---|---|---|---|---|
| with Co-Morbid AUD | without Co-Morbid AUD | ||||||
| Mean Age (years +/− SD) | 41.5 | +/− 12.0 | 42.4 | +/− 12.5 | T = 0.37, p = 0.72 | ||
| Sex | F (n = 31) | M (n = 13) | F (n = 25) | M (n = 19) | Χ2 = 1.77, p = 0.18 | ||
| Body Mass Index (+/− SD) | 28.8 +/− 7.9 | 26.7 +/− 4.4 | T = 0.86, p = 0.40 | ||||
| TSPO variant | AA/AG (n = 22) | GG (n = 22) | AA/AG (n = 25) | GG (n = 19) | Χ2 = 0.41, p = 0.52 | ||
| HAM-D | 7.4 +/− 6.8 | 7.5 +/− 7.7 | U = 0.29, p = 0.77 | ||||
| YMRS | 2.6 +/− 3.9 | 2.1 +/− 2.4 | U = −0.08, p = 0.94 | ||||
| Manic Episodes | AAO (years) | 20.5 +/− 12.4 | 25.6 +/− 11.8 | U = −1.88, p = 0.06 | |||
| Functional Severity | 3.9 | 3.6 | U = 1.03, p = 0.30 | ||||
| Number of Lifetime Episodes | 15.8 | 10.8 | U = 1.22, p = 0.22 | ||||
| Major Depressive Episodes | AAO (years) | 16.1 +/− 10.3 | 19.6 +/− 11.9 | U = −1.48, p = 0.14 | |||
| Functional Severity | 3.1 | 3.3 | U = −0.23, p = 0.82 | ||||
| Number of Lifetime Episodes | 16.4 | 12.3 | U = 0.14, p = 0.88 | ||||
Data on racial background was missing on n=2 subjects. Within the remainder (n=112), racial distribution of the TSPO polymorphism (rs6971) was similar to distributions previously reported (Thomas, 2014). The overwhelming majority of subjects (n=95) identified as Caucasian during the diagnostic interview. Caucasians were split fairly evenly (almost 50% per group) among the TSPO HAB group (n=46) and the non TSPO HAB group (n=49). The remaining subjects (n=17) included those who identified as either Asian (n=3), Black/AfricanAmerican (n=7), or more than one race (n=7). All subjects identifying as Asian were within the non TSPO HAB group. Other subjects were fairly evenly distributed between TSPO HAB (Black/AfricanAmerican: n=4; more than one race: n=3) and TSPO MAB/LAB Alan R. Prossin et al. group (Black/AfricanAmerican: n=3; more than one race: n=4). Given the small numbers within non Caucasian racial groups, these groups were collapsed into a group denoted non-Caucasian (n=19) for subsequent analyses. After dichotomizing the race variable, the TSPO HAB group was comprised of n=46 Caucasians and n=7 non-Caucasians. Similarly, the TSPO MAB/LAB group was comprised of n=49 Caucasians and n=12 non-Caucasians. Mann-Whitney U testing confirmed that the TSPO polymorphism impacted clinical presentation in BD-I volunteers. TSPO HAB volunteers (homozygous for the conserved G-allele) had lower age of onset of depression (U = −2.5, p = 0.01) and more life-time manic episodes (U = 2.3, p = 0.02) but no difference in age of onset of mania (p = 0.09) or in the number of life-time depressive episodes (p > 0.10) as compared to MAB (AG variant) and LAB (AA variant) volunteers. Additional, post-hoc testing showed functional severity of mania was lower in TSPO HAB volunteers (U = −2.9, p = 0.004) compared to MAB and LAB volunteers, but neither functional severity of depression, HAM-D, or YMRS scores differed between groups (p > 0.10).
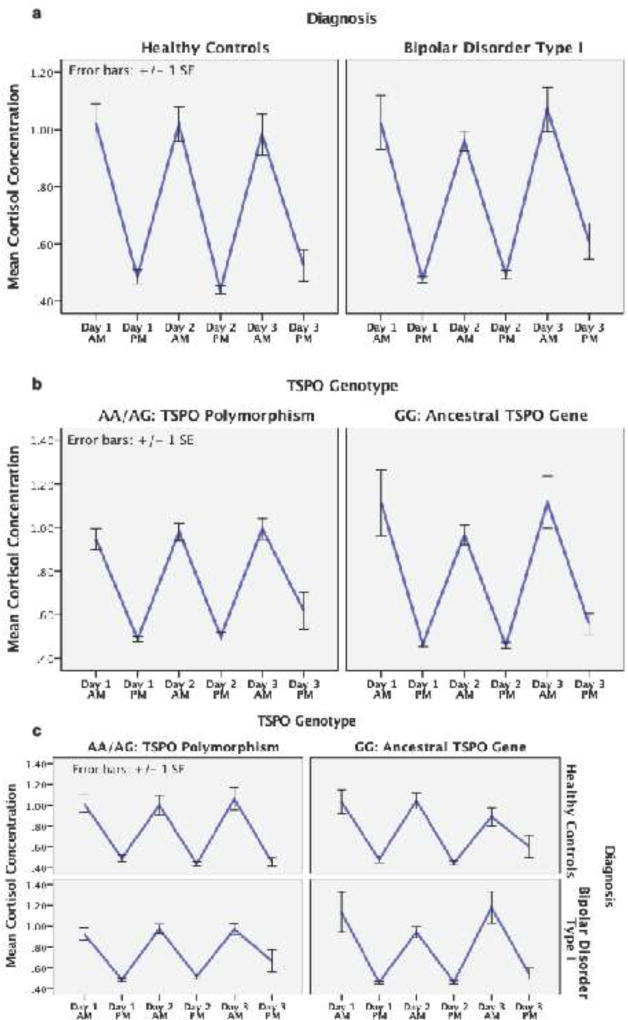
