A small molecule mimics the CD4 receptor for human immunodeficiency virus type 1, binds the viral envelope glycoprotein gp120, and blocks vaginal infection of BLT humanized mice.
Keywords: HIV-1, prevention, sexual transmission, envelope glycoprotein
Abstract
Background
Small-molecule CD4-mimetic compounds (CD4mc) inhibit human immunodeficiency virus (HIV-1) entry by blocking binding to the CD4 receptor and by premature triggering of the viral envelope glycoprotein (Env) spike.
Methods
The efficacy of a CD4mc in protecting bone marrow–liver–thymus (BLT) humanized mice from vaginal HIV-1 challenge was evaluated.
Results
Intravaginal application of the CD4mc JP-III-48, either before or simultaneously with virus challenge, protected BLT humanized mice from HIV-1JR-CSF infection in a dose- dependent manner.
Conclusion
The direct antiviral effects of a CD4mc prevent HIV-1 infection in a murine model of sexual transmission.
Without treatment, the vast majority of individuals infected with human immunodeficiency virus type 1 (HIV-1) develop AIDS. Combinations of antiretroviral drugs have greatly extended the length and quality of life of HIV-1–infected people. Efforts to prevent new HIV-1 infections, a goal important for curbing the AIDS pandemic, have been less successful. Over 36 million people are currently infected with HIV-1, and 2–3 million new infections occur annually [1]. Most new HIV-1 infections result from sexual transmission of the virus. In many regions of the world, young women are at particular risk of HIV-1 infection, and AIDS-related illnesses remain the leading cause of death in women of reproductive age (ie, those aged 15–44 years). Despite extensive efforts over the last 30 years, a vaccine to prevent HIV-1 infection has not been developed. Therefore, the development and application of prophylactic modalities to stop HIV-1 transmission represent major global health priorities.
HIV-1 infection would be most effectively prevented by interrupting early events in the virus life cycle that occur prior to the formation of the provirus. HIV-1 entry into host cells is mediated by the envelope glycoprotein (Env) trimer, which is composed of 3 gp120 exterior glycoproteins and 3 gp41 transmembrane glycoproteins [2]. The metastable Env spike is triggered to undergo conformational changes required for viral entry by sequential binding to the host cell receptors, CD4 and either CCR5 or CXCR4. The lability and accessibility of Env on the virion surface make it an attractive target for prophylactic interventions.
Small-molecule CD4-mimetic compounds (CD4mc) bind HIV-1 gp120 within a well-conserved pocket used for the interaction with CD4 [3–6]. In addition to inhibiting CD4 binding, CD4mc prematurely trigger Env, leading to irreversible inactivation [7, 8]. Subneutralizing concentrations of CD4mc sensitize HIV-1 and HIV-1–infected cells to antibodies mediating virus neutralization and antibody-dependent cell cytotoxicity, respectively. Here we compare the antiviral breadth and potency of 2 CD4mc, JP-III-48 [9] and one of its early predecessors, JRC-II-191 [3], in tissue culture. We also evaluate the contribution of the direct antiviral effect of CD4mc to protection of humanized mice from vaginal HIV-1 infection.
METHODS
CD4mc Synthesis
JRC-II-191 and JP-III-48 were designed and synthesized from commercially available materials as previously described [3, 9]. Spectroscopic and physical data were in agreement with previously reported values.
Recombinant HIV-1 Expressing Luciferase
293T human embryonic kidney cells were cotransfected with a plasmid expressing the pCMVΔP1Δenv HIV-1 Gag-Pol packaging construct, a plasmid expressing the HIV-1 envelope glycoproteins (from the HIV-1 strains BG505, 191, JR-FL, YU2, AD8, JR-CSF, 1012, C5, Ce0393, and 3016) or the envelope glycoprotein of the control amphotropic murine leukemia virus (A-MLV), and the firefly luciferase-expressing vector at a DNA ratio of 1:1:3 μg, using the Effectene transfection reagent (Qiagen), as described previously [8]. Cotransfection produced recombinant, luciferase-expressing viruses capable of a single round of infection. The virus-containing supernatants were harvested 36–40 hours after transfection and cleared of debris by low-speed centrifugation. Aliquots of the virus preparations were frozen at −80°C until further use. The reverse transcriptase levels of all virus stocks were measured as described previously [4, 8].
