Abstract
Background
Previous studies suggested that inhibition of apoptosis prevents the dysfunction of ischemia-reperfusion injury. In the pathogenesis of ischemia-reperfusion injury, JNK/ERK1/2 and p38 play an essential role in regulation of cell apoptosis. Electroacupuncture (EA), a form of acupuncture, has demonstrated superiority in preventing ischemia-reperfusion injury, but the underlying mechanism is unclear. In the present study, we explored the effects of electroacupuncture at Shenting (GV24) and Baihui (GV20) acupoints on focal cerebral ischemia-reperfusion (MCAO) rats, and explored whether JNK/ERK1/2- and p38-mediated cell apoptosis are involved.
Material/Methods
The rats were divided into a sham operation control group, an ischemia group, and an electroacupuncture group with acupuncture applied for 10 days (30 min per day). TTC staining was used to calculate the ischemic brain volume. TUNEL staining and transmission electron microscopy were used to detect cell apoptosis. Western blot analysis and Bio-Plex were used to detect JNK, p38, ERK1/2, Bcl-2, and Bax protein expression.
Results
We found that electroacupuncture at day 10 significantly reduced cerebral infarction. In addition, electroacupuncture suppressed activation of JNK and p38, while enhancing the activation of ERK1/2 in the peri-ischemic regions. Consequently, the effect of electroacupuncture on these pathways resulted in the inhibition of apoptosis, which was demonstrated by TUNEL and transmission electron microscopy. We found that electroacupuncture upregulated the anti-apoptotic Bcl-2/Bax ratio in peri-ischemic regions.
Conclusions
Our findings suggest that inhibition of cell apoptosis via regulating multiple signaling pathways might be a mechanism whereby electroacupuncture has a positive therapeutic effect on post-stroke impairment.
MeSH Keywords: Acupuncture, Apoptosis Regulatory Proteins, Stroke
Background
Apoptosis, a genetically programmed cell death process, can be regulated by pro- and anti-apoptotic proteins such as the Bcl-2 family and Fas system [1,2] and plays an important role in the progression of cerebral ischemia-reperfusion injury. Several studies have confirmed that the inhibition cell apoptosis can reduce ischemia-reperfusion injury [3,4]. In the mitochondrial signaling pathway, Bcl-2 family members play a key role in regulating apoptosis, and changes in the balance of Bcl-2 and Bax may result in either inhibition or induction of cell apoptosis [5,6]. There are many activating signaling pathways that regulate apoptosis during post-stroke, such as the ERK1/2, JNK, and p38 signaling pathways [7–9].
Ischemia-reperfusion injury causes overproduction of free radicals, which can include triggers of apoptosis by activating lipid peroxidation and mediating Ca2+ channel signals, altering membrane phospholipids [10]. In the mitochondrial signaling pathway, overproduction of free radicals is followed by release of apoptotic proteins such as cytochrome c (CytC) into the cytosol. CytC interacts with the apoptotic protease to form an apoptosome, which serves as a scaffold to bring the initiator caspase molecules together. Caspase-9 and caspase-8 are activated in the pathway, leading to cleavage and activation of procaspase-3 [11–13], which is one of the important apoptosis-related regulatory factors. In the process, the Bcl-2 protein family plays an important role in regulating apoptosis [5]. Many apoptosis-regulating signaling pathways are activated during cerebral ischemia-reperfusion, such as ERK1/2, JNK, and p38 signaling pathways. These pathways play a key role in regulating apoptosis [14–16]. ERK is a signaling molecule that activates gene expression [17]. Many studies have reported that ERK1/2 activation promotes cerebral cell proliferation and regulates ischemia-induced apoptosis by adjusting Bcl-2 family expression [18,19]. C-Jun N-terminal kinases (JNK) also play an important role in apoptosis by interacting with the mitochondrial apoptotic machinery [20,21]. p38 is responsive to stress stimuli and is involved in cell apoptosis [22] through activation of poly-ADP-ribose polymerase (PARP) and caspase-9-mediated mechanisms [23]. These signaling pathways regulate apoptosis, which mainly involves the Bcl-2 family, such as Bcl-2/Bax proteins, which plays an important role in anti-apoptosis.
In preclinical studies, activation of anti-apoptotic mechanisms has become a promising strategy for treatment of post-stroke impairment [24]. In Traditional Chinese Medicine (TCM), acupuncture has relatively significant benefits and play an important role in the treatment of central nervous system diseases [25]. Electroacupuncture (EA), with electro-stimulation [26] in which 2 needles are inserted as electrodes to pass an electric current, is one style of acupuncture [27]. EA has the advantage of setting stimulation frequency and intensity objectively and quantifiably. Previous study have suggested that, compared to manual acupuncture, EA was more effective in some situations, particularly when strong, continued stimulation is required [28]. It has been reported that EA can produce more widespread functional magnetic resonance imaging signal increase in anterior middle cingulate cortex [29]. Wu et al. have found that, compared to sham EA, real EA elicited significantly higher activation over the hypothalamus and primary somatosensory-motor cortex and deactivation over the rostral segment of anterior cingulate cortex [30]. Shenting (GV24) and Baihui (GV20) are Du Meridian acupoints and play a crucial in the nervous system modulation in TCM. It is generally believed that the physiological effects of electroacupuncture are caused by the mechanical or electrical stimulation of the autonomic nervous system via induction of the somatosensory pathway or release of neuroactive mediators from immune cells [31].
