Abstract
Our objective was to determine the associations of peripheral bone strength and microarchitecture with incident clinical and major osteoporotic fracture among older men after adjusting for major clinical risk factors. We used a prospective cohort study design with data from 1794 men (mean age 84.4 years) in the Osteoporotic Fractures in Men (MrOS) study. Eligible men attended the Year 14 visit, had high resolution peripheral quantitative computed tomography (HR-pQCT) scans of the distal radius and distal or diaphyseal tibia, DXA measured BMD, and were followed for mean 1.7 years for incident fracture. Failure load was estimated using finite element analysis. We used Cox proportional hazards models with standardized HR-pQCT parameters as exposure variables. Primary outcome was clinical fracture (n=108). Covariates included either FRAX major osteoporotic fracture probability calculated with BMD (FRAX-BMD), or individual clinical risk factors (CRF) including age, total hip BMD, race, falls, prevalent fracture after age 50. Lower failure load was associated with higher risk of incident clinical fracture and incident major osteoporotic fracture. For clinical fracture with FRAX-BMD adjustment, the associations ranged from HR=1.58 (95% CI: 1.25, 2.01) to 2.06 (95% CI: 1.60, 2.66) per SD lower failure load at the diaphyseal tibia and distal radius. These associations were attenuated after adjustment for individual CRFs, but remained significant at the distal sites. Associations of volumetric BMD with these outcomes were similar to those for failure load. At the distal radius, lower trabecular BMD, number, and thickness, and lower cortical BMD, thickness, and area were all associated with higher risk of clinical fracture, but cortical porosity was not. Among community-dwelling older men, HR-pQCT measures including failure load, volumetric BMD, and microstructure parameters at peripheral sites (particularly distal radius) are robust independent predictors of clinical and major osteoporotic fracture.
Keywords: Osteoporosis, older men, bone strength, bone microarchitecture, FRAX, fracture
INTRODUCTION
While BMD is a strong independent predictor of fracture risk in older women and men, prospective studies have consistently reported that fracture risk prediction is further improved by the combination of BMD and clinical risk factors [1–3]. Among fracture risk assessment tools, The Fracture Risk Assessment Tool (FRAX) is widely-used instrument that uses BMD in combination with clinical risk factors to assess the 10-year probabilities of major osteoporotic fracture (hip, forearm, humerus, or spine) and hip fracture [4]. However, FRAX was developed and validated using cohort studies of predominantly women. A recent prospective study in older men that evaluated simple and complex fracture risk assessment tools found that models based on age plus femoral neck BMD T-score alone classified men with and without incident hip fracture as accurately as FRAX and other fracture risk assessment tools[5]. Thus, there is a need to better characterize older men at increased fracture risk.
It has been hypothesized that the age-related increase in fracture risk is only partially explained by decline in bone mass and bone mineral density, and that bone microarchitecture and distribution may play a key role. High-resolution peripheral quantitative computed tomography (HR-pQCT) enables the assessment of bone density, structure and microarchitecture of cortical and trabecular bone of the tibia and radius. Furthermore, these detailed assessments can be used to estimate bone strength using finite element analysis (FEA). FEA enables a global prediction of bone strength by decomposing complex HR-pQCT imaging data into a network of smaller elements and computing forces and failure for these elements. Estimated failure load depends on the distribution of mineralized tissue (global geometry, global and local density, cortical porosity, local geometry of trabecular network) and therefore might be a better proxy of fracture risk than areal or volumetric BMD.
Previous retrospective studies based on analysis of prevalent fracture cases have suggested that parameters of HR-pQCT, including bone strength as measured by failure load, volumetric BMD, and compartment specific BMD, are related to clinical fracture outcomes [6–11]. These studies have been primarily conducted in post-menopausal women, but a few have evaluated older men. One case-control study in older men that included 177 fractures cases and 743 controls reported that HR-pQCT parameters were related to fracture (both vertebral and non-vertebral), but these associations were not statistically significant after adjustment for areal BMD [10]. Another case-control study in older Swedish men that included 87 fractures cases and 421 controls reported that fracture cases had lower cortical thickness, higher cortical porosity, and lower trabecular bone volume fraction at the distal tibia[11]. A subsequent prospective study noted that HR-pQCT parameters of the tibia were associated with incident fracture in older men, but notably only cortical thickness, cortical area, and cortical bone mineral content were stronger predictors of fracture risk than femoral neck areal BMD[12]. Thus, the role of HR-pQCT as a predictor of incident fracture independent of BMD remains uncertain among older men.
Our primary aim was to determine and compare the associations of bone strength, density, structure, and microarchitecture at the distal radius and distal and diaphyseal tibia with incident clinical fracture (primary outcome) and major osteoporotic fracture (secondary outcome) among a large population of community-dwelling older men after adjustment for major clinical risk factors (CRFs). Our secondary aim was to determine which HR-pQCT parameters at a given skeletal site most robustly predicted clinical fracture risk after mutual adjustment for other parameters at the same skeletal site, thus we included multiple HR-pQCT parameters from each skeletal site in a single model with clinical fracture as the outcome.
METHODS
Study Population
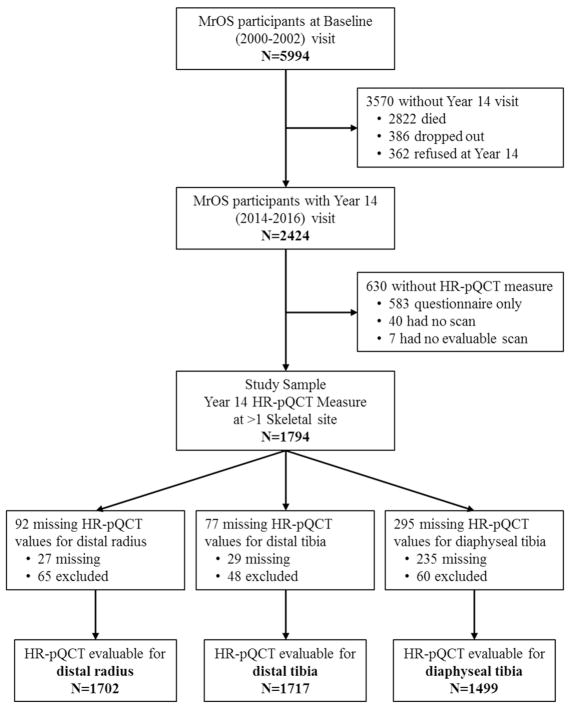
From 2000 to 2002, the Osteoporotic Fractures in Men (MrOS) study enrolled 5994 ambulatory community-dwelling men, aged 65 years and older living in one of six U.S. metropolitan areas[13,14]. Men with a history of bilateral hip replacement or the inability to walk without the assistance of another person were not eligible to participate. The institutional review board at each participating institution approved the study protocol and written informed consent was obtained from all participants. All active MrOS participants were invited to participate in the Year 14 visit between May 2014 and May 2016). Of the 2424 participants who completed at least the questionnaire component of this visit, 1801 completed high-resolution peripheral quantitative computed tomography (HR-pQCT) dataset and 1794 have evaluable scan data from at least one skeletal site (Figure 1). Men were eligible for the present study if they had HR-pQCT data at the Year 14 visit. The analytic sample for the multivariate analysis was based on complete cases, i.e. those in the study sample who had non-missing covariates, in particular non-missing DXA-BMD.