Quantified from saliva samples, diurnal pattern of volunteers’ salivary cortisol is depicted in Figure 1. Log normalized mean salivary cortisol concentrations are depicted on the vertical axis and day/time of salivary sample is depicted on the horizontal axis. The graphs depict cortisol concentration in the morning and evening on each of 3 consecutive days. Using repeated measures ANOVA (rmANOVA), we tested whether significant inter-individual variance existed in the diurnal cortisol cycle. Salivary cortisol (6 timepoints: morning and evening on each of three consecutive days) was modeled as the repeated measure and BD-I, AUD, and TSPOHAB were modeled as specific factors of interest, controlling for both Age and Sex, as reported by subjects at time of DIGS completion. Results showed significant variance in the diurnal cortisol cycle over three consecutive days (F5,525 = 13.8, p < 0.001). Neither Age or sex had a significant effect on the diurnal cortisol cycle (p > 0.05 for each). Significant effects were confirmed for the factors of interest with greater variance in BD-I volunteers (F5,525 = 3.0, p = 0.010), as well as in the subset of BD-I volunteers with AUD (F5,525 = 2.9, p = 0.012), each compared to healthy volunteers. While the presence of the TSPOHAB phenotype on its own was not associated with significant variance in the diurnal cortisol cycle (p > 0.05), significant interaction effects between TSPOHAB and diagnoses were identified. Presence of TSPOHAB phenotype together with a BD-I diagnosis accounted for significant inter-individual variance in cortisol’s diurnal cycle (F5,525 = 3.9, p = 0.002). A similar interaction was identified in volunteers with an AUD diagnosis, wherein the diurnal cortisol cycle in volunteers with both TSPOHAB and AUD differed significantly from those without both TSPOHAB variant and AUD (F5,525 = 2.8, p = 0.017). Follow-up testing for effects Alan R. Prossin et al. of race included similar rmANOVA testing while controlling for race (dichotomized as Caucasian or non-Caucasian) as a covariate in those subjects for whom data on race was available (n=117). Controlling for race in this manner had minimal effect on the aforementioned results. Additionally, Race did not have a significant effect on the diurnal cortisol cycle (p = 0.33).
Figure 1.
Figure 1 provides graphical illustration of diurnal cortisol rhythm (also known as cycle) (1 saliva sample in the morning, 1 saliva sample in the evening) on each of 3 consecutive days in study volunteers. Time/Day is represented on the x-axis and mean cortisol concentration on the y-axis. Figure 1a depicts cortisol data in each diagnostic group (i.e. healthy control volunteers on the left, BD-I volunteers on the right). Repeated Measures ANOVA confirmed greater variance in the diurnal cortisol rhythm in BD-I volunteers (F5,525 = 3.0, p = 0.010) compared to healthy volunteers. Figure 1b depicts cortisol data in 2 separate groups based on presence/absence of the TSPO polymorphism. The TSPO polymorphism homo/heterozygotes (AA/AG) on the left and individuals homozygous for the conserved/ancestral allele (GG) on the right. Without considering diagnostic factors, TSPO Genotype did not significantly impact the diurnal cortisol cycle in study volunteers (p > 0.05). Figure 1c depicts cortisol data in 4 separate groups based on presence/absence of BD-I diagnosis and presence/absence of TSPO polymorphism. Healthy controls are depicted in the top row of graphs and BD-I individuals are depicted in the bottom row. Results from individuals with the TSPO polymorphism (AA/AG) (TSPO LAB/MAB) are depicted the left and individuals with the conserved/ancestral allele (GG) (TSPO HAB) are depicted on the right. Presence of TSPOHAB phenotype together with a BD-I diagnosis accounted for significant variance in cortisol’s diurnal cycle (F5,525 = 3.9, p = 0.002).
