The envelope glycoprotein B of the human cytomegalovirus is a ligand for the lectin DC-SIGN. Here, the authors characterized this interaction at the molecular level. This should help to design new specific antiviral drugs.
Keywords: antibody-mediated blockade, attachment, cytomegalovirus, DC-SIGN, glycoprotein B
Abstract
Background
Human cytomegalovirus (HCMV) is a leading cause of virally induced congenital disorders and morbidities in immunocompromised individuals, ie, transplant, cancer, or acquired immune deficiency syndrome patients. Human cytomegalovirus infects virtually all cell types through the envelope glycoprotein complex gH/gL/gO with or without a contribution of the pentameric gH/gL/pUL128L. Together with gH/gL, the HCMV envelope glycoprotein B (gB) contributes to the viral fusion machinery.
Methods
We previously showed that gB is a ligand for the C-type lectin dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) contributing to HCMV attachment to and infection of DC-SIGN-expressing cells. However, the features of the DC-SIGN/gB interaction remain unclear. To address this point, the role of glycans on gB and the consequences of mutagenesis and antibody-mediated blockades on both partners were examined in this study.
Results
We identified DC-SIGN amino acid residues involved in this interaction through an extensive mutagenesis study. We also showed the importance of high-mannose N-glycans decorating the asparagine residue at position 208, demonstrating that the antigenic domain 5 on gB is involved in the interaction with DC-SIGN. Finally, antibody-mediated blockades allowed us to identify DC-SIGN as a major HCMV attachment receptor on monocyte-derived dendritic cells.
Conclusions
Taken together, these results have permitted us to fine-map the interaction between DC-SIGN and HCMV gB.
Human cytomegalovirus (HCMV) is a highly prevalent beta-herpesvirus, ie, 40–90 worldwide. The virus is usually asymptomatic, yet it causes diseases in newborns and immunocompromised hosts, eg, transplant, cancer, and acquired immune deficiency syndrome patients [1]. Although neutralizing antibodies (NAb) were shown to target the pentameric complex [2, 3], the HCMV envelope glycoprotein B (gB) remains a target for the humoral response [4, 5]. Human cytomegalovirus gB was recently shown to assemble with gH/gL to form the fusion machinery that enables virus to penetrate cells [6, 7]. Five gB antigenic domains ([AD] 1 to 5) are recognized by NAb [8]. Human cytomegalovirus gB belongs to the class III fusion proteins with the herpes simplex virus (HSV)-1 gB [9], the Epstein-Barr virus gB [10], the baculovirus gp64 [11], the Thogotovirus glycoprotein [12], as well as the prototypical vesicular stomatitis virus (VSV)-G envelope glycoprotein [13].
Human cytomegalovirus gB interacts with the dendritic cell-specific intercellular adhesion molecule (ICAM)-3-grabbing nonintegrin (DC-SIGN) [14]. Dendritic cell-SIGN is expressed by immature monocyte-derived dendritic cells (MDDC) and macrophage subsets [15, 16]. It is a type II C-type lectin composed of a short N-terminal intracytoplasmic tail, seven and a half conserved 23-amino acid repeats forming the “neck” region involved in tetramerization [17–19], and a C-terminal calcium-dependent carbohydrate-recognition domain (CRD) containing 2 calcium sites enabling ligand binding [15]. Dendritic cell-SIGN tetramers display a high avidity for fucose or high-mannose sugars [20]. It recognizes endogenous ligands such as ICAM-2 and -3 known to stabilize interactions between DC and endothelial cells or naive T-cells, respectively [15, 21]. It also functions as a receptor for many viral glycoproteins (for review, see [22]). Nevertheless, the interaction between DC-SIGN and gB remains poorly characterized. To address this point, we examine the role of glycans, mutagenesis, and antibody-mediated blockades.
METHODS
Ethic Statements
Blood samples from anonymous healthy volunteers were obtained from the Etablissement Français du Sang, the French blood bank (EFS, Nantes, France) in accordance with authorizations by the “Ministère français de l’enseignement supérieur et de la recherche” and the “Comité de protection des personnes” (Agreement numbers: DC-2013-1832/DC-2014-2206).
Cells
Monocytes were isolated by immunomagnetic separation (Miltenyi Biotec, Bergisch Gladbach, Germany) or by elutriation (DTC, Nantes University Hospital/Biogen Ouest, Nantes, France) from healthy donors (>95% purity). Monocytes were differentiated into MDDC as previously described [23] with 20 ng/mL recombinant human interleukin-4 (CellGenix, Freiburg, Germany), 100 ng/mL granulocyte-macrophage colony-stimulating factor (Gentaur, Paris, France), and 2% human serum albumin (Vialebex, LFB, Coutaboeuf, France). U937, HEK293T cells (American Type Culture Collection number CRL-3216; LGC Standards, Teddington, UK), and MRC-5 (RD Biotech, Besançon, France) were propagated as described elsewhere [14].
Viruses and Reagents
TRI, an in-house clinical isolate (no sequence data available yet), 3 low passage HCMV laboratory strains (Toledo, TB40/E-GFP and VHL/E; a gift from Dr. Christian Sinzger) [24, 25], and BAC4-based HCMV gB-mutated viruses were propagated on MRC-5 cells and used as clarified viral supernatants for trans-infection experiments at a multiplicity of infection (MOI) ranging from 2 to 10. Cytomegalovirus (CMV) supernatants were also tittered by quantitative polymerase chain reaction (qPCR) as described elsewhere [26]. Chinese hamster ovary (CHO)-derived recombinant HCMV gB and human immunodeficiency virus (HIV)-1 IIIB gp120 were purchased from Sanofi Pasteur (Marcy l’Etoile, France) and ImmunoDx (Woburn, MA), respectively. Glycoproteins were used either as purified or fluorescence-conjugated reagents (Molecular Probes, Thermo Fisher Scientific, Waltham, MA). Antibodies against AD-4/5 (clones SM5-1 and 1G2) and AD-1/2 (clones ITC33, ITC39, ITC48, ITC52, ITC63, and ITC88) [27] were kindly provided by Professor Michael Mach and Dr. Mats Ohlin, respectively. A polyclonal antibody (pAb) against gB was purchased from Sino Biological ([10202-RP01-100], Beijing, China). Fifteen antihuman DC-SIGN antibodies from Dendritics SA (Lyon, France) were used in this study. Four clones, 103G2.07, 111E3.04, 102E11.06, and 111H2.02, are directed against the neck region (amino acide [AA] 96–257), whereas 9 (clones 104B4.01, 103F12.01, 109H12.03, 113B11.02, 106A4.01, 120E12.03, 108H8.05, 105E9.01, and 114F1.08) were targeting the CRD (AA, 258–404). We considered that clone 108C7.01 recognized both the extracellular proximal region ([ECPR] AA 59–95) and the CRD, whereas clone 102F10.04 bound to the ECPR alone (see Supplementary Table 2). Four other commercially available anti-DC-SIGN monoclonal antibodies (mAb), clones MR1 (Bio-Rad AbDSerotec, Oxford, UK), AZN-D1 (Beckman Coulter France, Villepinte, France), 1B10, and an anti-neck (H200, Santa Cruz Biotechnology Inc., Heidelberg, Germany), were also used here. Antibodies targeting platelet-derived growth factor receptor (PDGFR)α (aR1), the β1 chain integrin (MAR4), and the αVβ3 integrin (23C6) were purchased from BD Biosciences (Franklin Lakes, NJ). Antibodies were used at 20 µg/mL for blockade experiments.
Viral Envelope Glycoprotein Binding Assay
Cells were resuspended in a binding buffer (Tris-buffered saline, 1 mM CaCl2, 2 mM MgCl2; 0.1% bovine serum albumin) and seeded in 96-well plates at 1 × 105 cells/wells. Dendritic cell-SIGN/gB interaction blockade was performed by incubating cells with antibodies for 30 minutes at 4°C. Conjugated gB or HIV-1 IIIB gp120 (2 µg/mL) were added to cells for 20 minutes at 4°C without washing. After 3 washes with ice-cold binding buffer, cells were analyzed on a LSR II flow cytometer (BD Biosciences, Franklin Lakes, NJ) with FlowJo (Tree Star, Ashland, OR). Surface plasmon resonance was carried out with soluble gB and recombinant DC-SIGN extracellular domain [28].