Figure 1.
Participant Flow
Measurement of HR-pQCT
HR-pQCT scans were done using Scanco XtremeCT II machines (Scanco Medical AG, Brüttisellen, Switzerland), which have a nominal voxel size of 61μm. Centrally trained operators acquired scans of the distal radius (9 mm from the articular surface), distal tibia (22 mm from the articular surface), and diaphyseal tibia (centered at 30% of tibial length, as measured externally from the tibial plateau to the tibial malleolus[15]. The radius from the non-dominant arm and the tibia from the ipsilateral leg were scanned except in the case of prior fracture, metal shrapnel or implant, or recent non-weight bearing loads >6 weeks. Machines were calibrated prior to being used in the present study, and a single cross-calibration density phantom was circulated among the study sites. The between site calibration coefficients were all <0.6%, and therefore pooled data was used without transformations [16]. The standard local density phantom was scanned on a daily basis to monitor for values that fall outside of the nominal range (8 mg HA/cm3). Centralized quality assurance and standard analysis of all image data, including micro finite element analysis (μFEA), was performed.
A central observer read all images for motion artifacts and used an established semi-quantitative 5-point grading system (1=superior, 5=poor) to score image quality and images with 4 or 5 were deemed to be of insufficient quality and were excluded from the analytic data set (97% of scans image grade ≤3) [17]. A fully automated analysis pipeline was developed to segment the radius and tibia for quantification of bone density and structure [18]. Segmentation failures were detected automatically by measuring slice-wise variation in total cross-sectional area; cases with an absolute slice-wise difference of 2 mm2 at the diaphyseal tibia, and 4 mm2 at the distal sites, were visually reviewed and manually corrected, as needed. Observed failure rates were <2% and <6%, for diaphyseal and distal scans, respectively.
Volumetric BMD and cross sectional area of the total, cortical, and trabecular compartments were measured. Cortical porosity and thickness, and trabecular thickness, separation and number were calculated directly [19,20]. Linear elastic micro-finite element analysis of a 1% uniaxial compression was performed using a homogenous elastic modulus of 10 GPa and a Poisson’s ratio of 0.3 (Scanco FE Software v1.12, Scanco Medical). The failure load was estimated by calculation of the reaction force at which 7.5% of the elements exceed a local effective strain of 0.7% [21].
All participants with outliers (difference from mean of greater than 3 standard deviations) were reviewed and those with abnormal anatomic findings at a given skeletal site (e.g. severe inflammatory arthritis, osteolytic lesions, injuries with ossification, unreported fracture) were excluded from the analysis for that skeletal site.
Fracture Outcomes
Participants were contacted every 4 months and queried whether they had a fracture. A proxy or next of kin was contacted in case of death in order to ascertain fracture outcomes resulting in 99% of cohort with complete follow-up for fracture outcomes. The radiographic reports pertaining to the fracture event and imaging for vertebral fractures were obtained and reviewed at the MrOS Coordinating Center by a physician to adjudicate incident fracture outcomes. Self-reported vertebral fractures were confirmed by the MrOS Coordinating Center study radiologist by comparing the community clinical imaging study (spine radiographs, CT, and/or MRI) to the MrOS Year 5 lateral spine radiographs. The primary fracture outcome was any incident clinical fracture which occurred after the Year 14 visit, regardless of precipitating trauma or skeletal site including fractures of head, hands, and feet. The secondary fracture outcome was incident major osteoporotic fracture (hip, clinical vertebral, forearm/wrist, or humerus).
Other variables
Other CRFs assessed for inclusion in adjusted models were: age, race/ethnicity (non-Hispanic white vs. other), clinic center, education, smoking, alcohol consumption, physical activity, falls, prior fracture after age 50, bisphosphonate use and oral corticosteroid use. Information on demographics, lifestyle, and medical and family history was obtained by questionnaire and interview by trained clinical staff. Race/ethnicity (non-Hispanic white vs. other) was self-identified. Participants were classified into ever smoker (100+ cigarettes) vs. never smokers. Self-reported alcohol intake was divided into three categories: <1 drink/week, 1–13 drinks/week, ≥14 drinks/week. Fall history (past-year) was queried on the Year 14 questionnaire. Physical activity was measured by computing the Physical Activity Scale for the Elderly (PASE)[22]. Participants were asked to bring all current (any use within the past 30 days) prescription medications with them to the clinic. All non-prescription and prescription medications were recorded in an electronic medication inventory database and matched to their ingredients(s) based on the Iowa Drug Information Service drug vocabulary (College Pharmacy, University of Iowa, Iowa City, IA)[23].
Participants had BMD at the hip measured using QDR 4500 fan-beam densitometers (Hologic, Bedford, MA, USA). The study used standardized procedures for position and scanning and certification of machine operators to ensure reproducibility. Scans were performed on the right hip unless there was a fracture, implant, hardware or other condition precluding accurate BMD assessment. A set of whole body, spine, hip, and linearity phantoms were circulated between centers; however, variation between centers was within acceptable limits and no corrections were required.
Statistical Analysis
We used t-tests for comparisons of continuous Year 14 participant variables by fracture outcome status and chi-square tests for comparisons of categorical variables by fracture outcome status. We used a time-to-event (Cox) model to determine the association between HR-pQCT measures and risk of clinical fracture (primary outcome) and major osteoporotic fracture (secondary outcome). Failure load was the primary predictor variable and the remaining HR-pQCT measures were secondary predictors. Participants were followed until a fracture outcome, death, loss to follow-up, or end of study follow-up, whichever occurred first. All HR-pQCT measures were standardized to have mean = 0 and SD = 1.
We considered a series of adjusted models. The first model series adjusted for clinical site, limb length, and major osteoporotic fracture 10-year probability as estimated by the FRAX tool (FRAX w/BMD). This first model series implicitly includes age, race, and CRFs as these are risk factors that are a part of the FRAX tool. For the next two models series we considered both femoral neck BMD and total hip BMD as covariates in place of the FRAX score in the first model. We reported the model series with total hip BMD since it was the predictor with the highest gradient of risk and contributed the most to attenuation of the HR-pQCT parameters. The final model series further adjusted for age, race and individual risk factors found to be associated with incident clinical fracture (i.e. falls and prior fracture). We ran the models with failure loads as predictors and clinical fracture as outcomes, but excluding men with BMD T-score −2.5 or less (based on female reference norms at the femoral neck, total hip, or lumbar spine) as a post-hoc sensitivity analysis. Finally, to assess how HR-pQCT alone predicts fracture risk using multiple parameters we included selected HR-pQCT parameters from each skeletal site in a single model with clinical fracture as the outcome. These models included compartmental specific BMD and area and cortical porosity in addition to limb length and center.
We tested and found no violation of the proportional hazards assumption. Since the aim of the present analysis was to identify and compare independent predictors of incident fracture as risk factors analogous to BMD, we chose to include all HR-pQCT variables as continuous standardized variables without transformation or higher order terms. FRAX 10-year major osteoporotic fracture probability was log-transformed to achieve better model fit for this adjustment variable. The main analyses were performed using a complete case approach.