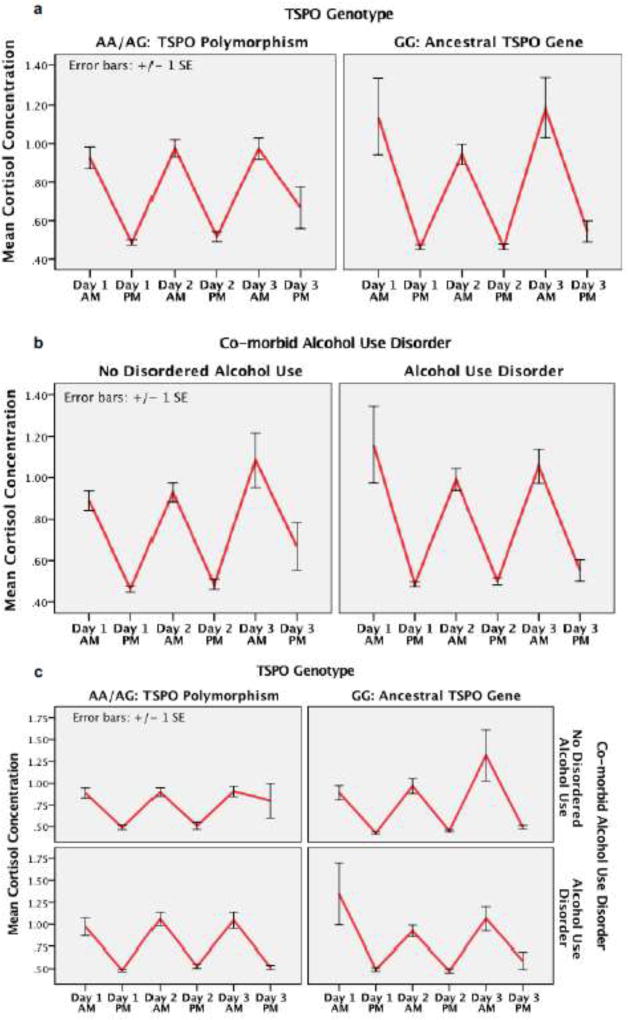
In figure 2 we depict diurnal cortisol cycle in BD-I volunteers, removing the healthy control volunteers from analyses. Using rmANOVA we tested for the effect of specific factors of interest (TSPOHAB, AUD) on variance in the diurnal cortisol cycle in BD-I volunteers. Within this group of BD-I volunteers, signifant variance in the diurnal cortisol cycle was confirmed (F5,420 = 119, p < 0.001). Neither TSPOHAB phenotype or AUD had significant effect on the diurnal cortisol cycle (P > 0.05 for each). A significant interaction effect was confirmed between TSPOHAB phenotype and diagnosis of AUD, wherein the diurnal cortisol cycle differed significantly between volunteers with both TSPOHAB phenotype and AUD as compared to those wtihout (F5,420 = 2.5, p = 0.030). Subsequently, in an attempt to control for medication effects, the rmANOVA was repeated, controlling for medication effects (each entered as covariate in the model). Neither antidepressant, mood stabilizer, antipsychotic, sedative hypnotic, or thyroid hormone treatment had a significant effect on diurnal cortisol cycle (P > 0.05 for each). After controlling for these medication effects, results from rmANOVA testing yielded a similar interaction effect between TSPOHAB phenotype or AUD (F5,395 = 2.3, p = 0.040).
Figure 2.
Figure 2 illustrates the diurnal cortisol rhythm (1 saliva sample in the morning, 1 saliva sample in the evening) on each of 3 consecutive days in volunteers with a diagnosis of BD-I (e.g. no healthy controls). Time/Day is represented on the x-axis and mean cortisol concentration on the y-axis.
Figure 2a depicts cortisol data in 2 separate graphs based on presence/absence of TSPO polymorphism. Individuals homozygous or heterozygous for the TSPO polymorphism (AA/AG) on the left and individuals homozygous for the conserved/ancestral allele (GG) on the right. Repeated Measures ANOVA confirmed that without considering AUDs, TSPO Genotype alone did not significantly impact the diurnal cortisol cycle in BD-I volunteers (p > 0.05). Figure 2b illustrates the diurnal cortisol rhythm over the same time interval, but with separate graphs based on presence (graph on the right) of absence (graph on the left) of AUD. Presence/absence of AUD didn’t have a significant effect on diurnal cortisol rhythm (p > 0.05). Figure 2c illustrates the diurnal cortisol rhythm over the same time interval, but with separate graphs based on presence/absence of AUD and presence/absence of TSPO HAB variant. The diurnal cortisol rhythm differed significantly between volunteers with both TSPOHAB phenotype and AUD and those wtihout (F5,445 = 3.5, p = 0.004).
Discussion
Production of steroids (neurosteroids, cortisol, etc.) in humans is essential for homeostatic survival, particularly in the setting of enhanced environmental and/or psychosocial stressors (de Kloet et al., 1999). Identifying factors underlying both inter-individual variance and diagnostic differences in HPA stress reactivity will likely contribute critical understanding to pathophysiology associated with dysregulated stress response processing in psychiatric illnesses.
Within the field, there remains some debate as to the role of TSPO in steroid formation (Papadopoulos et al., 2017). However, it should be noted that recent data obtained in new mouse and rat knockout models as well as in clinical trials support the role of TSPO in adrenal steroid formation (Barron et al., 2018; Owen et al., 2017). Substantial evidence suggests steroid production is impacted by the common TSPO polymorphism (rs6971). This 10 Alan R. Prossin et al. ala147thr functional polymorphism confers a deletion of the C-terminus on TSPO’s fifth transmembrane domain, reducing cholesterol entry into mitochondria (Lacapere and Papadopoulos, 2003; Li and Papadopoulos, 1998), consequently blunting production of steroid precursors. Providing additional support to the argument that TSPO plays a role in steroid synthesis, the data we presented shows that cortisol production in BD-I individuals homozygous for the conserved/ancestral, G-allele, was unabated, resulting in an aberrant diurnal cortisol pattern in BD-I individuals without the TSPO functional polymorphism. In HABs with BD-I, we found both higher clinical severity and increased variance in the diurnal cortisol rhythm, particularly in those without AUD. The diurnal patterns of cortisol differ between volunteers with AUD and those without AUD and may reflect underlying pathophysiology that places BD-I patients at risk for co-morbid AUD. However, an alternate hypothesis suggests these differences could be a product of alcohol use in AUD, blunting the cortisol response from use of alcohol over prolonged periods. Unfortunately, the temporal limitations associated with our study design (lack of life course follow-up) limit our determination of such cause/effect relationships.