Transinfection Experiments
Cells were incubated with blockers for 30 minutes at 4°C if necessary before adding virus (MOI = 2) without washing. After a subsequent 2-hour incubation at 37°C, cells were washed and cultured with 70%–80% confluent MRC-5 cells for 48–72 hours. Cells were fixed in acetone/water 9:1 (volume/volume) and further stained with an Alexa Fluor 488-conjugated anti-IE/E antigen antibody (8B1.2; Millipore, Burlington, MA). Percentages of IE/E+ cells were determined on 4 fields per condition with Fiji (SciJava consortium; http://scijava.org/).
Statistical Analysis
Statistical tests were performed using the Graph Pad Prism 5.0 software (Graph Pad Software Inc., La Jolla, CA). On-way analysis of variance was applied to all data of this manuscript. P values below or equal to .05 were considered significant. Additional materials and methods are available in Supplementary Materials.
RESULTS
Dendritic Cell-Specific Intercellular Adhesion Molecule-3-Grabbing Nonintegrin Binds to Glycoprotein B Through Its Carbohydrate Recognition Domain
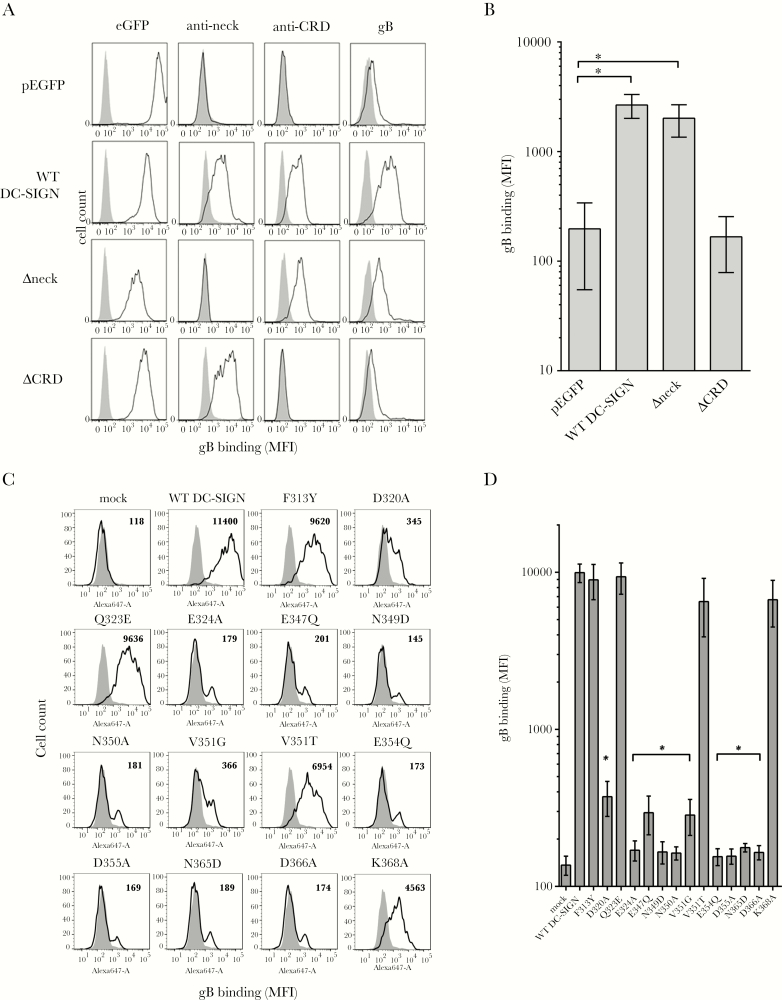
Although HCMV gB is known as a DC-SIGN ligand, it is not clear whether this interaction is restricted to the DC-SIGN CRD [14]. To that purpose, HEK293T cells were modified to express wild-type (WT) DC-SIGN (AA 1–404; UnitProtKB, Q9NNX6) or 2 deletion mutants, respectively, lacking neck repeats (AA 1–80 in frame with AA 253–404, called Δneck) or the CRD (AA 1–252, called ΔCRD) in fusion with the enhanced green fluorescent protein (eGFP) [29]. All cells expressed comparable eGFP levels and DC-SIGN cell surface expression as well (Figure 1A). We showed that gB interacts with CRD-containing DC-SIGN molecules and does not require the neck repeats (Figure 1A and B).
Figure 1.
Dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) binds the glycoprotein B (gB) through its carbohydrate recognition domain. (A) Histograms showing DC-SIGN expression of wild-type (WT) DC-SIGN or deletion mutants lacking the DC-SIGN neck repeat (Δneck) or the carbohydrate-recognition domain ([CRD] ΔCRD) regions fused to enhanced green fluorescent protein (eGFP). The eGFP allowed a rapid quantitation of the DC-SIGN expression level on stably transfected HEK293T (left panels), except for the pEGFP-transfected cells (first line). The 2 centered columns represent extracellular staining of DC-SIGN with an antineck (clone H-200) and an anti-CRD (clone 1B10) antibody, respectively. The ability of DC-SIGN variants to bind recombinant biotinylated human cytomegalovirus (HCMV) gB is represented in right panels. Gray histograms display nontransfected HEK293T cell fluorescence background. (B) Quantitative measurements of the binding of recombinant biotinylated HCMV gB (2 µg/mL) onto WT DC-SIGN or Δneck- and ΔCRD-expressing cells compared with a control cell line (pEGFP). Biotinylated HCMV gB was revealed with 1 µg/mL antigen-presenting cell-conjugated streptavidin. Values are expressed as mean fluorescence intensities (n = 4; *, P < .05; one-way analysis of variance [ANOVA] with multiple comparison tests). (C) Histograms showing the binding of recombinant Alexa Fluor 647-conjugated HCMV gB (4 µg/mL, mean fluorescence intensity [MFI]) on HEK293T cell lines expressing WT or mutated DC-SIGN on their surface. Values indicated for each histogram represent MFI. These results are representative of 3 independent experiments. (D) Quantitative results showing the behavior of mutated DC-SIGN compared with the WT form towards the binding of recombinant Alexa Fluor 647-conjugated HCMV gB (n = 3). Statistically significant results were marked by an asterisk (*, P < .05; one-way ANOVA with multiple comparison tests).
Then, we sought to identify CRD AA involved in this interaction. We hypothesized that AA taking part to the calcium ion coordination or sugar binding could be detrimental [20, 30]. Single-point mutants were generated and further expressed in HEK293T cells. Antineck staining showed similar DC-SIGN expression across all cell lines (Supplementary Figure 1). Their ability to bind gB was then assessed by flow cytometry (Figure 1C). E347, N349, E354, N365, and D366 form the calcium binding “site 2” and enable contact with high-mannose sugars as well [30, 31]. Expectedly, mutations at these positions precluded interaction with gB (Figure 1D). Similarly, mutants D320A, E324A, N350A, and D355A lost their ability to optimally bind gB, assuming that it was likely due to substantial fold changes in the calcium binding “site 1” as proposed for HIV-1 gp120 [32]. Here, F313Y, Q323E, and K368A DC-SIGN mutations were ineffective (Figure 1D). Moreover, we confirmed that the E354Q within site 2 broke the interaction [33]. The V351 residue was shown to discriminate between endogenous and pathogen-derived ligands such as ICAM-3 and HIV-1 gp120 or hepatitis C virus E1/E2, respectively [32, 34, 35]. In this study, we analyzed 2 mutations, ie, V351G and V351T. The V351G mutant lost its binding capacity to gB, suggesting that this AA is as important as its human herpesvirus (HHV)-8 counterpart and ICAM-3 [36]. It is interesting to note that a methyl group substitution of the V351 by a hydroxyl group of a threonine conserved a nominal binding activity, suggesting that subtle variations in the DC-SIGN fold could determine its ability to recognize unrelated ligands. Taken together, these results demonstrate that gB utilizes the same CRD AA as the HHV-8 gB or even ICAM-3.