Sensitivity analyses were performed to test robustness of findings to the study sample, showing that inclusion of covariates was not sample dependent, and thus change in point estimates reflected correlation of main variable with covariates. Finally we assessed the performance of the two best predictive HR-pQCT variables (distal radius failure load and BMD) in comparison to the standard areal BMD variables (femoral neck and total BMD) using receiver operator curves. In post hoc analysis, we also considered the combined prediction of distal radius failure load and total hip BMD. We determined clinical cutoffs associated with the maximal Youden index[24]. Analysis was performed using Stata Version 14.0 (College Station, TX).
RESULTS
There were 1794 men (mean age 84.4 years, range 77–101 years) at the Year 14 exam with HR-pQCT measures. There were 108 men with a clinical fracture and 48 men with major osteoporotic fracture over a mean follow-up time of 1.7 (IQR=1.4–2.3, max 2.9) years. More specifically, there were 127 fractures (in 108 men) including: hip (22), vertebral (17), wrist (9), other arm (11), ribs/sternum (24), pelvis (4), shoulder (6), other leg (7), ankle (11), hands/fingers (7), feet/toes (4), and facial bones (5). There were 100 men who died without incident fracture and were censored at time of death and another 26 who died post-fracture. The characteristics of the analytic sample stratified by outcome status are shown in Table 1. Using a female peak bone reference values for femoral neck, total hip, or lumbar spine, only 91 (5.1%) men had minimum T-score ≤ −2.5 while 812 (45.3%) had low bone mass (−2.5< minimum T-score ≤ −1). Men with subsequent clinical fracture were older, had lower DXA BMD, and were more likely to be non-Hispanic white and to have a history of falling and fracture. Fracture cases were not significantly different from non-cases by limb length, weight, education, smoking, alcohol use, bisphosphonate or corticosteroid use.
Table 1.
Characteristics of Study Sample Stratified by Incident Fracture Status
| Mean ± SD N (%) |
Analytic cohort | Without incident FX | Incident FX | p-value* |
|---|---|---|---|---|
| N=1794 | N=1686 | N=108 | ||
| Age, years | 84.4 ± 4.2 | 84.3 ± 4.1 | 86.0 ± 4.9 | <0.001 |
| Radius length, mm | 286 ±15 | 286 ± 15 | 286 ± 12 | 0.944 |
| Tibia length, mm | 404 ± 25 | 404 ± 25 | 405 ± 23 | 0.621 |
| Weight, kg | 79.8 ± 12.6 | 79.9 ± 12.6 | 78.1 ± 11.6 | 0.153 |
| Total hip BMD, g/cm2 | 0.932 ± 0.151 | 0.937 ± 0.150 | 0.846 ± 0.140 | <0.001 |
| Osteoporosis (T-score ≤ −2.5) | 91 (5.1) | 75 (4.5) | 16 (14.8) | <0.001 |
| Osteopenia (−2.5< T-score ≤ −1) | 812 (45.3) | 749 (44.4) | 63 (58.3) | <0.001 |
| MOF risk (FRAX w/BMD) | 9.5% ± 6.0% | 9.3% ± 5.8% | 12.8% ± 7.8% | <0.001 |
| Physical activity (PASE score) | 114 ± 66 | 114 ± 66 | 106 ± 67 | 0.210 |
| Race/ethnicity (non-Hispanic white) | 1621 (90.4) | 1517 (90.0) | 104 (96.3) | 0.031 |
| College education | 1121 (62.5) | 1049 (62.2) | 72 (66.7) | 0.355 |
| Smoker (100+ cigarettes) | 1023 (57.2) | 961 (57.0) | 62 (57.4) | 0.934 |
| Alcohol (1+ drink/wk) | 881 (49.4) | 824 (48.2) | 57 (52.8) | 0.474 |
| Any fall in past year | 690 (38.5) | 621 (36.9) | 69 (63.9) | <0.001 |
| Fracture after age 50 | 598 (33.3) | 546 (32.4) | 52 (48.1) | 0.001 |
| Bisphosphonate use | 52 (2.9) | 47 (2.8) | 5 (4.6) | 0.272 |
| Oral corticosteroid use | 51 (2.9) | 45 (2.7) | 6 (5.6) | 0.082 |
Abbreviations: BMD, bone mineral density; MOF, major osteoporotic fracture risk; PASE, physical activity scale
p-value comparing fracture cases vs. non-cases using chi-squared or t-test; comparisons with p > 0.05 are italicized
Number missing: radius length, n=27; tibia length, n=28; weight, n=1; total hip BMD, n=46; MOF risk, n=47; PASE score, n=5; alcohol, n=12; falls, n=2; medications, n=6
The pairwise correlations of the HR-pQCT parameters are shown in Supplemental Table 1. Failure load and volumetric BMD were strongly correlated at the distal sites and moderately correlated at the diaphyseal tibia. Failure load and cross-sectional area were positively and moderately correlated at the diaphyseal tibia, but not correlated at the distal radius and very weakly correlated at the distal tibia. Greater total cross-sectional area was in general moderately associated with lower total and compartmental BMD. Greater total cross-sectional area also was associated with lower cortical thickness at the distal radius and tibia, but not at the diaphyseal tibia. The associations between cortical and trabecular parameters were stronger at the distal radius than at the distal tibia. Cortical porosity was at most weakly correlated with other parameters at the distal radius and tibia, but was strongly correlated with cortical BMD at the diaphyseal tibia.
Association of Failure Load and Total Volumetric BMD with Incident Clinical Fracture
Lower failure loads at the distal radius, distal tibia and diaphyseal (proximal) tibia in FRAX-BMD adjusted models were each associated with a higher risk of incident clinical fracture, with the associations varying from HR=1.58 (95% CI: 1.25–2.01) per SD decrease at the diaphyseal tibia to HR=2.06 (95% CI:1.60–2.66) per SD decrease at the distal radius (Table 2). These associations were somewhat attenuated, but still significant, after adjustment for total hip BMD, and further attenuated after additional adjustment for CRF, at which point associations were significant for the distal sites but not the diaphyseal tibia. Lower total volumetric BMDs at all peripheral sites, like failure loads, were each associated with a similarly increased risk of incident fracture in FRAX-BMD adjusted models. These associations were again attenuated, but still significant, after adjustment for total hip BMD, and further attenuated after additional adjustment for CRF, at which point associations were significant for the distal sites but not the diaphyseal tibia. We found very similar associations relating failure load and clinical fracture when men with T-scores ≤−2.5 were excluded from the analyses (Supplemental Table 2).
Table 2.
