Abstract
Background
Early diagnosis and treatment of rheumatoid synovitis can reduce the progression of rheumatoid arthritis (RA). However, in the early stages of rheumatoid synovitis, patients may only have non-specific musculoskeletal symptoms, and plain film radiographs may not detect early synovial changes. The aim of this study was to compare the sensitivity and specificity of ultrasound with radiography, and clinical investigations in the detection of rheumatoid synovitis in patients presenting with nonspecific musculoskeletal symptoms.
Material/Methods
This was a non-randomized, cross-sectional, clinical study that included 189 patients who had nonspecific musculoskeletal symptoms. All patients underwent clinical investigations, postero-anterior and dorsal radiographic imaging, and bilateral grey-scale ultrasound examinations of the third and second metacarpophalangeal (MCP) joints, the third and the second proximal interphalangeal (PIP) joints, the second and the fifth metatarsophalangeal (MTP) joints, and the wrist.
Results
There was no clear predictive value for detection of early synovitis by clinical investigations alone. Plain film radiography of patients only provided accurate information of joint erosions but less information for synovitis. Grey-scale ultrasound was more effective at detecting early synovitis compared with clinical investigations (p=0.00015; q=4.548) and compared with plain film radiography (p=0.0002; q=4.537), and quantified the synovial changes. The predictive values of plain film radiography and clinical investigations had 0.43 and 0.24 sensitivity and 0 specificity compared with ultrasound.
Conclusions
The findings of this study support the use of grey-scale ultrasound in the detection of early rheumatoid synovitis of the fingers and the wrist.
MeSH Keywords: Metacarpophalangeal Joint, Metatarsophalangeal Joint, Radiography, Synovitis, Ultrasonography, Wrist Joint
Background
Early diagnosis and treatment of synovitis in rheumatoid arthritis (RA) reduce the progression of the disease, reduce the loss of joint function, joint damage, and inflammation [1]. Therefore, there is a need for identification of synovitis in RA at an early stage [2].
There are currently evidence-based clinical guidelines available for the clinical diagnosis and management of early RA, including the 2010 guidelines from the American College of Rheumatology (ACR), and the 2013 guidelines from the European League Against Rheumatism (EULAR) [3]. Detection of rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA) are used for the diagnosis of RA [4]. In addition to clinical laboratory investigations, plain film radiography can detect bone erosion and is helpful in the diagnosis of advanced joint disease [5]. However, in the very early stage of RA, patients have non-specific musculoskeletal symptoms only, and symptoms and signs of synovitis may not be easy to detect, inflammatory biomarkers may not be detectable, and X-ray films can be found to be normal [6].
The use of ultrasound is non-invasive and does not cause discomfort to patients, it is cheap, and widely accessible [7]. Also, ultrasound can assess of synovitis by quantifying the thickening of the synovial membrane [8], including targeted tendon sheaths, bursae, and joints [9] by B-mode (grey-scale) ultrasound [10]. Ultrasound has site-specific sensitivity [11], and has been reported to have 44% sensitivity and 99% specificity, which compare favorably with magnetic resonance imaging (MRI), which is considered to be the ‘gold-standard’ method for evaluating tenosynovitis in RA [12].
Ultrasound is more accurate and sensitive in identifying rheumatoid synovitis than clinical laboratory investigations alone [13,14] and than X-rays [15]. Ultrasound with grey-scale imaging can also evaluate joint effusion and thickening of the synovium can be characterized and quantified. Doppler ultrasound, particularly power Doppler, facilitates the recognition of synovial tissue hyperemia [16]. Also, ultrasound is a more reliable diagnostic modality than plain film radiography in RA and can detect inflammation in RA as well as joint erosions [15]. Ultrasound has a high sensitivity for the detection of small bone erosions and high specificity for the detection of tenosynovitis, synovitis, bursitis, and clinical swelling [17]. At this time, there have been no previously reported studies that have followed the 2015 Standards for Reporting Diagnostic Accuracy (STARD) guidelines (http://www.stard-statement.org) in a cross-sectional study on the diagnosis of rheumatoid synovitis using grey-scale ultrasound radiography, without reference to ACR and EULAR guidelines [3,17].