Infection of Single-Round Recombinant Viruses
Cf2Th-CD4/CCR5 target cells were seeded at a density of 6 × 103 cells/well in 96-well luminometer-compatible tissue culture plates (PerkinElmer) 24 hours before infection. To test the direct antiviral activity of the CD4mc, on the day of infection, JRC-II-191 or JP-III-48 (0–100 μM) was incubated with recombinant viruses (10000 reverse transcriptase units) at 37°C for 30 minutes. The mixtures were then added to the target cells and incubated for 48 hours at 37°C. After this time, the medium was removed from each well, and the cells were lysed by the addition of 30 μL of passive lysis buffer (Promega), with 3 freeze-thaw cycles. An EG&G Berthold LB 96V microplate luminometer was used to measure the luciferase activity of each well after the addition of 100 μL of luciferin buffer (15 mM MgSO4, 15 mM KPO4, pH 7.8, 1 mM ATP, and 1 mM dithiothreitol) and 50 μL of 1 mM 99% firefly d-luciferin free acid (Prolume).
BLT Humanized Mice
BLT humanized mice were NOD/SCID/IL2Rγ-/- (NSG) mice bearing humanized bone marrow following reconstitution with CD34+ cells from human fetal liver and a surgical human thymic graft, as described previously [10]. Consistency of HIV-1 infection after virus challenge was achieved by using only mice with high levels of human immune reconstitution (ie, those for which >25% of peripheral blood cells were within a lymphocyte gate on forward-versus-side scatter plots, >50% of cells in the lymphocyte gate are human [hCD45+/mCD45−], and >40% of human cells in the lymphocyte gate are T cells [hCD3+]). In absolute numbers, each mouse in the study had >200 human CD4+ cells per 100 μL of murine blood. To achieve more-uniform susceptibility to infection by the mucosally applied HIV, mice were treated with 2 mg of medroxyprogesterone subcutaneously (Besse, New York, NY) approximately 1 week prior to HIV-1 challenge. This treatment synchronizes the estrus cycle of the mice, allowing their vaginal epithelium to be at a comparable thickness at the time of HIV-1 challenge [11]. Equal numbers of mice generated from a single human fetal donor were assigned to each experimental group. Occasionally in our experimental system, some mice die from a transplantation-related wasting syndrome. These deaths occur equally in HIV-1–infected and –uninfected animals and are not related to viral load, CD4+ T-cell counts, or treatment. In the 6-week course of the HIV-1 challenge studies reported here, none of the mice died from this wasting syndrome. Animal work was approved by the Institutional Animal Care and Use Committee of the Massachusetts General Hospital.
HIV-1 Infection of BLT Humanized Mice
BLT humanized mice were infected with HIV-1JR-CSF [12, 13] with an Env sequence that matches GenBank sequence M38429, with 2 exceptions (R525A and G666D). For HIV-1 infection of BLT humanized mice, 2 protocols were followed: (1) 105 50% tissue culture infective doses (TCID50) of HIV-1JR-CSF diluted in phosphate-buffered saline (PBS) was mixed with different concentrations of JP-III-48 in a 20-μL final volume and applied atraumatically to the vaginal vault, using 20-μL XL pipette tips (Neptune Scientific, San Diego, CA); and (2) 30 minutes after 10 μL of different concentrations of JP-III-48 in a dimethyl sulfoxide (DMSO)/PBS mixture was applied intravaginally, a 10-μL virus suspension (105 TCID50) was applied, both atraumatically. In both protocols, the negative control consisted of a solution of DMSO in PBS at a concentration equivalent to that in the 104-µM dose of JP-III-48; the negative control was used at the same volume and the anesthesia applied for the same length of time as for the JP-III-48 samples. Pilot experiments indicated that 100% of control mice became infected when 105 TCID50 of HIV-1JR-CSF was used. Plasma viral RNA was monitored using reverse-transcription quantitative polymerase chain reaction analysis weekly for 8 weeks following inoculation. Mice that exhibited viremia on >1 occasion were considered infected.
