Abstract
Fitness costs of incubation ensue whenever the trade-off between incubation and foraging leads to suboptimal incubation or decreased parental body condition. We examined the costs of incubation in a wild population of house wrens, Troglodytes aedon, by experimentally extending or decreasing the incubation period by cross-fostering eggs between nests at different stages of incubation (eggs from control nests were cross-fostered at the same stage of incubation). We determined whether parents or offspring bear the costs of incubation by measuring effects on females and offspring within the same breeding season during which the manipulation occurred, but also by evaluating potential trade-offs between current and future reproduction by monitoring return rates of experimental females and recruitment rates of offspring in subsequent breeding seasons. There was no difference in hatching or fledging success across treatments. There was also no effect of incubation duration on female size-corrected mass, and females from different treatments were equally likely to produce a second brood. Nestlings produced by females did not differ in body mass, tarsus length or residual mass. Neither return rates of females, nor the number of offspring recruited, differed across treatments. We conclude, therefore, that although prolonged incubation entails increased energy expenditures, females are able to offset these losses while foraging, thereby mitigating the costs of incubation. This resiliency is more likely to be seen in income breeders, such as house wrens, that retain some ability to recoup energy expended in incubation, than in capital breeders that are constrained by stored energy reserves.
Keywords: bird, cost of reproduction, incubation, life-history trade-offs, Troglodytes aedon
Incubation, once regarded as a relatively innocuous stage of avian reproduction, often entails significant energy expenditures (Nord & Williams, 2015). Incubating birds must meet these energetic demands either by foraging or by using stored energy reserves (Reid, Monaghan, & Nager, 2002). Income breeders, individuals that adjust their food intake according to their immediate needs (Drent & Daan, 1980; Jönsson, 1997), must balance the time spent foraging against the time required to maintain optimal incubation temperatures (Hepp, DuRant, & Hopkins, 2015). Fitness costs of incubation ensue, therefore, whenever the trade-off between incubation and foraging leads to suboptimal incubation or decreased parental body condition (Reid, Monaghan, & Nager, 2002). Although this trade-off is widely believed to exact a significant cost of reproduction, any such cost can only be revealed through experimental manipulation of the energy required for incubation, or the amount of food available to incubating birds (Reid, Monaghan, & Nager, 2002).
Various types of manipulations have been employed to explore the trade-off between incubation and foraging, among them, experimental manipulation of clutch size, altering the energy required for incubation by heating or cooling the nest, providing supplementary food, or experimentally extending the period of incubation (reviewed in Reid, Monaghan, & Nager, 2002). Of these, arguably the most widely used is an experimental increase in clutch size, predicated on the well-founded assumption that larger clutch sizes require greater energy expenditures during incubation (Tinbergen & Williams, 2002). Any decrease in hatching success or increase in the incubation period upon such an increase is often taken as evidence of a cost of reproduction associated with incubation (reviews in Reid, Monaghan, & Nager, 2002; Thomson, Monaghan, & Furness, 1998), but such an inference can be misleading. Parents may be physically constrained in their ability to optimally incubate their eggs by the increased surface area presented by an enlarged clutch (Reid, Monaghan, & Nager, 2002; Reid, Monaghan, & Ruxton, 2000a).
Heating or cooling of the nest can be a particularly effective means to decrease or increase the energetic demands of incubation, respectively. Experimental heating of nests has been shown to result in decreased incubation effort, higher offspring body condition and greater fledging success (Pérez, Ardia, Chad, & Clotfelter, 2008; Reid, Monaghan, & Ruxton, 2000b), whereas experimental cooling of nests can lead to extended incubation periods and lower offspring body condition (Ardia, Pérez, & Clotfelter, 2010; Nilsson, Stjernman, & Nilsson, 2008). Although these results are consistent with a trade-off between incubation and foraging, other studies report opposing effects of heating (or cooling) on incubation effort (reviewed in Álvarez & Barba, 2014). Moreover, experimental heating or cooling does not always affect nestling development or condition (Álvarez & Barba, 2014), suggesting that under normal conditions, incubating parents may be able to offset energy expenditures during incubation while foraging off the nest, while still managing to maintain adequate incubation temperatures.
The provision of supplemental food to incubating birds offers another means of altering the presumed trade-off between incubation and foraging, the primary expectation being that increased availability of food should lead to an increase in incubation effort as the need to forage is correspondingly reduced. Although this basic expectation has been met in several studies (e.g. Barnett & Briskie, 2010; Chalfoun & Martin, 2007; Pearse, Cavitt, & Cully, 2004), a critical test of the presumed trade-off requires that greater incubation attentiveness actually enhances reproductive performance of the supplemented parent or some aspect of offspring fitness. Studies on house wrens, Troglodytes aedon, suggest that this does not always follow (Lothery, Thompson, Lawler, & Sakaluk, 2014; Pearse, Cavitt, & Cully, 2004).
Arguably the most direct way of probing the costs of incubation is to force incubating individuals to alter their incubation effort by manipulating the length of the incubation period, which can be accomplished by cross-fostering eggs at different stages of embryonic development (Reid, Monaghan, & Nager, 2002). A review of such cross-fostering experiments (Verhulst & Nilsson, 2008) reveals that parents with shortened incubation periods generally produce nestlings that grow faster, fledge at higher body mass and are more likely to recruit to the breeding population than those produced by parents forced to incubate for longer periods. Although these results are consistent with the hypothesis that incubation imposes significant costs of reproduction, their interpretation is obscured by a potential confound with the effects of time of season on reproductive performance (Brinkhof, Cavé, Daan, & Perdeck, 2002; Verhulst & Nilsson, 2008). The reproductive success of birds breeding in seasonal environments typically declines over the course of the season (Verhulst & Nilsson, 2008), so that any differences in reproductive success of individuals experiencing experimentally manipulated incubation periods could be due as much or more to a seasonal effect than to any effect of incubation effort per se. Consequently, great care must be taken in establishing appropriate controls with which to compare reproductive performance of birds with experimentally delayed and experimentally advanced hatching dates.
