Abstract
Protocols for photoreceptor outer segment (POS) isolation that can be used in phagocytosis assays of retinal pigment epithelium (RPE) cells have routinely used a large number of cow or pig eyes. However, when working with large animal models (e.g., dog, cats, transgenic pigs) of inherited retinal degenerative diseases, access to retinal tissues may be limited. An optimized protocol is presented in this paper to isolate sufficient POS from a single canine retina for use in RPE phagocytosis assays.
Keywords: Inherited retinal degeneration, Photoreceptor outer segment isolation, Retinal pigment epithelium, Phagocytosis, Canine
72.1 Introduction
The RPE has a number of important functions in the retina including transport of nutrients, secretion of neurotrophic factors, recycling of 11-cis retinal in the visual cycle, and phagocytosis and degradation of outer segment discs shed from the photoreceptors (Strauss 2005). Since RPE cells interact closely with the photoreceptors, mutations that affect RPE cells compromise photoreceptor function and vice versa. In recent years, there has been increased interest in characterizing RPE function and assessing RPE health in large animal models of diseases that either directly affect the RPE, such as in the RPE65 form of Leber congenital amaurosis (LCA) (Gu et al. 1997; Marlhens et al. 1997; Aguirre et al. 1998), bestrophinopathies (Guziewicz et al. 2007; Xiao et al. 2010), or in diseases with mutations that affect primarily the photoreceptors with a secondary impact on RPE health and viability.
Phagocytosis of POS by either RPE primary cultures or cell lines is often used as a measure of RPE function in vitro. Current published protocols utilize a large number of rodent, cattle, or pig eyes to isolate the necessary amounts of POS for phagocytosis assays. Such protocols frequently require >20 cattle or pig eyes (Martin et al. 2005; Mao and Finnemann 2013) and are not optimal for use with large animal models of inherited retinal diseases for which retinal tissue resources are usually limited. In this paper, we present an optimized protocol for isolation of POS from a single retina of an adult dog. In addition, we present a simple protocol for primary canine RPE cell culture and show that these can be combined for use in RPE phagocytosis assays.
72.2 Isolation of POS from a Single Canine Retina
This protocol has been modified from the POS preparation protocol presented in Martin et al. (2005) and scaled down for preparation of POS from a single neuroretina of a large animal such as that of a dog.
72.2.1 Materials Required for POS Isolation
Phosphate-buffered saline (PBS) (Thermo Scientific, catalog no. 28372)—500 ml.
Sucrose solutions prepared in PBS: 47%, 37%, 32%, and 10%—up to 10 ml per retina.
2 ml tissue grinder (Wheaton, catalog no. 358003).
Beckman polyallomer tubes (9/16 × 31/2 in., catalog no. 331372).
Beckman Coulter Optima L-90 K Ultracentrifuge.
Beckman SW 41 TI swinging-bucket rotor.
Disposable transfer pipettes (7.5 ml capacity) (Samco Scientific Corporation, catalog no. 225).
5 and 10 ml disposable serological pipettes (Fisherbrand).
Neubauer hemocytometer.
Chill all solutions to 4 °C. Rotor should be pre-cooled to 4 °C.
72.2.2 Protocol for POS Isolation
Place a single, fresh or frozen (stored at −80 °C) canine neuroretina in a tissue grinder. Place the grinding chamber on ice. Add 1 ml of cold 47% sucrose-PBS solution. Use eight passes of the pestle to completely homogenize the tissue.
Transfer homogenized retinal extract to a Beckman polyallomer tube (9/16 × 31/2 in.) using a disposable transfer pipette.
Add 1.5 ml of cold 47% sucrose-PBS solution and mix well using the transfer pipette.
Carefully layer 6 ml of cold 37% sucrose-PBS solution on top. Slowly release the solution along the wall of the tube using a 10 ml serological pipette.
Next, layer 1.5 ml of cold 32% sucrose-PBS solution on top of the 37% sucrose- PBS solution layer.
Transfer the tube to a pre-cooled SW 41 TI rotor, taking care not to disturb the sucrose layers. Balance the rotor carefully.
Centrifuge in a Beckman Ultracentrifuge at 25,000 rpm for 90 min at 4 °C.
After centrifugation, POS layer will be formed at the junction of the 37% and 32% sucrose-PBS layer. Carefully remove the 32% sucrose-PBS solution using a disposable transfer pipette.
Use a new disposable transfer pipette to aspirate the isolated POS and transfer to a new Beckman polyallomer tube. Add 10 ml of 10% sucrose-PBS solution. Mix gently by pipetting.
Balance the rotor carefully and spin at 25,000 rpm in the ultracentrifuge for 20 min at 4 °C.
Remove the supernatant and resuspend the POS pellet in 0.5 ml of 10% sucrose-PBS solution.