Recombinant Glycoprotein B Contains High-Mannose Sugars
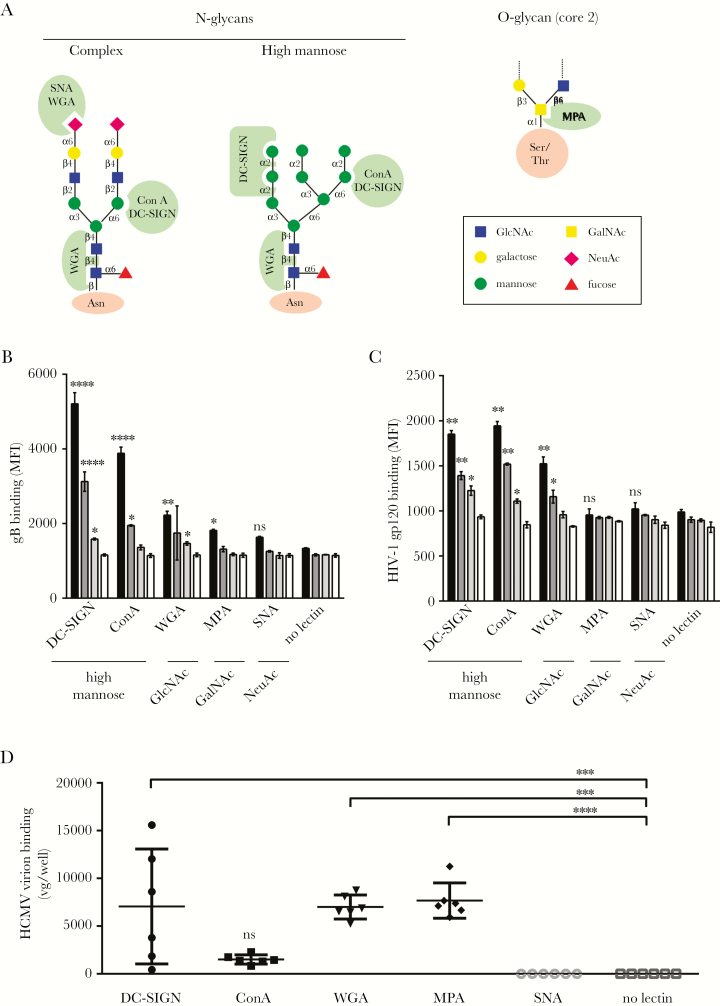
Glycoprotein B and HIV-1 gp120 are both able to bind to DC-SIGN, although their respective folds are distinct. We postulated that their glycan covers might be similar. Prototypical N- or O-glycans are depicted in the Figure 2A. Culture wells were coated with various lectins from plant or mammalian origins to characterize glycans on CHO-derived gB. Human immunodeficiency virus-1 gp120 was used as a control of a high mannose-containing glycoprotein. In line with other pioneering studies on gp120 [20], we observed dose-dependent binding of both glycoproteins to DC-SIGN, concanavalin A, and, to a lesser extent, to wheat germ agglutinin (WGA), suggesting that high mannose-containing N-glycans were decorating gB and gp120 (Figures 2B and C). Binding to Datura stramonium Agglutinin, and also to a lesser extent to WGA (due to its double specificity), confirmed the presence of N-acetylglucosamine. A weak binding to Maclura pomifera agglutinin (MPA) at the highest gB concentration suggested that O-glycans were almost absent. No significant signal was observed with Sambucus nigra agglutinin.
Figure 2.
Recombinant soluble or particle-associated human cytomegalovirus (HCMV) glycoprotein B (gB) preferentially interacts with high mannose-specific and N-glycan-specific lectins. (A) Schematic representation of typical N- or O-glycans potentially harbored by glycoproteins. Sugar linkage is indicated between sugar residues. Green zones represent lectin specificities. Lectin binding assay revealing interactions between Alexa 488-conjugated gB (B) or human immunodeficiency virus (HIV)-1 IIIB gp120 (C) and various recombinant plant and animal lectins of known specificities immobilized on plastic 96-well plates. Serial dilutions of each viral glycoprotein were used in this assay: 10 µg/mL (black), 2.5 µg/mL (dark gray), 0.4 µg/mL (light gray), and no gB (open bars). Lectin specificities are indicated beneath each lectin. Results are shown as mean fluorescence intensity ([MFI] n = 3). The “no lectin” condition was used for both recombinant glycoproteins as a negative control providing a basal level of unspecific binding for fluorescence-labeled glycoproteins. (D) Binding of HCMV infectious particles (TB40/E strain, BAC4) to plastic-immobilized plant and animal lectins. A single input of virus was equivalent to 8 × 106 viral genomes/well (vg/well). Results are expressed as vg/well (n = 6). Mean values are represented by horizontal bars for each type of lectin (****, P < .0001; ***, P < .001; **, P < .01; *, P < .05; one-way analysis of variance with multiple comparison tests). Abbreviations: DC-SIGN, dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin; ConA, concanavalin A; MPA, Maclura pomifera agglutinin; ns, nonsignificant; SNA, Sambucus nigra agglutinin; WGA, wheat germ agglutinin.
Next, we used whole HCMV virions to perform the same kind of experiments. Here, virus attachment to lectins was quantified by qPCR. Again, DC-SIGN and WGA retained HCMV particles, suggesting the presence of high-mannose sugars on viral particles. It is interesting to note that O-glycans were readily recognized by MPA on virions most likely on glycoproteins other than gB. Apparently, no sialic acid (SA) residues could be detected on virions. These results demonstrated that CHO-derived gB and HIV-1 gp120 are decorated with high-mannose sugars on N-glycans. We also showed that gB and whole HCMV particles share similar glycans contents except for O-glycans.
High-Mannose Sugars Decorating Glycoprotein B Enable Its Binding to Dendritic Cell-Specific Intercellular Adhesion Molecule-3-Grabbing Nonintegrin
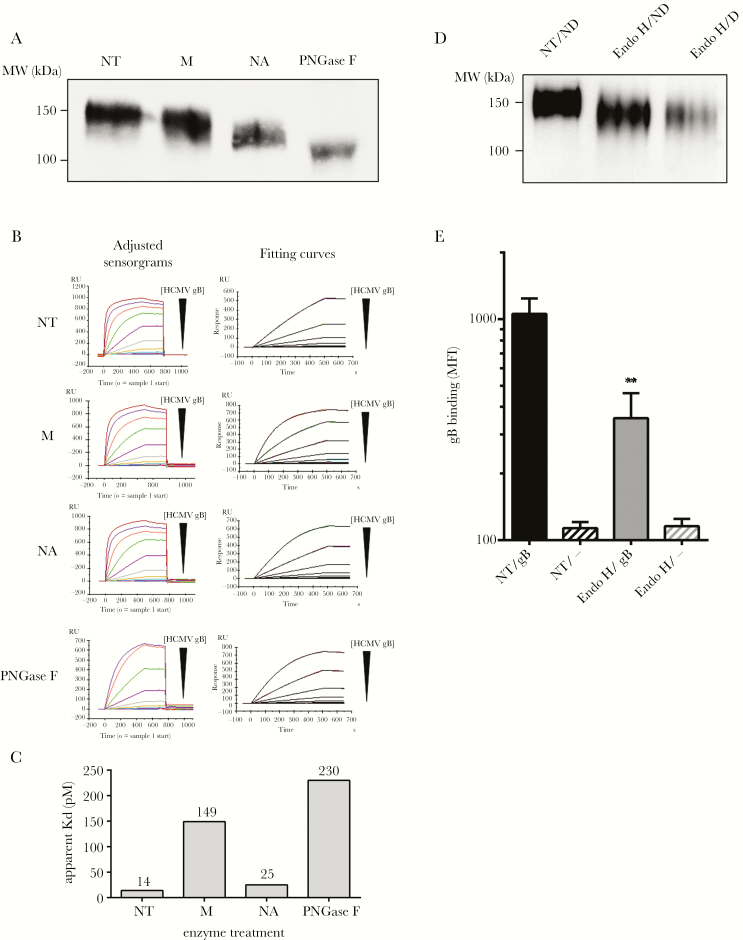
Next, we examined the contribution of gB glycans to the interaction with DC-SIGN. Hence, various glycosidases were used to selectively remove sugars from gB. Although a moderate decrease of the gB molecular weight (MW) was observed after a mannosidase treatment, the PNGase F digestion induced a clear shift in the apparent gB MW, confirming that N-glycans account for a substantial part of the molecule (Figure 3A). Neuraminidase (NA)-treated gB also exhibited an altered electrophoretic mobility compared with nontreated gB, likely indicating an unexpected presence of SA based on results shown in Figure 3.
Figure 3.