House wrens are an ideal study species with which to investigate costs of reproduction because females are easily manipulated into laying more eggs than they normally would (Bowers, Sakaluk, & Thompson, 2012; Hodges, Bowers, Thompson, & Sakaluk, 2015), and thus it is possible to manipulate each stage of the reproductive cycle independently of the other stages. These studies have revealed that the costs of increased egg production fall most heavily on the females, which are less likely to reproduce again (or at a lower reproductive output than control females; Bowers, Sakaluk, & Thompson, 2012), whereas the costs of rearing supernumerary young appear to fall most heavily on the nestlings, which fledge at a lower body mass or lower body condition (Bowers, Nietz, Thompson, & Sakaluk, 2014; Finke, Milinkovich, & Thompson, 1987; but see Harper, Juliano, & Thompson,1992). The costs of incubation are, however, less clear. An experimental increase in clutch size resulted in a slight increase in the incubation period, but no effect on hatching success or early nestling survival (Baltz & Thompson, 1988; Dobbs, Styrsky, & Thompson, 2006). Supplemental feeding of females during incubation increased nest attentiveness (Pearse, Cavitt, & Cully, 2004; Lothery, Thompson, Lawler, & Sakaluk, 2014), but had no effect on female condition or reproductive success (Lothery, Thompson, Lawler, & Sakaluk, 2014).
Here we examine the costs of incubation in a wild population of house wrens by experimentally extending or decreasing the incubation period by cross-fostering eggs between nests at different stages of incubation. We attempted to determine whether parents or offspring bear the costs of incubation by measuring effects on females and offspring not only within the same breeding season during which the manipulation occurred, but also by evaluating potential trade-offs between current and future reproduction by monitoring return rates of experimental females and recruitment rates of offspring in subsequent breeding seasons. We predicted that if there is a trade-off between incubation and foraging, females forced to incubate for longer periods should have lower reproductive success than control females, whereas females incubating over experimentally shortened periods should enjoy higher reproductive success.
METHODS
House wrens are small (10–12 g), insectivorous songbirds and, as secondary cavity nesters, readily nest in nestboxes. Upon arrival at our study area in north-central Illinois (40°40’N, 88°53’W) following spring migration, females select a male in attendance at a nestbox and, after completing the nest, lay a clutch of four to eight eggs. In our study population, house wrens are double-brooded, with approximately half of the females producing a second brood within the same breeding season (Bowers, Sakaluk, & Thompson, 2012; Bowers, Smith, Hodges, Zimmerman, Thompson, & Sakaluk, 2012; Drilling & Thompson, 1991). Only females incubate the eggs and brood the nestlings, but both adults provision nestlings and fledglings. Parents bring one prey item back to the nest at a time (Barnett, Clairardin, Thompson, & Sakaluk, 2011; Barnett, Thompson, & Sakaluk, 2012). Additional information concerning the breeding biology of house wrens is provided in Johnson (2014).
This study was initiated during the 2014 breeding season, with survival and reproductive success of experimental individuals monitored through the end of the 2016 breeding season. In all 3 years, we captured and banded nearly all the adults and nestlings produced on the study area. Nestboxes (N = 820) of uniform construction (Lambrechts et al., 2010) were spaced 30 m apart along north–south transects separated by 60 m. All nestboxes were mounted on 1.5 m metal poles and protected with 48.3 cm diameter aluminium baffles mounted below nestboxes to discourage terrestrial nest predators. We visited nestboxes twice weekly to determine the beginning of nest building and, once egg laying had begun, visited active nests daily until clutch completion. We deemed incubation to have begun when the clutch size remained the same over two consecutive days and the eggs were warm to the touch. Adults were captured inside nestboxes or by employing mist nets near the box during incubation or shortly after hatching, and uniquely banded with a U.S. Geological Survey leg band; in addition, males received three coloured bands in a unique combination to visually identify and distinguish them from females during provisioning.
Females normally incubate eggs for 12–13 days before they hatch. In 2014, we experimentally extended or decreased this period by cross-fostering eggs between nests at different stages of the incubation period. Specifically, eggs of females that had been incubating for 6 days were cross-fostered with eggs of females that had been incubating for 1 day. Thus, experimental females that had been incubating for only 1 day at the time of cross-fostering would have had their incubation period decreased by approximately 5 days, whereas those that had been incubating for 6 days at the time of cross-fostering would have had their incubation period increased by approximately 5 days. To control for the effects of cross-fostering, we established a control group of females in which eggs were cross-fostered at the same stage of incubation. Within this control group, eggs were cross-fostered either 1 day after incubation had begun or 6 days after, to control for the possibility that the time at which eggs are cross-fostered influences hatching success. This cross-fostering protocol was applied to pairs of females throughout the first month of the breeding season; within each pair, one female was randomly assigned to the incubation-lengthened treatment whereas the other was assigned to the incubation-shortened treatment, or else, both females were assigned to the control treatment. These assignments were made opportunistically depending on when females began incubating, although we attempted to balance control and incubation-manipulated replicates over time. In some instances, the clutch size of paired replicates differed (but never by more than one egg); in these cases, we added a single egg, taken from the nest of a nonexperimental female at the same point in the incubation cycle, to the nest of the female with the smaller clutch size. In total (excluding a few nests that failed due to depredation or premature abandonment; see Supplementary Material for details), we established a total of 42 replicates including 16 incubation-shortened females, 14 incubation-lengthened females and 12 control females.
Females were captured up to three times during the breeding period, once on incubation-day 6 (incubation-day 1 is the day the last egg of the clutch is laid), once on brood-day 2 (brood-day 0 is the day hatching begins), and, if they produced a second brood, once during incubation of their second-brood clutch. Upon their capture, females were weighed to the nearest 0.1 g using a digital scale (Acculab Pocket Pro PP 201) and their tarsus length measured to the nearest 0.1 mm using dial callipers.
After the eggs in a nest had hatched, we weighed the young twice during the nestling period, once on brood-day 4 and again on brood-day 11, when they were also banded with a U.S. Geological Survey leg band and their tarsus length measured. Beginning on brood-day 13, nests were visited daily to document the day of fledging or the loss of any offspring.
Provisioning of nestlings by both parents at nests was video-recorded twice during the first brood, once on brood-day 4 or 5 and once on brood-day 8 or 9. Parental provisioning at brood-days 4–5 is especially germane, as this is the age at which nestling growth is most rapid, and the amount of food delivered by parents at this age is positively correlated with nestling growth, fledging success and the recruitment of offspring to the breeding population (Bowers et al., 2014; Bowers, Bowden, Sakaluk, & Thompson, 2015). Recordings were made using pocket-sized digital video cameras (Kodak Sport Zx5, Eastman Kodak, Rochester, NY, U.S.A.) positioned about 1.5 m from the nestbox and secured at the end of a 1.5 m length of metal conduit (1.5 cm diameter) using a cell-phone holster (Barnett, Clairardin, Thompson, & Sakaluk, 2011; DeMory, Thompson, & Sakaluk, 2010; Dorset, Sakaluk, & Thompson, 2017; Will, Dorset, Thompson, Sakaluk, & Bowers, 2017). Adults were habituated to the camera by setting out a dummy camera 24 h before the actual recording began. We video-recorded nests for approximately 100–120 min between 0630 and 1100 hours; this ensured that we had at least 60 min of undisturbed provisioning for subsequent analysis. Previous studies have shown that parental provisioning quantified over this duration is a reliable proxy for parental behaviour over longer periods (Bowers et al. 2014; Murphy, Chutter, & Redmond, 2015). Videos were scored for the frequency of provisioning visits by each parent by an observer blind to the treatment.