The Associations* between Global HR-pQCT Measures and Incident Clinical Fracture after Consideration of Clinical Risk Factors Including FRAX and Total Hip BMD
| HR (95% CI)
|
||||
|---|---|---|---|---|
| Crude | Model 1 (FRAX) | Model 2 (TH BMD) | Model 3 (CRF) | |
| Distal Radius | N=1702; # Fx=100 | N=1656; # Fx=95 | N=1657; # Fx=95 | N=1655; # Fx=95 |
| Failure load | 2.31 (1.82, 2.93) | 2.06 (1.60, 2.66) | 1.86 (1.40, 2.48) | 1.81 (1.36, 2.41) |
| Total vBMD | 2.20 (1.76, 2.76) | 2.05 (1.60, 2.61) | 1.83 (1.40, 2.41) | 1.76 (1.34, 2.32) |
| Distal Tibia | N=1717; # Fx=105 | N=1677; # Fx=101 | N=1678; # Fx=101 | N=1676; # Fx=101 |
| Failure load | 1.98 (1.60, 2.45) | 1.82 (1.44, 2.30) | 1.55 (1.17, 2.06) | 1.49 (1.12, 1.97) |
| Total vBMD | 1.90 (1.55, 2.34) | 1.75 (1.39, 2.21) | 1.48 (1.13, 1.94) | 1.41 (1.08, 1.85) |
| Diaphyseal Tibia | N=1499; # Fx=91 | N=1464; # Fx=87 | N=1465; # Fx=87 | N=1463; # Fx=87 |
| Failure load | 1.67 (1.35, 2.06) | 1.58 (1.25, 2.01) | 1.30 (1.00, 1.70) | 1.16 (0.88, 1.51) |
| Total BMD | 1.69 (1.40, 2.04) | 1.50 (1.23, 1.84) | 1.24 (0.98, 1.57) | 1.16 (0.92, 1.47) |
Associations given per SD decrease in the HR-pQCT predictor variable; those with p > 0.05 are italicized. Model 1 adjusted for clinical site, limb length, major osteoporotic fracture risk (FRAX w/BMD). Model 2 adjusted for clinical site, limb length, total hip BMD. Model 3 adjusted for clinical site, limb length, total hip BMD, age, race, falls, prevalent fracture after age 50.
Associations of Other Distal Radius HR-pQCT Parameters with Incident Clinical Fracture
At the radius, lower trabecular BMD, trabecular number and trabecular thickness were each associated with a higher risk of incident fracture, while lower trabecular area was associated with a lower risk of fracture (Table 3). Lower cortical BMD, cortical thickness and cortical area at the radius were each associated with a higher risk of incident fracture. As with the global parameters of failure load and total vBMD, these associations were attenuated after adjusting for total hip BMD instead of FRAX-BMD and further attenuated after adjusting for both total hip BMD and CRFs. Of note, cortical porosity was not a risk factor for fracture in any of the models.
Table 3.
The Associations* between Compartmental HR-pQCT Measures and Incident Clinical Fracture after Consideration of Clinical Risk Factors Including FRAX and Total Hip BMD
| HR (95% CI)
|
||||
|---|---|---|---|---|
| Crude | Model 1 (FRAX) | Model 2 (TH BMD) | Model 3 (CRF) | |
| Distal Radius | N=1702; # Fx=100 | N=1656; # Fx=95 | N=1657; # Fx=95 | N=1655; # Fx=95 |
| Trabecular vBMD | 1.98 (1.60, 2.45) | 1.79 (1.42, 2.26) | 1.58 (1.22, 2.04) | 1.63 (1.26, 2.10) |
| Trabecular # | 1.68 (1.41, 2.01) | 1.52 (1.25, 1.85) | 1.34 (1.07, 1.67) | 1.39 (1.11, 1.75) |
| Trabecular thickness | 1.40 (1.13, 1.73) | 1.27 (1.02, 1.59) | 1.21 (0.97, 1.51) | 1.25 (1.01, 1.56) |
| Trabecular area | 0.75 (0.62, 0.90) | 0.72 (0.58, 0.89) | 0.73 (0.59, 0.90) | 0.75 (0.61, 0.93) |
| Cortical vBMD | 1.73 (1.46, 2.05) | 1.63 (1.36, 1.96) | 1.49 (1.22, 1.82) | 1.36 (1.10, 1.66) |
| Cortical thickness | 1.93 (1.54, 2.42) | 1.74 (1.37, 2.22) | 1.54 (1.20, 1.99) | 1.43 (1.11, 1.85) |
| Cortical porosity | 1.08 (0.88, 1.33) | 1.04 (0.85, 1.28) | 0.99 (0.80, 1.21) | 1.02 (0.83, 1.26) |
| Cortical bone area | 1.91 (1.51, 2.42) | 1.70 (1.33, 2.17) | 1.46 (1.12, 1.91) | 1.35 (1.03, 1.77) |
| Distal Tibia | N=1717; # Fx=105 | N=1677; # Fx=101 | N=1678; # Fx=101 | N=1676; # Fx=101 |
| Trabecular vBMD | 1.45 (1.19, 1.76) | 1.30 (1.05, 1.62) | 1.08 (0.86, 1.36) | 1.16 (0.93, 1.45) |
| Trabecular # | 1.29 (1.07, 1.56) | 1.17 (0.96, 1.43) | 0.99 (0.80, 1.23) | 1.04 (0.84, 1.29) |
| Trabecular thickness | 1.09 (0.90, 1.32) | 1.03 (0.84, 1.26) | 0.99 (0.81, 1.21) | 1.04 (0.85, 1.28) |
| Trabecular area | 0.78 (0.65, 0.94) | 0.78 (0.62, 0.98) | 0.82 (0.65, 1.03) | 0.83 (0.66, 1.05) |
| Cortical vBMD | 1.76 (1.49, 2.08) | 1.61 (1.35, 1.93) | 1.43 (1.17, 1.75) | 1.26 (1.02, 1.56) |
| Cortical thickness | 1.82 (1.48, 2.23) | 1.63 (1.31, 2.04) | 1.40 (1.09, 1.78) | 1.28 (1.01, 1.64) |
| Cortical porosity | 0.98 (0.81, 1.19) | 0.98 (0.80, 1.19) | 0.98 (0.81, 1.19) | 1.02 (0.84, 1.23) |
| Cortical bone area | 1.89 (1.55, 2.31) | 1.68 (1.36, 2.07) | 1.45 (1.14, 1.83) | 1.31 (1.03, 1.66) |
| Diaphyseal Tibia | N=1499; # Fx=91 | N=1464; # Fx=87 | N=1465; # Fx=87 | N=1463; # Fx=87 |
| Cortical BMD | 1.38 (1.14, 1.66) | 1.25 (1.03, 1.52) | 1.13 (0.92, 1.38) | 1.09 (0.89, 1.34) |
| Cortical thickness | 1.71 (1.39, 2.09) | 1.56 (1.25, 1.94) | 1.27 (0.99, 1.63) | 1.16 (0.90, 1.49) |
| Cortical porosity | 0.69 (0.60, 0.80) | 0.74 (0.63, 0.87) | 0.80 (0.68, 0.95) | 0.86 (0.73, 1.01) |
| Cortical area | 1.58 (1.27, 1.95) | 1.50 (1.18, 1.90) | 1.22 (0.94, 1.59) | 1.09 (0.83, 1.43) |
Associations given per SD decrease in the HR-pQCT predictor variable; those with p > 0.05 are italicized. Model 1 adjusted for clinical site, limb length, major osteoporotic fracture risk (FRAX w/BMD). Model 2 adjusted for clinical site, limb length, total hip BMD. Model 3 adjusted for clinical site, limb length, total hip BMD, age, race, falls, prevalent fracture after age 50.