RESULTS
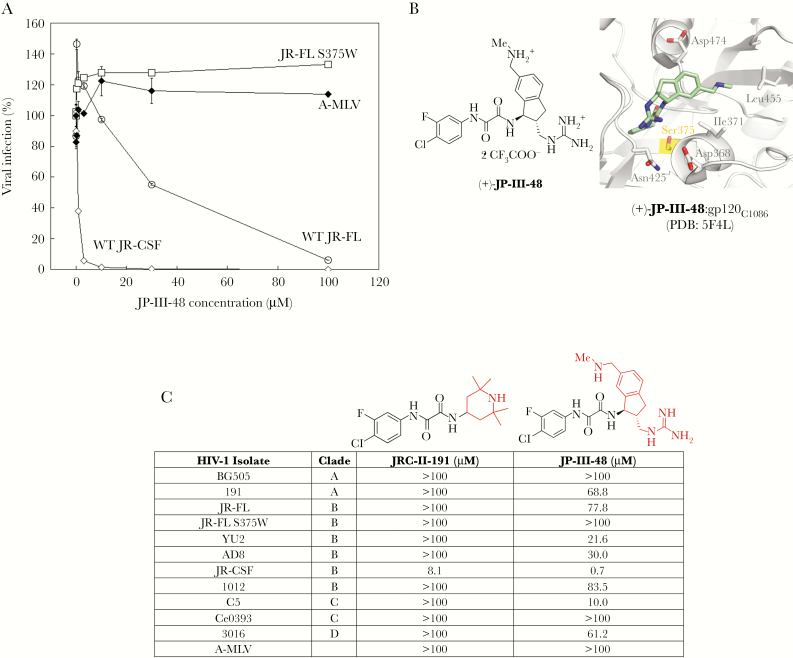
The ability of the CD4mc JP-III-48 to inhibit a single round of HIV-1 infection was evaluated. JP-III-48 specifically inhibited the wild-type HIV-1JR-CSF and HIV-1JR-FL, with 50% inhibitory concentrations of 0.7 μM and 37 μM, respectively (Figure 1A). As expected, the HIV-1JR-FL S375W mutant, in which the gp120 Phe 43 cavity is occupied by the indole ring of the substituted tryptophan residue [4–6], was resistant to the JP-III-48 inhibitory activity. This result is consistent with the binding of JP-III-48 to the Phe 43 cavity (Figure 1B). Although HIV-1JR-CSF is derived from the same HIV-1–infected subject as HIV-1JR-FL (with 92.3% amino acid identity) [12, 13], the former virus was more sensitive to JP-III-48. Apparently, some of the sequence differences between HIV-1JR-CSF and HIV-1JR-FL Envs account for the increased triggerability of the former Env by CD4mc. No inhibition was observed for HIV-1 pseudotyped with the A-MLV Env (Figure 1A). JP-III-48 exhibited a greater breadth of antiviral activity than the early CD4mc predecessor, JRC-II-191 (Figure 1C).
Figure 1.
Inhibition of human immunodeficiency virus type 1 (HIV-1) infection by the small-molecule CD4-mimetic compound (CD4mc) JP-III-48. A, Recombinant HIV-1 encoding luciferase was pseudotyped with envelope glycoproteins from HIV-1JR-CSF, HIV-1JR-FL (wild type [WT] or the S375W mutant), or the amphotropic murine leukemia virus (A-MLV). Viruses were incubated with Cf2Th-CD4/CCR5 cells in the presence of the indicated concentrations of the CD4mc JP-III-48. After 48 hours, the luciferase activity in the target cells was measured. The level of infection relative to that seen in the absence of the compound is reported. The means and standard errors from triplicate samples in a typical experiment are reported. The findings shown are representative of those from 3 independent experiments. B, The chemical structure of JP-III-48 is shown on the left. On the right is shown the crystal structure of a complex of JP-III-48 and the HIV-1C1086 gp120 glycoprotein core (PDB 5F4L) [5]. Some of the gp120 residues near the Phe 43 cavity are shown. Note that substitution of Ser 375, highlighted in yellow, with amino acid residues with large side chains fills the Phe 43 cavity and creates steric barriers to the binding of CD4mc. C, Fifty percent inhibitory concentrations (IC50) of 2 CD4mc for recombinant HIV-1 pseudotyped with Envs from the indicated HIV-1 strains or A-MLV are reported. The activity of JP-III-48, which has an indane ring, is compared to that of its predecessor, JRC-II-191, which has a piperidine ring.
