Abstract
BACKGROUND
Both hypertensive and atherosclerotic processes contribute to common carotid artery intima-media thickness (CCA-IMT). Elevated CCA-IMT may be indicative of subclinical cerebrovascular disease; however, its role in the absence of concomitant carotid artery plaque is uncertain, and few studies have examined associations in Black populations.
MATERIALS AND METHODS
At cohort visit 3 (1993–1995) a subset of stroke-free participants (641 Blacks and 702 Whites, mean age 63) from the Atherosclerosis Risk in Communities (ARIC) study was imaged by brain MRI and carotid ultrasound. A CCA-IMT >0.9 mm was considered elevated. Asymptomatic brain lesions ≥3 mm were considered silent brain infarctions (SBI). Subcortical SBI measuring 3 to <20 mm were considered lacunes. Associations between elevated CCA-IMT and SBI were analyzed with Poisson regression.
RESULTS
Elevated CCA-IMT was identified in 168 participants (16% of Blacks, 10% of Whites), and SBI were observed in 156 (15% of Blacks, 8% of Whites). Elevated CCA-IMT was strongly related to anterior circulation SBI, posterior circulation SBI, and lacunes. After adjustments, elevated CCA-IMT remained associated with greater number of lacunes in Blacks ([prevalence ratio, PR] = 1.60; 95% confidence interval [CI]: 1.02–2.51), but not Whites (PR = 0.85; 95% CI: 0.35–2.04); P value for interaction = 0.12. Among Black participants without concomitant carotid plaque, elevated CCA-IMT was associated with twice the number of lacunes (PR = 2.00; 95% CI: 1.05–3.82).
CONCLUSIONS
In older Black adults, elevated CCA-IMT is independently associated with lipohyalinosis of the cerebral small vessels, irrespective of concomitant carotid plaque and vascular risk factors.
Keywords: blood pressure, carotid intima-media thickness, epidemiology, hypertension, silent brain infarction
Carotid artery intima-media thickness (IMT) is easily quantified by ultrasonography and has long been considered a marker of subclinical atherosclerosis. However, IMT measurements encompass not only intimal thickening, but also medial hypertrophy, the result of long-standing hypertension.1 Although the common carotid artery (CCA) provides the most reproducible IMT measurements,2 it is more influenced by hypertension than measurements from either the bifurcation or internal carotid artery (ICA), which are more atherogenic.1,3 Furthermore, the etiology and implications of elevated IMT may differ by race. Previous research suggests elevated CCA-IMT is more strongly related to hypertension in Black compared to White individuals.4
Despite ambiguity in its interpretation, elevated CCA-IMT has been shown to predict incident myocardial infarction and cerebrovascular disease.5 Silent brain infarctions (SBI), a precursor to stroke and cognitive decline,6,7 have also been associated with elevated CCA-IMT in cohort studies primarily composed of White populations.8–10 However, SBI etiologies are heterogenous and vary by lesion location. Infarcts of the large vessels are typically atheroembolic, while infarcts of the small deep vessels are more often attributable to hypertensive processes and lipohyalinosis.11 We hypothesized that elevated CCA-IMT in Black individuals is more reflective of long-standing hypertension than atherosclerosis, and a better indicator of lipohyalinosis of the small deep cerebral vessels than in White individuals. To test this, we examined imaging data from the Atherosclerosis Risk in Communities (ARIC) Study.
METHODS
The ARIC study
The ARIC study is an observational, prospective cohort of 15,792 adults, primarily Blacks and Whites, in 4 US communities: Forsyth County, NC; Washington County, MD; Jackson, MS; and 8 northwest suburbs of Minneapolis, MN. Participants were recruited with informed consent at the study onset in 1987–1989. Carotid ultrasound was offered to all participants at cohort visit 3 (1993–1995), with brain MRI offered at the same visit to a subset of participants from the MS and NC clinical sites. All study protocols were approved by the local Institutional Review Boards.
Cerebrovascular history
History of stroke was determined at the study onset by self-reported signs and symptoms analyzed by a validated algorithm.12,13 Following study enrollment, cerebrovascular events were identified through hospital surveillance, with diagnoses ascertained by physician review.14 For the purposes of this analysis, any participants with history of stroke prior to cohort study visit 3 were excluded.