Many studies have shown that acupuncture at Baihui and Shenting acupoints can significantly improve impaired functions such as motor control and cognition of post-stroke patients [32]. Our previous study indicated that electroacupuncture at Baihui and Shenting can improve neurological deficit scores and ameliorate cognitive impairment in ischemic rats via inhibiting NF-κB-mediated neuronal cell apoptosis, which ameliorated cognitive impairment in ischemic rats [33]. However, since TCMs are considered to be multi-component and multi-targeted agents [34], it is unclear whether other signaling pathways are activated. In this study, we used an MCAO model to investigate the mechanisms underlying the effects of electroacupuncture at Baihui and Shenting acupoints. In additional, Huang et al. conducted a systematic review and found mild stimulation, which means the reinforcing method in TCM theory is adopted widely including improving functional impairment of post-stroke [35]. Shu et al. found EA at GV26 acupoints and right sulcus auriculae posterior with the frequency of 2 and 20 Hz for 30 min, alternatively (disperse-dense waves), and intensity strong enough to only elicit slight twitches of the orofacial areas can significantly decreased neurological deficit scores and the volume of cerebral infarction, and relax the autophagy and apoptosis in cerebral ischemia reperfusion injury rats [36]. Our previous study suggested electroacupuncture at Shenting and Baihui with the parameter of 30 min/d and disperse-dense waves of 1/20 Hz of frequency can significantly downregulated the expression of Nogo-A and NgR in the hippocampus of the cerebral ischemia side [37], improve neurological deficit score, and reduce cerebral infarct volumes in cerebral ischemia-reperfusion injured rats [38,39]. In our previous study, we also found the number of times that rats crossed the location of the platform in the Morris water maze test was significantly increased compared to the MCAO group, which suggests that electroacupuncture ameliorated cognitive impairment in cerebral ischemia-reperfusion–injured rats by EA at Shenting (GV24) and Baihui (GV20) with the above parameter for 10 days, and also significantly suppressed the ischemia-reperfusion induced activation of NF-κB signaling in ischemic cerebral tissues [39]. Therefore, EA at Shenting and Baihui with the parameter of 30 min/d and disperse-dense waves of 1/20 Hz of frequency for 10 days may be an effective parameter to improve post-stroke dysfunction. In the present study, we used electroacupuncture using the above parameter to explore the underlying mechanisms. We hypothesized that electroacupuncture at Shenting (GV24) and Baihui (GV20) acupoints inhibits cell apoptosis in cerebral ischemia-reperfusion injury rats via modulating p38, ERK½, and JNK.
Material and Methods
Material and reagents
TUNEL assay kits were obtained from Promega (Madison, WI, USA). All antibodies and horseradish peroxidase (HRP)-conjugated secondary antibodies were provided by Cell Signaling Technology, Inc (Beverly, MA, USA). Bio-Plex phosphoprotein assay kits were purchased from Bio-Rad Laboratories (Hercules, CA, USA). 2,3,5-Triphenyltetrazolium chloride (TTC) and all the additional chemicals used, unless otherwise stated, were obtained from Sigma Chemicals (St. Louis, MO, USA).
Animals and ethics
Sixty adult male Sprague–Dawley rats (250–280 g) were supplied by SLAC Laboratory Animal Co., Ltd. (Shanghai, China) and were housed in specific pathogen-free rooms in the Central Laboratory of Fujian University of Traditional Chinese Medicine (Fuzhou, China) at 22°C with a 12–12 h light/dark cycle. Food and water were provided freely. The rats were randomly divided into a sham operation control group (sham group, n=20) and an ischemia group (n=40) by use of a computer-generated randomization schedule. All animals were treated strictly according to the National Institutes of Health guide concerning the Care and Use of Laboratory Animals and international ethical guidelines. The study was approved by the Institutional Animal Care and Use Committee of Fujian University of Traditional Chinese Medicine (Ethics Committee reference number: Fujian university of Traditional Chinese Medicine [2015] Ethics Committee reference number [008]; Fuzhou, China).
The middle cerebral artery occlusion model (MCAO), which was develop by Longa et al. as a reliable method for studying reversible regional ischemia in rats without craniectomy model [40], has been widely used in ischemia-reperfusion injury studies [41,42]. In the present study, we established the cerebral ischemia-reperfusion model by MCAO. Briefly, all rats were anesthetized with 10% chloral hydrate (300 mg/Kg; i.p.). The left common carotid artery (CCA), the left external carotid artery (ECA), and the internal carotid artery (ICA) were exposed via a neck incision. The ECA was blocked proximal to the branch of the ECA and ICA. The middle cerebral artery (MCA) was occluded with an embolus made from fishing line with 0.28–0.30-mm diameter through a small incision in the ICA. Reperfusion was achieved by removing the line after 120 min. The body temperatures of the animals were maintained at 37°C during surgery. Only the CCA, ICA, and ECA were isolated, without endovascular occlusion in the sham group.
Twenty-four hours after reperfusion, the rats were evaluated in blinded fashion using the neurological deficit score, which is used to exam the neurologic function of the rats after MCAO [40]: 0=no deficit, 1=failure to extend the right forepaw fully, 2=circling to the right, 3=falling to the right, and 4=no walking and depressed level of consciousness. The rats with scores of 1–3 were included into the experiment and were randomly divided into the ischemia control group (MCAO group, n=19) or the electroacupuncture group (EA+MCAO group, n=19) (1 rat was excluded due to the neurological deficit score=0 and 1 rat died during the operation) by use of a computer-generated randomization schedule. In the EA group, electroacupuncture at Shenting (GV24) and Baihui (GV20) acupoints with dense disperse wave (1 or 20 HZ, 1mA, adjusted to the muscle twitch threshold) was performed for 10 days (30 min per day) after the MCAO operation with the electroacupuncture apparatus (SDZ-II, Huatuo, Suzhou, China). In the MCAO and the sham groups, no intervention was given to the rats. There were 56 rats at the end of the experiment (n=20 in sham group, n=17 in MCAO group, n=19 in EA+MCAO group), and 2 rats died during the experiment in MCAO group. Four rats each group were used for TTC staining, 4 rats in each group were used for TUNEL staining, 4 rats each group were used for transmission electron microscopy, and 4 rats each group were used in both Western blotting analysis and Bio-Plex phosphoprotein assay.