The aim of this cross-sectional clinical study was to compare the sensitivity and specificity of ultrasound with radiography, and clinical laboratory investigations in the detection of rheumatoid synovitis in the fingers and the wrist of patients presenting with nonspecific musculoskeletal symptoms. This study was designed using the STARD guidelines but without using ACR and EULAR remission criteria [3,17].
Material and Methods
Ethical approvals, patients consents, study registrations, and study guidelines
This clinical study, and its design were approved by the Rencheng District First Maternal and Child Health Care Hospital Review Board (Registration No. RD/Re/Hu/15/14; dated 15 December 2014) and was approved for clinical research in human subjects. The study was registered in the Research Registry (http://www.researchregistry.com), (UID No. 3486; dated 16 December 2014). The protocol of the study adhered to the legal requirements of the Peoples’ Republic of China, the1964 Declaration of Helsinki, and the 2015 Standards for Reporting Diagnostic Accuracy (STARD) guidelines (http://www.stard-statement.org). All patients signed an informed consent form before radiology and pathology investigations. All patients enrolled in the study signed an informed consent to have their clinical data analyzed and used in a manuscript for publication, and provided consent for the analysis and use of radiological images and data in all formats.
Inclusion criteria
A total of 189 patients were included in the study, who had been referred to the Department of Rheumatology of the Rencheng District First Maternal and Child Health Care Hospital, Heze Municipal Hospital, Heze, Yanzhou Hospital of Traditional Chinese Medicine, Yanzhou, and Jining No.1 People’s Hospital, Jining, China between January 2015 to December 2017. Patients who were >18 years-of-age and who presented with nonspecific musculoskeletal symptoms were included in the study. The demographical characteristics of enrolled patients are presented in Table 1.
Table 1.
Demographical characteristics and working habits of enrolled patients.
| Characters | Population (n=189) | |
|---|---|---|
| Age (year) | 18–40 | 11 (6) |
| 41–45 | 60 (31) | |
| 46–50 | 55 (29) | |
| 51–55 | 54 (29) | |
| 56–60 | 6 (3) | |
| ≥61 | 3 (2) | |
| Gender | Female | 112 (59) |
| Male | 77 (41) | |
| BMI (kg/m2) | 22.48±1.71 | |
| Nonspecific musculoskeletal symptoms time (days) | 56±17 | |
| Ethnicity | Chinese | 185 (98) |
| Non-Chines | 4 (2) | |
| Systemic lupus erythematosus | 41 (22) | |
| Morning stiffness | 11 (6) | |
| Evening stiffness | 7 (4) | |
| VAS | 1.08±0.72 | |
| Overall hand function* | 82.76±8.56 | |
| Daily living activity# | 80.96±9.84 | |
| Hand grip strength (kg) | Nondominant | 23.43±3.44 |
| Dominant | 33.4±4.58 | |
| Sensation of tingling | 35 (19) | |
| Laptop/desktop usage (h/day) | No | 17 (9) |
| <3 | 87 (46) | |
| 3–5 | 58 (31) | |
| ≥5 | 27 (14) | |
| Driving (h/day) | <1 | 75 (40) |
| 1–2 | 89 (47) | |
| ≥3 | 25 (13) | |
Continuous parameters are represented as mean ±SD and constant parameters are represented as number (percentage). BMI – Body Mass Index; VAS – Visual analog scale, 0: Absent pain, 10: Possible worst pain.
0–100 scale, 0: worst function, 100: Normal function.
0–100 scale, 0: worst activity, 100: Normal activity.