Carotid ultrasound
Carotid ultrasounds were performed by registered sonographers following a standardized imaging protocol. All images were acquired with a Biosound 2000 II (Esaote S.p.A; Genoa, Italy) ultrasound machine interfaced with an 8 MHz transducer, which achieved an axial resolution of 0.1 mm. The carotid artery was imaged bilaterally in the longitudinal orientation, using the internal and external carotid artery flow divider as a visual landmark. After image digitization from sVHS tapes, CCA-IMT distances were measured from the far wall with electronic calipers, using the lumen-intima to media-adventitia boundaries. The CCA-IMT was averaged from 12 equally spaced measurements along a 1 cm segment proximal to the carotid bifurcation. Measurements were made irrespective of the presence of focal plaque. Carotid plaque in the CCA, bifurcation, or ICA was defined by a focal IMT measurement >1.5 mm, or a hyperechoic region with luminal protrusion.15 A global CCA-IMT value was derived, by averaging the left and right arteries. Consistent with European Society of Cardiology definitions,16 we considered a CCA-IMT >0.9 mm to be “elevated”.
Brain magnetic resonance imaging
Brain MRI was offered to participants over the age of 55 during ARIC visit 3, at the Jackson, MS and Forsyth, NC sites. Examinations were performed by certified technologists using a standardized imaging protocol and either a GE Signa 1.5 T or Picker 1.5 T scanner (General Electric; Boston, MA). As previously described,17 the axial imaging plane was aligned parallel to the anterior/posterior commissure line, and images were captured in contiguous 5 mm slices. T1 weighted images were acquired with a repetition time of 500 ms and an echo time of 20 ms. T2 weighted images were captured using a 3,000 ms repetition time and echo time of 100 ms. All images were read by board-certified ARIC neuroradiologists.
Silent brain infarctions
SBI were identified by focal lesions hyperintense to gray matter on spin-density and T2 weighted MRI. Lesions of the white matter and brain stem additionally required T1 hypointensity for classification. SBI diameters were measured with electronic calipers, in the axial (anterior to the posterior), and lateral (right to left) planes, using the largest dimension within the contiguous MRI slices. SBI thickness (superior to inferior diameter) was calculated by multiplying the number of MRI slices by the slice thickness (5 mm). Consistent with previous analyses, a minimum 3 mm diameter was required for SBI classification, to differentiate SBI from pervivascular space.8–10 Interreader and intrareader agreement of SBI ≥3mm was 79% and 82%, respectively.17
Infarcts were classified into 23 categories by ARIC neuroradiologists, based on the location and vascular distribution. These included the anterior cerebral artery (ACA) frontal lobe, ACA parietal lobe, middle cerebral artery (MCA) frontal lobe, MCA parietal lobe, MCA temporal lobe, posterior cerebral artery (PCA) posterior lobe, PCA temporal lobe, PCA occipital lobe; territories of the superior cerebellar artery, anterior inferior cerebellar artery, and posterior inferior cerebellar artery; and regions of the deep cerebellar white matter, caudate nucleus, lentiform nucleus, internal capsule anterior limb and internal capsule posterior limb, thalamus, midbrain, pons, medulla, ACA-MCA watershed, MCA-PCA watershed, and deep cerebral white matter. Because lesions occasionally spanned more than one region, infarcts were allowed up to 4 categories. The majority of lesions (85%) were assigned a single location. Multiple locations most often resulted from SBI spanning the small regions of the caudate nucleus, lentiform nucleus, or internal capsule anterior limb.
Lesions within territories of the ACA, MCA, and ICA, or their respective branches, were considered “anterior SBI”. These included infarcts of the cortex (frontal, parietal, and temporal lobes), the watershed region of the ACA-MCA, the deep cerebral white matter, and infarcts localized to the caudate nucleus, lentiform nucleus, internal capsule anterior limb, and internal capsule posterior limb (within territories of the lenticulostriate arteries branching from the MCA, artery of Heubner branching from the ACA, and anterior choroidal branch of the ICA). Lesions arising from the PCA, superior cerebellar artery, anterior inferior cerebellar artery, or posterior inferior cerebellar artery, and their respective branches were considered “posterior SBI”. These included infarcts of the cortex (posterior, temporal, and occipital lobes), deep cerebellar white matter, thalamus, midbrain, pons, and medulla. Infarcts within the MCA-PCA watershed could not be classified as either “anterior” or “posterior”.