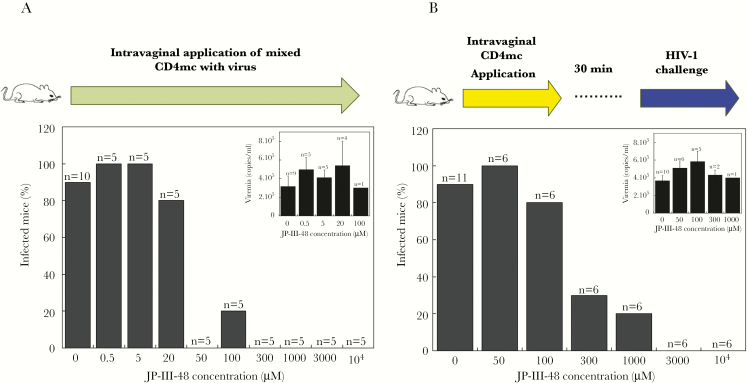
To test the ability of JP-III-48 to block HIV-1 acquisition, we used a murine model of HIV-1 sexual transmission. BLT humanized mice contain human immune cells (macrophages, dendritic cells, and CD4+ T cells) in many tissues, including the female reproductive tract [10]. Vaginal exposure of BLT humanized mice to 105 TCID50 HIV-1JR-CSF has been shown to result in 50%–100% of the inoculated animals exhibiting viremia as early as 2 weeks later [10]. A high-dose inoculum of HIV-1JR-CSF was either mixed with different concentrations of JP-III-48 for 30 minutes before atraumatic application to the vaginal tract (Figure 2A), or it was applied vaginally 30 minutes after vaginal application of different concentrations of JP-III-48 (Figure 2B). In both HIV-1 challenge experiments, JP-III-48 reduced the percentage of infected mice in a dose-dependent manner. Higher concentrations of JP-III-48 were required to block HIV-1JR-CSF infection when the virus inoculation was delayed for 30 minutes after application of JP-III-48 to the vaginal tract. The average levels of viremia observed in the infected mice were similar in the animals treated with different JP-III-48 concentrations (Figure 2A and 2B). These results indicate that the presence of the CD4mc at the site of sexual exposure to HIV-1 protects BLT humanized mice from HIV-1 infection.
Figure 2.
Protection of bone marrow–liver–thymus (BLT) humanized mice from vaginal human immunodeficiency virus type 1 (HIV-1) strain JR-CSF challenge. Ten microliters of a saline solution of the small-molecule CD4-mimetic compound (CD4mc) JP-III-48 at the indicated concentrations was applied intravaginally to female BLT humanized mice. A, JP-III-48 and the HIV-1JR-CSF challenge virus were mixed and incubated at room temperature for 30 minutes before intravaginal application. B, JP-III-48 was applied to the vaginal tract first, and 30 minutes later, mice were challenged vaginally with 10 μL of an HIV-1JR-CSF suspension. The percentage of infected mice is shown; n values denote the number of mice per group. The inset in each panel shows the average level of viremia in the infected mice treated with different concentrations of CD4mc; n values denote the number of infected mice.
Discussion
This study explored the potential of a CD4mc, JP-III-48, to inhibit HIV-1 acquisition following atraumatic exposure of the vaginal mucosa to a virus inoculum. JP-III-48 is a CD4mc analogue that contains an indane ring; we previously demonstrated that earlier-generation indane-bearing CD4mc analogues exhibit higher antiviral potency and breadth than their piperidine-bearing congeners [3, 9]. This result is consistent with our comparison of JRC-II-191 (with a piperidine moiety) and JP-III-48 (with an indane ring) (Figure 1C). JP-III-48 indeed inhibits more HIV-1 variants than JRC-II-191. Some natural HIV-1 variants, like HIV-1BG505 in clade A and HIV-1Ce0393 in clade C, were not inhibited by JP-III-48. This observation highlights the importance of further modifying CD4mc to optimize their binding to Env, antiviral potency, and breadth of coverage.
HIV-1JR-CSF, the challenge virus in the BLT humanized mouse model, was particularly sensitive to JP-III-48, with an 50% inhibitory concentration of 700 nM. Nonetheless, considerably higher JP-III-48 concentrations were required to achieve complete protection of the BLT humanized mice. This was true even in the experimental format where JP-III-48 was premixed with the challenge virus prior to application to the vaginal tract. This suggests that the events leading to infection in the BLT humanized mouse model may be even more efficient than those in our single-round tissue culture infection assay. This underscores the need to improve the potency of CD4mc if we are to rely only on their direct antiviral effects for protection. Nonetheless, our results establish the proof of principle that these direct anti-HIV-1 effects of CD4mc can contribute to protection from a vaginal HIV-1 challenge.