Associations of Other Tibial HR-pQCT Parameters with Incident Clinical Fracture
Lower trabecular BMD at the distal tibia was associated with a higher risk of incident fracture, while no association was noted for trabecular number or thickness. Lower trabecular area of the distal tibia was associated with a lower risk of fracture. Lower cortical BMD and cortical thickness and area at the distal tibia were each associated with a higher risk of incident fracture. As with the global parameters of failure load and total vBMD, these associations were attenuated after adjustment for total hip BMD instead of FRAX and further attenuated, but still statistically significant, after adjusting for total hip BMD and CRFs. Of note, cortical porosity was not a risk factor for fracture in any of the models.
At the diaphyseal tibia, lower cortical BMD and cortical thickness and area were each associated with a higher risk of incident fracture, while lower cortical porosity at the diaphyseal tibia was associated with lower risk of fracture. These associations were attenuated and non-significant after adjustment for total hip BMD and CRFs.
Association of Failure Load and Total vBMD with Risk of Major Osteoporotic Fracture
Lower failure loads at the distal radius, distal tibia and diaphyseal (proximal) tibia in FRAX-BMD adjusted models were each associated with a higher risk of incident major osteoporotic fracture, with the associations varying from HR=2.05 (95% CI: 1.45–2.90) per SD decrease at the diaphyseal tibia to HR=3.48 (95% CI:2.32–5.21) per SD decrease at the distal radius (Table 4). These associations were attenuated when adjusted for total hip BMD; associations remained at the distal sites but were no longer statistically significant for the diaphyseal tibia. The associations were further attenuated with additional adjustment for clinical risk factors, but remained significant at the distal radius. The patterns of associations for total vBMD by skeletal site were similar to those noted for failure load.
Table 4.
The Associations* between Global HR-pQCT Measures and Incident Major Osteoporotic Fracture after Consideration of Clinical Risk Factors Including FRAX and Total Hip BMD
| HR (95% CI)
|
||||
|---|---|---|---|---|
| Crude | Model 1 (FRAX) | Model 2 (TH BMD) | Model 3 (CRF) | |
| Distal Radius | N=1702; # Fx=43 | N=1656; # Fx=41 | N=1657; # Fx=41 | N=1655; # Fx=41 |
| Failure load | 3.90 (2.64, 5.77) | 3.48 (2.32, 5.21) | 2.45 (1.55, 3.86) | 2.38 (1.51, 3.75) |
| Total vBMD | 3.66 (2.55, 5.25) | 3.19 (2.18, 4.66) | 2.19 (1.44, 3.33) | 2.16 (1.43, 3.28) |
| Distal Tibia | N=1717; # Fx=47 | N=1677; # Fx=46 | N=1678; # Fx=46 | N=1676; # Fx=46 |
| Failure load | 2.80 (2.02, 3.88) | 2.52 (1.79, 3.56) | 1.56 (1.02, 2.39) | 1.45 (0.96, 2.19) |
| Total vBMD | 2.70 (1.97, 3.71) | 2.48 (1.75, 3.50) | 1.55 (1.03, 2.33) | 1.50 (1.01, 2.22) |
| Diaphyseal Tibia | N=1499; # Fx=41 | N=1464; # Fx=40 | N=1465; # Fx=40 | N=1463; # Fx=40 |
| Failure load | 2.20 (1.60, 3.03) | 2.05 (1.45, 2.90) | 1.21 (0.81, 1.82) | 1.00 (0.66, 1.50) |
| Total BMD | 2.01 (1.54, 2.62) | 1.78 (1.34, 2.36) | 1.11 (0.78, 1.57) | 1.00 (0.71, 1.42) |
Associations given per SD decrease in the HR-pQCT predictor variable; those with p > 0.05 are italicized. Model 1 adjusted for clinical site, limb length, major osteoporotic fracture risk (FRAX w/BMD). Model 2 adjusted for clinical site, limb length, total hip BMD. Model 3 adjusted for clinical site, limb length, total hip BMD, age, race, falls, prevalent fracture after age 50.
Compartmental HR-pQCT Parameters as Independent Predictors of Incident Clinical Fracture
We considered the associations of bone compartment specific parameters with risk of clinical fracture when mutually adjusted for other HR-pQCT parameters (Table 5). In these models we did not include FRAX-BMD, total hip BMD, or CRFs, since the goal was to determine the independent risks of specific compartmental HR-pQCT parameters. Lower cortical BMD and lower trabecular BMD at the distal radius and distal tibia were both independently associated with increased risk of clinical fracture after mutual adjustment and adjustment for other skeletal site specific parameters, with associations ranging from HR=1.34 (95% CI: 1.09, 1.65) per SD trabecular BMD decrease at the distal tibia to HR=1.92 (95% CI: 1.48, 2.49) per SD trabecular BMD decrease at the distal radius. Cortical area at the distal radius and distal tibia and trabecular area at the distal radius were not risk factors after adjustment for compartmental BMD. Cortical porosity at the diaphyseal tibia was an independent risk factor for clinical fracture after adjustment for other diaphyseal parameters, but cortical porosity and the distal radius and distal tibia were not risk factors after adjustment for skeletal site specific parameters.
Table 5.
The Multivariate Associations* between Trabecular and Cortical HR-pQCT Measures and Incident Clinical Fracture (Adjusted for Other HR-pQCT Measures at Same Skeletal Site)
| HR (95% CI) | |
|---|---|
| Distal Radius | N=1702; # Fx=100 |
| Trabecular vBMD | 1.92 (1.48, 2.49) |
| Trabecular area | 1.13 (0.79, 1.39) |
| Cortical vBMD | 1.58 (1.12, 2.19) |
| Cortical area | 1.01 (0.68, 1.49) |
| Cortical porosity | 0.87 (0.68, 1.10) |
| Distal Tibia | N=1717; # Fx=105 |
| Trabecular vBMD | 1.34 (1.09, 1.65) |
| Trabecular area | 1.13 (0.87, 1.45) |
| Cortical vBMD | 1.58 (1.22, 2.06) |
| Cortical area | 1.26 (0.94, 1.68) |
| Cortical porosity | 1.06 (0.87, 1.30) |
| Diaphyseal Tibia | N=1499; # Fx=91 |
| Cortical BMD | 1.05 (0.79, 1.39) |
| Cortical area | 1.63 (1.30, 2.04) |
| Cortical porosity | 0.78 (0.62, 0.99) |
Associations given per SD decrease in the HR-pQCT predictor variable; those with p> 0.05 are italicized. Multivariate models further adjusted for clinical site, limb length.