Cortical infarcts were classified by lesions of the frontal, parietal, temporal, posterior, and occipital lobes. Because the watershed territories encompass both the cortex and deep white matter, infarcts within these regions could not be classified as either “cortical’ or “subcortical”. We considered focal, nonmass lesions measuring <20 mm in diameter to be “lacunes” when situated in subcortical regions of the vascular distribution. Subcortical regions were delineated by the lentiform nucleus, caudate nucleus, anterior and posterior limbs of the internal capsule, the thalamus, midbrain and pons, and the deep cerebral and cerebellar white matter.
Clinical covariates
Medical histories and clinical values were collected at ARIC visit 3 by standardized protocols. Seated blood pressures were measured by random-zero mercury manometers, with the average of the second and third measurements recorded for analysis. Hypertension was considered a systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or antihypertensive medication use. Blood samples were processed at a central laboratory, with diabetes defined by a fasting blood glucose ≥126 mg/dl, a nonfasting blood glucose ≥200 mg/dl, or prior physician diagnosis of diabetes. Hyperlipidemia was considered a total cholesterol ≥200 mg/dl or statin use within 2 weeks of the clinic visit; however, statin medications in 1993–1995 were uncommon (4%). Age, sex, race, and smoking were self-reported.
Statistical analysis
Continuous variables were assessed for normality and compared by 2-sample t-tests or Wilcoxon rank sums tests, as appropriate. Categorical variables were compared using χ2 tests. Categorical variables with expected cell counts <5 were analyzed using Fisher’s exact test. Prevalence ratios (PRs) for the respective numbers (ranging from 0 to 5) of total SBI, anterior SBI, posterior SBI, and lacunes in participants with vs. without elevated IMT were analyzed using Poisson regression. Separate, race-stratified models were constructed for each outcome and were additionally tested within the subset of participants without carotid plaque. The association between elevated CCA-IMT and number of lacunes was further interrogated by multivariable Poisson models, adjusted for demographics (age, race, sex, and ARIC center), and clinical predictors associated with cerebral small vessel disease (smoking, hypertension, and diabetes). Racial modification was analyzed by assessing multiplicative statistical interaction between race, elevated CCA-IMT, and number of lacunes. A P <0.20 was considered evidence of significant interaction, accounting for the diminished statistical power inherent with stratification.18 To account for possible etiologic heterogeneity, a sensitivity analysis limited to lacunes <7 mm was also conducted, with the assumption that small lacunes are more attributable to lipohyalinosis than microatheroma.11 Appropriate fit was ascertained for all models by the deviance to degree of freedom ratio. All statistical analyses were carried out using SAS 9.4 (SAS Institute; Cary, NC).
RESULTS
A total of 1,823 ARIC participants underwent brain MRI and carotid ultrasound at study visit 3. Of these, 477 were missing bilateral carotid ultrasound images, and 3 self-identified as a race other than Black or White. After applying these exclusions, 1,343 participants remained (Supplementary Appendix Figure 1). An elevated CCA-IMT (>0.9 mm) was identified in 168 of the included participants (16% of Blacks, 10% of Whites). For both races, individuals with elevated CCA-IMT were more likely to be men and older, with a greater prevalence of hypertension and diabetes (Table 1). Other vascular risk factors, such as hyperlipidemia and smoking, were similar, irrespective of CCA-IMT.
Table 1.