In addition to their direct antiviral effects, CD4mc sensitize HIV-1 and HIV-1–infected cells to neutralization and lysis, respectively, by otherwise ineffectual antibodies [14, 15]. Recently, a CD4mc has been shown to synergize with gp120-induced antibodies to protect monkeys from a high-dose mucosal challenge with a heterologous transmitted/founder simian- human immunodeficiency virus (Madani et al, unpublished observations). Thus, particularly if used in combination with Env vaccines, CD4mc could afford multiple levels of protection against HIV-1 acquisition.
Notes
Acknowledgments. We are grateful to the Ragon Institute Virology Core for supplying the viral stocks for BLT humanized mouse infections.
N. M. and J. S. designed the study. B. M., J. P., and A. B. S. III synthesized the CD4mc. A. M. P., V. D. V., A. M. T., and N. M. performed the BLT humanized mouse experiments. N. M. and J. S. wrote the first draft of the manuscript. All authors approved the final draft of the manuscript.
This manuscript is dedicated to the memory of our beloved colleague and collaborator Andrew M. Tager.
Disclaimer. The funder had no role in study design, data collection, analysis, interpretation of the results, and the writing of the manuscript.
Financial support. This study was supported by the National Institutes of Health (grants AI134494, AI124982, and GM56550; grant AI90682 to N. M.), the late William F. McCarty-Cooper, amfAR (grant 107431-45-RFNT to N. M.), and the Ragon Institute (Innovation Award to N. M.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global health observatory data 2017. http://www.who.int/gho/hiv/en/. Accessed 8 August 2017.
- 2. Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 1998; 280:1884–8. [DOI] [PubMed] [Google Scholar]
- 3. Courter JR, Madani N, Sodroski J, et al. Structure-based design, synthesis and validation of CD4-mimetic small molecule inhibitors of HIV-1 entry: conversion of a viral entry agonist to an antagonist. Acc Chem Res 2014; 47:1228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Madani N, Schön A, Princiotto AM, et al. Small-molecule CD4 mimics interact with a highly conserved pocket on HIV-1 gp120. Structure 2008; 16:1689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kwon YD, LaLonde JM, Yang Y, et al. Crystal structures of HIV-1 gp120 envelope glycoprotein in complex with NBD analogues that target the CD4-binding site. PLoS One 2014; 9:e85940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Curreli F, Kwon YD, Belov DS, et al. Synthesis, antiviral potency, in vitro ADMET, and X-ray structure of potent CD4 mimics as entry inhibitors that target the Phe43 cavity of HIV-1 gp120. J Med Chem 2017; 60:3124–53. [DOI] [PubMed] [Google Scholar]
- 7. Haim H, Si Z, Madani N, et al. Soluble CD4 and CD4-mimetic compounds inhibit HIV-1 infection by induction of a short-lived activated state. PLoS Pathog 2009; 5:e1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Madani N, Princiotto AM, Zhao C, et al. Activation and inactivation of primary human immunodeficiency virus envelope glycoprotein trimers by CD4-mimetic compounds. J Virol 2017; 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Melillo B, Liang S, Park J, et al. Small-molecule CD4-mimics: structure-based optimization of HIV-1 entry inhibition. ACS Med Chem Lett 2016; 7:330–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brainard DM, Seung E, Frahm N, et al. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J Virol 2009; 83:7305–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vargas G, Shilagard T, Johnston R, et al. Use of high- resolution confocal imaging of the vaginal epithelial microstructure to detect microbicide toxicity. J Infect Dis 2009; 199:1546–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koyanagi Y, Miles S, Mitsuyasu RT, Merrill JE, Vinters HV, Chen IS. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science 1987; 236:819–22. [DOI] [PubMed] [Google Scholar]
- 13. O’Brien WA, Koyanagi Y, Namazie A, et al. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature 1990; 348:69–73. [DOI] [PubMed] [Google Scholar]
- 14. Madani N, Princiotto AM, Schön A, et al. CD4-mimetic small molecules sensitize human immunodeficiency virus to vaccine-elicited antibodies. J Virol 2014; 88:6542–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richard J, Veillette M, Brassard N, et al. CD4 mimetics sensitize HIV-1-infected cells to ADCC. Proc Natl Acad Sci U S A 2015; 112:E2687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]