In contrast, the presence of the TSPO polymorphism (AA/AG; LAB/MAB) in BD-I individuals had a significant impact on cortisol concentration, appearing to limit variance in diurnal cortisol rhythm in these BD-I individuals. This is consistent with previous findings wherein animals subjected to TSPO deletion in steroidogenic cells using a steroidogenic cell specific Cre (Sf1) retained their ability to produce basal levels of corticosterone, but lost their ability to react to external ACTH by producing additional corticosterone. Subsequent histochemical analysis showed that cholesterol, unused for steroidogenesis, accumulated in lipid droplets, a phenotype seen in congenital adrenal hyperplasia (Fan et al., 2015). In our figures, the pattern of diurnal cortisol rhythm in BD-I individuals with the TSPO functional polymorphism was similar to that in the healthy controls, possibly reflecting an adaptive function in these BD-I individuals. Additionally, its specific impact on our salivary cortisol data needs clarification. We show associations between alcohol abuse/dependence and blunting of cortisol, visible in Figure 3c as primarily affecting BD-I individuals homozygous for the conserved (G) allele (i.e. TSPO HABs). This data suggests the TSPO functional polymorphism may contribute, amongst other factors (Frank et al., 2000; Prossin A, 2010; Prossin et al., 2013), to reduction in homeostatic, neuroprotective mechanisms as well as reduced control over immune function, each with potential for wide ranging impact on human physiology and behavior. If validated, our results suggest TSPO screening may have a role in predicting enhanced biological vulnerabilities, and in triggering early implementation of patient oriented prevention strategies in those BD-I individuals at greatest Alan R. Prossin et al. risk for stress-related clinical decompensation. However, replication and validation of this data is required as is a greater understanding of the clinical/behavioral ramifications of blunted diurnal cortisol rhythm in BD-I patients.
The data presented also informs microglia based PET research, specifically since radioactive [11C]PBR-28 binds to the microglial TSPO protein (Fujita et al., 2008; Hannestad et al., 2012). While binding of older generation TSPO ligands ([11C]PK11195) were less impacted by the TSPO polymorphism, binding of newer ligands ([11C]PBR-28) is quite vulnerable, the A-allele conferring a significantly reduced binding affinity as compared to the G-allele (Owen et al., 2012). The data presented helps to reduce clinical confounders associated with the requisite genetic pre-screening for high affinity binders (HAB) and improve the clinical translational potential of microglial based, [11C]PBR-28 PET research. While its specific role in brain immune function is unclear, upregulation of brain microglial TSPO proteins appears to reflect phenotypic changes to microglial cells associated with activation and release of IL-1 family cytokines as well as regulation of cell death and production of oxygen free radicals (Venneti et al., 2006). As such, non-invasive [11C]PBR-28 PET imaging of brain microglial activity is uninterpretable in the presence of this functional polymorphism (A-allele) of the TSPO protein. However, whether the TSPO protein (or its functional polymorphism) contribute to immune-related illness susceptibility is currently under investigation.
The three-way analyses conducted in this study were not directional. We were mainly concerned with understanding whether presence or absence of the TSPO HAB variant (GG genotype) would explain a significant portion of cortisol’s variance as illustrated in figures 2 and 3. While our results suggest the presence or absence of TSPO HAB may explain some variance attributed to HPA dysfunction in bipolar disorder and alcohol use disorder, we did not confirm whether the presence of the TSPO functional polymorphism (GA and AA genotypes), or the conserved/ancestral (GG) variant, individually explain significant portions of the total variance. Therefore, this study takes a step in the right direction, but further interaction analyses should be conducted to tease apart the TSPO variants in order to confirm a significant interaction, if any, between either a low or high affinity binding TSPO phenotype, difference in diurnal cortisol rhythm over time and BD/AUD diagnoses. Due to limitations in study design we did not factor additional measures (mood, medication, etc.) into analyses involving cortisol. Although some evidence suggests cortisol may not be a reliable biomarker of specific mood states in BD (Cervantes et al., 2001), other evidence suggests measurable changes in plasma cortisol may be related to non-specific changes in mood and/or affective state (Prossin et al., 2016a, b), suggesting clinical translation of similar future studies will be enhanced if greater consideration to impact of mood changes are incorporated into study design. Further, while asserting their compliance, volunteers’ actual adherence is unknown, posing potential confounds. The relatively small sample size (and lack of equivalency between control and BD-I sample sizes) pose potential confounds and future studies incorporating larger samples and greater attention to potential confounders will likely provide more clinically relevant and translatable data.
Combined with existing evidence, the data we present suggest the low binding affinity TSPO variant impacts HPA axis output by reducing the normally wide range of capacity for steroid production. Depending on the presence/absence of risk for underlying stress-related illness, impact of this polymorphism could be either homeostatic or disruptive. If validated, these findings are poised to facilitate improved medical precision by guiding treatment with TSPO ligands (or other clinically relevant benzodiazepines) particularly in individuals at risk for stress-related clinical exacerbation. Such treatment strategies could help restore dysregulated HPA functioning in these individuals and specifically, in individuals with bipolar and comorbid disorders. However, Further testing in an expanded clinical sample (including BD Type II, BD NOS, Major Depression) is needed to validate associations between TSPO rs6971 genotype and downstream steroid synthesis and to delineate associated diagnostic interactions.