The majority of experimental females (32 of 42) produced a second brood. We also measured the reproductive success of these females (i.e. clutch size, hatching success, brood size, nestling mass and size, fledging success) in their unmanipulated second-brood nests.
Return rates of adults and recruitment of any offspring to the breeding population were documented in the 2015 and 2016 breeding seasons. We also measured the reproductive success of these individuals to determine any long-lasting effects of our experimental manipulation. Lifetime reproductive success of females was measured as the total number of descendants produced, determined as the sum of the number of offspring produced over the 3 years of the study, the number of grandoffspring produced in 2015 and 2016, and the number of great-grandoffspring produced in 2016. We also computed each female’s lifetime allelic fitness based on the allelic value of each offspring (0.5), grandoffspring (0.25) and great-grandoffspring (0.125), assuming no inbreeding in the population (Reid, Arcese, Bocedi, Duthie, Wolak, & Keller, 2015). Lifetime allelic fitness was calculated as the sum of the allelic values for all descendants; strictly speaking, fitness should be measured only from one generation to the next (Hunt & Hodgson, 2010; Wolf & Wade, 2001), but owing to the small number of offspring recruited to the breeding population, we opted to use all descendants as a proxy for fitness in this study.
Statistical Analyses
All analyses were conducted using SAS statistical software (SAS 9.4; SAS Institute, Cary, NC, U.S.A.), and all tests were two tailed (α = 0.05). All means reported are least-squares means ± 1 SE unless otherwise noted. In analyses in which multiple measurements were taken from the same nest, nest was included as a random effect to account for the statistical nonindependence of nestlings within a brood. In all mixed models, degrees of freedom were calculated using the Satterthwaite method, which can result in noninteger denominator degrees of freedom. Where appropriate, we included hatching date (or clutch initiation date) and brood size (or clutch size) as covariates in analyses of treatment effects, but these were retained in the final models only when significant.
We utilized general linear models (PROC GLM) to determine the effect of incubation treatment on initial clutch size, duration of the incubation period, hatching success, residual female body mass after hatching (i.e. body mass conditioned on tarsus length), average nestling mass on brood-day 4, duration of the nestling period and the number of young that fledged.
We used contingency table analysis in PROC FREQ to compare treatments with respect to the proportion of females that produced a second brood. To compare treatments with respect to the time taken to produce a second brood, we used a proportional hazards regression in PROC PHREG. Females that did not produce a second brood were included as right-censored observations and assigned a clutch initiation date of 212 for the second brood, the last clutch initiation date on which a breeding pair succeeded in fledging offspring in 2014.
We analysed parental provisioning rates by females (food items delivered per h) on brood-days 4 and 8 using a repeated measures general linear mixed model (PROC MIXED), with treatment and time as main effects, nest as a random effect, and female identity as the repeated factor. We also controlled in this analysis for brood size and hatching date. The interaction between treatment and time in their effect on maternal provisioning was not significant (F2,38.7 = 0.93, P = 0.40) and removed from the final model. We also tested for a correlation (PROC CORR) between maternal and paternal provisioning rates with nests from each treatment pooled.
Effects of incubation treatment on mass, tarsus length, and residual mass of offspring in first and second broods of experimental females were assessed using a repeated measures, general linear mixed model (PROC MIXED), with treatment and brood (nested within female) as main effects, nest as a random effect, and female identity as the repeated factor.
To determine the effect of the experimental manipulation on adult return rates the following breeding season, we used a generalized linear mixed model (PROC GLIMMIX) with a binary response (returned or did not), with treatment and sex as main effects, and nest as a random effect. Similarly, to assess the effect of treatment on recruitment of offspring to the breeding population, we used a generalized linear mixed model (PROC GLIMMIX) with a binary response (recruited or did not), with nest as a random effect.
To determine the effect of incubation treatment on female reproductive success (total number of descendants and lifetime allelic fitness), we used a generalized linear mixed model (PROC GLIMMIX) with treatment as a main effect, and clutch initiation date and female residual body mass prior to the experimental manipulation as covariates.
To assess the magnitude of the effect of incubation treatment on the dependent variables, we determined effect sizes ± 95% confidence intervals (Nakagawa & Cuthill, 2007) using F statistics from the above analyses to calculate correlation coefficients as , where DDF represents the error degrees of freedom from an F test (Rosenthal, 1994). Confidence limits were calculated using the z transformation (Sokal & Rohlf, 1995). Values of r equal to 0.1, 0.3 and 0.5 are considered to represent small, medium and large effects, respectively.
Ethical Note
Although house wrens are highly tolerant of human disturbance around their nest site, we took steps to minimize the disturbance caused to breeding pairs during cross-fostering of eggs. Eggs removed from a nest were immediately replaced with dummy eggs, which were left in the nest until we had retrieved the eggs from the donor nest. Females readily incubated the fake eggs in the period during which the transfer of eggs between nests took place (as evidenced by the warmth of the dummy eggs when replaced with real eggs), and we made every effort to complete both ends of the cross-fostering between nests within 30 min. All research activities were performed in accordance with the Illinois State University Institutional Animal Care and Use Committee (Protocol No. 04–2013) and United States Geological Survey banding permit 09211.
RESULTS
Effects on Within-season Reproductive Success
Within the control group, there was no difference in the hatching success of females whose eggs were cross-fostered 1 day after incubation and those whose eggs were cross-fostered 6 days after (F1,10 = 1.36, P = 0.27); thus, for all remaining comparisons, control females of both types were pooled as a single control treatment.
There was no difference in the initial clutch size of females across treatments (F2,39 = 0.78, P = 0.46); the mean clutch size of all females was 6.88 ± 0.1. As anticipated, there was a significant effect of treatment on the duration of the incubation period across treatments (F2,39 = 682.9, P < 0.0001, r = 0.97 (0.95–0.98)), with incubation-shortened females spending 5 days fewer, and incubation-lengthened females, 5 days longer, respectively, than control females incubating their eggs (Fig. 1a).