High mannose-containing N-glycans on glycoprotein B (gB) are crucial for its interaction with dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN). (A) Western blot analysis showing the detection of recombinant soluble gB with or without enzymatic treatments to remove specifically particular glycan residues. M, NA, and PNGase F stand for the α (1-2,3,6)-mannosidase, the α (2 to 3,6)-neuraminidase, and the peptide-N-glycosidase F, respectively. (B) Adjusted sensorgrams and fitting curves (kinetic model 1:1 binding) for Kd app (apparent Kd) measurements. Black triangles indicate decreasing human cytomegalovirus (HCMV) gB concentration ([HCMV gB]) with 10-fold dilution steps. (C) The affinity of HCMV gB for the extracellular domain of DC-SIGN was determined by surface plasmon resonance (Kd app) before and after deglycosylation with Man, Neu, or PNGase F. (D) Western blot analysis showing from left to right untreated gB, Endo Hf-digested gB in nondenaturing conditions (ND; O/N, 37°C, no denaturation buffer), and Endo Hf-digested gB in denaturing conditions, ie, 1 hour at 37°C with denaturation buffer. (E) Biotinylated HCMV gB was treated with Endo Hf in nondenaturing conditions or left untreated (NT) and further incubated with wild-type DC-SIGN-expressing HEK293T cells. The HCMV gB fixation was revealed as described in the Supplementary Materials. Abbreviation: MW, molecular weight.
We then measured the affinity of DC-SIGN for untreated or deglycosylated gB by surface plasmon resonance spectroscopy. Apparent dissociation constants (Kd app) were calculated from the sensorgrams presented in Figure 3B. The Kd app values were extrapolated using a kinetic model (Figure 3B). Untreated gB had a Kd app of 14 pM (Figure 3C). Sialic acid removal did not seem to alter the binding to immobilized DC-SIGN extracellular domain (Kd app = 25 pM), indicating that SA are not important, in line with our results in Figure 2D. In contrast, the mannosidase activity led to a marked Kdapp increase (149 pM), suggesting a function for terminal mannose residues. The PNGase F-treated gB had an even higher Kd app (230 pM) in that setting, confirming that N-glycans are crucial (Figure 3C). The relatively limited Kd app increase upon removal of N-glycans was associated to an incomplete PNGase F digestion in regular conditions [37]. By adding sodium dodecyl sulfate to the PNGase F or using the trifluoromethanesulfonic acid to chemically deglycosylate gB, we confirmed our hypothesis (Supplementary Figure 2).
Dendritic cell-SIGN is a high-mannose sugar specific lectin. We looked for the presence of high-mannose sugars on gB. Electrophoretic mobilities of Endo Hf-treated or untreated gB were compared (Figure 3D). Endo Hf removed high mannoses on gB in denaturing (D) and in non-denaturing (ND) conditions but with an extended incubation time (O/N) compared with untreated gB. We showed that Endo Hf-treated gB significantly lost its ability to interact with WT DC-SIGN-expressing HEK293T cells compared with untreated gB (Figure 3E). Altogether, these data show that high-mannose on N-glycans but not SA are crucial for the gB/DC-SIGN interaction.
Antibodies Against Antigenic Domains 4 and 5 Impair the Glycoprotein B/Dendritic Cell-Specific Intercellular Adhesion Molecule-3-Grabbing Interaction
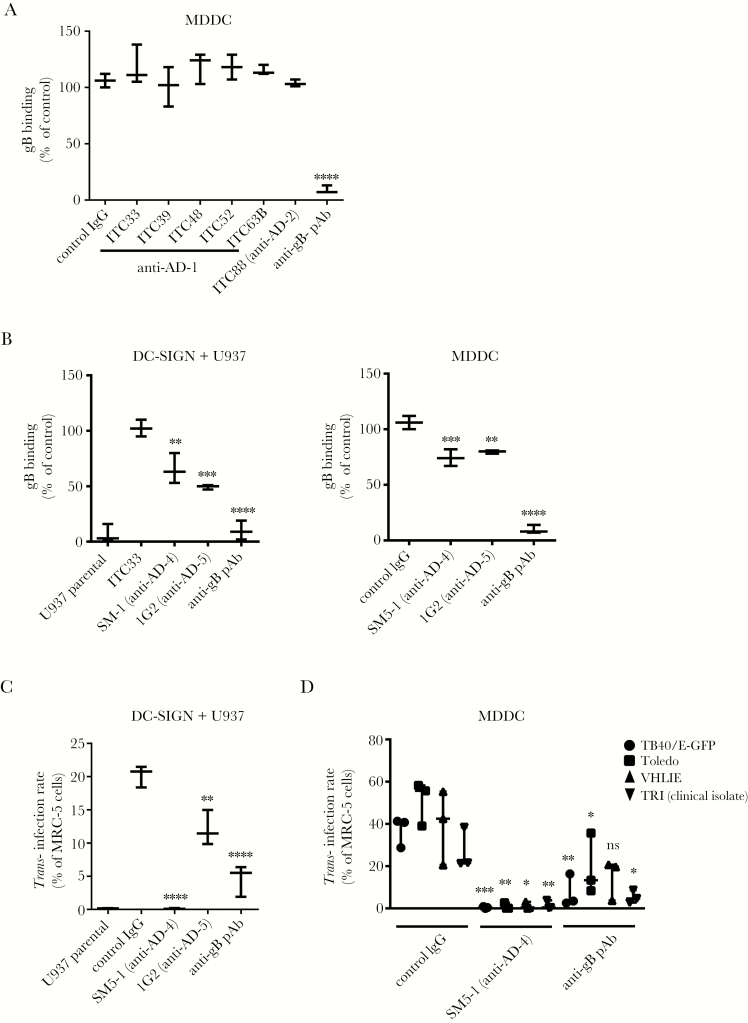
To better define the DC-SIGN/gB interaction, we first used mAbs against AD-1 (clones ITC33, ITC39, ITC48, ITC52, and ITC63B) and AD-2 (clone ITC88) for blockade experiments [27]. None of these mAbs blocked the binding except the polyclonal, which demonstrated that AD-1 or -2 were not involved (Figure 4A). Two mAbs, clones SM5-1 and 1G2 recognizing AD-4 and AD-5, respectively [8], were further tested. Results showed a partial but significant blocking activity of both antibodies up to 48% loss of binding on DC-SIGN+ U937 or MDDC, suggesting that AD-4 and AD-5 could be responsible for the interaction with DC-SIGN (Figure 4B). It is noticeable that DC-SIGN appeared as the only cellular receptor for gB because the pAb-mediated blockade totally abrogated binding on DC-SIGN+ U937 compared with parental cells (Figure 4B, left graph). Those antibodies were also used to block HCMV trans-infection by DC-SIGN+ cells, which relies on the transfer of DC-SIGN+ cells-immobilized HCMV virions, ie, either stuck on the plasma membrane or endocytosed, to HCMV permissive cells. We were surprisinged to find that although 1G2 consistently blocked half of the trans-infection by DC-SIGN+ U937 cells, the SM5-1 clone totally abrogated it (Figure 4C). We hypothesized that this was due to the SM5-1 ability to block the pre-fusion to intermediate or postfusion transition, thus impairing fusion and eventually the infection of MCR-5 cells [38]. Similar results were obtained with 4 low-passage HCMV strains, ie, TB40/E-GFP, VHL/E, Toledo, and a clinical isolate from our center (TRI) on MDDC (Figure 4D). Therefore, we concluded that AD-5 and AD-4 were both responsible for the interaction with DC-SIGN.
Figure 4.