Nevertheless, additional factors not controlled in our study may pose potential confounds to the data presented. Clinical translation of this data may be limited by the sample size (relatively small for a genetic study) and lack of replication in an independent sample. Additionally, while we attempted to control for medication effects by dichotomizing medication subgroups based on their clinical use (i.e. anti-depressant, mood stabilizer, etc.), potential confounds related to the putative effects of individual medications on steroid production may persist when applying this approach. However, none of the study volunteers was in receipt of treatment with cortisol, hydrocortisone, or other cortisol medications at the time of the study. Further, while study volunteers were actively queried regarding their medication treatment, it is possible that lack of recollection and/or report of specific medication treatment may have confounded the data. Additionally, disrupted sleep patterns, present at an increased rate in Bipolar individuals, could pose additional confounds. Unfortunately, time of collection was not recorded and as such, was not available for subsequent analyses and we cannot eliminate variability within our data due to non-adherence to instructions. Future studies that incorporate a larger sample size and control of additional factors will facilitate reduction/alleviation of these potential confounds, permitting more robust statistical control for individual medication and dose effects and enhancing clinical translational potential of resulting data.
Highlights.
Structural changes to the TSPO protein, as occur with a common, functional, single nucleotide polymorphism (rs6971), may be clinically impactful in humans suffering with bipolar disorder, a stress exacerbated illness.
Inter-individual variation in cortisol’s diurnal rhythm may be partially explained by this common TSPO polymorphism (rs6971).
Within Bipolar patients, presence or absence of the common, functional, TSPO polymorphism (rs6971) may contribute to co-morbidity of stress-exacerbated illnesses including alcohol use disorders.
Acknowledgments
We would like to thank Christine Brucksh, Gloria Harrington, and the staff of the Heinz C. Prechter Bipolar Research Program for their efforts in maintaining the repository and making the data available to us.
Role of Funding Sources:
Funding for this study was provided by Heinz C. Prechter Bipolar Research Fund at the University of Michigan Depression Center and the Richard Tam Foundation and grant support from the National Institute on Drug Abuse and the National Center for Advancing Translational Sciences, both of the National Institutes of Health under award numbers DA033454 (AP) and 2KL2TR000434 (KR) respectively. None of the funding sources were involved (or exerted tangible influence) in development of the hypotheses, data collection, data analyses of this study or in development of the manuscript.
One author (MK) reports to have received research funding from Janssen, Assurex Health and Intra-Cellular Therapies, Inc. in the past three years.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Statement:
I, Alan Prossin, verify that, to the best of my knowledge, the results presented in this manuscript are truly representative of the data obtained as outlined in the methods section of this manuscript.
Contributors:
Alan Prossin – developing and editing manuscript from start to completion, data analysis, assist with data curation, interview of subjects
Matthew Chandler – editing of manuscript draft, help with figures
Kelly Ryan - editing of manuscript draft, assist with data curation, interview of subjects
Masoud Kamali – assist with data curation, editing of manuscript draft, interview of subjects
Erica Saunders – assist with data curation, editing of manuscript draft, interview of subjects
Sebastian Zoellner – curation of genetics data, consulted on genetics aspects of manuscript, edited manuscript
Robert Dantzer – consulted on psychoneuroimmunology (and cortisol) aspects of data, edited manuscript
Vassillios Papadopoulos – assisted in developing framework of study, consulted on aspects of study directly related to TSPO and cortisol, edited manuscript
Melvin McInnis – lead PI of Prechter Research Program (where data originated from), interviewed study subjects, assisted with saliva collection, edited manuscript, contributed to development of main study objectives/hypotheses.
Financial Disclosures:
The authors do not report additional financial or other potential conflicts of interests.
References
- Abelson JL, Erickson TM, Mayer SE, Crocker J, Briggs H, Lopez-Duran NL, Liberzon I. Brief cognitive intervention can modulate neuroendocrine stress responses to the Trier Social Stress Test: buffering effects of a compassionate goal orientation. Psychoneuroendocrinology. 2014;44:60–70. doi: 10.1016/j.psyneuen.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman S, Haeri S, Cohen LJ, Ten A, Barron E, Galynker II, Duhamel KN. Predictors of relapse in bipolar disorder: A review. J Psychiatr Pract. 2006;12:269–282. doi: 10.1097/00131746-200609000-00002. [DOI] [PubMed] [Google Scholar]