Area Under ROC Curve and Maximal Youden Index
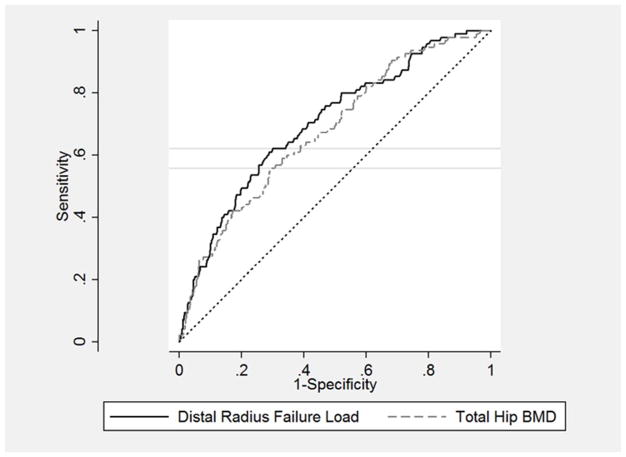
We calculated the area under the curve (AUC), the cut-off value for the maximal Youden index and the sensitivity and specificity at the identified cut-off value for the prediction of incident clinical fracture. The AUC for distal radius failure load and for prediction of clinical fracture was 0.70 (95% CI: 0.65–0.76), which was nearly identical (within rounding) to that for distal radius volumetric BMD. The AUC for total hip BMD for prediction of clinical fracture was 0.68 (95% CI: 0.62–0.73), which was nearly identical (within rounding) to that that for femoral neck BMD. While the AUCs for the HR-pQCT parameters were slightly higher than those for the DXA variables, there were no statistically significant differences between any of these AUCs. In post-hoc analysis, we also considered the combined prediction of total hip BMD and distal radius failure load from the Cox proportional hazards parameter estimates. The bias-corrected AUC for this predictor variable was 0.71 (95% CI: 0.66–0.76), which was slightly higher but not statistically different from the distal radius parameters alone. The maximal Youden index for distal radius failure load was 0.32 at failure load of 4170 N, with associated sensitivity of 0.62 and specificity of 0.70. The maximal Youden index for total hip BMD was 0.27 at failure load of 0.850 g/cm2, with associated sensitivity of 0.56 and specificity of 0.71 (see Figure 2).
Figure 2. Receiver Operator Curve for Prediction of Incident Clinical Fracture using Distal Radius Failure Load vs. Total Hip BMD.
AUC for distal radius failure load= 0.70 (95% CI: 0.65–0.76)
AUC for total hip BMD= 0.68 (95% CI: 0.62–0.73)
Horizontal gray lines show sensitivity for cut-off at maximal Youden index
Distal radius failure cut-off=4170 N, with maximal Youden index=0.32, sensitivity=0.62 and specificity= 0.70
Total BMD cut-off= 0.850 g/cm2, with maximal Youden index=0.27. sensitivity=0.56 and specificity=0.71
DISCUSSION
We found that HR-pQCT estimated failure load at the distal radius and distal tibia each were strong independent predictors of incident clinical and major osteoporotic fractures among older men after adjustment for FRAX probability, TH BMD, or TH BMD and major CRFs. The association of failure load at each of the three skeletal sites with incident clinical fracture had the highest gradient of risk after adjusting for FRAX probability. These associations were somewhat attenuated after adjustment for areal total hip BMD and further attenuated but still significant at distal sites after further adjustment for individual CRFs. The predictive value of HR-pQCT total volumetric BMD was similar to that of the estimated failure load at the same skeletal site. At the distal radius, lower trabecular BMD, number, and thickness, and lower cortical BMD, thickness, and area were all associated with higher risk of clinical fracture, but cortical porosity was not. In multivariable models based on multiple HR-pQCT parameters, both cortical and trabecular vBMD at distal radius and distal tibia were independent predictors of incident clinical fracture risk when included in the same model. In contrast, while trabecular and cortical area at the distal radius were predictors of clinical fracture in models with only a single HR-pQCT variable, they were not predictors of clinical fracture in the model adjusting for other HR-pQCT variables (i.e. cortical and trabecular BMD).
Our main findings were in general agreement with previous studies in older men. For example, a prospective study of older Swedish men found that lower failure load and total bone mineral density at the distal tibia were associated with a higher risk of incident clinical fracture and incident major osteoporotic fracture after adjustment for femoral neck BMD or FRAX major osteoporotic fracture probability[12]. However, this previous study of HR-pQCT parameters did not examine measures at the distal radius, a non-weight bearing site. This study also did not examine the robustness of the association in the presence of total hip BMD, or adjustment for major individual clinical risk factors. A related cross-sectional study in the same cohort found that HR-pQCT compartmental parameters were associated with prevalent clinical fracture, but did not consider failure load as a parameter [11]. In another cross-sectional study of 920 older men, Szulc et al reported that HR-pQCT parameters were related to prevalent fracture, but these associations were only statistically significant for the subset of vertebral fractures after adjustment for areal BMD[10]. The discrepant findings could be attributable to ascertainment of incident vs. prevalent fracture, older mean age of the MrOS cohort, and the overall distribution of skeletal site of fracture (e.g. 22 hip fractures in 108 men in the MrOS cohort vs. 6 hip fractures in 177 men in the STRAMBO cohort).
Our findings are also generally consistent with those of previous prospective studies in postmenopausal women. In particular, a study of younger postmenopausal women (mean age 67 years) found that total vBMD of the distal radius and tibia were a risk factors for major osteoporotic fracture after adjustment for traditional risk factors[25]. However, there were some discrepant findings as well. This study found no association between bone size and fracture risk, whereas in our study larger bone size (greater trabecular or total cross-sectional area) was associated with increased risk of clinical fracture. Another study in younger postmenopausal women found that lower failure load was associated with a higher risk of incident fracture and incident major osteoporotic fracture after adjusting for femoral neck BMD or FRAX[26]. Again, there was no assessment as to whether these associations were independent of major individual clinical risk factors. An international multicenter cross-sectional study in older women found that HR-pQCT measures of the distal radius and distal tibia had similar associations with history of fragility fracture [6]. In the current study, associations of HR-pQCT parameters at the distal radius with fracture risk appeared to be more robust than those at the tibial sites suggesting improved fracture prediction using radial parameters. The discrepancy between our findings and finding based on younger postmenopausal women may be related to patterns of age-related bone loss. Using cross-sectional data, the estimated mean total decrement comparing old vs young men between the ages 20 and 90 years is 32% at the distal radius versus 22% at the tibia[27], thus suggesting that there may be preferential bone loss at the distal radius and this loss would be more prominent in older men.
Patterns of bone strength and bone loss are also known to be different between men and women [27–29]. A longitudinal study using pQCT in older men showed that there are concurrent changes in bone area and volumetric density at the distal radius whereby increasing bone area is associated with decreasing BMD [30]. In our study, larger bones did have lower volumetric BMD and thinner cortices, both of which were risk factors for fracture. Larger bones are in general stronger; however, among these oldest of old men, the increased bone size may have been due to a compensatory periosteal reaction to bone loss and thinning cortices that ultimately compromise bone strength. In this study, bone size was inversely related to failure load and volumetric BMD. Thus, larger bone size might indicate that further assessment is warranted, at least among the oldest men.