Antigenic domains (AD)-4- and AD-5- but not AD-1/2-specific antibodies inhibit the dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN)/human cytomegalovirus (HCMV) glycoprotein B (gB) interaction and the DC-SIGN-dependent trans-infection of various HCMV strains. (A) Anti-AD-1 (ITC33, ITC39, ITC48, ITC52, and ITC63B) monoclonal antibodies (mAbs), anti-AD-2 (ITC88) mAbs, or a polyclonal anti-HCMV gB serum were used as potential competitors of the HCMV gB binding to immature DC-SIGN+ monocyte-derived dendritic cells (MDDCs). Binding of HCMV gB was assessed by flow cytometry. Binding intensities are represented as mean percentages of the maximum binding (n = 3), ie, without any antibody, and compared with a control immunoglobulin (Ig)G. (B) Inhibition of gB binding on DC-SIGN+ U937 cells compared with the parental counterpart (left panel) or on day 6 immature MDDCs (right panel) by anti-AD-4 (clone SM5-1), anti-AD-5 (clone 1G2), and an anti-gB polyclonal antibody (pAb). Binding intensities are represented as mean percentages of the maximum binding, ie, without any antibody (n = 5). (C) Graph showing trans-infection results obtained with parental vs DC-SIGN+ U937 cells and the TB40/E-GFP strain (multiplicity of infection [MOI] = 2). (D) Similar trans-infection experiments with MDDCs loaded with various low-passage HCMV strains (VHL/E, Toledo, and TB40/E) and a clinical isolate (TRI) (MOI = 2). Asterisks represent significant results compared with the control IgG condition (****, P < .0001; ***, P < .001; **, P < .01; *, P < .05; one-way analysis of variance with multiple comparison tests). Abbreviation: ns, nonsignificant.
Mutating the Asparagine At Position 208 on Glycoprotein B Impairs Its Interaction With Dendritic Cell-Specific Intercellular Adhesion Molecule-3 and Subsequent Transinfection
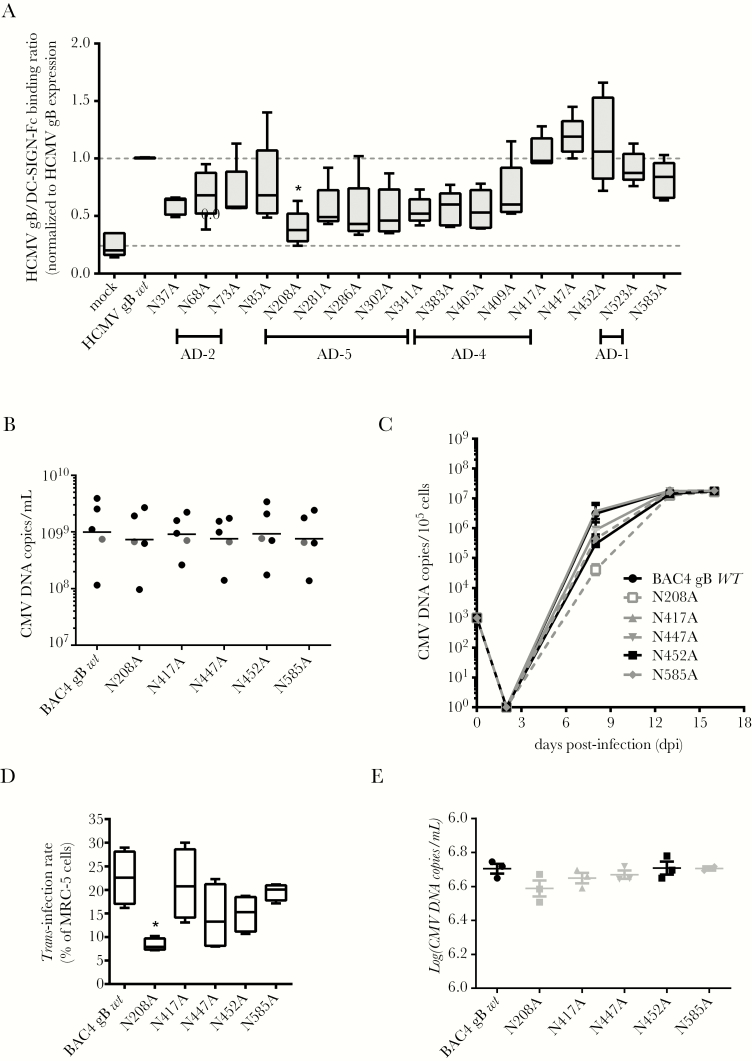
Antigenic domain-4 and AD-5 are heavily N-glycosylated on HCMV gB [39]. To identify the high-mannose sugar-containing N-glycans recognized by DC-SIGN on gB, all putative N-glycosylation sites were mutated and expressed in HEK293T cells (see Methods, Supplementary Figure 3, and Supplementary Table 1). Wild-type or mutated gB in non-denatured cell lysates were immobilized on a nitrocellulose membrane. The capacity of DC-SIGN-Fc to bind to gB in all conditions was calculated on a normalized gB expression. Values ranged from 0.24 for the negative control (ie, empty pRC-CMV vector) to 1.15 for WT gB. The N208A mutation located in AD-5 led to a significant DC-SIGN-Fc binding decrease, suggesting that N-glycans on this residue are important, although other mutants found in AD-4 and AD-5 (from N281A to N409A) behaved similarly (Figure 5A).
Figure 5.
Mutating the asparagine into an alanine at the position 208 of the human cytomegalovirus (HCMV) gBAA sequence selectively impairs both the HCMV glycoprotein B (gB)/dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) interaction and the DC-SIGN-dependent HCMV trans-infection. (A) Quantitative assessment of the interaction of DC-SIGN-Fc (2 µg/mL) on membrane-immobilized cell lysates of HCMV gB either mutated or wild-type (WT)-expressing HEK293T cells (n = 5). Mean ratios measuring the interaction between WT HCMV gB and DC-SIGN-Fc were normalized to 1 (top gray dashed line), whereas the “mock” condition determines unspecific DC-SIGN-Fc binding on cells transfected with an empty pRC-CMV plasmid (bottom gray dashed line). (B) Five independent viral supernatants were reconstituted for BAC4 gB wt and its mutated versions, ie, N208A, N417A, N447A, N452A, and N585A from BAC-transfected MRC-5 (see Supplementary Materials). The CMV deoxyribonucleic acid (DNA) copy quantification per volume unit by quantitative polymerase chain reaction (qPCR) for all stocks produced in this work is shown here. Grey dots represent stocks from 1 reconstitution. (C) The growth kinetics of the parental HCMV strain (gB WT) or the virus variants with the indicated mutations in the gB protein on MRC5 cells was assessed at day 2, 8, 13, and 16 postinfection. MRC-5 (105) cells were initially infected with a viral dose equivalent to 103 genome copies. Virus amounts are given as genome copies per 105 cells. (D) Wild-type (BAC4 gB wt) and mutated virions (N208A, N417A, N447A, N452A, and N585A) were used to load monocyte-derived dendritic cells (multiplicity of infection [MOI] = 2) for trans-infection experiments. Trans-infection rates represent the percentages of HCMV-infected MRC-5 cells, ie, IE/E antigen-positive among total MRC-5 cells stained at day 2 postinfection (*, P < .05; one-way analysis of variance with multiple comparison tests; n = 4). Results are displayed as a minimum to maximum representation; the box plots show the 10th percentile, the mean (intermediate bars in boxes), and the 90th percentile. (E) In parallel, we collected supernatants of MCR-5 infected with the same amount of virus compared with the one used in trans-infection experiments (MOI = 2). Quantifications by qPCR (CMV deoxyribonucleic acid [DNA] copies/mL) of these supernatants are shown here (n = 3 except for the N585A supernatant where n = 2).
We sought to determine whether such a mutation could alter the binding of a whole HCMV particle. To that end, we took advantage of the “en-passant” mutagenesis strategy developed by Tischer et al [40] to modify the UL55 sequence in the genome of the TB40 HCMV strain (BAC4) to generate gB-mutated virions. Virions bearing N208A, N417A, N447A, N452A, and N585A mutations were reconstituted with similar yields (Figure 5B) reaching a plateau at day 16 postinfection (approximately 1 × 107 genome copies/mL), indicating that mutations did not notably alter growth kinetics during reconstitution (Figure 5C). Then, we demonstrated that the N208A mutation exhibited a significantly altered trans-infection ability (Figure 5D). We showed that all mutated and WT virions were infecting MRC-5 cells similarly at the same time point, ie, day 2 postinfection (Figure 5E). We concluded from these results that gB interacts with DC-SIGN mainly through high-mannose containing N-glycans on the asparagine at position 208.