Figure 1.
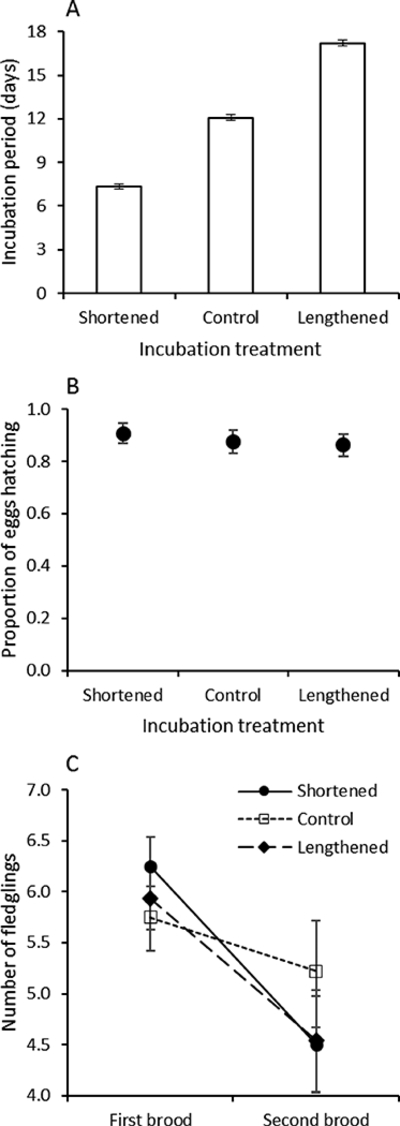
(a) Mean duration of incubation period (± SE) in relation to experimental treatment. (b) Proportion of eggs hatching in relation to treatment (mean ± SE). (c) Mean number of fledglings produced (± SE) in first and second broods in relation to treatment.
There was no difference in hatching success across treatments (Fig. 1b; F2,39 = 0.34, P = 0.71, r = 0.09 (−0.22–0.39)). The duration of the nestling period (the time from hatching to fledging) did not differ across treatments (F2,39 = 0.59, P = 0.16, r = 0.12 (−0.19–0.22)). There were also no effects of treatment on the number of offspring that fledged from first broods (Fig. 1c; F2,39 = 0.71, 0.50, r = 0.13 (−0.18–0.42)).
Although the duration of incubation varied greatly among treatments, there was no effect of incubation duration on female residual body mass measured shortly after hatching (i.e. brood-day 2) (Fig. 2a; F2,39 = 0.41, P = 0.67, r = 0.10 (−0.21–0.39)). There was no difference across treatments in the proportion of females that produced a second brood (likelihood ratio: = 0.07, P = 0.97). There was also no difference in the time taken to produce a second brood (Fig. 2b; Wald = 0.39, P = 0.82). However, the time taken to produce a second brood increased significantly with the time in the season at which the first clutch was initiated (Wald = 4.33, P = 0.037). There were no differences between treatments in the number of offspring that fledged from unmanipulated second broods (Fig. 1c; F2,39 = 0.58, P = 0.56, r = 0.12 (−0.14–0.37)).
Figure 2.
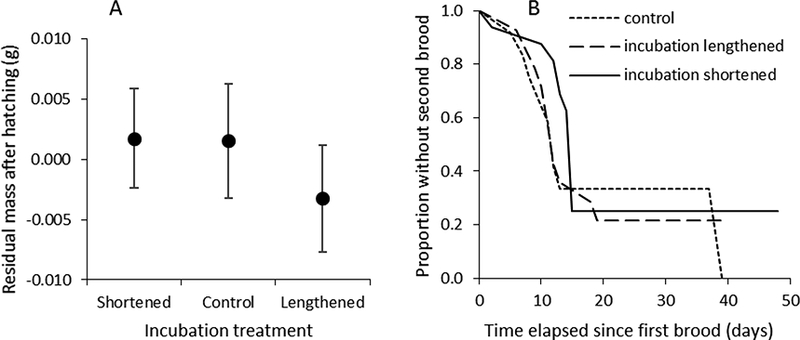
(a) Residual body mass of females (body condition) after hatching in relation to treatment. (b) Proportion of females without a second brood as a function of time elapsed since the first brood.
Effects on Parental Provisioning
Females provisioned offspring with food at a higher rate later in development on brood-day 8 than on brood-day 4 (F1,40.6 = 56.66, P < 0.0001), but there were no differences between maternal provisioning rates across treatments (Fig. 3a; F2,37 = 0.45, P = 0.64, r = 0.11 (−0.20–0.40)). Provisioning to young increased as the first brood of the season advanced (effect of hatching date: F1,36.4 = 9.06, P = 0.0047), and maternal provisioning increased with brood size (F1,36.7 = 4.66, P = 0.0375). Although maternal provisioning increased with nestling age, paternal feeding did not, and maternal and paternal provisioning rates within pairs were strongly negatively correlated both on brood-day 4 (Fig. 3b; Pearson correlation: r40 = –0.605, P < 0.0001) and on brood-day 8 (Fig. 3c; r38 = –0.479, P = 0.0018).
Figure 3.
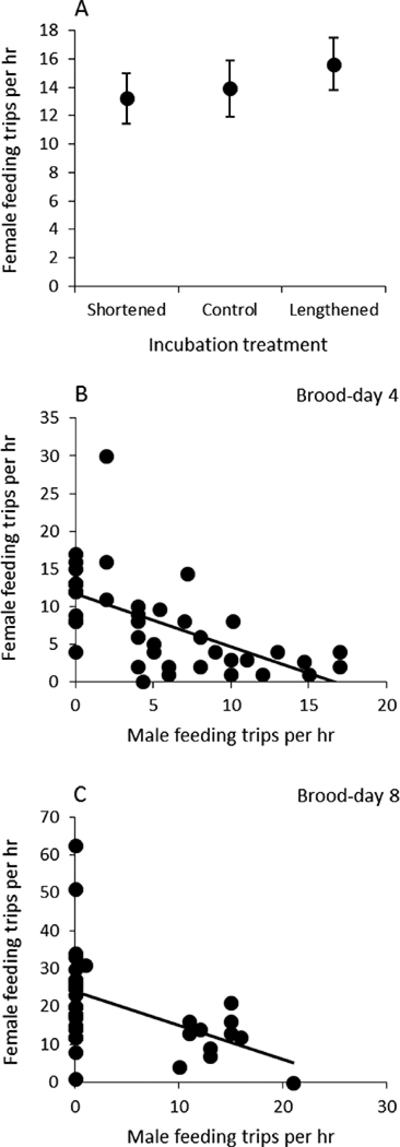
(a) Provisioning rate of females (mean ± SE) in relation to treatment. (b) Relationship between female and male provisioning rates on brood-day 4 pooled across all treatments. (c) Relationship between female and male provisioning rates on brood-day 8 pooled across all treatments.