Exclusion criteria
Patients who had a diagnosis of systemic lupus erythematosus (SLE) were excluded from the study. Patients with no joint tenderness, stiffness, pain, or joint swelling, detected by a medical interview and physical examinations by a rheumatologist, were excluded. Patients who had confirmed rheumatoid arthritis but who did not sign an informed consent form, who had a history of joint surgery, joint trauma, crystal-associated peri-arthritis, and infective bursitis in the previous year, were excluded from the study. Patients who had been already taken pain medication or steroids, or who were being treated for organ failure were excluded from the study.
Study design
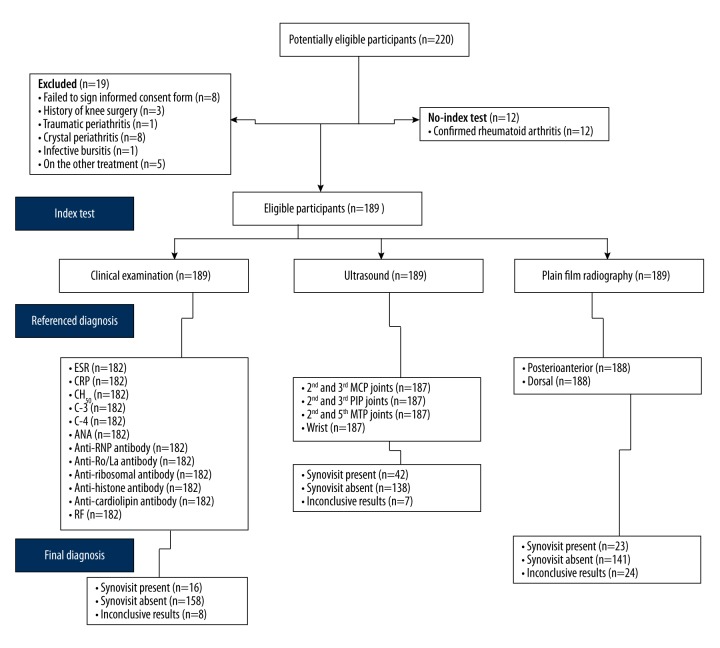
All 189 patients who were enrolled in the study participated in a clinical non-randomized cross-sectional study. The STARD 2015 flow diagram of the study is presented in Figure 1.
Figure 1.
Study design: the 2015 Standards for Reporting Diagnostic Accuracy (STARD) flow diagram of the cross-sectional study. ESR – erythrocyte sedimentation rate; CRP – C-reactive protein; CH50 – 50% hemolytic complement activity of serum; C – complement component; ANA – anti-nuclear antibody; RF – rheumatoid factor; MCP – metacarpophalangeal; PIP – proximal interphalangeal; MTP – metatarsophalangeal. The 2015 Standards for Reporting Diagnostic Accuracy (STARD) guidelines (http://www.stard-statement.org).
Clinical laboratory investigations
A venous blood sample was obtained from each study participant, which was stored in citrate-containing ampoules and used for the detection of erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), 50% hemolytic complement activity of serum (CH50), complement components C1, C3, C4. An enzyme-linked immunosorbent assay (ELISA) was used to measure serum antibodies to ribonucleoprotein (anti-RNP), antibodies to double-strand DNA (anti-dsDNA), anti-Ro/La antibody, antibody to smooth muscle (anti-Sm), anti-ribosomal P antibody, anti-β2 glycoprotein 1 (GPI) antibody, anti-cardiolipin antibody, anti-histone antibody, and rheumatoid factor (RF) [13].
Plain film radiography
After half an hour of clinical examination and investigations, postero-anterior and dorsal radiographic images were performed for both hands by a radiologist who was unaware of the clinical history or findings, using an Optima XR220amx X-ray machine (GE Healthcare, USA). The specifications for plain film radiography were single-screen, high-resolution, single-emulsion, focus field distance 104 cm (41 inches), 101 mA, 49–56 kV at 301 ms [18]. Any disagreement between radiological interpretation regarding erosion or synovitis was overcome by discussion among radiologists.