POS yield can be calculated by counting the number of POS using a Neubauer hemocytometer.
Store POS at −80 °C.
Note For experiments where unbleached POS are required, or when working with light-sensitive mutant retinas, collection and all steps in the POS isolation protocol must be carried out under dim-red illumination.
Using this protocol, yield from a single canine retina ranged from 10,000 to 50,000 full-length POS and between 10 and 25 million full-length and fragmented POS (n = 4) (Fig. 72.1).
Fig. 72.1. POS isolated from a single canine retina.
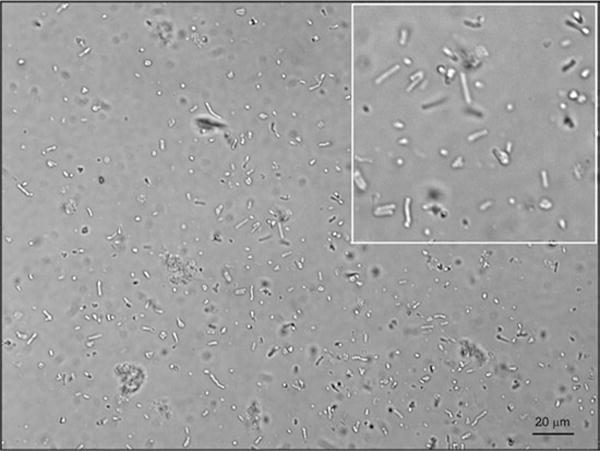
POS were isolated from single canine retinas (n = 4). Image was acquired using a Zeiss Axioplan microscope
72.3 Primary RPE Cell Culture
The protocol for primary RPE cell culture was modified slightly from Stramm et al. (1983, 1990).
72.3.1 Materials Required for Primary RPE Cell Culture
All reagents, media, instruments, and containers used for cell culture should be appropriately sterilized.
Transportation media: CO2-independent medium (Gibco, catalog no. 18045-088) containing 2% penicillin/streptomycin (Lonza, catalog no. 17-602E).
0.25% Trypsin-EDTA (Gibco, catalog no. 25200–056).
Growth medium: 20% FBS (Sigma, catalog no. F2442), 1% penicillin/streptomycin, 1% L-glutamine (Lonza, catalog no. 17-605E), 1% sodium pyruvate (Gibco, catalog no. 11360–070), 1% Eagle’s minimum essential medium (MEM), nonessential amino acids (Sigma, catalog no. M7145), DMEM/F-12(1:1) medium (Gibco, catalog no. 11320-033).
Dulbecco’s phosphate-buffered saline without Ca+2 and Mg+2 (DPBS) (Mediatech, catalog no. MT21-031-CV).
Transwell tissue culture plate (Corning Costar, catalog no. 3470).
70% ethanol.
50 ml specimen container.
Dissection tools: surgical prep blade, Noyes micro-scissors, microdissection tweezers, gauze.
Custom-made aluminum supports (see Fig. 72.2).
Sterile 15 ml disposable centrifuge tubes (Falcon, catalog no. 352196).
Fig. 72.2. Eyecup holders.

Custom-made aluminum supports of different diameters (indicated on the sides) to fit eyes of various sizes. The supports are sterilized by autoclaving before use
72.3.2 Protocol for RPE Cell Culture
Enucleate eye from a dog immediately following euthanasia and store in transportation medium in a specimen container for 8–10 h at 4 °C.
All following steps should be performed in the cell culture hood. Dip the eye in 70% ethanol for a couple of minutes, and then rinse twice with DPBS containing 2% penicillin/streptomycin.
Dissect eyes by cutting the sclera 3–5 mm away from the limbus. Make a nick using a surgical prep blade and cut using micro-scissors and a microdissecting tweezer.
Separate the anterior segment/lens and gently remove the vitreous completely by slowly dragging the posterior cup over a dry sterile gauze.
Place the posterior cup on a sterile aluminum support. Fill the eyecup with DPBS and gently remove the detached retina.
Rinse the RPE layer thoroughly with DPBS.
Fill the eyecup with 0.25% trypsin-EDTA and incubate at 37 °C in a 5% CO2 tissue culture incubator for 30 mins.
Collect detached RPE cells in trypsin-EDTA using a 1 ml pipette, transfer to a sterile 15 ml tube, and store at 37 °C in a 5% CO2 tissue culture incubator.
Refill the eyecup with trypsin-EDTA. Repeat incubation and collection four to five times.
Pellet cells by centrifugation (1000 rpm, 5 min) and resuspend in the growth medium.
Seed RPE cells on Transwell tissue culture plates at a density of 105 cells/cm2.
Incubate cells at 37 °C in a 5% CO2 tissue culture incubator for 2–3 weeks to form a confluent monolayer. Change medium 2–3 times a week during this period.