Demographics and clinical characteristics of stroke-free participants of the Atherosclerosis Risk in Communities (ARIC) Study who were imaged by carotid ultrasound and brain MRI at visit 3 (1993–1995)
| Black study participants | White study participants | |||||
|---|---|---|---|---|---|---|
| CCA-IMT ≤0.9 | CCA-IMT >0.9 | CCA-IMT ≤0.9 | CCA-IMT >0.9 | |||
| Characteristic | N = 541 | N = 100 | P value | N = 634 | N = 68 | P value |
| Demographics | ||||||
| Age | 62 ± 5 | 63 ± 4 | 0.009 | 63 ± 4 | 65 ± 4 | <0.0001 |
| Male | 195 (36%) | 48 (48%) | 0.02 | 248 (39%) | 33 (49%) | 0.1 |
| Medical history | ||||||
| Current smoking | 106 (20%) | 18 (18%) | 0.7 | 115 (18%) | 11 (16%) | 0.7 |
| Hypertension | 315 (59%) | 72 (72%) | 0.01 | 181 (29%) | 36 (53%) | <0.0001 |
| Systolic blood pressure | 131 ± 20 | 138 ± 24 | 0.006 | 122 ± 18 | 131 ± 19 | <0.0001 |
| Diastolic blood pressure | 75 ± 11 | 74 ± 11 | 0.4 | 68 ± 10 | 69 ± 10 | 0.7 |
| Diabetes mellitus | 106 (20%) | 35 (35%) | 0.0004 | 51 (8%) | 9 (13%) | 0.1 |
| Total cholesterol (mg/dl) | 209 ± 41 | 209 ± 40 | 0.9 | 211 ± 37 | 207 ± 36 | 0.4 |
| Common carotid artery (mm) | ||||||
| Intima-media thickness, right | 0.7 ± 0.1 | 1.1 ± 0.3 | 0.7 ± 0.1 | 1.1 ± 0.4 | ||
| Intima-media thickness, left | 0.7 ± 0.1 | 1.0 ± 0.2 | 0.7 ± 0.1 | 1.0 ± 0.3 | ||
Abbreviation: CCA-IMT, intima medial thickness of the common carotid artery.
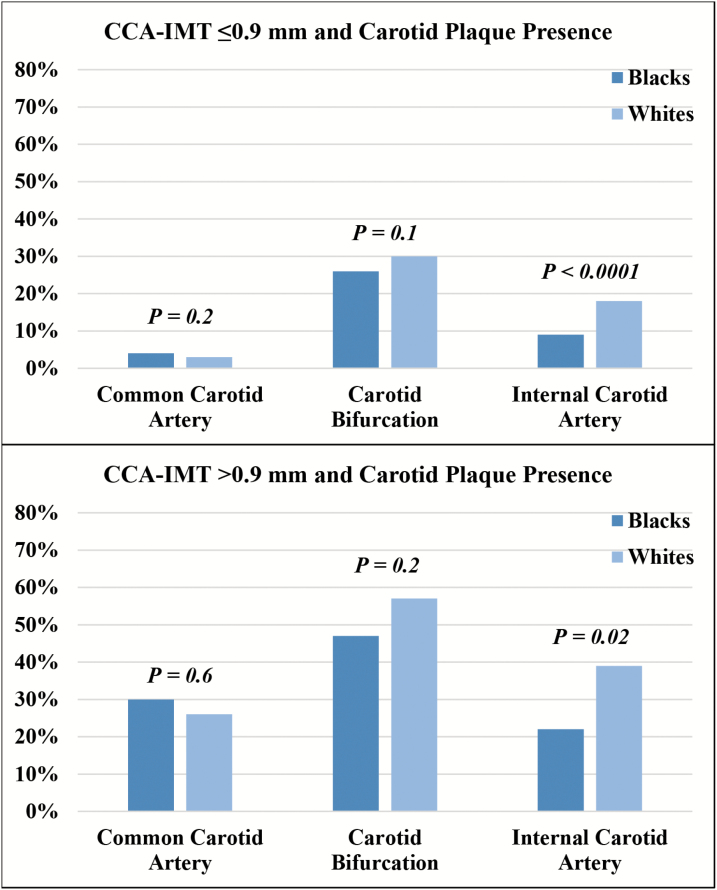
Although Black study participants had more comorbidities and vascular risk factors, carotid plaque was more common in White participants. Plaque was noted in either the right or left carotid artery in 507 participants (35% of Blacks, 40% of Whites), most frequently at the bifurcation (29% of Blacks, 33% of Whites), followed by the ICA (11% of Blacks, 20% of Whites) and CCA (8% of Blacks, 5% of Whites). When stratified by CCA-IMT elevation, carotid plaque was consistently more common in the carotid bifurcation and ICA of White compared to Black individuals (Figure 1).