Ultrasound imaging and quantitation
Ultrasound examinations were performed bilaterally for the third and the second metacarpophalangeal (MCP) joints, the third and the second proximal interphalangeal (PIP) joints, the second and the fifth metatarsophalangeal (MTP) joints, and the wrist using a Logiq P5 ultrasound machine (GE Healthcare, USA) with a linear probe at 6.95–11.95 MHz, and a Doppler frequency of 4.95–6.99 MHz [19]. The probe was kept parallel to the extensor digitorum tendons, and the synovium was examined in the wrist, the third and the second MCP, and PIP joints from palmar, ulnar, and dorsal sides [20]. The area of interest of the ultrasound assessment is shown in Table 2. The synovitis was scored as shown in Table 3, using the German US-7 seven-point scoring index to assess synovitis and erosions in patients with RA [18,21].
Table 2.
Grey-scale ultrasound for synovitis.
| Condition | Hand | ||
|---|---|---|---|
| Wrist | Fingers | Thumb | |
| Synovitis | MCP 2nd, 3rd | MTP 2nd, 5th | |
| Dorsal | Palmar | Dorsal | |
| Palmar | Dorsal | ||
| Ulnar | |||
| PIP 2nd, 3rd | |||
| Palmar | |||
| Dorsal | |||
| Joint | 1 | 4 | 2 |
Table 3.
Grading of grey scale for synovitis.
| Scale | Condition of maximal potential joint enlargement | Prediction of synovitis |
|---|---|---|
| 0 | 0 | Absent |
| 1 | <⅓ | Mild |
| 2 | ≥⅓ but <½ | Moderate |
| 3 | ≥½ but <⅔ | Moderate to severe |
| 4 | ≥⅔ but <9/10 | Sever |
| 5 | ≥½ | Extreme |
MCP – metacarpophalangeal; PIP – proximal interphalangeal; MTP – metatarsophalangeal.
Statistical analysis
One-way analysis of variance (ANOVA), following Tukey’s post hoc test (considering critical value q >4.143) was performed for predictive values between diagnostic modalities [13]. GraphPad InStat software (GraphPad, USA) was used for statistical analysis. Results were considered significant at 99% confidence level.
Results
From the 189 patients who were enrolled in the study and who had blood samples collected for laboratory investigations, seven samples of blood were unsuitable for analysis. The remaining 182 samples underwent laboratory investigation. The results showed that serological findings showed no was no clear predictive or diagnostic association with the presence of rheumatoid synovitis (Table 4).
Table 4.
Clinical examinations of enrolled patients.
| Parameter | Reference | Populations (n=182) |
|---|---|---|
| ESR (mm/h) | Normal (0–22 for M and 0–29 for F) | 180 (99) |
| Abnormal (>22 for M and >29 for F) | 2 (1) | |
| CRP (mg/dL) | <3 | 179 (98) |
| ≥3 | 3 (2) | |
| CH50 (U/mL) | >41 but <90 | 180 (99) |
| ≥90 | 2 (1) | |
| C1 (mg/dL) | >16 but <31 | 181 (99) |
| ≥31 | 1 (1) | |
| C-3 (mg/dL) | Normal (≥88 but <252 for M and ≥88 but <206 for F) | 179 (98) |
| Abnormal (≥252 for M and ≥206 for F) | 3 (2) | |
| C-4 (mg/dL) | Normal (≥12 but <72 for M and ≥13 but <75 for F) | 180 (99) |
| Abnormal (≥72 for M and ≥75 for F) | 2 (1) | |
| Anti-nuclear antibody | Positive | 0 (0) |
| Negative | 182 (100) | |
| Anti-RNP antibody | Positive | 0 (0) |
| Negative | 182 (100) | |
| Anti-Ro/La antibody | Positive | 0 (0) |
| Negative | 182 (100) | |
| Anti-Sm antibody | Positive | 0 (0) |
| Negative | 182 (100) | |
| Anti-ribosomal P antibody | Positive | 0 (0) |
| Negative | 182 (100) | |
| Anti-histone antibody | Positive | 0 (0) |
| Negative | 182 (100) | |
| Anti-β2 GPI antibody | Positive | 0 (0) |
| Negative | 182 (100) | |
| Anti-cardiolipin antibody | Positive | 0 (0) |
| Negative | 182 (100) | |
| Rheumatoid factor (IU/mL) | <15 | 3 (2) |
| ≥15 | 179 (98) |
Data were represented as number (percentage). M – Male; F – Female; ESR – erythrocyte sedimentation rate; CRP – C-reactive protein; CH50 – hemolytic complement; C – complement component.