We found that both cortical and trabecular parameters of the distal radius were associated with incident fracture, but that cortical HR-pQCT parameters of the distal tibia were more consistent predictors of clinical fracture than trabecular parameters at this site. Studies of longitudinal change in older men have shown that both total and cortical volumetric BMD of the distal radius and tibia decrease over time, whereas there was no concurrent change in trabecular BMD[27]. A subsequent longitudinal study in the same cohort compared fracture cases versus non-cases (albeit limited to women only) and reported modest changes in total and cortical BMD among cases and non-cases, but only marginal change in trabecular BMD at the distal tibia, but this study had to few fracture cases to assess whether there were differences by fracture status[31]. Thus, our findings that cortical BMD and trabecular BMD are both independent risk factors for future fracture indicate that it is likely that lifetime trajectories of bone gain and loss are relevant for fracture risk, and that those with low initial trabecular BMD may be at greatest risk due to loss of cortical bone.
We found that cortical porosity of the distal radius and distal tibia was not a predictor of fracture, either with or without adjustment for other risk factors. These results are discordant with some previous studies which have reported an association between cortical porosity and prevalent fractures [8,11,32]. Our study used the standard volumetric method that was originally developed for first generation HR-pQCT to compute the cortical porosity[18,33]. Even at the improved resolution of second generation HR-pQCT (spatial resolution ~90μm), this method does not capture all pores. Thus our assumption is that macro-porosity measured using HR-pQCT is highly correlated to integral porosity, as has been shown previously[34]. It may be that porosity measured in this population is less predictive of fracture because the oldest-old have largely trabecularized cortical bone, which is therefore not included in the cortical compartment using thresholding algorithms. The development of increased intra-cortical porosity may be followed by progressive trabecularization and cortical thinning, leading to a minimal cortical width with less measurable macro-porosity. Further longitudinal study is necessary to clarify this issue. It should be noted that there is in general a transition zone between the cortical and trabecular bone at these sites and that this transition zone is an active area of bone remodeling [35]. Various thresholding algorithms can be used to separate the bone compartment into cortical and trabecular bone with the exact demarcation lying somewhere in the transition zone. In particular both cortical thickness and cortical porosity could vary by choice of algorithm and threshold. Thus, decreased cortical thickness using one thresholding method might appear as increased cortical porosity by another.
The strengths of the present study include its longitudinal collection of incident fracture outcomes, 50% more fractures outcomes than the previous study examining HR-pQCT and incident fracture in older men, comparison with standard fracture risk assessment tools (areal hip BMD and FRAX), and consideration of individual traditional clinical risk factors. The present study included HR-pQCT assessment at three sites, whereas prior incident fracture studies have included at most two sites. The HR-pQCT assessments were performed on latest generation high-resolution scanners and had excellent quality control.
Our study has an observational design. Thus, limitations include the possibility of selection bias and residual confounding. The generalizability of the present study is limited to healthy community-dwelling very old men (mean age 84 years). The cohort was also mostly non-Hispanic white, and therefore we were unable to assess potential racial/ethnic differences. Based on study cohort and the length of follow-up our results are most relevant for the determination of short term fracture risk among older men. There were a limited number of fractures at specific skeletal sites (e.g. hip).
In summary, we found that lower failure load and lower total volumetric BMD as assessed by HR-pQCT are associated with increased risk of incident clinical and major osteoporotic fracture risk as risk factors with high gradient of risk even after accounting for total hip BMD and traditional clinical risk factors or estimated fracture risk as calculated by the FRAX tool (with BMD). In particular, the strong relationships between distal radius failure load and distal radius volumetric BMD might be useful both alone and as measures relevant after consideration of full risk factor profile, with the latter having lower computational burden but similar performance.
Supplementary Material
Acknowledgments
Source of Funding:
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128, R01 AR060700.
This manuscript is the result of work supported with resources and use of facilities of the Minneapolis VA Health Care System. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Footnotes
Authors’ roles:
Study concept and design: LL, AJB, KEE, JAC, ESO
Data collection: KEE, JAC, EBC, ESO
Data analysis and interpretation: LL, KWP
Drafting manuscript: LL
Critical review and final approval of manuscript content: LL, KWP, AJB, KEE, HAF, PMC, JAC, JTS, EBC, ESO
Statistical Analysis: Lisa Langsetmo and Kathy Peters performed the statistical analyses and are independent of any commercial funder. Lisa Langsetmo had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Disclosures:
The submitted work was supported by NIH grants (all authors).
Dr. Orwoll reports consultation with Radius and grants from Lilly and Mereo, outside the submitted work; Dr. Burghardt reports non-financial support from Scanco Medical AG, outside the submitted work; Dr. Cawthon reports grants from Eli Lilly, outside the submitted work. Lisa Langsetmo; Katherine W. Peters; Kristine E. Ensrud; Howard A. Fink; Jane A. Cauley; John T. Schousboe; Elizabeth Barrett-Connor have no further disclosures.
References
- 1.Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, Cauley J, Black D, Vogt TM. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–73. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 2.Taylor BC, Schreiner PJ, Stone KL, Fink HA, Cummings SR, Nevitt MC, Bowman PJ, Ensrud KE. Long-term prediction of incident hip fracture risk in elderly white women: study of osteoporotic fractures. J Am Geriatr Soc. 2004;52:1479–86. doi: 10.1111/j.1532-5415.2004.52410.x. [DOI] [PubMed] [Google Scholar]
- 3.Cauley JA, Cawthon PM, Peters KE, Cummings SR, Ensrud KE, Bauer DC, Taylor BC, Shikany JM, Hoffman AR, Lane NE, Kado DM, Stefanick ML, Orwoll ES. Risk Factors for Hip Fracture in Older Men: The Osteoporotic Fractures in Men Study (MrOS) J Bone Miner Res. 2016;31:1810–19. doi: 10.1002/jbmr.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanis JA, McCloskey E, Johansson H, Oden A, Leslie WD. FRAX((R)) with and without bone mineral density. Calcif Tissue Int. 2012;90:1–13. doi: 10.1007/s00223-011-9544-7. [DOI] [PubMed] [Google Scholar]