Figure 1.
Presence of plaque in either the left or right carotid artery of Black and White participants of the Atherosclerosis Risk in Communities Study, stratified by common carotid artery intima-media thickness ≤0.9 mm vs. >0.9 mm (elevated). Abbreviation: CCA-IMT, intima medial thickness of the common carotid artery.
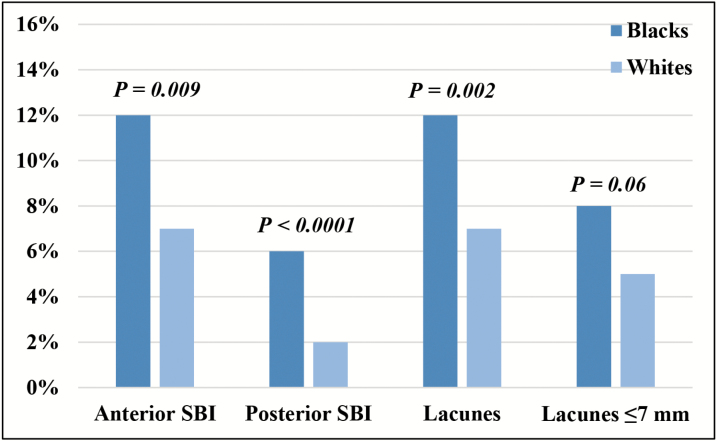
Overall, 156 participants (15% of Blacks, 8% of Whites) were identified with SBI, giving rise to a collective total of 245 lesions. Most SBI were in the anterior circulation (75%), totaling 183 anterior lesions in 128 individuals (12% of Blacks, 7% of Whites). Posterior SBI were almost always singular, observed in 51 individuals (6% of Blacks, 2% of Whites), for a total of 55 lesions. The majority were localized to the thalamus (52%), supplied by branches of the PCA and basilar artery. Lacunes were observed in 129 participants (12% of Blacks, 7% of Whites), yielding 191 infarctions. Of these, 87 measured <7 mm, strongly suggesting lipohyalinosis (8% of Blacks, 5% of Whites), Figure 2. Compared to White study participants, Blacks were twice as likely to have multiple SBI lesions (6% vs. 3%; P = 0.001).
Figure 2.
Prevalence of silent brain infarction lesions among Black and White participants of Atherosclerosis Risk in Communities Study. Abbreviation: SBI, silent brain infarctions.
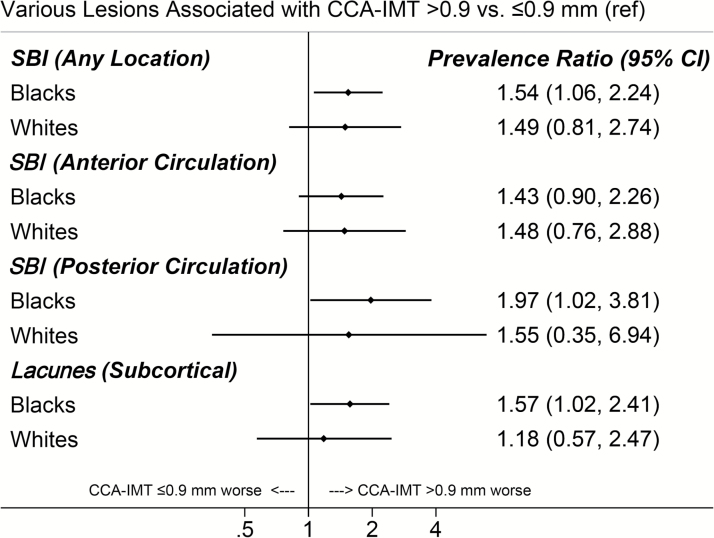
When the number of lesions per participant (ranging from 0 to 5) was analyzed, elevated CCA-IMT was significantly associated with greater number of anterior circulation SBI (PR = 1.54; 95% confidence interval [CI]: 1.06–2.24), posterior circulation SBI (PR = 1.57; 95% CI: 1.02–2.41), and lacunes (PR = 1.57; 95% CI: 1.09–2.26). When stratified by race, similar associations between elevated CCA-IMT and anterior SBI were observed for Whites and Blacks. However, elevated CCA-IMT was more strongly related to posterior SBI, lacunes, and small lacunes (<7 mm) in Black study participants (Figure 3).