Plain film radiographs of one patient were lost, and the sets of radiographs for the remaining 188 patients were used in the analysis. Plain film radiography of patients only provided accurate information of joint erosions but less information regarding the presence or degree of synovitis (Table 5).
Table 5.
Plain-film radiographic assessment of enrolled patients.
| Parameters | Populations (n=188) | |
|---|---|---|
| Dominant hand | Non-dominant hand | |
| Wrist joints erosion | 12 (6) | 11 (6) |
| Hand joints erosion | 15 (8) | 13 (7) |
| Wrist and hand joints erosion | 4 (2) | 3 (2) |
| Synovitis | 13 (7) | 7 (4) |
Data were represented as number (percentage).
Two patients were failed to undergo ultrasound examination, but ultrasound assessment of synovitis in the remaining 187 patients is shown in Table 6.
Table 6.
Ultrasound assessment of enrolled patients.
| Site | Synovitis detected (Dominant + non-dominant hand) |
|---|---|
| 2nd and 3rd MCP joints | 13 (7) |
| 2nd and 3rd PIP joints | 6 (3) |
| 2nd and 5th MTP joints | 7 (4) |
| Wrist | 9 (5) |
Populations (n)=187. Data were represented as number (percentage). MCP – metacarpophalangeal; PIP – proximal interphalangeal; MTP – metatarsophalangeal.
Grey-scale ultrasound was more effective at detecting early synovitis compared with clinical laboratory investigations (p=0.00015; q=4.548) and compared with plain film radiography (p=0.0002, q=4.537), and quantified the synovial changes (Figure 2). The predictive values of plain film radiography and clinical investigations had 0.43 and 0.24 sensitivity and 0 specificity when compared with ultrasound
Figure 2.
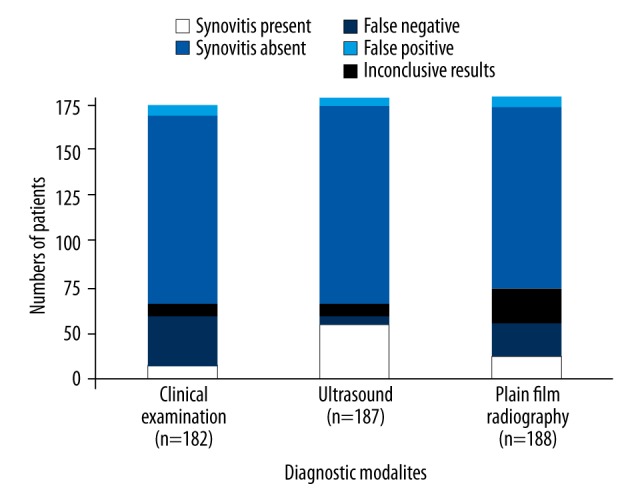
Comparison between the predictive values of the diagnostic modalities used in the study. The p-values and q-values of the comparison of clinical laboratory investigations with ultrasound imaging were 0.00015 and 4.548. The p-values and q-values of the comparison of plain film radiography with ultrasound imaging were 0.0002 and 4.537. One-way analysis of variance (ANOVA), following Tukey’s post hoc range test, were used for statistical analysis. Values of p<0.01 and q>4.143 were considered to be statistically significant.