Antibody-Mediated Targeting of Dendritic Cell-Specific Intercellular Adhesion Molecule-3 Blocks Glycoprotein B Binding and Neutralizes Trans-Infection
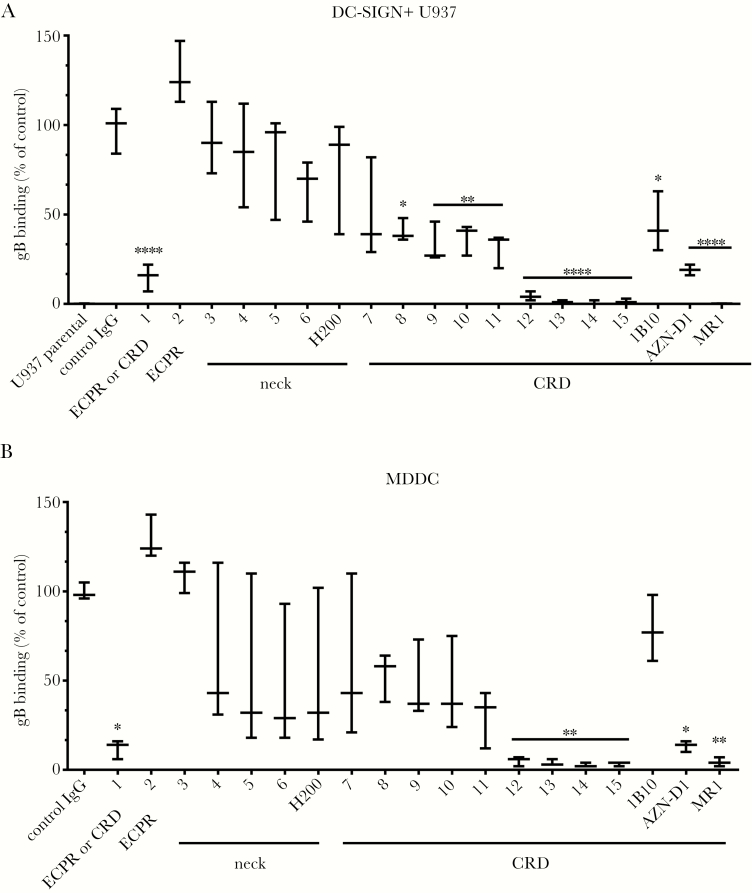
Next, we looked at the blockade of the DC-SIGN/gB interaction with specific Abs to DC-SIGN. First, we characterized the specificity of 15 anti-DC-SIGN mAbs by flow cytometry on WT DC-SIGN, Δneck- or ΔCRD-expressing cell lines. We identified 9 anti-CRD mAbs (7 to 15). Monoclonal Ab 1 was listed as an anti-CRD or -ECPR antibody (Supplementary Table 2). All mAbs were tested in parallel with commercial Abs, 1 anti-neck (H200), and 3 additional anti-CRD antibodies (1B10, AZN-D1, and MR-1), to block gB binding to DC-SIGN. Almost all anti-CRD mAbs including commercial ones were moderate to high inhibitors of this interaction. For mAbs 12 to 15, a total abrogation of gB binding was even observed (Figure 6A). Similar results were obtained with MDDC, confirming that targeting the DC-SIGN CRD almost totally abrogates gB binding (Figure 6B). To clarify whether other known HCMV receptors could participate to this interaction, we analyzed expression of PDGFRα [41], β1 chain integrin [42], and αVβ3 integrin [42] on U937 cells and MDDC. Whereas MDDC displayed low to moderate expression levels for all tested receptors, U937 expressed the β1 chain integrin and PDGFRα. However, none of these HCMV receptors could be linked to our observations. Therefore, we concluded that DC-SIGN is the major, if not the only, attachment receptor for gB on MDDC and DC-SIGN+ U937 cells.
Figure 6.
Anti-dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) carbohydrate-recognition domain (CRD) antibodies abrogate the DC-SIGN/human cytomegalovirus (HCMV) glycoprotein B (gB) interaction. The binding of conjugated gB (2 µg/mL) to (A) parental or DC-SIGN+ U937 cells or (B) immature monocyte-derived dendritic cells (MDDC) was assessed by flow cytometry. Fifteen available anti-DC-SIGN antibodies directed against the neck, extracellular proximal region (ECPR), or CRD regions were compared with the H200 (anti-neck), 1B10, AZN-D1, and MR-1 (anti-CRD) commercially available monoclonal antibodies for their ability to block the DC-SIGN/HCMV gB interaction. Binding intensities are represented as mean percentages of the maximum binding (n = 5), ie, in the absence of any antibody, and compared with treatment with an irrelevant control immunoglobulin (Ig)G. Asterisks represent significant results (****, P < .0001; **, P < .01; *, P < .05; one-way analysis of variance with multiple comparison tests).
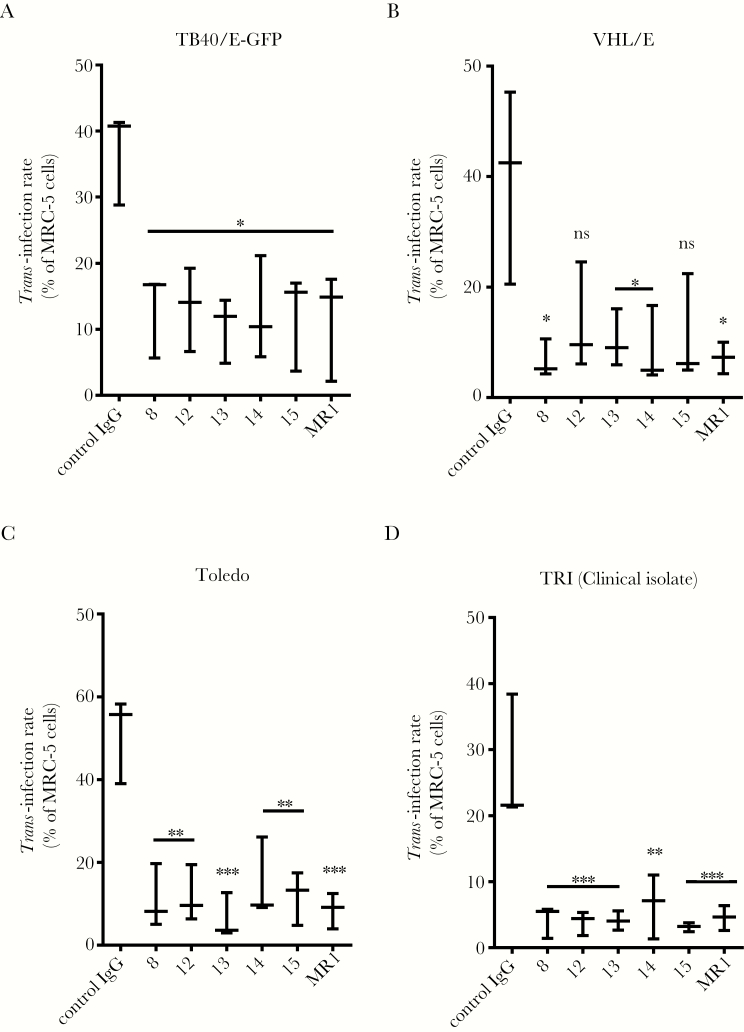
Finally, we tested some anti-DC-SIGN CRD mAbs in trans-infection blockade experiments. All were shown to drastically neutralize the TB40/E-GFP trans-infection by MDDC or DC-SIGN+ U937 cells (62% to 75% reduction; Figure 7A and Supplementary Figure 5). Similar results were obtained with VHL/E and Toledo and with an in-house-generated clinical isolate (TRI) (inhibitions ranging from 67% to 83%, 70% to 86%, and 79% to 87.5%, respectively; Figures 7B–D). Taken together, these results establish that blocking the DC-SIGN CRD prevents gB binding and almost completely neutralizes HCMV trans-infection.
Figure 7.
Blocking dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) carbohydrate-recognition domain (CRD) strongly impairs human cytomegalovirus (HCMV) trans-infection by monocyte-derived dendritic cells (MDDC). (A) Antibody-mediated blockade of HCMV trans-infection by MDDC. Six anti-DC-SIGN CRD antibodies were used to interfere with the capture and subsequent transmission to highly permissive MRC-5 cells. Monoclonal antibodies were used to block the capture and further transmission of HCMV (TB40/E-GFP strain, multiplicity of infection = 2). Similar experiments showing the trans-infection of the VHL/E (B) and Toledo (C) strains as well as a clinical isolate from our research center, TRI (D). Asterisks represent significant results compared with the control immunoglobulin (Ig)G condition (***, P < .001; **, P < .01; *, P < .05; one-way analysis of variance with multiple comparison tests). Abbreviation: ns, nonsignificant.