Figure 3.
Prevalence ratios for numbers of silent brain infarctions (SBI), comparing Black and White participants of the Atherosclerosis Risk in Communities Study with elevated common carotid artery intima-media thickness (>0.9 mm) to those without. Abbreviations: CCA-IMT, intima-media thickness of the common carotid artery; CI, confidence interval; SBI, silent brain infarctions.
After adjustment for demographics (age, sex, and ARIC field center) and small vessel disease risk factors (smoking, hypertension, diabetes mellitus), elevated CCA-IMT remained strongly related to the number of lacunes in Black study participants (PR = 1.60; 95% CI: 1.02–2.51), but not in White study participants (PR = 0.80; 95% CI: 0.38–1.69); P value for interaction = 0.12. A similar trend was observed when limiting the outcome to lacunes <7mm, with a stronger association observed in Blacks (PR = 1.72; 95% CI: 0.97–3.05) compared to Whites (PR = 0.85; 95% CI: 0.35–2.04); P value for interaction = 0.18.
Among the subset of 837 participants (414 Blacks and 423 Whites) without concomitant plaque of either the right or left CCA, bifurcation, or ICA, 57 (9% of Blacks and 5% of Whites) were classified with elevated CCA-IMT. Compared to CCA-IMT <0.9 mm, elevated CCA-IMT was associated with twice the number of lacunes in Black participants without concomitant plaque (PR = 1.99; 95% CI: 1.002–3.96), after adjustments for demographics and small vessel disease risk factors. In contrast, elevated CCA-IMT was not related to number of lacunes in White participants (PR = 0.86; 95% CI: 0.26–2.79).
DISCUSSION
In this examination of stroke-free, middle aged and older adults from the general population, we make the following previously unreported observations: (i). Elevated CCA-IMT is more strongly related to lipohyalinosis (i.e., subcortical lacunes and small lacunes measuring <7 mm) in Black compared to White individuals. (ii) The relationship between elevated CCA-IMT and lacunes remains robust after controlling for vascular risk factors in Black, but not White, individuals; and (iii) The relationship between elevated CCA-IMT and lacunes persists in Black, but not White, individuals when limiting the sample to subjects without concomitant carotid artery plaque. Racial differences in the etiology of elevated CCA-IMT may have contributed to these associations. Hypertension is more prevalent in Black populations; thus, CCA-IMT measurements may capture more arterial medial hypertrophy than atherosclerotic pathology. A protective effect preventing carotid plaque formation in Black individuals may also have an influence. Although Black participants of the ARIC study had a greater overall number of vascular risk factors, paradoxically, carotid plaque was observed more frequently in White study participants.
Elevated IMT has long been considered a biomarker of subclinical atherosclerosis. However, the validity of this assumption may depend on the location and the method of measurement, and to date, no consensus for standardized IMT measurements exists.1 The CCA provides the most reproducible IMT measurements,2 but likely is more influenced by hypertensive processes than IMT measured from either the bifurcation or ICA, which are more atherogenic.1,3 It is also debatable whether IMT measurements should include, or avoid, regions of focal plaque.1 In the present study, CCA-IMT was measured from the far wall, without exclusion of focal plaques. Similar to previous reports,4 we observed distal plaques in the carotid bifurcation and ICA more frequently in White than Black study participants with elevated CCA-IMT. Consistent with these observations, in the Multi-Ethnic Study of Atherosclerosis (MESA), a lower incidence of carotid plaque was observed among Black study participants, despite higher baseline CCA-IMT values compared to White study participants.19
Previous studies examining IMT and SBI in the general population have largely been limited to Asian or White study participants.20 The Cardiovascular Health Study reported an incremental association between increasing quintiles of CCA-IMT and prevalence of SBI.8 In contrast, an analysis from the Framingham Study reported no association between CCA-IMT and prevalent SBI after adjustment for stroke risk factors, but a significant association between SBI and IMT of the more atherogenic ICA.10 This suggests the relationship between IMT and SBI may be driven more by atherosclerosis than blood pressure. In support of this, a hospital-based study of asymptomatic Japanese patients reported no association between CCA-IMT and SBI in patients without concomitant plaque in the carotid bifurcation.21 However, SBI arises from both embolic and hypertensive occlusion, and pathophysiologic relationships between elevated CCA-IMT and SBI likely depend on not only the SBI subtype, but also the population.