Biochemical staining
TTC staining
Triphenyltetrazole oxide (TTC) staining was used to evaluate the cerebral infarction area from the morphological level. Ten days after the operation, the rats were anesthetized (i.p.) with 10% chloral hydrate, then the anesthetized animals were sacrificed by cervical dislocation. The brains were quickly removed and sectioned into five 2-mm coronal slices, and stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC) at 37°C for 20 min and fixed with 10% buffered formalin solution at 4°C, followed by scanning with a high-definition camera (Canon SX20, Tokyo, Japan). The infarct volume was determined with the Motic Med 6.0 System (Motic, China). The percentage of ischemic brain was calculated by taking the volume of infarcted ischemia, dividing by the total brain volume, and multiplying by 100.
TUNEL staining
In the present study, we used the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL), a method for detecting DNA fragmentation by labeling the 3′-hydroxyl termini in the double-strand DNA breaks generated during apoptosis, which is widely used to identify and quantify apoptotic cells [43,44] to examine effects of EA on the apoptosis level of the MCAO rats. The peri-ischemic region brain tissues were fixed with 4% paraformaldehyde and cut into 5-μm–thick coronal sections after being paraffin-embedded. The nuclei of all cells were stained by DAPI and the green fluorescence of apoptotic cells were detected using a confocal fluorescence Carl Zeiss microscope (LSM 710, Thornwood, NY, USA), according to the TUNEL assay kit manufacturer’s protocol. Apoptotic cells were counted in 4 randomly selected areas at a magnification of 400×, and the percent apoptotic cells were calculated as the ratio of green-stained cells to the blue fluorescence cells.
Transmission electron microscopy
Transmission electron microscopy was used to observe cellular and ultrastructural changes of neurons after EA intervention. The fixative-perfused brain specimens were fixed with a mixture of 5% glutaraldehyde and 10% paraformaldehyde for 72 h at 4°C, followed by 1% osmium tetroxide in cacodylate buffer for 2 h at room temperature. After washing with PBS, the samples were dehydrated with the standard samples and embedded in an Epon-Araldite 618 mixture. The brain was sectioned into 80-nm modular ultrathin slices and observed with a H70 electron microscope operating at 80 kV. The photos were obtained on an HP digital CCD camera (SIS4million Volex).
Western blotting analysis
To qualitatively assess the effective of EA on the BCL-2 and Bax protein expression, which plays a key role in the progress of the modulation of post-stroke, we used Western blotting analysis. After total protein of the peri-ischemic region brains tissues was collected and quantified by bicinchoninic acid (BCA) assay, the lysates were separated by SDS-PAGE gels and transferred onto PVDF. The membrane was then incubated with primary antibodies against Bcl-2, Bax, and β-actin (1: 1000; Cell Signaling Technology) overnight at 4°C. A computer scan was performed and processed by use of the Bio-Image Analysis System (Bio-Rad, Hercules, CA, USA) after the corresponding secondary antibody (1: 5000) was added (n=4/group).
Bio-Plex phosphoprotein assay
In the present study, we used the Bio-Plex phosphoprotein assay to test the phosphoprotein level of ERK1/2, JNK, and p38. Compared to other protein detection methods, the Bio-Plex assays was shown to be robust and reliable and can reduce sample volume and time of experiment [45]. After the total protein of peri-ischemic hippocampal tissue of the ischemic side was collected and quantified by bicinchoninic acid (BCA) assay, the presence of p-ERK1/2, p-JNK, and p-p38 was detected using a bead-based multiplex assay for phosphoproteins (Bio-Plex Phosphoprotein assay, Bio-Rad Laboratories). Data were collected and analyzed using the Bio-Plex 200 suspension array system (Bio-Rad).
Statistical analysis
Data are presented as means with standard errors of the mean (mean ±SEM). All data were analyzed using IBM SPSS 24.0 (IBM Inc., Armonk, NY, USA) statistics software. Statistical analysis was performed with the t test and one-way analysis of variance (ANOVA). We performed post hoc analysis by Fisher’s least significant difference (LSD) or Games-Howell test. Statistical significance was determined by p<0.05.
Results
Effects of electroacupuncture at Baihui and Shenting acupoints on cerebral infarct volumes
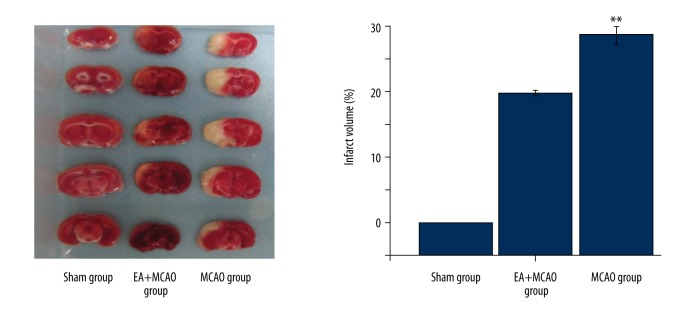
As shown in Figure 1, the cerebral infarct volumes in the MCAO and EA+MCAO groups were significantly increased compared to the sham group (p<0.01), indicating successful development of an I/R injury model. At day 10, there were significant decreases in cerebral infarction volumes of the EA+MCAO group (p<0.05) (p<0.05, vs. the MCAO group), showing that EA provided significant therapeutic efficacy in preventing cerebral I/R injury.
Figure 1.
Effects of electroacupuncture at Baihui and Shenting on the cerebral infarction in injured rats. The cerebral infarct volumes in the MCAO group and EA+MCAO group were significantly lower, while EA treatment for 10 days significantly reduced the cerebral infarction volumes (p<0.01, vs. the MCAO group) (*, EA+MCAO group vs. MCAO group; ** p<0.01) (n=4 in each group).