Effects on Offspring
There was no effect of incubation treatment on average nestling mass on brood-day 4 (F2,38 = 0.04, P = 0.96, r = 0.03 (−0.27–0.33)), but there was a significant effect of brood size on average mass on brood-day 4 (F1,38 = 5.97, P = 0.019), with average mass of young increasing with increased brood size. Mixed-model, repeated measures ANOVA of nestling mass on brood-day 11 for first and second broods revealed no differences between treatments (F2,39 = 1.10, P = 0.34, r = 0.17 (−0.15–0.45)), no differences across broods (F1,37.9 = 2.97, P = 0.09), and no treatment-by-brood interaction (F2,37.9 = 0.58, P = 0.45). Similar analyses showed no effect of treatment (F2,33.5 = 1.07, P = 0.35, r = 0.18 (−0.13–0.46)), brood (F1,36.4 = 0.33, P = 0.57), or treatment-by-brood interaction (F2,36.4 = 0.75, P = 0.48) on offspring tarsus length, and no effect of treatment (F2,39.3 = 0.53, P = 0.59, r = 0.11 (−0.19–0.40)), brood (F1,37.5 = 2.53, P = 0.12), or treatment-by-brood interaction (F2,37.6 = 0.40, P = 0.67) on offspring residual body mass (Fig. 4).
Figure 4.
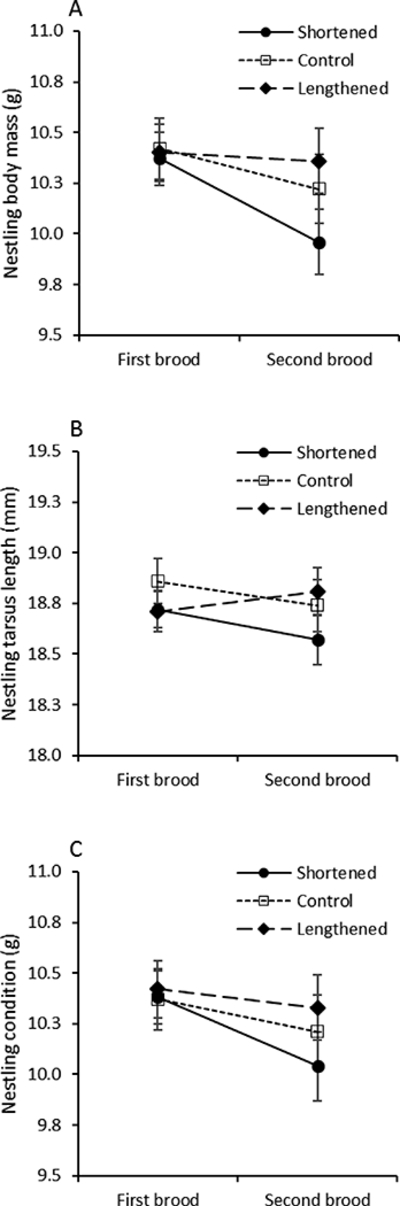
Size-related traits of nestlings in relation to treatment (all measures are shown as mean ± SE). (a) Body mass. (b) Tarsus length. (c) Residual body mass (body condition).
Effects on Adult Return Rates, Offspring Recruitment and Lifetime Reproductive Success
Of the 42 females manipulated in 2014, 11 returned to breed in 2015, one of which also returned to breed in 2016. Fourteen of the 37 males identified at these nests in 2014 (5 males were not identified) also returned to breed in 2015. There were no significant differences across treatments in adult return rates (Fig. 5; F2,41.35 = 0.00, P = 1.00, r = 0.00 (−0.22–0.22)). There was also no difference in return rates of male and female parents (F1,73 = 0.98, P = 0.32), nor any significant treatment-by-sex interaction (F2,73 = 0.99, P = 0.38).
Figure 5.
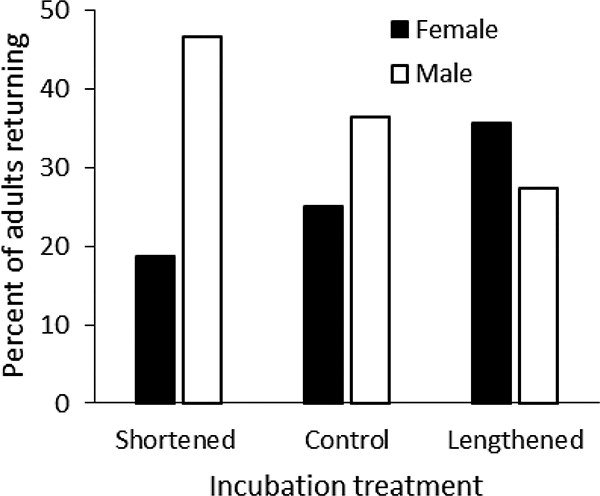
Return rates of experimental females and males in relation to treatment.
Of the 407 banded offspring produced in 2014, 13 were recruited to the breeding population, six of which bred in both 2015 and 2016, five of which bred in 2015 only, and two of which were not captured in 2015, but bred in 2016. There were no differences in offspring recruitment rates across treatments (F2,134.2 = 0.98, P = 0.38, r = 0.08 (−0.01–0.18)).
There were no differences between treatments in the total number of descendants produced by experimental females (Table 1; F2,37 = 1.32, P = 0.28, r = 0.19 (−0.12–0.46)). However, female residual body mass prior to experimental manipulation positively predicted the total number of descendants (Fig. 6a; F1,37 = 5.11, P = 0.0298). In addition, females initiating their first clutches earlier in the breeding season in 2014 produced more descendants than those initiating their first clutches later in the season (parameter estimate ± SE = −0.33 ± 0.16; F1,37 = 4.11, P = 0.0498).
Table 1.