Discussion
The aim of this cross-sectional study was to compare the sensitivity and specificity of ultrasound with radiography, and clinical laboratory investigations in the detection of rheumatoid synovitis in patients presenting with nonspecific musculoskeletal symptoms. There was no clear predictive value for detection of early synovitis by clinical examination and laboratory tests alone. Plain film radiography of patients only provided accurate information of joint erosions but less information for synovitis, but grey-scale ultrasound was more effective at detecting early rheumatoid synovitis compared with clinical investigations and compared with plain film radiography, and quantified the synovial changes
Several previously published diagnostic studies have shown that early detection is required in rheumatoid synovitis to prevent disease progression [1,5,22]. Imaging modalities are useful in the diagnosis of rheumatoid synovitis [13,15,16,23] and are also recommended by current clinical guidelines [17,24]. Clinical and laboratory investigations [13], plain film radiographs [25], ultrasound [20], and magnetic resonance imaging (MRI) [18] are used for the diagnosis of rheumatoid synovitis. However, clinical laboratory investigations and plain film radiographs are not sensitive for the diagnosis of early rheumatoid synovitis [18]. MRI has high signal-to-noise ratio and anatomical coverage [15] but has a high cost and may not be available in all clinical centers [18]. Ultrasound has been shown to have good intra-observer and inter-observer reproducibility and reliability for the detection of rheumatoid synovitis [7], but these properties for MRI are less clear [26]. Also, in the present study, the German US-7 seven-point scoring index to assess synovitis and erosions in patients with rheumatoid arthritis (RA) was used [18, 21]. All the methods used in this clinical study were non-invasive, rapid, non-costly, and reliable for the detection of rheumatoid synovitis.
In this study, a high frequency, linear probe was used for ultrasound diagnosis, whereas previously published ultrasound studies have used an L-shaped hockey stick probe (HSP) for real-time diagnostic ultrasonography [7,15,19,23]. Also, ultrasound provides a more sensitive diagnostic imaging technique for the assessment of inflammation [7]. Considering the selection of the ultrasound probe, the probe position, the ultrasound machine configuration, the study was helpful in demonstrating the value of grey-scale ultrasound in the detection of early rheumatoid synovitis.
The predictive values of plain film radiography and clinical laboratory investigations had 0.43 and 0.24 sensitivity and no specificity compared with ultrasound. Until this time, available ultrasound techniques have required an evaluation of seven joints in the wrist and fingers [13] for the diagnosis of rheumatoid synovitis and has required repeated examinations [19]. From the findings of the ultrasound procedure used for this study, the ultrasound method was shown to be rapid, easy to use and interpret, economical, and a reliable method for the diagnosis of early rheumatoid synovitis.
There were several limitations in this study. Color Doppler imaging was not performed and would be important to evaluate in future studies. No inter-observer or intra-observer quality control (QC) checks were performed. The final decision regarding the diagnosis of rheumatoid synovitis was taken by the authors of this study only, and no independent external expert opinion was sought. Also, this study was limited to RA involving the fingers and wrist only. A comparison between ultrasound and MRI was not undertaken, and if any of the patients had undergone MRI imaging, this data was not included in the study. Because age also has an effect on the onset and progression of rheumatoid synovitis, and this study did not include an analysis of the effect of age on the diagnostic modalities used, future studies should include analysis of the effects of age in the detection of early rheumatoid synovitis.
Conclusions
The findings of this cross-sectional study support the use of grey-scale ultrasound in the detection of early rheumatoid synovitis of the fingers and the wrist. Clinical laboratory investigations did not provide information regarding joint erosions and synovitis, plain film radiographs provided information of joint erosions, but did not provide information regarding synovitis. Grey-scale ultrasound is a validated, precise, cheap, and easily available method for the detection of early rheumatoid synovitis of the finger joints and the wrist.
Acknowledgements
The authors thank the medical staff, and non-medical technical staff of Jining No.1 Peoples’ Hospital, Jining, China, Heze Municipal Hospital, Heze, China, Yanzhou Hospital of Traditional Chinese Medicine, Yanzhou, China, and Rencheng District First Maternal and Child Health Care Hospital, Jining, China for their help during this clinical study.