Effects of electroacupuncture at Baihui and Shenting acupoints on inhibiting apoptosis
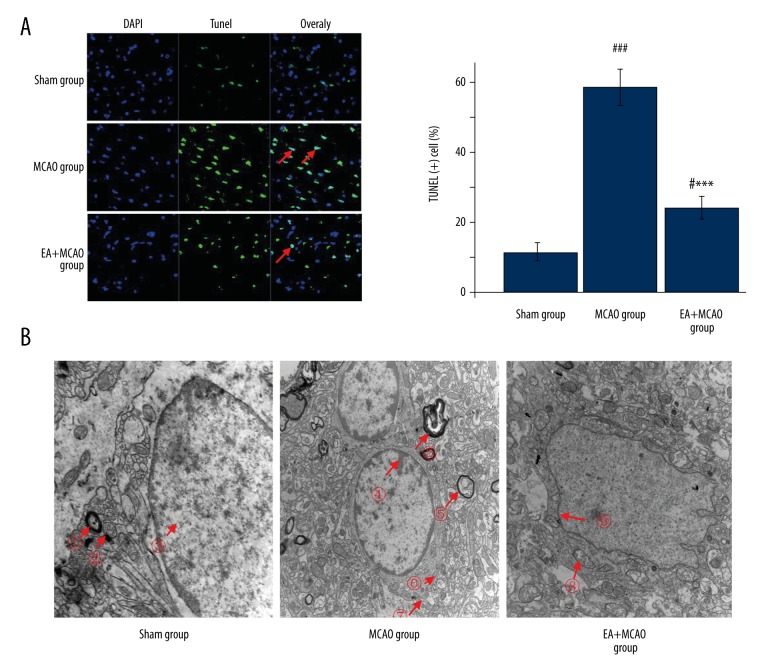
As shown in Figure 2A, the percentage of TUNEL-positive cells in the peri-ischemic region cortex in the MCAO group (70±0.8%) was significantly increased compared to the sham group (11±0.5%), indicating that ischemia-reperfusion can induce apoptosis in cells by day 10. The percentages were significantly decreased (21±0.9%) in the EA+MCAO group, suggesting the anti-apoptotic activity of EA in vivo.
Figure 2.
Effects of electroacupuncture on inhibiting cell apoptosis. (A) The percentage of TUNEL-positive cells in the peri-ischemic regions in the MCAO group was significantly increased compared with the sham group (p<0.001), However, the percentage was significantly decreased in the EA +MCAO group (p<0.001). (n=4 in each group; *, vs. MCAO group; ***p<0.001; #, vs. sham group; # p<0.05/### p<0.001. (B) In the sham group, ? large axon; ? mitochondria; ? chromatin. In the MCAO group, ? chromatin; ? cell myelin; ? mitochondria; ? cells mitochondria. In the EA+MCAO group, ? mitochondria; ? chromatin (n=4 in each group).
As shown by the SEM in Figure 2B in the sham group, there were abundant mitochondria and intact mitochondrial crests were found. The large axon can be seen and the chromatin was uniformly distributed. In the MCAO group, the euchromatin was found at the edge of the neuronal nuclei, the cells appeared smaller than in the sham group, and the electronic density was deep, which are the early apoptotic signals. In the EA+MCAO group, the cell membrane was partly ruptured and the mitochondria were slightly damaged. No changes in the rough endoplasmic reticulum and axon were observed in the EA+MCAO group. The cell nucleoli were complete and chromatin was uniformly distributed.
Effects of electroacupuncture at Baihui and Shenting acupoints at the translational level of Bcl-2 and Bax
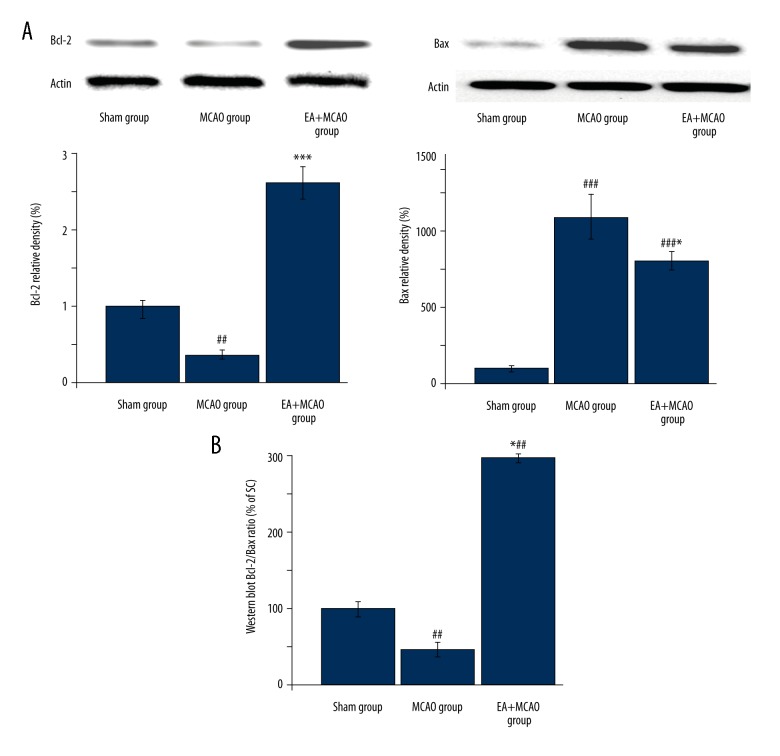
To further explore the mechanism of the anti-apoptotic effect of EA, we evaluated the protein levels of Bcl-2 and Bax, which are essential anti-apoptotic and pro-apoptotic mediators. As shown in Figure 3A and 3B, EA treatment profoundly upregulated the Bcl-2/Bax ratio at translational levels after cerebral injury (vs. MCAO group).
Figure 3.
Electroacupuncture modulated the apoptosis factors of Bcl-2 and Bax in injured rats. At translational levels, EA treatment profoundly inhibited the model construction-mediated down-regulation of Bcl-2 and Bax and the Bcl-2/Bax ratio (data are expressed as the percentage of the value of sham group) (*, vs. MCAO group; * p<0.05/*** p<0.001; #, vs. sham group; ## p<0.01/### p<0.001) (n=4 in each group).