DISCUSSION
In this study, we characterized the DC-SIGN/HCMV gB interaction. We identified DC-SIGN AA residues involved through an extensive mutagenesis study. We also demonstrated the importance of high mannose N-glycans decorating the asparagine residue in position 208 on gB, although others might be involved too. Finally, antibody-mediated blockades allowed us to identify DC-SIGN as a major attachment molecule for HCMV on MDDC.
Mutating DC-SIGN has confirmed a crucial role for the 2 Ca2+ binding sites and the glycan binding pocket in the recognition of gB, arguing in favor of a common interaction mode for all DC-SIGN viral ligands described to date, ie, HIV-1 gp120 or HSV-1/HHV-8 gB [36, 43]. Su et al [33] showed that turning the V351 into an alanine abrogated the HIV-1 gp120 binding. Our observation is consistent with this and indicates that a glycine residue instead of a valine at position 351 is deleterious. In contrast, replacement of this valine by a threonine, both having similar electron densities, has no effect, indicating that the fold matters at this position. In this study, we showed that the DC-SIGN neck is dispensable for gB binding, although 2 anti-neck mAbs, ie, 4 and 6 (Supplementary Figure 5), were shown to block HCMV trans-infection. We hypothesized that the neck-dependent DC-SIGN microclustering at the plasma membrane was dispensable for soluble gB fixation to DC-SIGN but mandatory for the HCMV attachment to its target cells by permitting multiple ligand/receptor interactions.
We identified gB AD-5 and, more precisely, high mannose-containing N-glycans harbored by the asparagine, at least in position 208, as responsible for the recognition of DC-SIGN. Based on structural homologies and functional studies, Sharma et al [44] proposed that HCMV gB could be classified as a class III fusion protein. Class III fusion proteins exist as pre- and postfusion states and possibly as conformational intermediates [45]. By analogy to a VSV-G pre-fusion crystal structure, a first model of a putative pre-fusion HCMV gB conformation was proposed by Spindler et al [38]. More recently, a study based on electron cryotomography showed for the first time that membrane-associated HSV-1 gB should exist because at least 2 distinct conformations reported as pre- and postfusion conformations [46]. Due to a high degree of similarity between HSV-1 and HCMV gB sequences, it is tempting to speculate that the latter exists at least as pre- and postfusion states. It is interesting to note that in the pre-fusion model reported by Zeev-Ben-Mordehai et al [46], AD-5 and AD-4 protrudes out of HCMV gB, potentially providing an accessible platform for DC-SIGN. In contrast, in line with a former work [47], Fontana et al [48] propose that the pre-fusion model described by Zeev-Ben-Mordehai et al [46] could be an intermediate between the pre- and postfusion states. They also suggested that the fusion loop points toward the viral membrane instead of pointing toward the cellular target membrane in the pre-fusion model of HSV-1 gB. In that setting, we can assume that DC-SIGN might be long enough to contact AD-4/AD-5 regardless of their respective locations into the pre-fusion model. Further investigations are needed to establish whether DC-SIGN can interact with HCMV gB pre-fusion conformation only or if it can adapt itself to distinct conformational states of HCMV gB.
CONCLUSIONS
In conclusion, we characterized the DC-SIGN/gB interaction in the present study. This work provides the molecular bases to understand how some anti-CRD mAbs can efficiently block the gB/DC-SIGN interaction. It should also help to rationally design other blockers of this interaction with antiviral properties. We propose that the DC-SIGN/gB interaction represents the most important HCMV attachment receptor on MDDC. Whether this holds true with other human tissue-resident DC or macrophages expressing DC-SIGN remains to be established. The relevance of our results now has to be challenged in appropriate models. To date, few single-nucleotide polymorphisms described in the 5’ untranslated region of the CD209 gene are associated with variable expression of DC-SIGN on MDDC [49]. Unexpectedly, the GG genotype (rs735240), which leads to a decreased DC-SIGN expression on MDDC, was shown to correlate (1) with increased CMV reactivation or disease in allogeneic stem cell transplantation patients [49] and (2) with decreased CMV infection-free survival in kidney transplant patients [50]. Further investigations are now required to reconcile our in vitro results with clinical observations.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Dr. Yann Guérardel (UMR8576 CNRS, Unité de Glycobiologiestructurale et Fonctionnelle, Université de Lille 1, Lille, France) and Dr. Ludovic Landemarre and Perrine Cancellieri (GLycoDiag, Orléans, France) for helpful discussions and Delphine Robert for technical contribution to this work. We also thank the Développement et Transfert à la Clinique platform (http://www.cicnantes.fr/fr/component/glossary/Glossaire-1/C/CIC-35/, CIC Biothérapies CBT 0503, CHU Nantes, France) for providing elutriated monocytes, clinical grade cytokines, and human serum albumin. Finally, we acknowledge the platforms of the Grenoble Instruct Center (ISBG: UMS 3518 CNRS-CEA-UGA-EMBL), notably the MP3 and SPR platforms.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was funded by the ARMINA Consortium (no. 2012 09680). This work was done in the context of the IHU-CESTI (ANR-10-ibhu-005) and the Labex IGO (ANR-11-LABX-0016-01) projects funded by the Investissements d’Avenir French Government program, managed by the French National Research Agency (ANR). Nantes Métropole and Région Pays de la Loire also supports the IHU-Cesti project. The fellowship of V. P. was provided by a grant from la Région Rhône‐Alpes, France. The French Infrastructure supports MP3 and SPR platforms for Integrated Structural Biology Initiative FRISBI (ANR-10-INSB-05-02) and the Grenoble Alliance for Integrated Structural Cell Biology GRAL (ANR-10-LABX-49-01) within the Grenoble Partnership for Structural Biology.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Dioverti MV, Razonable RR. Cytomegalovirus. Microbiol Spectr 2016; 4:Aug; 4(4). [DOI] [PubMed] [Google Scholar]
- 2. Kabanova A, Perez L, Lilleri D et al. Antibody-driven design of a human cytomegalovirus gHgLpUL128L subunit vaccine that selectively elicits potent neutralizing antibodies. Proc Natl Acad Sci U S A 2014; 111:17965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wussow F, Chiuppesi F, Martinez J et al. Human cytomegalovirus vaccine based on the envelope gH/gL pentamer complex. PLoS Pathog 2014; 10:e1004524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kirchmeier M, Fluckiger AC, Soare C et al. Enveloped virus-like particle expression of human cytomegalovirus glycoprotein B antigen induces antibodies with potent and broad neutralizing activity. Clin Vaccine Immunol 2014; 21:174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang Z, La Rosa C, Maas R et al. Recombinant modified vaccinia virus Ankara expressing a soluble form of glycoprotein B causes durable immunity and neutralizing antibodies against multiple strains of human cytomegalovirus. J Virol 2004; 78:3965–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schultz EP, Lanchy JM, Ellerbeck EE, Ryckman BJ. Scanning mutagenesis of human cytomegalovirus glycoprotein gH/gL. J Virol 2015; 90:2294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vanarsdall AL, Ryckman BJ, Chase MC, Johnson DC. Human cytomegalovirus glycoproteins gB and gH/gL mediate epithelial cell-cell fusion when expressed either in cis or in trans. J Virol 2008; 82:11837–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pötzsch S, Spindler N, Wiegers AK et al. B cell repertoire analysis identifies new antigenic domains on glycoprotein B of human cytomegalovirus which are target of neutralizing antibodies. PLoS Pathog 2011; 7:e1002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heldwein EE, Lou H, Bender FC, Cohen GH, Eisenberg RJ, Harrison SC. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 2006; 313:217–20. [DOI] [PubMed] [Google Scholar]
- 10. Backovic M, Longnecker R, Jardetzky TS. Structure of a trimeric variant of the Epstein-Barr virus glycoprotein B. Proc Natl Acad Sci U S A 2009; 106:2880–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kadlec J, Loureiro S, Abrescia NG, Stuart DI, Jones IM. The postfusion structure of baculovirus gp64 supports a unified view of viral fusion machines. Nat Struct Mol Biol 2008; 15:1024–30. [DOI] [PubMed] [Google Scholar]