- 5.Gourlay ML, Ritter VS, Fine JP, Overman RA, Schousboe JT, Cawthon PM, Orwoll ES, Nguyen TV, Lane NE, Cummings SR, Kado DM, Lapidus JA, Diem SJ, Ensrud KE. Comparison of fracture risk assessment tools in older men without prior hip or spine fracture: the MrOS study. Arch Osteoporos. 2017;12:91. doi: 10.1007/s11657-017-0389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boutroy S, Khosla S, Sornay-Rendu E, Zanchetta MB, McMahon DJ, Zhang CA, Chapurlat RD, Zanchetta J, Stein EM, Bogado C, Majumdar S, Burghardt AJ, Shane E. Microarchitecture and Peripheral BMD are Impaired in Postmenopausal White Women With Fracture Independently of Total Hip T-Score: An International Multicenter Study. J Bone Miner Res. 2016;31:1158–66. doi: 10.1002/jbmr.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boutroy S, van RB, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res. 2008;23:392–99. doi: 10.1359/jbmr.071108. [DOI] [PubMed] [Google Scholar]
- 8.Sundh D, Nilsson AG, Nilsson M, Johansson L, Mellstrom D, Lorentzon M. Increased cortical porosity in women with hip fracture. J Intern Med. 2017;281:496–506. doi: 10.1111/joim.12587. [DOI] [PubMed] [Google Scholar]
- 9.Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res. 2007;22:425–33. doi: 10.1359/jbmr.061206. [DOI] [PubMed] [Google Scholar]
- 10.Szulc P, Boutroy S, Vilayphiou N, Chaitou A, Delmas PD, Chapurlat R. Cross-sectional analysis of the association between fragility fractures and bone microarchitecture in older men: the STRAMBO study. J Bone Miner Res. 2011;26:1358–67. doi: 10.1002/jbmr.319. [DOI] [PubMed] [Google Scholar]
- 11.Sundh D, Mellstrom D, Nilsson M, Karlsson M, Ohlsson C, Lorentzon M. Increased Cortical Porosity in Older Men With Fracture. J Bone Miner Res. 2015;30:1692–700. doi: 10.1002/jbmr.2509. [DOI] [PubMed] [Google Scholar]
- 12.Ohlsson C, Sundh D, Wallerek A, Nilsson M, Karlsson M, Johansson H, Mellstrom D, Lorentzon M. Cortical Bone Area Predicts Incident Fractures Independently of Areal Bone Mineral Density in Older Men. J Clin Endocrinol Metab. 2017;102:516–24. doi: 10.1210/jc.2016-3177. [DOI] [PubMed] [Google Scholar]
- 13.Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Bonaretti S, Majumdar S, Lang TF, Khosla S, Burghardt AJ. The comparability of HR-pQCT bone measurements is improved by scanning anatomically standardized regions. Osteoporos Int. 2017;28:2115–28. doi: 10.1007/s00198-017-4010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burghardt AJ, Pialat JB, Kazakia GJ, Boutroy S, Engelke K, Patsch JM, Valentinitsch A, Liu D, Szabo E, Bogado CE, Zanchetta MB, McKay HA, Shane E, Boyd SK, Bouxsein ML, Chapurlat R, Khosla S, Majumdar S. Multicenter precision of cortical and trabecular bone quality measures assessed by high-resolution peripheral quantitative computed tomography. J Bone Miner Res. 2013;28:524–36. doi: 10.1002/jbmr.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pialat JB, Burghardt AJ, Sode M, Link TM, Majumdar S. Visual grading of motion induced image degradation in high resolution peripheral computed tomography: impact of image quality on measures of bone density and micro-architecture. Bone. 2012;50:111–18. doi: 10.1016/j.bone.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK. Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone. 2010;47:519–28. doi: 10.1016/j.bone.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hildebrand T, Laib A, Muller R, Dequeker J, Ruegsegger P. Direct three-dimensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest, and calcaneus. J Bone Miner Res. 1999;14:1167–74. doi: 10.1359/jbmr.1999.14.7.1167. [DOI] [PubMed] [Google Scholar]
- 20.Manske SL, Zhu Y, Sandino C, Boyd SK. Human trabecular bone microarchitecture can be assessed independently of density with second generation HR-pQCT. Bone. 2015;79:213–21. doi: 10.1016/j.bone.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Mueller TL, Christen D, Sandercott S, Boyd SK, van RB, Eckstein F, Lochmuller EM, Muller R, van Lenthe GH. Computational finite element bone mechanics accurately predicts mechanical competence in the human radius of an elderly population. Bone. 2011;48:1232–38. doi: 10.1016/j.bone.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 22.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 23.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–11. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 24.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 25.Sornay-Rendu E, Boutroy S, Duboeuf F, Chapurlat RD. Bone Microarchitecture Assessed by HR-pQCT as Predictor of Fracture Risk in Postmenopausal Women: The OFELY Study. J Bone Miner Res. 2017;32:1243–51. doi: 10.1002/jbmr.3105. [DOI] [PubMed] [Google Scholar]
- 26.Biver E, Durosier-Izart C, Chevalley T, van RB, Rizzoli R, Ferrari S. Evaluation of Radius Microstructure and Areal Bone Mineral Density Improves Fracture Prediction in Postmenopausal Women. J Bone Miner Res. 2018;33:328–37. doi: 10.1002/jbmr.3299. [DOI] [PubMed] [Google Scholar]
- 27.Burt LA, Hanley DA, Boyd SK. Cross-sectional Versus Longitudinal Change in a Prospective HR-pQCT Study. J Bone Miner Res. 2017;32:1505–13. doi: 10.1002/jbmr.3129. [DOI] [PubMed] [Google Scholar]
- 28.Macdonald HM, Nishiyama KK, Kang J, Hanley DA, Boyd SK. Age-related patterns of trabecular and cortical bone loss differ between sexes and skeletal sites: a population-based HR-pQCT study. J Bone Miner Res. 2011;26:50–62. doi: 10.1002/jbmr.171. [DOI] [PubMed] [Google Scholar]
- 29.Shanbhogue VV, Brixen K, Hansen S. Age- and Sex-Related Changes in Bone Microarchitecture and Estimated Strength: A Three-Year Prospective Study Using HRpQCT. J Bone Miner Res. 2016;31:1541–49. doi: 10.1002/jbmr.2817. [DOI] [PubMed] [Google Scholar]
- 30.Pye SR, Ward KA, Cook MJ, Laurent MR, Gielen E, Borghs H, Adams JE, Boonen S, Vanderschueren D, Wu FC, O’Neill TW. Bone turnover predicts change in volumetric bone density and bone geometry at the radius in men. Osteoporos Int. 2017;28:935–44. doi: 10.1007/s00198-016-3816-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burt LA, Manske SL, Hanley DA, Boyd SK. Lower Bone Density, Impaired Microarchitecture, and Strength Predict Future Fragility Fracture in Postmenopausal Women: 5-Year Follow-up of the Calgary CaMos Cohort. J Bone Miner Res. 2018 doi: 10.1002/jbmr.3347. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed LA, Shigdel R, Joakimsen RM, Eldevik OP, Eriksen EF, Ghasem-Zadeh A, Bala Y, Zebaze R, Seeman E, Bjornerem A. Measurement of cortical porosity of the proximal femur improves identification of women with nonvertebral fragility fractures. Osteoporos Int. 2015;26:2137–46. doi: 10.1007/s00198-015-3118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burghardt AJ, Kazakia GJ, Ramachandran S, Link TM, Majumdar S. Age- and gender-related differences in the geometric properties and biomechanical significance of intracortical porosity in the distal radius and tibia. J Bone Miner Res. 2010;25:983–93. doi: 10.1359/jbmr.091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tjong W, Kazakia GJ, Burghardt AJ, Majumdar S. The effect of voxel size on high-resolution peripheral computed tomography measurements of trabecular and cortical bone microstructure. Med Phys. 2012;39:1893–903. doi: 10.1118/1.3689813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zebaze R, Ghasem-Zadeh A, Mbala A, Seeman E. A new method of segmentation of compact-appearing, transitional and trabecular compartments and quantification of cortical porosity from high resolution peripheral quantitative computed tomographic images. Bone. 2013;54:8–20. doi: 10.1016/j.bone.2013.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.