Effects of electroacupuncture at Baihui and Shenting acupoints on the levels of ERK1/2, JNK, and p38 in MCAO rats
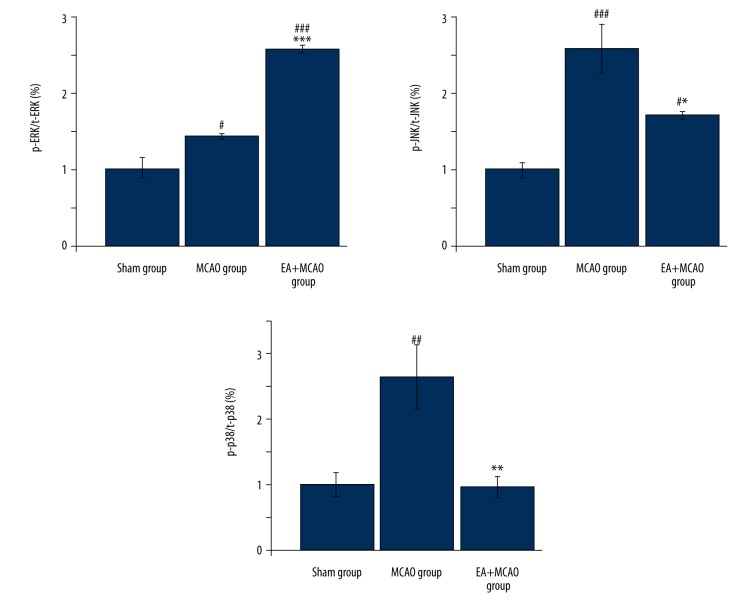
To further investigate the mechanism of the anti-apoptotic activity of electroacupuncture, we investigated the phosphorylation level of ERK1/2, JNK, and p38 in the peri-ischemic region cortex. As shown in Figure 4, the phosphorylation level of ERK1/2 (the ratio of phosphorylation ERK1/2 and total ERK 1/2, p-ERK1/2/t-ERK1/2) was significantly increased, whereas that of JNK (p-JNK/t-JNK) and p38 (p-p38/t-p38) was significantly reduced (Figure 4, p<0.05, vs. MCAO group). These data suggest that EA modulated the activation of multiple apoptotic signaling pathways.
Figure 4.
Electroacupuncture at Baihui and Shenting modulated ERK, JNK, and p38 pathways in focal cerebral ischemia-reperfusion (MCAO) rats. After EA treatment, the phosphorylation level of ERK1/2 (p-ERK1/2/t-ERK1/2) in peri-ischemic regions was significantly increased, whereas that of JNK (p-JNK/t-JNK) and p38 (p-p38/t-p38) was significantly reduced (n=4 in each group) (*, vs. MCAO group; * p<0.05/** p<0.01/*** p<0.001; #, vs. sham group; # p<0.05/## p<0.01/### p<0.001).
Discussion
In the present study we investigated the therapeutic efficacy of EA at Shenting and Baihui acupoints after ischemia-reperfusion injury and explored the possible mechanism by which it affects apoptosis. We found that EA significantly decreased cerebral suppressed activation of the JNK and p38 signaling pathways while enhancing the activation of ERK1/2. Consequently, the effect of electroacupuncture at Baihui and Shenting acupoints on these pathways resulted in the inhibition of apoptosis, which was demonstrated by transmission electron microscopy and Western blot analysis.
Electroacupuncture, a mainstay treatment in TCM for a variety of diseases, is a special form of acupuncture therapy, and is based on traditional acupuncture [46–48]. Compared with traditional acupuncture, electroacupuncture has advantages of being readily controlled, standardized, and objectively measured. Electroacupuncture preconditioning attenuates ischemic brain injury by activation of the adenosine monophosphate-activated protein kinase signaling pathway. Several studies showed that the physiological effects of electroacupuncture are caused by the mechanical and electrical stimulation of the autonomic nervous system via induction of robust axon reflex or release neuroactive mediators from immune cells [49,50]. Baihui and Shenting, 2 important acupoints of the Du meridian, have been demonstrated in the improvement in post-stroke functional impairment [51], which was also confirmed by our previous study [33]. Based on Traditional Chinese Medical theory, Du meridians are associated with the regulation of a number of brain functions and are used to treat brain disease [53,54]. The Baihui acupoint has 3 Yang and 5 convergences, and Shenting is the convergence of qi and blood, and both of them can activate spirit and resuscitate the brain in Traditional Chinese Medicine [55].
Oxidative stress after stroke leads to an increase in reactive species production, resulting in DNA fragmentation, and is thought to be one of the major factors that induce apoptosis in the peri-ischemic region [56]. Apoptosis in the ischemic penumbra or peri-infarct region play a key role in the progress of cerebral ischemia-reperfusion injury [57]. After acute ischemic stroke, oxidative stress immediately occurred and then oxidative stress induced lipid peroxidation and mediating Ca2+ channel signals. Apoptotic proteins such as cytochrome c (CytC) are released into the cytosol, which can interacts with the apoptotic protease to form an apoptosome, and finally results in neuronal apoptosis in post-ischemic stroke [58,59]. In the present study, we showed that the number of TUNEL-positive apoptotic cells and the phenomenon of small cells and the electronic density deep in the peri-inchemic regions were increased after MCAO and were inhibited by electroacupuncture. This indicates that electroacupuncture might inhibit apoptosis.
To further explore the underlying anti-apoptotic mechanism of electroacupuncture, we examined the effects of EA on several signaling pathways that modulate apoptosis after ischemia-reperfusion injury. Ischemia and reperfusion generate large quantities of ROS products, which activates a number of signaling pathways such as ERK, JNK, and p38 [60]. These pathways play a key role in regulating apoptosis. ERK1/2/C-Jun N-terminal kinases (JNKs) and the p38 mitogen-activated protein kinases activation play a major role in ischemia-induced apoptosis through regulation of Bax/Bcl-2/Bcl-xL expression [61,62]. In the present study, ERK1/2, JNK, and p38 pathways were activated after cerebral I/R injury, which was demonstrated by Bio-Plex analysis. The results are consistent with previous results [62], while EA significantly further upregulated ERK1/2 in I/R-injured brain tissues and inhibited JNK and p38 activation. Activation of these signaling pathways upregulated the ratio of Bcl-2/Bax, which plays an important anti-apoptotic role. The results demonstrated that inhibiting multiple cellular pathways regulating cell apoptosis is one of the possible mechanisms by which electroacupuncture reduces injury in post-stroke rats.