- Amir D, Ellison PT, Hill KR, Bribiescas RG. Diurnal variation in salivary cortisol across age classes in Ache Amerindian males of Paraguay. Am J Hum Biol. 2015;27:344–348. doi: 10.1002/ajhb.22645. [DOI] [PubMed] [Google Scholar]
- Anholt RR, De Souza EB, Kuhar MJ, Snyder SH. Depletion of peripheral-type benzodiazepine receptors after hypophysectomy in rat adrenal gland and testis. Eur J Pharmacol. 1985;110:41–46. doi: 10.1016/0014-2999(85)90026-3. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Vieta E, Calabrese JR, Tohen M, Bowden CL. Bipolar depression: overview and commentary. Harv Rev Psychiatry. 2010;18:143–157. doi: 10.3109/10673221003747955. [DOI] [PubMed] [Google Scholar]
- Barron AM, Ji B, Kito S, Suhara T, Higuchi M. Steroidogenic abnormalities in translocator protein knockout mice and significance in the aging male. Biochem J. 2018;475:75–85. doi: 10.1042/BCJ20170645. [DOI] [PubMed] [Google Scholar]
- Belvederi Murri M, Prestia D, Mondelli V, Pariante C, Patti S, Olivieri B, Arzani C, Masotti M, Respino M, Antonioli M, Vassallo L, Serafini G, Perna G, Pompili M, Amore M. The HPA axis in bipolar disorder: Systematic review and meta-analysis. Psychoneuroendocrinology. 2016;63:327–342. doi: 10.1016/j.psyneuen.2015.10.014. [DOI] [PubMed] [Google Scholar]
- Braestrup C, Squires RF. Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H)diazepam binding. Proc Natl Acad Sci U S A. 1977;74:3805–3809. doi: 10.1073/pnas.74.9.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes P, Gelber S, Kin FN, Nair VN, Schwartz G. Circadian secretion of cortisol in bipolar disorder. J Psychiatry Neurosci. 2001;26:411–416. [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Huang KC, Chiu WC, Huang KC, Tang CH, Su KP. Change in employment status in bipolar disorder: a longitudinal study using national claims data. J Clin Psychiatry. 2016;77:e429–435. doi: 10.4088/JCP.14m09576. [DOI] [PubMed] [Google Scholar]
- Colasanti A, Owen DR, Grozeva D, Rabiner EA, Matthews PM, Craddock N, Young AH. Bipolar Disorder is associated with the rs6971 polymorphism in the gene encoding 18kDa Translocator Protein (TSPO) Psychoneuroendocrinology. 2013;38:2826–2829. doi: 10.1016/j.psyneuen.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B, Pini S, Gabelloni P, Da Pozzo E, Abelli M, Lari L, Preve M, Lucacchini A, Cassano GB, Martini C. The spontaneous Ala147Thr amino acid substitution within the translocator protein influences pregnenolone production in lymphomonocytes of healthy individuals. Endocrinology. 2009;150:5438–5445. doi: 10.1210/en.2009-0752. [DOI] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics, C. Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Perlis RH, Mowry BJ, Thapar A, Goddard ME, Witte JS, Absher D, Agartz I, Akil H, Amin F, Andreassen OA, Anjorin A, Anney R, Anttila V, Arking DE, Asherson P, Azevedo MH, Backlund L, Badner JA, Bailey AJ, Banaschewski T, Barchas JD, Barnes MR, Barrett TB, Bass N, Battaglia A, Bauer M, Bayes M, Bellivier F, Bergen SE, Berrettini W, Betancur C, Bettecken T, Biederman J, Binder EB, Black DW, Blackwood DH, Bloss CS, Boehnke M, Boomsma DI, Breen G, Breuer R, Bruggeman R, Cormican P, Buccola NG, Buitelaar JK, Bunney WE, Buxbaum JD, Byerley WF, Byrne EM, Caesar S, Cahn W, Cantor RM, Casas M, Chakravarti A, Chambert K, Choudhury K, Cichon S, Cloninger CR, Collier DA, Cook EH, Coon H, Cormand B, Corvin A, Coryell WH, Craig DW, Craig IW, Crosbie J, Cuccaro ML, Curtis D, Czamara D, Datta S, Dawson G, Day R, De Geus EJ, Degenhardt F, Djurovic S, Donohoe GJ, Doyle AE, Duan J, Dudbridge F, Duketis E, Ebstein RP, Edenberg HJ, Elia J, Ennis S, Etain B, Fanous A, Farmer AE, Ferrier IN, Flickinger M, Fombonne E, Foroud T, Frank J, Franke B, Fraser C, Freedman R, Freimer NB, Freitag CM, Friedl M, Frisen L, Gallagher L, Gejman PV, Georgieva L, Gershon ES, Geschwind DH, Giegling I, Gill M, Gordon SD, Gordon-Smith K, Green EK, Greenwood TA, Grice DE, Gross M, Grozeva D, Guan W, Gurling H, De Haan L, Haines JL, Hakonarson H, Hallmayer J, Hamilton SP, Hamshere ML, Hansen TF, Hartmann AM, Hautzinger M, Heath AC, Henders AK, Herms S, Hickie IB, Hipolito M, Hoefels S, Holmans PA, Holsboer F, Hoogendijk WJ, Hottenga JJ, Hultman CM, Hus V, Ingason A, Ising M, Jamain S, Jones EG, Jones I, Jones L, Tzeng JY, Kahler AK, Kahn RS, Kandaswamy R, Keller MC, Kennedy JL, Kenny E, Kent L, Kim Y, Kirov GK, Klauck SM, Klei L, Knowles JA, Kohli MA, Koller DL, Konte B, Korszun A, Krabbendam L, Krasucki R, Kuntsi J, Kwan P, Landen M, Langstrom N, Lathrop M, Lawrence J, Lawson WB, Leboyer M, Ledbetter DH, Lee PH, Lencz T, Lesch KP, Levinson DF, Lewis CM, Li J, Lichtenstein P, Lieberman JA, Lin DY, Linszen DH, Liu C, Lohoff FW, Loo SK, Lord C, Lowe JK, Lucae S, MacIntyre DJ, Madden PA, Maestrini E, Magnusson PK, Mahon PB, Maier W, Malhotra AK, Mane SM, Martin CL, Martin NG, Mattheisen M, Matthews K, Mattingsdal M, McCarroll SA, McGhee KA, McGough JJ, McGrath PJ, McGuffin P, McInnis MG, McIntosh A, McKinney R, McLean AW, McMahon FJ, McMahon WM, McQuillin A, Medeiros H, Medland SE, Meier S, Melle I, Meng F, Meyer J, Middeldorp CM, Middleton