72.4 RPE Phagocytosis Assay
POS isolated from a single canine retina and RPE cells obtained from canine eyes can be used for RPE phagocytosis assay. In typical experiments, POS isolated from a single canine retina were used for feeding confluent RPE cells grown on six out of eight wells of an eight-chambered glass slide. Results from RPE phagocytosis assay are shown in Figs. 72.3 and 72.4.
Fig. 72.3. Phagocytosis of canine POS by ARPE-19 cells.
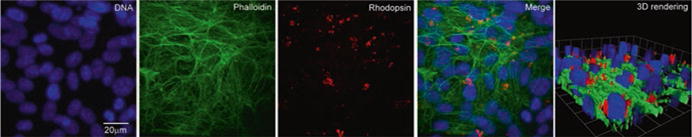
Confluent polarized ARPE-19 cells were stained with DAPI nuclear stain, Alexa Fluor 488 phalloidin, and anti-rhodopsin antibody after POS phagocytosis. Images were acquired using a Leica DMI 4000 inverted microscope equipped with a Yokogawa spinning disk confocal head and a Hamamatsu EMCCD 510 camera. Over 50 fields each containing ~50 cells were counted to evaluate the percentage of cells showing ROS phagocytosis. 65 ± 5% of the ARPE-19 cells were found to phagocytize ROS
Fig. 72.4. Phagocytosis of canine POS by primary canine RPE cells.
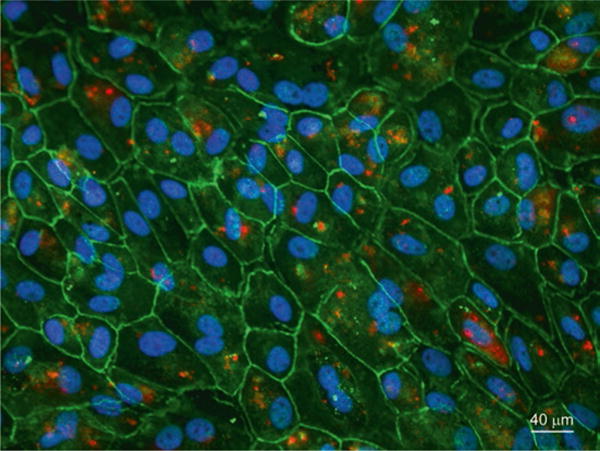
Confluent polarized canine primary RPE cells were stained with anti-rhodopsin and anti-ZO-1 antibody after POS phagocytosis. Images were acquired using a Zeiss Axioplan epifluorescence microscope
72.4.1 Materials Required for RPE Phagocytosis Assay
Primary RPE monolayer or ARPE-19 cells (ATCC, catalog no. CRL-2302) grown on 8-chambered glass slide (Lab-Tek, catalog no. 155411) in growth medium.
Polarization medium: Dulbecco’s MEM (DMEM) (Mediatech, catalog no. MT10-013-CV) with 1% FBS.
POS.
Neubauer hemocytometer.
72.4.2 Materials Required for Antibody Staining After Phagocytosis
Phosphate-buffered saline (PBS) (Thermo Scientific, catalog no. 28372).
Fixative: 4% paraformaldehyde prepared in PBS.
Permeabilization: 0.2% Triton X-100 (Fisher Scientific, catalog no. BP151-100) in PBS.
Blocking buffer: 5% BSA and 4.5% fish gelatin (Sigma, catalog no. G7765-250ML) in PBS.
Dilution buffer for Alexa Fluor 488 phalloidin: 1% BSA in PBS.
- Antibodies:
- Mouse anti-rhodopsin antibody (Millipore, catalog no. MAB5356).
- Rabbit anti-ZO-1 antibody (Invitrogen, catalog no. 402200).
- Alexa labeled anti-mouse and anti-rabbit secondary antibodies (Invitrogen).
Alexa Fluor 488 phalloidin (Molecular probes, catalog no. A12379).
DAPI (4′,6-Diamidino-2-Phenylindole, Dihydrochloride) (ThermoFisher Scientific, catalog no. D1306).
72.4.3 Protocol for RPE Phagocytosis Assay
Day 1: Seed primary RPE cells or ARPE-19 cells in growth medium on 8-chambered glass slides for next-day confluence. Incubate at 37 °C in 5% CO2 tissue culture incubator for 24 h.
Day 2: Change cell growth medium to polarization medium. Incubate overnight at 37 °C in the 5% CO2 tissue culture incubator.
Day 3: Thaw POS; calculate POS density using a hemocytometer. Centrifuge to pellet POS, and then resuspend in polarization medium.
Overlay POS suspension on the RPE cells in each chamber at an approximate density of 10 POS per cell.