Conclusions
This study showed possible mechanisms by which electroacupuncture at GV20 and GV24 promotes functional recovery in post-stroke rats. Electroacupuncture mediates neuronal cell apoptosis via multiple cellular pathways such as JNK, ERK, and P38. Further studies are needed to determine other protective mechanisms of electroacupuncture.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by the Natural Science Foundation of China (No. 81373778 and No. 81273835). It was also supported by the Fujian Collaborative Innovation Center for Rehabilitation Technology, Traditional Chinese Medicine Rehabilitation Research Center of State Administration of Traditional Chinese Medicine and National-Local Joint Engineering Research Center of Rehabilitation Medicine Technology
References
- 1.Siddiqui WA, Ahad A, Ahsan H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update. Arch Toxicol. 2015;89(3):289–317. doi: 10.1007/s00204-014-1448-7. [DOI] [PubMed] [Google Scholar]
- 2.Villa-Morales M, Cobos MA, González-Gugel E, et al. FAS system deregulation in T-cell lymphoblastic lymphoma. Cell Death Dis. 2014;5:e1110. doi: 10.1038/cddis.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, Jiang X, Zhang Q, et al. Neuroprotective effects of Kukoamine A against cerebral ischemia via antioxidant and inactivation of apoptosis pathway. Neurochem Int. 2017;107:191–97. doi: 10.1016/j.neuint.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 4.Li P, Shen M, Gao F, et al. An antagomir to microRNA-106b-5p ameliorates cerebral ischemia and reperfusion injury in rats via inhibiting apoptosis and oxidative stress. Mol Neurobiol. 2016;54(4):2901–21. doi: 10.1007/s12035-016-9842-1. [DOI] [PubMed] [Google Scholar]
- 5.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21(1):92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashkenazi A, Fairbrother WJ, Leverson JD, Souers AJ. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat Rev Drug Discov. 2017;16:273–84. doi: 10.1038/nrd.2016.253. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Zhang H, Xu X, et al. BFGF inhibits ER stress induced by ischemic oxidative injury via activation of the PI3K/Akt and ERK1/2 pathways. Toxicol Lett. 2012;212(2):137–46. doi: 10.1016/j.toxlet.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Fan J, Li X, Yan YW, et al. Curcumin attenuates rat thoracic aortic aneurysm formation by inhibition of the c-Jun N-terminal kinase pathway and apoptosis. Nutrition (Burbank, Los Angeles County, Calif) 2012;28(10):1068–74. doi: 10.1016/j.nut.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Zhou H, Yang WS, Li Y, et al. Oleoylethanolamide attenuates apoptosis by inhibiting the TLR4/NF-κB and ERK1/2 signaling pathways in mice with acute ischemic stroke. Naunyn Schmiedebergs Arch Pharmacol. 2017;390(1):77–84. doi: 10.1007/s00210-016-1309-4. [DOI] [PubMed] [Google Scholar]
- 10.Fan W, Dai Y, Xu H, et al. Caspase-3 modulates regenerative response after stroke. Stem Cells. 2014;32(2):473–86. doi: 10.1002/stem.1503. [DOI] [PubMed] [Google Scholar]
- 11.Glushakova OY, Glushakov AA, Wijesinghe DS, et al. Prospective clinical biomarkers of caspase-mediated apoptosis associated with neuronal and neurovascular damage following stroke and other severe brain injuries: Implications for chronic neurodegeneration. Brain Circulation. 2017;3(2):87–108. doi: 10.4103/bc.bc_27_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estaquier J, Vallette F, Vayssiere JL, Mignotte B. The mitochondrial pathways of apoptosis. Adv Exp Med Biol. 2012;942:157–83. doi: 10.1007/978-94-007-2869-1_7. [DOI] [PubMed] [Google Scholar]
- 13.Hu Q, Wu D, Chen W, et al. Proteolytic processing of the caspase-9 zymogen is required for apoptosome-mediated activation of caspase-9. J Biol Chem. 2013;288(21):15142–47. doi: 10.1074/jbc.M112.441568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu X, Li L, Zhang L, et al. Inhibition of thioredoxin-1 with siRNA exacerbates apoptosis by activating the ASK1-JNK/p38 pathway in brain of a stroke model rats. Brain Res. 2015;1599:20–31. doi: 10.1016/j.brainres.2014.12.033. [DOI] [PubMed] [Google Scholar]
- 15.Lu Z, Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life. 2006;58:621–31. doi: 10.1080/15216540600957438. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson BS, Harrison BC, Jeong MY, et al. Signal-dependent repression of DUSP5 by class I HDACs controls nuclear ERK activity and cardiomyocyte hypertrophy. Proc Natl Acad Sci USA. 2013;110(24):9806–11. doi: 10.1073/pnas.1301509110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan Y, Guo Q, Ye Z, et al. Ischemic postconditioning protects brain from ischemia/reperfusion injury by attenuating endoplasmic reticulum stress-induced apoptosis through PI3K-Akt pathway. Brain Res. 2011;1367:85–93. doi: 10.1016/j.brainres.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Feng D, Wang B, Wang L, et al. Pre-ischemia melatonin treatment alleviated acute neuronal injury after ischemic stroke by inhibiting endoplasmic reticulum stress-dependent autophagy via PERK and IRE1 signalings. J Pineal Res. 2017;62(3):e12395. doi: 10.1111/jpi.12395. [DOI] [PubMed] [Google Scholar]
- 19.Liebelt B, Papapetrou P, Ali A, et al. Exercise preconditioning reduces neuronal apoptosis in stroke by up-regulating heat shock protein-70 (heat shock protein-72) and extracellular-signal-regulated-kinase 1/2. Neuroscience. 2010;166(4):1091–100. doi: 10.1016/j.neuroscience.2009.12.067. [DOI] [PubMed] [Google Scholar]