Similar to the Rotterdam Scan Study,9 we observed the majority of SBI within subcortical regions and the basal ganglia. Infarcts of these regions, or lacunes, are thought to arise from lipohyalinosis of the small deep penetrating vessels, particularly when lesions measure <7 mm in diameter.22 Thus, the common factor relating elevated CCA-IMT to lacunes may be hypertension.23 In support of this, lacunar SBI were observed in both brain hemispheres in a study of asymptomatic patients with unilateral carotid steno-occlusive disease,24 suggesting an etiology distinct from atheroembolism. In our analysis from the ARIC study, elevated CCA-IMT was related not only to SBI in the downstream anterior cerebral circulation, but also SBI of the posterior cerebral circulation, a vascular system separate from the carotid arteries. Because both the anterior and posterior circulation supply subcortical regions of the brain, lipohyalinosis of the small cerebral vessels may account for this finding. Of note, elevated CCA-IMT was more strongly related to posterior SBI in Black than White individuals.
However, our study should be viewed in the context of its limitations. Black study participants were recruited almost entirely from Jackson, MS, and may not generalize to all Black populations. We were also limited by the cross-sectional study design, which does not take duration of hypertension into account. Our investigation also relied upon somewhat dated imaging modalities, which have realized several technological advancements in recent years. On the other hand, our analysis has several important strengths. The ARIC Study allows a unique examination of brain MRI in stroke-free Black and White individuals from the general population, with sufficient sample to analyze CCA-IMT and several subtypes of SBI. Data were collected with quality assurance using standardized protocols, and all MRI scans were read and adjudicated by board-certified neuroradiologists.
In conclusion, elevated CCA-IMT is independently associated with lipohyalinosis in older Black adults, irrespective of vascular risk factors or presence of concomitant carotid artery plaque.
SUPPLEMENTARY DATA
Supplementary materials are available at American Journal of Hypertension online.
ACKNOWLEDGEMENTS
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN26820 1100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The authors thank the staff and participants of the ARIC study for their important contributions.
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging 2014; 7:1025–1038. [DOI] [PubMed] [Google Scholar]
- 2. Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation 1986; 74:1399–1406. [DOI] [PubMed] [Google Scholar]
- 3. Bots ML, Visseren FL, Evans GW, Riley WA, Revkin JH, Tegeler CH, Shear CL, Duggan WT, Vicari RM, Grobbee DE, Kastelein JJ; RADIANCE 2 Investigators . Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double-blind trial. Lancet 2007; 370:153–160. [DOI] [PubMed] [Google Scholar]
- 4. Mackinnon AD, Jerrard-Dunne P, Porteous L, Markus HS. Carotid intima-media thickness is greater but carotid plaque prevalence is lower in Black compared with White subjects. AJNR Am J Neuroradiol 2010; 31:1951–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation 1997; 96:1432–1437. [DOI] [PubMed] [Google Scholar]
- 6. Longstreth WT Jr, Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol 1998; 55:1217–1225. [DOI] [PubMed] [Google Scholar]
- 7. Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM; Rotterdam Scan Study . Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke 2003; 34:1126–1129. [DOI] [PubMed] [Google Scholar]