Footnotes
Conflict of interest
None.
Source of support: Self financing
References
- 1.Barhamain AS, Magliah RF, Shaheen MH, et al. The journey of rheumatoid arthritis patients: A review of reported lag times from the onset of symptoms. Open Access Rheumatol. 2017;9:139–50. doi: 10.2147/OARRR.S138830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuriya B, Xiong J, Boire G, et al. CATCH Investigators. Earlier time to remission predicts sustained clinical remission in early rheumatoid arthritis – results from the Canadian Early Arthritis Cohort (CATCH) J Rheumatol. 2014;41:2161–66. doi: 10.3899/jrheum.140137. [DOI] [PubMed] [Google Scholar]
- 3.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 4.Alemao E, Guo Z, Frits ML, et al. Association of anti-cyclic citrullinated protein antibodies, erosions, and rheumatoid factor with disease activity and work productivity: A patient registry study. Semin Arthritis Rheum. 2017 doi: 10.1016/j.semarthrit.2017.10.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Nam JL, D’Agostino MA. Role of ultrasound imaging in individuals at risk of RA. Best Pract Res Clin Rheumatol. 2017;31:71–79. doi: 10.1016/j.berh.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Schueller-Weidekamm C, Lodemann KP, Grisar J, et al. Contrast-enhanced MR imaging of hand and finger joints in patients with early rheumatoid arthritis: Do we really need a full dose of gadobenate dimeglumine for assessing synovial enhancement at 3 T? Radiology. 2013;268:161–69. doi: 10.1148/radiol.13121733. [DOI] [PubMed] [Google Scholar]
- 7.Ostergaard M, Pedersen SJ, Dohn UM. Imaging in rheumatoid arthritis-status and recent advances for magnetic resonance imaging, ultrasonography, computed tomography and conventional radiography. Best Pract Res Clin Rheumatol. 2008;22:1019–44. doi: 10.1016/j.berh.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Furtado RN, Pereira DF, da Luz KR, et al. Effectiveness of imaging-guided intra-articular injection: A comparison study between fluoroscopy and ultrasound. Rev Bras Reumatol. 2013;53:476–82. doi: 10.1016/j.rbr.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Ogura T, Hirata A, Hayashi N, et al. Comparison of ultrasonographic joint and tendon findings in hands between early, treatment-naive patients with systemic lupus erythematosus and rheumatoid arthritis. Lupus. 2017;26:707–14. doi: 10.1177/0961203316676375. [DOI] [PubMed] [Google Scholar]
- 10.Xiao H, Liu M, Tan L, et al. Value of ultrasonography for diagnosis of synovitis associated with rheumatoid arthritis. Int J Rheum Dis. 2014;17:767–75. doi: 10.1111/1756-185X.12390. [DOI] [PubMed] [Google Scholar]
- 11.Takase-Minegishi K, Horita N, Kobayashi K, et al. Diagnostic test accuracy of ultrasound for synovitis in rheumatoid arthritis: Systematic review and meta-analysis. Rheumatology. 2018;57:49–58. doi: 10.1093/rheumatology/kex036. [DOI] [PubMed] [Google Scholar]
- 12.Wakefield RJ, O’Connor PJ, Conaghan PG, et al. Finger tendon disease in untreated early rheumatoid arthritis: A comparison of ultrasound and magnetic resonance imaging. Arthritis Rheum. 2007;57:1158–64. doi: 10.1002/art.23016. [DOI] [PubMed] [Google Scholar]
- 13.Yoon HS, Kim KJ, Baek IW, et al. Ultrasonography is useful to detect subclinical synovitis in SLE patients without musculoskeletal involvement before symptoms appear. Clin Rheumatol. 2014;33:341–48. doi: 10.1007/s10067-014-2502-9. [DOI] [PubMed] [Google Scholar]