Effects of experimentally manipulated incubation periods on female lifetime reproductive success
| N | Least-squares | SE | |
|---|---|---|---|
| Total number of descendants | |||
| Control | 12 | 11.7 | 2.08 |
| Incubation lengthened | 14 | 14.9 | 1.98 |
| Incubation shortened | 16 | 16.1 | 1.85 |
| Lifetime allelic fitness | |||
| Control | 12 | 5.67 | 0.75 |
| Incubation lengthened | 14 | 6.52 | 0.71 |
| Incubation shortened | 16 | 7.15 | 0.67 |
Figure 6.
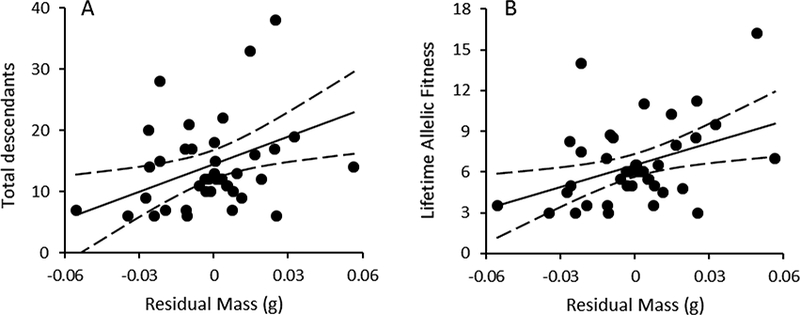
(a) Total number of descendants produced by females as a function of residual body mass prior to experimental manipulation pooled across all treatments. (b) Lifetime allelic fitness of females as a function of residual body mass prior to experimental manipulation pooled across all treatments.
There were also no differences between treatments in female lifetime allelic fitness (Table 1; F2,37 = 1.09, P = 0.35, r = 0.17 (−0.14–0.45)). However, female residual body mass prior to experimental manipulation positively predicted lifetime allelic fitness (Fig. 6b; F1,37 = 5.04, P = 0.031). In addition, females initiating their first clutches earlier in the breeding season in 2014 exhibited a higher lifetime allelic fitness than those initiating their first clutches later in the season (parameter estimate ± SE = −0.13 ± 0.06; F1,37 = 4.65, P = 0.0375).
DISCUSSION
Although our experimental manipulation succeeded in imposing vastly different incubation periods on our subjects, there was no discernible effect on the within-season reproductive success of experimental females. Differences in the duration of incubation across treatments had no effect on hatching success, and there were no effects of treatment on the number of offspring that fledged from first broods. There was also no effect of incubation duration on female residual body mass measured shortly after hatching, and females from the different treatments were equally likely to produce a second brood. There were also no differences between treatments in the number of offspring that fledged from these unmanipulated second broods.
It seems evident, therefore, that any immediate costs of reproduction arising from experimentally increased incubation effort do not fall on the females. It is equally clear, however, that any such costs are not paid by offspring either. Nestlings produced by females did not differ in body mass, tarsus length or residual mass, and nestlings from the different treatments were equally likely to recruit to the breeding population in subsequent years.
Even though there were no immediate costs of reproduction associated with increases in female incubation effort, it remains possible, of course, that such costs could be manifest in a trade-off between current and future reproduction. Here too, however, evidence of such costs was elusive. There were no significant differences across treatments in adult return rates, and no differences in fitness as measured by the total number of descendants produced or lifetime allelic fitness. Although we cannot be certain that some individuals did not disperse outside of the study site, dispersal distances from year to year are remarkably short, at least for adults: males and females shift their breeding location, on average, 67 and 134 m, respectively, from their location the year before (Drilling & Thompson, 1988). Given the absence of a treatment effect on the various proxies of within-season reproductive success, female residual body mass and fitness-related traits of offspring, we can see no obvious reason why treatment might differentially influence dispersal probability. Thus, if any experimental females or their offspring returned, but bred outside the study site in subsequent years, we expect that this would introduce noise, but no bias to our results.
There have been few studies that have examined the effect of cross-fostering eggs between nests at different stages of incubation on female fitness beyond the season in which the treatment has been imposed (Verhulst & Nilsson, 2008). A notable exception is a study by Brinkhof, Cavé, Daan, and Perdeck (2002), who employed cross-fostering to simultaneously advance and delay hatching date in European coots, Fulica atra. In contrast to our study, they found that birds experiencing shortened incubation periods were more like to produce a second brood than those experiencing extended incubation periods; incubation-shortened adults also exhibited lower survival than incubation-lengthened adults, which the authors attributed to the higher cost of rearing two broods. It seems likely that had we imposed our experimental manipulation on females nesting for the first time later in the season, it too might have resulted in a decreased probability of producing a second brood. Previous work on our study population has revealed that the likelihood of a female attempting a second brood decreases as the breeding season advances (Bowers, Sakaluk, & Thompson, 2013), so the extent to which prolonged incubation influences the probability of a second brood likely depends on when in the breeding season the manipulation is imposed.
The potential cost of an extended incubation period on the prospect of producing a second brood may explain the unexpected effect of treatment on the rate of female provisioning. Perhaps counterintuitively given the presumed cost of incubation, incubation-lengthened females tended to provision at a higher rate than either incubation-shortened females or controls (albeit not significantly so). It may be that females experiencing an unexpectedly long period of incubation attempted to compensate by feeding their young at an increased rate, thereby shortening the nestling period and enhancing their prospects of producing a second brood. However, our results show no effect of treatment on the duration of the nestling period, and so, if females were attempting to compensate for an overly long incubation period, they were largely unsuccessful in doing so. One possible clue as to why they did not succeed in decreasing the nestling period can be discerned by the relationship between male and female provisioning rates within pairs, which was significantly negative across all treatment groups; increased female provisioning rates were accompanied by lower provisioning rates by the male parent, and vice versa, a pattern also observed in previous work (Will, Dorset, Thompson, Sakaluk, & Bowers, 2017). We cannot, obviously, disentangle cause and effect in this correlation because males could be responding to changes in female provisioning effort or vice versa, but whatever the underlying cause, it does suggest that females are constrained in their ability to alter the length of the nestling period via adjustments in their feeding rate.