L, Milanova V, Miranda A, Monaco AP, Montgomery GW, Moran JL, Moreno-De-Luca D, Morken G, Morris DW, Morrow EM, Moskvina V, Muglia P, Muhleisen TW, Muir WJ, Muller-Myhsok B, Murtha M, Myers RM, Myin-Germeys I, Neale MC, Nelson SF, Nievergelt CM, Nikolov I, Nimgaonkar V, Nolen WA, Nothen MM, Nurnberger JI, Nwulia EA, Nyholt DR, O'Dushlaine C, Oades RD, Olincy A, Oliveira G, Olsen L, Ophoff RA, Osby U, Owen MJ, Palotie A, Parr JR, Paterson AD, Pato CN, Pato MT, Penninx BW, Pergadia ML, Pericak-Vance MA, Pickard BS, Pimm J, Piven J, Posthuma D, Potash JB, Poustka F, Propping P, Puri V, Quested DJ, Quinn EM, Ramos-Quiroga JA, Rasmussen HB, Raychaudhuri S, Rehnstrom K, Reif A, Ribases M, Rice JP, Rietschel M, Roeder K, Roeyers H, Rossin L, Rothenberger A, Rouleau G, Ruderfer D, Rujescu D, Sanders AR, Sanders SJ, Santangelo SL, Sergeant JA, Schachar R, Schalling M, Schatzberg AF, Scheftner WA, Schellenberg GD, Scherer SW, Schork NJ, Schulze TG, Schumacher J, Schwarz M, Scolnick E, Scott LJ, Shi J, Shilling PD, Shyn SI, Silverman JM, Slager SL, Smalley SL, Smit JH, Smith EN, Sonuga-Barke EJ, St Clair D, State M, Steffens M, Steinhausen HC, Strauss JS, Strohmaier J, Stroup TS, Sutcliffe JS, Szatmari P, Szelinger S, Thirumalai S, Thompson RC, Todorov AA, Tozzi F, Treutlein J, Uhr M, van den Oord EJ, Van Grootheest G, Van Os J, Vicente AM, Vieland VJ, Vincent JB, Visscher PM, Walsh CA, Wassink TH, Watson SJ, Weissman MM, Werge T, Wienker TF, Wijsman EM, Willemsen G, Williams N, Willsey AJ, Witt SH, Xu W, Young AH, Yu TW, Zammit S, Zandi PP, Zhang P, Zitman FG, Zollner S, Devlin B, Kelsoe JR, Sklar P, Daly MJ, O'Donovan MC, Craddock N, Sullivan PF, Smoller JW, Kendler KS, Wray NR, International Inflammatory Bowel Disease Genetics C Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45:984–994. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren A, Kecklund G, Akerstedt T. Different levels of work-related stress and the effects on sleep, fatigue and cortisol. Scand J Work Environ Health. 2005;31:277–285. doi: 10.5271/sjweh.883. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- Ellenbogen MA, Hodgins S, Walker CD, Couture S, Adam S. Daytime cortisol and stress reactivity in the offspring of parents with bipolar disorder. Psychoneuroendocrinology. 2006;31:1164–1180. doi: 10.1016/j.psyneuen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Fan J, Campioli E, Midzak A, Culty M, Papadopoulos V. Conditional steroidogenic cell-targeted deletion of TSPO unveils a crucial role in viability and hormone-dependent steroid formation. Proc Natl Acad Sci U S A. 2015;112:7261–7266. doi: 10.1073/pnas.1502670112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Lindemann P, Feuilloley MG, Papadopoulos V. Structural and functional evolution of the translocator protein (18 kDa) Curr Mol Med. 2012;12:369–386. doi: 10.2174/1566524011207040369. [DOI] [PubMed] [Google Scholar]
- Frank E, Swartz HA, Kupfer DJ. Interpersonal and social rhythm therapy: managing the chaos of bipolar disorder. Biol Psychiatry. 2000;48:593–604. doi: 10.1016/s0006-3223(00)00969-0. [DOI] [PubMed] [Google Scholar]
- Fujita M, Imaizumi M, Zoghbi SS, Fujimura Y, Farris AG, Suhara T, Hong J, Pike VW, Innis RB. Kinetic analysis in healthy humans of a novel positron emission tomography radioligand to image the peripheral benzodiazepine receptor, a potential biomarker for inflammation. Neuroimage. 2008;40:43–52. doi: 10.1016/j.neuroimage.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, Gallezot JD, Schafbauer T, Lim K, Kloczynski T, Morris ED, Carson RE, Ding YS, Cosgrove KP. Endotoxin-induced systemic inflammation activates microglia: [(1)(1)C]PBR28 positron emission tomography in nonhuman primates. Neuroimage. 2012;63:232–239. doi: 10.1016/j.neuroimage.2012.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamali M, Saunders EF, Prossin AR, Brucksch CB, Harrington GJ, Langenecker SA, McInnis MG. Associations between suicide attempts and elevated bedtime salivary cortisol levels in bipolar disorder. J Affect Disord. 2012;136:350–358. doi: 10.1016/j.jad.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Lacapere JJ, Papadopoulos V. Peripheral-type benzodiazepine receptor: structure and function of a cholesterol-binding protein in steroid and bile acid biosynthesis. Steroids. 2003;68:569–585. doi: 10.1016/s0039-128x(03)00101-6. [DOI] [PubMed] [Google Scholar]
- Larsson CA, Gullberg B, Rastam L, Lindblad U. Salivary cortisol differs with age and sex and shows inverse associations with WHR in Swedish women: a cross-sectional study. BMC Endocr Disord. 2009;9:16. doi: 10.1186/1472-6823-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Liu J, Liu N, Kuhn LA, Garavito RM, Ferguson-Miller S. Translocator Protein 18 kDa (TSPO): An Old Protein with New Functions? Biochemistry. 2016;55:2821–2831. doi: 10.1021/acs.biochem.6b00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Papadopoulos V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology. 1998;139:4991–4997. doi: 10.1210/endo.139.12.6390. [DOI] [PubMed] [Google Scholar]