- 14.Emamifar A, Hess S, Gildberg-Mortensen R, Jensen Hansen IM. Association of remitting seronegative symmetrical synovitis with pitting edema, polymyalgia rheumatica, and adenocarcinoma of the prostate. Am J Case Rep. 2016;17:60–64. doi: 10.12659/AJCR.895717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baillet A, Gaujoux-Viala C, Mouterde G, et al. Comparison of the efficacy of sonography, magnetic resonance imaging and conventional radiography for the detection of bone erosions in rheumatoid arthritis patients: A systematic review and meta-analysis. Rheumatology. 2011;50:1137–47. doi: 10.1093/rheumatology/keq437. [DOI] [PubMed] [Google Scholar]
- 16.Karimzadeh H, Seyedbonakdar Z, Mousavi M, Karami M. Comparison the percentage of detection of periarthritis in patients with rheumatoid arthritis using clinical examination or ultrasound methods. J Res Med Sci. 2016;21:134. doi: 10.4103/1735-1995.196616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colebatch AN, Edwards CJ, Ostergaard M, et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis. 2013;72:804–14. doi: 10.1136/annrheumdis-2012-203158. [DOI] [PubMed] [Google Scholar]
- 18.Taouli B, Zaim S, Peterfy CG, et al. Rheumatoid arthritis of the hand and wrist: Comparison of three imaging techniques. Am J Roentgenol. 2004;182:937–43. doi: 10.2214/ajr.182.4.1820937. [DOI] [PubMed] [Google Scholar]
- 19.Backhaus M, Ohrndorf S, Kellner H, et al. Evaluation of a novel 7-joint ultrasound score in daily rheumatologic practice: A pilot project. Arthritis Rheum. 2009;61:1194–201. doi: 10.1002/art.24646. [DOI] [PubMed] [Google Scholar]
- 20.Ozer PK, Sahin O, Ozer Z, et al. Ultrasound-defined remission for good functional status in rheumatoid arthritis. Indian J Med Res. 2017;146:230–36. doi: 10.4103/ijmr.IJMR_548_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skacelova M, Martina S, Pavel H, et al. Relationship between rheumatoid arthritis disease activity assessed with the US7 score and quality of life measured with questionnaires (HAQ, EQ-5D, WPAI) Curr Rheumatol Rev. 2017;13(3):224–30. doi: 10.2174/1573397113666170517160726. [DOI] [PubMed] [Google Scholar]
- 22.Evans J, Negoescu A. Early referral improves long-term outcomes in rheumatoid arthritis. Practitioner. 2017;261:21–25. [PubMed] [Google Scholar]
- 23.Wang Y, Geng Y, Deng XR, Zhang ZL. Relationship between wrist bone mineral density and synovitis, erosion by ultrasonography in female rheumatoid arthritis patients. J Peking Univ (Health Sci) 2015;47:774–80. [PubMed] [Google Scholar]
- 24.Wakefield RJ, Balint PV, Szkudlarek M, et al. OMERACT 7 Special Interest Group. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32:485–87. [PubMed] [Google Scholar]
- 25.Ahmadzadeh A, Dehghan P, Rajaee A, et al. Assessing rheumatologists and radiologists’ agreement rate regarding the diagnosis of focal bone erosions and osteopenic changes using hand X-rays radiography in patients with rheumatoid arthritis. Rheumatol Int. 2013;33:2019–23. doi: 10.1007/s00296-012-2645-4. [DOI] [PubMed] [Google Scholar]
- 26.Ji L, Li G, Xu Y, et al. Early prediction of rheumatoid arthritis by magnetic resonance imaging in the absence of anti-cyclic citrullinated peptide antibodies and radiographic erosions in undifferentiated inflammatory arthritis patients: a prospective study. Int J Rheum Dis. 2015;18:859–65. doi: 10.1111/1756-185X.12420. [DOI] [PubMed] [Google Scholar]