- 12. Peng R, Zhang S, Cui Y, Shi Y, Gao GF, Qi J. Structures of human-infecting Thogotovirus fusogens support a common ancestor with insect baculovirus. Proc Natl Acad Sci U S A 2017; 114:E8905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Backovic M, Jardetzky TS. Class III viral membrane fusion proteins. Curr Opin Struct Biol 2009; 19:189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Halary F, Amara A, Lortat-Jacob H et al. Human cytomegalovirus binding to DC-SIGN is required for dendritic cell infection and target cell trans-infection. Immunity 2002; 17:653–64. [DOI] [PubMed] [Google Scholar]
- 15. Geijtenbeek TB, Torensma R, van Vliet SJ et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 2000; 100:575–85. [DOI] [PubMed] [Google Scholar]
- 16. Soilleux EJ, Morris LS, Leslie G et al. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J Leukoc Biol 2002; 71:445–57. [PubMed] [Google Scholar]
- 17. Feinberg H, Guo Y, Mitchell DA, Drickamer K, Weis WI. Extended neck regions stabilize tetramers of the receptors DC-SIGN and DC-SIGNR. J Biol Chem 2005; 280:1327–35. [DOI] [PubMed] [Google Scholar]
- 18. Mitchell DA, Fadden AJ, Drickamer K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J Biol Chem 2001; 276:28939–45. [DOI] [PubMed] [Google Scholar]
- 19. Yu QD, Oldring AP, Powlesland AS et al. Autonomous tetramerization domains in the glycan-binding receptors DC-SIGN and DC-SIGNR. J Mol Biol 2009; 387:1075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feinberg H, Mitchell DA, Drickamer K, Weis WI. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 2001; 294:2163–6. [DOI] [PubMed] [Google Scholar]
- 21. Geijtenbeek TB, Krooshoop DJ, Bleijs DA et al. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat Immunol 2000; 1:353–7. [DOI] [PubMed] [Google Scholar]
- 22. Van Breedam W, Pöhlmann S, Favoreel HW, de Groot RJ, Nauwynck HJ. Bitter-sweet symphony: glycan-lectin interactions in virus biology. FEMS Microbiol Rev 2014; 38:598–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 1994; 179:1109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Plotkin SA, Starr SE, Friedman HM, Gönczöl E, Weibel RE. Protective effects of Towne cytomegalovirus vaccine against low-passage cytomegalovirus administered as a challenge. J Infect Dis 1989; 159:860–5. [DOI] [PubMed] [Google Scholar]
- 25. Sinzger C, Hahn G, Digel M et al. Cloning and sequencing of a highly productive, endotheliotropic virus strain derived from human cytomegalovirus TB40/E. J Gen Virol 2008; 89:359–68. [DOI] [PubMed] [Google Scholar]
- 26. Bressollette-Bodin C, Coste-Burel M, Besse B, André-Garnier E, Ferre V, Imbert-Marcille BM. Cellular normalization of viral DNA loads on whole blood improves the clinical management of cytomegalovirus or Epstein Barr virus infections in the setting of pre-emptive therapy. J Med Virol 2009; 81:90–8. [DOI] [PubMed] [Google Scholar]
- 27. Ohlin M, Sundqvist VA, Mach M, Wahren B, Borrebaeck CA. Fine specificity of the human immune response to the major neutralization epitopes expressed on cytomegalovirus gp58/116 (gB), as determined with human monoclonal antibodies. J Virol 1993; 67:703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tabarani G, Thépaut M, Stroebel D et al. DC-SIGN neck domain is a pH-sensor controlling oligomerization: SAXS and hydrodynamic studies of extracellular domain. J Biol Chem 2009; 284:21229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu P, Wang X, Itano MS, Neumann AK, Jacobson K, Thompson NL. The formation and stability of DC-SIGN microdomains require its extracellular moiety. Traffic 2012; 13:715–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Geijtenbeek TB, van Duijnhoven GC, van Vliet SJ et al. Identification of different binding sites in the dendritic cell-specific receptor DC-SIGN for intercellular adhesion molecule 3 and HIV-1. J Biol Chem 2002; 277:11314–20. [DOI] [PubMed] [Google Scholar]
- 31. Guo Y, Feinberg H, Conroy E et al. Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nat Struct Mol Biol 2004; 11:591–8. [DOI] [PubMed] [Google Scholar]
- 32. Engering A, Geijtenbeek TB, van Kooyk Y. Immune escape through C-type lectins on dendritic cells. Trends Immunol 2002; 23:480–5. [DOI] [PubMed] [Google Scholar]
- 33. Su SV, Hong P, Baik S, Negrete OA, Gurney KB, Lee B. DC-SIGN binds to HIV-1 glycoprotein 120 in a distinct but overlapping fashion compared with ICAM-2 and ICAM-3. J Biol Chem 2004; 279:19122–32. [DOI] [PubMed] [Google Scholar]
- 34. Feinberg H, Castelli R, Drickamer K, Seeberger PH, Weis WI. Multiple modes of binding enhance the affinity of DC-SIGN for high mannose N-linked glycans found on viral glycoproteins. J Biol Chem 2007; 282:4202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ludwig IS, Lekkerkerker AN, Depla E et al. Hepatitis C virus targets DC-SIGN and L-SIGN to escape lysosomal degradation. J Virol 2004; 78:8322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hensler HR, Tomaszewski MJ, Rappocciolo G, Rinaldo CR, Jenkins FJ. Human herpesvirus 8 glycoprotein B binds the entry receptor DC-SIGN. Virus Res 2014; 190:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ma BJ, Alam SM, Go EP et al. Envelope deglycosylation enhances antigenicity of HIV-1 gp41 epitopes for both broad neutralizing antibodies and their unmutated ancestor antibodies. PLoS Pathog 2011; 7:e1002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spindler N, Diestel U, Stump JD et al. Structural basis for the recognition of human cytomegalovirus glycoprotein B by a neutralizing human antibody. PLoS Pathog 2014; 10:e1004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Burke HG, Heldwein EE. Crystal structure of the human cytomegalovirus glycoprotein B. PLoS Pathog 2015; 11:e1005227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tischer BK, Smith GA, Osterrieder N. En passant mutagenesis: a two step markerless red recombination system. Methods Mol Biol 2010; 634:421–30. [DOI] [PubMed] [Google Scholar]
- 41. Soroceanu L, Akhavan A, Cobbs CS. Platelet-derived growth factor-alpha receptor activation is required for human cytomegalovirus infection. Nature 2008; 455:391–5. [DOI] [PubMed] [Google Scholar]
- 42. Feire AL, Koss H, Compton T. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc Natl Acad Sci U S A 2004; 101:15470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Jong MA, de Witte L, Bolmstedt A, van Kooyk Y, Geijtenbeek TB. Dendritic cells mediate herpes simplex virus infection and transmission through the C-type lectin DC-SIGN. J Gen Virol 2008; 89:2398–409. [DOI] [PubMed] [Google Scholar]
- 44. Sharma S, Wisner TW, Johnson DC, Heldwein EE. HCMV gB shares structural and functional properties with gB proteins from other herpesviruses. Virology 2013; 435:239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roche S, Rey FA, Gaudin Y, Bressanelli S. Structure of the prefusion form of the vesicular stomatitis virus glycoprotein G. Science 2007; 315:843–8. [DOI] [PubMed] [Google Scholar]
- 46. Zeev-Ben-Mordehai T, Vasishtan D, Hernández Durán A et al. Two distinct trimeric conformations of natively membrane-anchored full-length herpes simplex virus 1 glycoprotein B. Proc Natl Acad Sci U S A 2016; 113:4176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gallagher JR, Atanasiu D, Saw WT et al. Functional fluorescent protein insertions in herpes simplex virus gB report on gB conformation before and after execution of membrane fusion. PLoS Pathog 2014; 10:e1004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fontana J, Atanasiu D, Saw WT et al. The fusion loops of the initial prefusion conformation of herpes simplex virus 1 fusion protein point toward the membrane. MBio 2017; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mezger M, Steffens M, Semmler C et al. Investigation of promoter variations in dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN) (CD209) and their relevance for human cytomegalovirus reactivation and disease after allogeneic stem-cell transplantation. Clin Microbiol Infect 2008; 14:228–34. [DOI] [PubMed] [Google Scholar]
- 50. Fernández-Ruiz M, Corrales I, Arias M et al. Association between individual and combined SNPs in genes related to innate immunity and incidence of CMV infection in seropositive kidney transplant recipients. Am J Transplant 2015; 15:1323–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.