- 20.Chan JY, Luzuriaga J, Maxwell EL, et al. The balance between adaptive and apoptotic unfolded protein responses regulates β-cell death under ER stress conditions through XBP1, CHOP and JNK. Mol Cell Endocrinol. 2015;413:189–201. doi: 10.1016/j.mce.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 21.Zhong M, Ma W, Zhang X, et al. Tetramethyl pyrazine protects hippocampal neurons against anoxia/reoxygenation injury through inhibiting apoptosis mediated by JNK/MARK signal pathway. Med Sci Monit. 2016;22:5082–90. doi: 10.12659/MSM.898921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greggio S, de Paula S, de Oliveira IM, et al. NAP prevents acute cerebral oxidative stress and protects against long-term brain injury and cognitive impairment in a model of neonatal hypoxia-ischemia. Neurobiol Dis. 2011;44(1):152–59. doi: 10.1016/j.nbd.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Quan H, Kim JM, Lee HJ, et al. AICAR enhances the phagocytic ability of macrophages towards apoptotic cells through P38 mitogen activated protein kinase activation independent of AMP-activated protein kinase. PLoS One. 2015;10(5):e0127885. doi: 10.1371/journal.pone.0127885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akpan N, Troy CM. Caspase inhibitors: Prospective therapies for stroke. Neuroscientist. 2013;19(2):129–36. doi: 10.1177/1073858412447875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang A, Wu HM, Tang JL, et al. Acupuncture for stroke rehabilitation. Cochrane Database Syst Rev. 2016;(8):CD004131. doi: 10.1002/14651858.CD004131.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsui P, Leung MCP. Comparison of the effectiveness between manual acupuncture and electro-acupuncture on patients with tennis elbow. Acupunct Electrother Res. 2002;27(2):107–17. doi: 10.3727/036012902816026040. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. Acupuncture: Review and analysis of reports on controlled clinical trials. World Health Organization; 2002. p. 31. [Google Scholar]
- 28.Mayor DF. Electroacupuncture: An introduction and its use for peripheral facial paralysis. Journal of Chinese Medicine. 2007:52–63. [Google Scholar]
- 29.Napadow V, Makris N, Liu J, et al. Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum Brain Mapp. 2005;24(3):193–205. doi: 10.1002/hbm.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu MT, Sheen JM, Chuang KH, et al. Neuronal specificity of acupuncture response: A fMRI study with electroacupuncture. Neuroimage. 2002;16(4):1028–37. doi: 10.1006/nimg.2002.1145. [DOI] [PubMed] [Google Scholar]
- 31.Kim MH, Park YC, Namgung U. Acupuncture-stimulated activation of sensory neurons. J Acupunct Meridian Stud. 2012;5(4):148–55. doi: 10.1016/j.jams.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Zhao L, Zhang FW, Zhang H, et al. [Mild cognitive impairment disease treated with electroacupuncture: A multi-center randomized controlled trial]. Zhongguo Zhen Jiu. 2012;32(9):779–84. [in Chinese] [PubMed] [Google Scholar]
- 33.Feng X, Yang S, Liu J, et al. Electroacupuncture ameliorates cognitive impairment through inhibition of NF-κB-mediated neuronal cell apoptosis in cerebral ischemia-reperfusion injured rats. Mol Med Rep. 2013;7(5):1516–22. doi: 10.3892/mmr.2013.1392. [DOI] [PubMed] [Google Scholar]
- 34.Zhuang Q, Hong F, Shen A, et al. Pien Tze Huang inhibits tumor cell proliferation and promotes apoptosis via suppressing the STAT3 pathway in a colorectal cancer mouse model. Int J Oncol. 2012;40(5):1569–74. doi: 10.3892/ijo.2012.1326. [DOI] [PubMed] [Google Scholar]
- 35.Huang K-Y, Liang S, Yu ML, et al. A systematic review and meta-analysis of acupuncture for improving learning and memory ability in animals. BMC Complement Altern Med. 2016;16(1):297. doi: 10.1186/s12906-016-1298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shu S, Li C-M, You Y-L, et al. Electroacupuncture ameliorates cerebral ischemia-reperfusion injury by regulation of autophagy and apoptosis. Evid Based Complement Alternat Med. 2016;2016:7297425. doi: 10.1155/2016/7297425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jixiang C, Yukun L, Yunan W, et al. [Effects of electroacupuncture on learning and memory and the expression of Nogo-A and NgR in rats with focal cerebral ischemia-reperfusion]. Chinese Journal of Rehabilitation Medicine. 2015;30(3):219–23. [in Chinese] [Google Scholar]
- 38.Jiao L, Chunyan L, Hongwei P, et al. Electroacupuncture attenuates learning and memory impairment via activation of α7nAChR-mediated anti-inflammatory activity in focal cerebral ischemia/reperfusion injured rats. Experimental and Therapeutic Medicine. 2017;14:939–46. doi: 10.3892/etm.2017.4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng X, Yang S, Liu J, et al. Electroacupuncture ameliorates cognitive impairment through inhibition of NF-kappaB-mediated neuronal cell apoptosis in cerebral ischemia-reperfusion injured rats. Mol Med Rep. 2013;7(5):1516–22. doi: 10.3892/mmr.2013.1392. [DOI] [PubMed] [Google Scholar]
- 40.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 41.Gupta S, Gupta YK. Combination of Zizyphus jujuba and silymarin showed better neuroprotective effect as compared to single agent in MCAo-induced focal cerebral ischemia in rats. J Ethnopharmacol. 2017;197:118–27. doi: 10.1016/j.jep.2016.07.060. [DOI] [PubMed] [Google Scholar]