Although the study was conducted over a 3-year period, the experimental manipulation itself was imposed in only the first year. Average temperature and precipitation at the study site during the month of May is highly variable from year to year, and so too is the average number of fledglings produced per egg (see Figure 1 in Lothery, Thompson, Lawler, & Sakaluk, 2014). April temperatures in 2014 were below the long-term average, and breeding started later than average (see Figure 2 in Bowers et al., 2016); however, during the time that the birds were incubating their clutches (May–June), temperatures were near the average for the 2007–2015 period (see Figure 3 in Bowers et al., 2016). Nevertheless, we cannot rule out the possibility that the absence of a cost of incubation in the present study could be the result of an extraordinarily good year with respect to the amount of food available to the breeding birds. The use of artificial nestboxes to provide nesting sites may also have greatly reduced one potentially important cost of an extended incubation period, and that is, an increased risk of predation on the eggs. Compared with house wrens nesting in natural cavities, nesting success of pairs breeding in artificial nestboxes is considerably higher (Kaluthota & Rendall, 2017), reflective of lower predation rates on nestboxes, which offer the advantage of smaller entrances compared with natural cavities. Thus, any increase in predation risk accruing to an increase in the incubation period was likely attenuated by the protection afforded by the nestbox and its associated predator guard.
Although there were no differences between treatments in the total number of descendants produced or female lifetime allelic fitness, females initiating their first broods earlier in the season experienced higher fitness, and so too did those females of significantly higher residual body mass. These results reinforce previous findings in the study population. Analysis of a 36-year data set revealed strong selection for early breeding, with those females initiating their first clutches earliest in the breeding season producing the highest number of recruits in most years (Bowers et al., 2016). Size-adjusted body mass of nestling house wrens near the time of nest leaving is positively predictive of offspring recruitment and subsequent longevity (Bowers et al., 2014), and so it is not surprising that adult females of higher residual body mass in the current study produced the most descendants over the 3-year study period.
In conclusion, female house wrens were surprisingly resilient to sizeable alterations in the duration of their incubation periods. There were no discernible with-season costs of incubation to females or their young, nor were there any apparent costs to future reproduction of experimentally extended incubation periods. It seems, likely, then, that despite the significant energy expenditures that occur during incubation (Nord & Williams, 2015), females can offset these costs through foraging without any apparent harm to their developing embryos. We anticipate that this ability is more likely to be observed in income breeders, such as house wrens, than in capital breeders, as the latter are constrained by stored energy reserves whereas the former retain some ability to recoup the energy expended in incubation. A comparative study could be highly informative in this respect, but must await the results of additional cross-fostering studies extended beyond the roughly dozen or so species that have been subject to similar manipulations of the incubation period (Verhulst & Nilsson, 2008).
Supplementary Material
Acknowledgments
We thank Will Cresswell and an anonymous referee for helpful comments on the manuscript, the 2014–2016 Wren Crews for field assistance, and the ParkLands Foundation (Merwin Nature Preserve), the Illinois Great Rivers Conference of the United Methodist Church and the Sears and Butler families for the use of their properties. This work was supported by the National Institutes of Health (R15HD076308) and a Faculty Research Award from the College of Arts and Sciences, Illinois State University.
References
- Álvarez E, & Barba E (2014). Behavioural responses of great tits to experimental manipulation of nest temperature during incubation. Ornis Fennica, 91, 220–230. [Google Scholar]
- Ardia DR, Pérez JH, & Clotfelter ED (2010). Experimental cooling during incubation leads to reduced innate immunity and body condition in nestling tree swallows. Proceedings of the Royal Society B: Biological Sciences, 277, 1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltz ME, & Thompson CF (1988). Successful incubation of experimentally enlarged clutches by house wrens. Wilson Bulletin, 100, 70–79. [Google Scholar]
- Barnett CA, & Briskie JV (2010). Silvereyes Zosterops lateralis increase incubation attentiveness in response to increased food availability. Ibis, 152, 169–172. [Google Scholar]
- Barnett CA, Clairardin SG, Thompson CF, & Sakaluk SK (2011). Turning a deaf ear: A test of the manipulating androgens hypothesis in house wrens. Animal Behaviour, 81, 113–120 [Google Scholar]
- Barnett CA, Thompson CF, & Sakaluk SK (2012). Aggressiveness, boldness and parental food provisioning in male house wrens (Troglodytes aedon). Ethology, 118, 984–993. [Google Scholar]
- Bowers EK, Bowden RM, Sakaluk SK, & Thompson CF (2015). Immune activation generates corticosterone-mediated terminal reproductive investment in a wild bird. American Naturalist, 185, 769–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Grindstaff JL, Soukup SS, Drilling NE, Eckerle KP, Sakaluk SK, et al. (2016). Spring temperatures influence selection on breeding date and the potential for phenological mismatch in a migratory bird. Ecology, 97, 2880–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Hodges CJ, Forsman AM, Vogel LA, Masters BS, Johnson BGP, et al. (2014). Neonatal body condition, immune responsiveness, and hematocrit predict longevity in a wild bird population. Ecology, 95, 3027–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Nietz D, Thompson CF, & Sakaluk SK (2014). Parental provisioning in house wrens: Effects of varying brood size and consequences for offspring. Behavioral Ecology, 25, 1485–1493. [Google Scholar]
- Bowers EK, Sakaluk SK, Thompson CF (2012). Experimentally increased egg production constrains future reproduction of female house wrens. Animal Behaviour, 83, 495–500. [Google Scholar]
- Bowers EK, Sakaluk SK, & Thompson CF (2013). Sibling cooperation influences the age of nest leaving in an altricial bird. American Naturalist, 181, 775–786. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Smith RA, Hodges CJ, Zimmerman LM, Thompson CF, & Sakaluk SK (2012). Sex-biased terminal investment in offspring induced by maternal immune challenge in the house wren (Troglodytes aedon). Proceedings of the Royal Society B: Biological Sciences, 279, 2891–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkhof MWG, Cavé AJ, Daan S, & Perdeck AC (2002). Timing of current reproduction directly affects future reproductive output in European coots. Evolution, 56, 400–411. [DOI] [PubMed] [Google Scholar]