Incubate in the cell culture incubator for 5 h.
Remove medium and wash cells three times with DPBS.
Fix cells with 4% paraformaldehyde at room temperature for 20 min. Wash three times with DPBS. Store at 4 °C or proceed with antibody and phalloidinalexa- 488 labeling.
72.4.4 Labeling POS with Anti-rhodopsin Antibody and Counterlabeling with ZO-1 Antibody or Alexa Fluor 488 phalloidin
Permeabilize cells in the 8-chambered glass slide with 0.2% Triton X-100 in PBS for 5 min. Wash three times with PBS.
Incubate cells in blocking buffer for 30 min at room temperature.
Incubate with anti-rhodopsin antibody alone or along with anti-ZO-1 antibody diluted in blocking buffer (mouse-anti-rhodopsin 1:400, rabbit-anti-ZO-1 antibody 1:100) for 1 h at 37 °C.
Wash three times with PBS.
Incubate with appropriate alexa-labeled secondary antibodies diluted 1:400 in blocking buffer for 1 h at room temperature.
Wash three times with PBS.
If performing phalloidin staining, incubate with Alexa Fluor 488 phalloidin diluted 1:50 in 1% BSA-PBS for 30 min at room temperature.
Wash three times with PBS.
Counterstain nuclei with DAPI stain. Wash once with PBS.
Detach media chamber from the slide. Apply appropriate mounting medium and coverslip and image using widefield epifluorescence or confocal microscopy.
Acknowledgments
The authors are grateful to the staff of RDSF facility for animal husbandry, Ms. Lydia Melnyk, for research coordination. This work is supported by NEI/NIH grants R24EY022012, R24EY023937, EY06855, EY017549, P30EY-001583, P30-EY021725, the Foundation Fighting Blindness, Hope for Vision, an unrestricted grant from Research to Prevent Blindness, and Van Sloun Fund for Canine Genetic Research.
Contributor Information
Raghavi Sudharsan, Division of Experimental Retinal Therapies, Department of Clinical Sciences and Advanced Medicine, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Michael H. Elliott, Department of Ophthalmology, University of Oklahoma Health Science Centre, Oklahoma City, OK, USA
Natalia Dolgova, Division of Experimental Retinal Therapies, Department of Clinical Sciences and Advanced Medicine, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Gustavo D. Aguirre, Division of Experimental Retinal Therapies, Department of Clinical Sciences and Advanced Medicine, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, PA, USA
William A. Beltran, Division of Experimental Retinal Therapies, Department of Clinical Sciences and Advanced Medicine, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, PA, USA
References
- Aguirre GD, Baldwin V, Pearce-Kelling S, et al. Congenital stationary night blindness in the dog: common mutation in the RPE65 gene indicates founder effect. Mol Vis. 1998;4:23. [PubMed] [Google Scholar]
- Gu SM, Thompson DA, Srikumari CR, et al. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997;17:194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- Guziewicz KE, Zangerl B, Lindauer SJ, et al. Bestrophin gene mutations cause canine multifocal retinopathy: a novel animal model for best disease. Invest Ophthalmol Vis Sci. 2007;48:1959–1967. doi: 10.1167/iovs.06-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Finnemann SC. Analysis of photoreceptor outer segment phagocytosis by RPE cells in culture. Methods Mol Biol. 2013;935:285–295. doi: 10.1007/978-1-62703-080-9_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlhens F, Bareil C, Griffoin JM, et al. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat Genet. 1997;17:139–141. doi: 10.1038/ng1097-139. [DOI] [PubMed] [Google Scholar]
- Martin RE, Elliott MH, Brush RS, et al. Detailed characterization of the lipid composition of detergent-resistant membranes from photoreceptor rod outer segment membranes. Invest Ophthalmol Vis Sci. 2005;46:1147–1154. doi: 10.1167/iovs.04-1207. [DOI] [PubMed] [Google Scholar]
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- Stramm LE, Haskins ME, McGovern MM, Aguirre GD. Tissue culture of cat retinal pigment epithelium. Exp Eye Res. 1983;36:91–101. doi: 10.1016/0014-4835(83)90092-1. [DOI] [PubMed] [Google Scholar]
- Stramm LE, Wolfe JH, Schuchman EH, et al. Beta-glucoronidase mediated pathway essential for retinal pigment epithelial degradtion of glycosaminoglycans. Disease expression and in vito disease correction using retroviral mediated cDNA transfer. Exp Eye Res. 1990;50:521–532. doi: 10.1016/0014-4835(90)90041-r. [DOI] [PubMed] [Google Scholar]
- Xiao Q, Hartzell HC, Yu K. Bestrophins and retinopathies. Pflugers Arch. 2010;460:559–569. doi: 10.1007/s00424-010-0821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