- 42.Loris ZB, Hynton JR, Pieper AA, Dietrich WD. Beneficial effects of delayed P7C3-A20 treatment after transient MCAO in rats. Transl Stroke Res. 2017 doi: 10.1007/s12975-017-0565-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Gorczyca W, Traganos F, Jesionowska H, Darzynkiewicz Z. Presence of DNA strand breaks and increased sensitivity of DNA in situ to denaturation in abnormal human sperm cells: Analogy to apoptosis of somatic cells. Exp Cell Res. 1993;207(1):202–5. doi: 10.1006/excr.1993.1182. [DOI] [PubMed] [Google Scholar]
- 44.Chumboatong W, Thummayot S, Govitrapong P. Neuroprotection of agomelatine against cerebral ischemia/reperfusion injury through an antiapoptotic pathway in rat. Neurochem Int. 2017;102:114–22. doi: 10.1016/j.neuint.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 45.Houser B. Bio-rad’s Bio-Plex® suspension array system, xMAP technology overview. Arch Physiol Biochem. 2012;118:192–96. doi: 10.3109/13813455.2012.705301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang H, Zhao L, Yang S, et al. Clinical observation on effect of scalp electroacupuncture for mild cognitive impairment. J Tradit Chin Med. 2013;33(1):46–50. doi: 10.1016/s0254-6272(13)60099-0. [DOI] [PubMed] [Google Scholar]
- 47.Lei H, Toosizadeh N, Schwenk M, et al. A pilot clinical trial to objectively assess the efficacy of electroacupuncture on gait in patients with Parkinson’s disease using body worn sensors. PLoS One. 2016;11(5):e0155613. doi: 10.1371/journal.pone.0155613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y, Zhou J, Li J, et al. Electroacupuncture pretreatment prevents cognitive impairment induced by limb ischemia-reperfusion via inhibition of microglial activation and attenuation of oxidative stress in rats. Brain Res. 2012;1432:36–45. doi: 10.1016/j.brainres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Zhang ZJ, Wang XM, McAlonan GM. Neural acupuncture unit: A new concept for interpreting effects and mechanisms of acupuncture. Evid Based Complement Alternat Med. 2012;2012:429412. doi: 10.1155/2012/429412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim M-H, Park Y-C, Namgung U. Acupuncture-stimulated activation of sensory neurons. J Aacupunct Meridian Stud. 2012;5(4):148–55. doi: 10.1016/j.jams.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 51.Lin R, Chen J, Li X, et al. Electroacupuncture at the Baihui acupoint alleviates cognitive impairment and exerts neuroprotective effects by modulating the expression and processing of brain-derived neurotrophic factor in APP/PS1 transgenic mice. Mol Med Rep. 2016;13(2):1611–17. doi: 10.3892/mmr.2015.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang WW, Xie CL, Lu L, Zheng GQ. A systematic review and meta-analysis of Baihui (GV20)-based scalp acupuncture in experimental ischemic stroke. Sci Rep. 2014;4(3981):1–16. doi: 10.1038/srep03981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li X, Guo F, Zhang Q, et al. Electroacupuncture decreases cognitive impairment and promotes neurogenesis in the APP/PS1 transgenic mice. BMC Complement Altern Med. 2014;14(1):37. doi: 10.1186/1472-6882-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu Y, Zeng Y, Wang X, Ye X. Effect of electroacupuncture on the expression of mTOR and eIF4E in hippocampus of rats with vascular dementia. Neurol Sci. 2013;34(7):1093–97. doi: 10.1007/s10072-012-1209-4. [DOI] [PubMed] [Google Scholar]
- 55.Chuang C-M, Hsieh CL, Li TC, Lin JG. Acupuncture stimulation at Baihui acupoint reduced cerebral infarct and increased dopamine levels in chronic cerebral hypoperfusion and ischemia-reperfusion injured sprague-dawley rats. Am J Chin Med. 2007;35(5):779–91. doi: 10.1142/S0192415X07005260. [DOI] [PubMed] [Google Scholar]
- 56.Ritzenthaler T, Lhommeau I, Douillard S, et al. Dynamics of oxidative stress and urinary excretion of melatonin and its metabolites during acute ischemic stroke. Neurosci Lett. 2013;544:1–4. doi: 10.1016/j.neulet.2013.02.073. [DOI] [PubMed] [Google Scholar]
- 57.Wei N, Xiao L, Xue R, et al. MicroRNA-9 mediates the cell apoptosis by targeting Bcl2l11 in ischemic stroke. Mol Neurobiol. 2016;53(10):6809–17. doi: 10.1007/s12035-015-9605-4. [DOI] [PubMed] [Google Scholar]
- 58.Baxter P, Chen Y, Xu Y, Swanson RA. Mitochondrial dysfunction induced by nuclear poly(ADP-Ribose) polymerase-1: A treatable cause of cell death in stroke. Transl Stroke Res. 2014;5:136–44. doi: 10.1007/s12975-013-0283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manzanero S, Santro T, Arumugam TV. Neuronal oxidative stress in acute ischemic stroke: Sources and contribution to cell injury. Neurochem Int. 2013;62:712–18. doi: 10.1016/j.neuint.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 60.Zhao H, Steinberg GK, Sapolsky RM. General versus specific actions of mild-moderate hypothermia in attenuating cerebral ischemic damage. J Cereb Blood Flow Metab. 2007;27(12):1879–94. doi: 10.1038/sj.jcbfm.9600540. [DOI] [PubMed] [Google Scholar]
- 61.Kim EK, Choi E-J. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802(4):396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 62.Anand SS, Babu PP. c-Jun N terminal kinases (JNK) are activated in the brain during the pathology of experimental cerebral malaria. Neurosci Lett. 2011;488(2):118–22. doi: 10.1016/j.neulet.2010.11.012. [DOI] [PubMed] [Google Scholar]