- Chalfoun AD, & Martin TE (2007). Latitudinal variation in avian incubation attentiveness and a test of the food limitation hypothesis. Animal Behaviour, 73, 579–585. [Google Scholar]
- DeMory ML, Thompson CF, & Sakaluk SK (2010). Male quality influences male provisioning in house wrens independent of attractiveness. Behavioral Ecology, 21, 1156–1164. [Google Scholar]
- Dobbs RC, Styrsky JD, & Thompson CF (2006). Clutch size and the costs of incubation in the house wren. Behavioral Ecology, 17, 849–856. [Google Scholar]
- Dorset EE, Sakaluk SK, & Thompson CF (2017). Behavioral plasticity in response to perceived predation risk in breeding house wrens. Evolutionary Biology, 44, 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drent RH, & Daan S (1980). The prudent parent: Energetic adjustments in avian breeding. Ardea, 68, 225–252. [Google Scholar]
- Drilling NE, & Thompson CF (1988). Natal and breeding dispersal in house wrens (Troglodytes aedon). Auk, 105, 480–491. [Google Scholar]
- Drilling NE, & Thompson CF (1991). Mate switching in multibrooded house wrens. Auk, 108, 60–70. [Google Scholar]
- Finke MA, Milinkovich DJ, & Thompson CF (1987). Evolution of clutch size: An experimental test in the house wren (Troglodytes aedon). Journal of Animal Ecology, 56, 99–114. [Google Scholar]
- Harper RG, Juliano SA, & Thompson CF (1992). Hatching asynchrony in the house wren, Troglodytes aedon: A test of the brood-reduction hypothesis. Behavioral Ecology, 3, 76–83. [Google Scholar]
- Hepp GR, DuRant SE, & Hopkins WA (2015). Influence of incubation temperature on offspring phenotype and fitness in birds In Deeming DC & Reynolds SJ (Eds.), Nests, eggs, and incubation: New ideas about avian reproduction (pp. 171–178). Oxford, U.K.: Oxford University Press. [Google Scholar]
- Hodges CJ, Bowers EK, Thompson CF, & Sakaluk SK (2015). Cascading costs of reproduction in female house wrens induced to lay larger clutches. Journal of Evolutionary Biology, 28, 1383–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J, & Hodgson D (2010). What is fitness, and how do we measure it? In Westneat DE & Fox CW (Eds.), Evolutionary behavioral ecology (pp. 46–70). Oxford, U.K: Oxford University Press. [Google Scholar]
- Johnson LS (2014). House wren (Troglodytes aedon) In Rodewald PG (Ed.), The birds of North America. Ithaca, NY: Cornell Lab of Ornithology; Retrieved from the Birds of North America: https://birdsna.org/Species-Account/bna/species/houwre. doi: 10.2173/bna.380. [DOI] [Google Scholar]
- Jönsson KI (1997). Capital and income breeding as alternative tactics of resource use in reproduction. Oikos, 78, 57–66. [Google Scholar]
- Kaluthota CD, & Rendall D (2017). Nest site selection and breeding biology of house wrens (Troglodytes aedon parkmanii) using natural cavities in western Canada. Canadian Journal of Zoology, 95, 505–514. [Google Scholar]
- Lambrechts MM, Adriaensen F, Ardia DR, Artemyev AV, Atiénzar F, Bańbura J, et al. (2010). The design of artificial nestboxes for the study of secondary hole-nesting birds: A review of methodological inconsistencies and potential biases. Acta Ornithologica, 45, 1–26. [Google Scholar]
- Lothery CJ, Thompson CF, Lawler ML, & Sakaluk SK (2014). Food supplementation fails to reveal a trade-off between incubation and self-maintenance in female house wrens. PLoS One, 9, e106260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MT, Chutter CM, & Redmond LJ (2015). Quantification of avian parental behavior: What are the minimum necessary sample times? Journal of Field Ornithology, 86, 41–50. [Google Scholar]
- Nakagawa S, & Cuthill IC (2007). Effect size, confidence interval and statistical significance: A practical guide for biologists. Biological Reviews, 82, 591–605. [DOI] [PubMed] [Google Scholar]
- Nilsson JF, Stjernman M, & Nilsson J-Å (2008). Experimental reduction of incubation temperature affects both nestling and adult blue tits Cyanistes caeruleus. Journal of Avian Biology, 39, 553–559. [Google Scholar]
- Nord A, & Williams JB (2015). The energetic costs of incubation In Deeming DC & Reynolds SJ (Eds.), Nests, eggs, and incubation: New ideas about avian reproduction (pp. 152–170). Oxford, U.K: Oxford University Press. [Google Scholar]
- Pearse AT, Cavitt JF, & Cully JF (2004). Effects of food supplementation on female nest attentiveness and incubation mate feeding in two sympatric wren species. Wilson Bulletin, 116, 23–30. [Google Scholar]
- Pérez JH, Ardia DR, Chad EK, & Clotfelter ED (2008). Experimental heating reveals nest temperature affects nestling condition in tree swallows (Tachycineta bicolor). Biology Letters, 4, 468–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JM, Monaghan P, & Ruxton GD (2000a). The consequences of clutch size for incubation conditions and hatching success in starlings. Functional Ecology, 14, 560–565. [Google Scholar]
- Reid JM, Monaghan P, & Ruxton GD (2000b). Resource allocation between reproductive phases: The importance of thermal conditions in determining the cost of incubation. Proceedings of the Royal Society of London B: Biological Sciences, 267, 37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JM, Arcese P, Bocedi G, Duthie AB, Wolak ME, & Keller LF (2015). Resolving the conundrum of inbreeding depression but no inbreeding avoidance: Estimating sex-specific selection on inbreeding by song sparrows (Melospiza melodia). Evolution, 69, 2846–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JM, Monaghan P, & Nager RG (2002). Incubation and the costs of reproduction In Deeming DC (Ed.), Avian incubation: Behaviour, environment, and evolution (pp. 314–325). Oxford, U.K.: Oxford University Press. [Google Scholar]
- Rosenthal R (1994). Parametric measures of effect size In Cooper H & Hedges LV (Eds.), The handbook of research synthesis (pp. 231–244). New York, NY: Russell Sage Foundation. [Google Scholar]
- Sokal RR, & Rohlf FJ (1995). Biometry (3rd ed.). New York, NY: W. H. Freeman. [Google Scholar]
- Thomson DL, Monaghan P, & Furness RW (1998). The demands of incubation and avian clutch size. Biological Reviews, 73, 293–304. [Google Scholar]
- Tinbergen JM, & Williams JB (2002). Energetics of incubation In Deeming DC (Ed.), Avian incubation: Behaviour, environment, and evolution (pp. 299–313). Oxford, U.K.: Oxford University Press. [Google Scholar]
- Verhulst S, & Nilsson J-Å (2008). The timing of birds’ breeding seasons: A review of experiments that manipulated timing of breeding. Philosophical Transactions of the Royal Society B: Biological Sciences, 363, 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will DS, Dorset EE, Thompson CF, Sakaluk SK, & Bowers EK (2017). Size of nest-cavity entrance influences male attractiveness and paternal provisioning in house wrens. Journal of Zoology, 302, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf JB, & Wade MJ (2001). On the assignment of fitness to parents and offspring: Whose fitness is it and when does it matter? Journal of Evolutionary Biology, 14, 347–356. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