- 8. Manolio TA, Burke GL, O’Leary DH, Evans G, Beauchamp N, Knepper L, Ward B. Relationships of cerebral MRI findings to ultrasonographic carotid atherosclerosis in older adults: the Cardiovascular Health Study. CHS Collaborative Research Group. Arterioscler Thromb Vasc Biol 1999; 19:356–365. [DOI] [PubMed] [Google Scholar]
- 9. Vermeer SE, Den Heijer T, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM; Rotterdam Scan Study . Incidence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke 2003; 34:392–396. [DOI] [PubMed] [Google Scholar]
- 10. Romero JR, Beiser A, Seshadri S, Benjamin EJ, Polak JF, Vasan RS, Au R, DeCarli C, Wolf PA. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: the Framingham study. Stroke 2009; 40:1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fisher CM. Capsular infarcts: the underlying vascular lesions. Arch Neurol 1979; 36:65–73. [DOI] [PubMed] [Google Scholar]
- 12. Toole JF, Lefkowitz DS, Chambless LE, Wijnberg L, Paton CC, Heiss G. Self-reported transient ischemic attack and stroke symptoms: methods and baseline prevalence. The ARIC Study, 1987-1989. Am J Epidemiol 1996; 144:849–856. [DOI] [PubMed] [Google Scholar]
- 13. Karanjia PN, Nelson JJ, Lefkowitz DS, Dick AR, Toole JF, Chambless LE, Hayes R, Howard VJ. Validation of the ACAS TIA/stroke algorithm. Neurology 1997; 48:346–351. [DOI] [PubMed] [Google Scholar]
- 14. Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke 1999; 30:736–743. [DOI] [PubMed] [Google Scholar]
- 15. Duncan BB, Metcalf P, Crouse JR 3rd, Li R, Sharrett AR, Tegeler C, Tyroler HA, Heiss G. Risk factors differ for carotid artery plaque with and without acoustic shadowing. Atherosclerosis Risk in Communities study investigators. J Neuroimaging 1997; 7:28–34. [DOI] [PubMed] [Google Scholar]
- 16. Taylor J. 2013 ESH/ESC guidelines for the management of arterial hypertension. Eur Heart J 2013; 34:2108–2109. [PubMed] [Google Scholar]
- 17. Bryan RN, Cai J, Burke G, Hutchinson RG, Liao D, Toole JF, Dagher AP, Cooper L. Prevalence and anatomic characteristics of infarct-like lesions on MR images of middle-aged adults: the Atherosclerosis Risk in Communities study. AJNR Am J Neuroradiol 1999; 20:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- 18. Greenland S. Tests for interaction in epidemiologic studies: a review and a study of power. Stat Med 1983; 2:243–251. [DOI] [PubMed] [Google Scholar]
- 19. Tattersall MC, Gassett A, Korcarz CE, Gepner AD, Kaufman JD, Liu KJ, Astor BC, Sheppard L, Kronmal RA, Stein JH. Predictors of carotid thickness and plaque progression during a decade: the Multi-Ethnic Study of Atherosclerosis. Stroke 2014; 45:3257–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Finn C, Giambrone AE, Gialdini G, Delgado D, Baradaran H, Kamel H, Gupta A. The Association between carotid artery atherosclerosis and silent brain infarction: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis 2017; 26:1594–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Inoue K, Matsumoto M, Shono T, Toyokawa S, Moriki A. Increased intima media thickness and atherosclerotic plaques in the carotid artery as risk factors for silent brain infarcts. J Stroke Cerebrovasc Dis 2007; 16:14–20. [DOI] [PubMed] [Google Scholar]
- 22. Fisher CM. Lacunar strokes and infarcts: a review. Neurology 1982; 32:871–876. [DOI] [PubMed] [Google Scholar]
- 23. Blanco PJ, Müller LO, Spence JD. Blood pressure gradients in cerebral arteries: a clue to pathogenesis of cerebral small vessel disease. Stroke Vasc Neurol 2017; 2:108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baradaran H, Gialdini G, Mtui E, Askin G, Kamel H, Gupta A. Silent brain infarction in patients with asymptomatic carotid artery atherosclerotic disease. Stroke 2016; 47:1368–1370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.