- Lucki NC, Li D, Sewer MB. Sphingosine-1-phosphate rapidly increases cortisol biosynthesis and the expression of genes involved in cholesterol uptake and transport in H295R adrenocortical cells. Mol Cell Endocrinol. 2012;348:165–175. doi: 10.1016/j.mce.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, Kessler RC. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormont MC, Levi F. Circadian-system alterations during cancer processes: a review. Int J Cancer. 1997;70:241–247. doi: 10.1002/(sici)1097-0215(19970117)70:2<241::aid-ijc16>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Archives of general psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863-844. [DOI] [PubMed] [Google Scholar]
- Ostacher MJ, Perlis RH, Nierenberg AA, Calabrese J, Stange JP, Salloum I, Weiss RD, Sachs GS, Investigators S-B. Impact of substance use disorders on recovery from episodes of depression in bipolar disorder patients: prospective data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Am J Psychiatry. 2010;167:289–297. doi: 10.1176/appi.ajp.2009.09020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DR, Fan J, Campioli E, Venugopal S, Midzak A, Daly E, Harlay A, Issop L, Libri V, Kalogiannopoulou D, Oliver E, Gallego-Colon E, Colasanti A, Huson L, Rabiner I, Suppiah P, Essagian C, Matthews PM, Papadopoulos V. TSPO mutations in rats and a human polymorphism impair the rate of steroid synthesis. Biochem J. 2017 doi: 10.1042/BCJ20170648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DR, Lewis AJ, Reynolds R, Rupprecht R, Eser D, Wilkins MR, Bennacef I, Nutt DJ, Parker CA. Variation in binding affinity of the novel anxiolytic XBD173 for the 18 kDa translocator protein in human brain. Synapse. 2011;65:257–259. doi: 10.1002/syn.20884. [DOI] [PubMed] [Google Scholar]
- Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, Rhodes C, Pulford DJ, Bennacef I, Parker CA, StJean PL, Cardon LR, Mooser VE, Matthews PM, Rabiner EA, Rubio JP. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab. 2012;32:1–5. doi: 10.1038/jcbfm.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Fan J, Zirkin B. Translocator protein (18 kDa): an update on its function in steroidogenesis. J Neuroendocrinol. 2017 doi: 10.1111/jne.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossin A, M M, Anand A, Heitzeg M, Zubieta J-K. Tackling the Kraepelinian Dichotomy: A Neuroimaging Review. Psychiatr Ann. 2010;40:162–167. doi: 10.3928/00485713-20100303-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossin AR, Koch AE, Campbell PL, Barichello T, Zalcman SS, Zubieta JK. Acute experimental changes in mood state regulate immune function in relation to central opioid neurotransmission: a model of human CNS-peripheral inflammatory interaction. Mol Psychiatry. 2016a;21:243–251. doi: 10.1038/mp.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossin AR, Koch AE, Campbell PL, Barichello T, Zalcman SS, Zubieta JK. Experimental sadness induces relevant interactions between central endogenous opioid activation and plasma IL-18 concentrations in depressed volunteers. Mol Psychiatry. 2016b;21:151. doi: 10.1038/mp.2015.213. [DOI] [PubMed] [Google Scholar]
- Prossin AR, Zalcman SS, Evans SJ, McInnis MG, Ellingrod VL. A pilot study investigating tumor necrosis factor-alpha as a potential intervening variable of atypical antipsychotic-associated metabolic syndrome in bipolar disorder. Ther Drug Monit. 2013;35:194–202. doi: 10.1097/FTD.0b013e31827e18d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders EF, Fitzgerald KD, Zhang P, McInnis MG. Clinical features of bipolar disorder comorbid with anxiety disorders differ between men and women. Depress Anxiety. 2012;29:739–746. doi: 10.1002/da.21932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton SE, Lush E, Dedert EA, Floyd AR, Rebholz WN, Dhabhar FS, Spiegel D, Salmon P. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav Immun. 2013;30(Suppl):S163–170. doi: 10.1016/j.bbi.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Spencer RL, Hutchison KE. Alcohol, aging, and the stress response. Alcohol Res Health. 1999;23:272–283. [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Maruyama Y, Ishitobi Y, Kawano A, Ando T, Ikeda R, Inoue A, Imanaga J, Okamoto S, Kanehisa M, Ninomiya T, Tsuru J, Akiyoshi J. Salivary alpha-amylase and cortisol responsiveness following electrically stimulated physical stress in bipolar disorder patients. Neuropsychiatr Dis Treat. 2013;9:1899–1905. doi: 10.2147/NDT.S48722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. Inflammation in Parkinson's disease. Springer; New York: 2014. [Google Scholar]
- Venneti S, Lopresti BJ, Wiley CA. The peripheral benzodiazepine receptor (Translocator protein 18kDa) in microglia: from pathology to imaging. Prog Neurobiol. 2006;80:308–322. doi: 10.1016/j.pneurobio.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Nho K, Risacher SL, Kim S, Shen L, Saykin AJ. Influence of TSPO genotype on 11C-PBR28 standardized uptake values. J Nucl Med. 2013;54:1320–1322. doi: 10.2967/jnumed.112.118885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. The British journal of psychiatry : the journal of mental science. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]