Human protothecosis is a rare microalgae infection, and its dissemination typically occurs in immunocompromised individuals. We describe a child with Prototheca zopfii pancolitis who harbors a novel recessive frameshift mutation in CARD9, suggesting a new mechanistic insight into anti-Prototheca immunity.
Keywords: human protothecosis, inherited CARD9 deficiency, whole exome sequencing, colitis
Abstract
Human protothecosis is a rare microalgae infection, and its dissemination typically occurs in immunocompromised individuals, but no specific immune defect has been reported. Here, we describe an 8-year-old daughter of a consanguineous union with abdominal pain and bloody diarrhea for 3 months who was found to have pancolitis with numerous microalgae identified as Prototheca zopfii. In the absence of a known immunodeficiency, exome sequencing was performed, which uncovered a novel recessive frameshift mutation in CARD9 (p.V261fs). This report highlights that CARD9 deficiency should be investigated in patients with unexplained systemic/visceral protothecosis and suggests a new mechanistic insight into anti-Prototheca immunity.
Prototheca species are achlorophyllic microalgae. Despite being ubiquitous in nature and endemic in cattle, they rarely cause infection in humans. Human protothecosis is thought to result from contact with potential sources or traumatic inoculation, leading to cutaneous, subcutaneous, and articular bursae infections, whereas dissemination and visceral involvement is thought to occur mainly in immunocompromised subjects. Humans with cellular immunodeficiency have been recognized to be at risk for protothecosis, and it has been proposed that quantitative and qualitative [1] defects in neutrophil function play an important role in the host defense against Prototheca species, but its pathogenesis remains poorly understood.
Caspase recruitment domain-containing protein 9 (CARD9) is an intracellular adapter protein primarily expressed in myeloid-lineage cells. It plays an important role in antifungal immunity. In humans, chronic mucocutaneous candidiasis, deep dermatophytosis, and other fungal infections have been described in patients with inherited CARD9 deficiency [2–11]. Here, we provide evidence to suggest an unrecognized link between disseminated/visceral human protothecosis, a rare microalgae infection, and inherited CARD9 deficiency.
METHODS
Blood Samples, DNA Isolation, and Exome Capture and Sequencing
This study was performed in accordance with the Gazi University and Yale University Institutional Review Board standards. All subjects provided written informed consent. Genomic DNA from the proband, her parents, and her 5 siblings was isolated from peripheral blood leukocytes using standard procedures. Exome sequencing of the proband and both parents was performed using the SeqCap EZ MedExome Target Enrichment Kit (from Roche), followed by 100 base paired-end sequencing on Illumina HiSeq platform.
Exome Analysis
Exome sequence data were aligned to the reference human genome (build 19), and variants were called using GATK. Allele frequencies of identified variants were determined in the National Heart, Lung, and Blood Institute’s Exome Variant Server (4300 European subjects and 2203 African American subjects; last accessed August 2017), 1000 Genomes (1094 subjects of various ethnicities), and the Exome Aggregation Consortium (ExAC; 61000 subjects of various ethnicities; 2016 release). Variants with overall ExAC allele frequency <1% were annotated using ANNOVAR.
Sanger Sequencing of Genomic DNA
Sanger sequencing of the identified CARD9 frameshift mutation (p.V261fr) was performed by polymerase chain reaction (PCR) amplification of genomic DNA of the proband, her parents, and 5 healthy siblings using the following primers: forward, 5’-CAGTACCTGGATGTAGGGGC-3’; reverse, 5’-TTGACCAGCTCAAGCACAGC-3’. The nomenclature of the CARD9 variant is based on National Center for Biotechnology Information reference NM_052813.
Histology, Histochemistry, and Transmission Electron Microscopy
Biopsy samples were fixed in 10% neutral buffered formalin and routinely processed for paraffin embedding, sectioning, and staining with hematoxylin and eosin, Grocott’s methenamine silver, and periodic acid–Schiff stains. On-slide embedding and transmission electron microscopic were performed as previously described [12].
Prototheca zopfii Genotyping
Genotyping was performed using the following PCR primers: forward, 5’-CGCGCAAATTACCCAATCC-3’; reverse, 5’-AACGGGACGGCCAGAGT-3’, as previously reported [13]. Prototheca zopfii genotype 2 was further confirmed by restriction fragment length polymorphism (RFLP), as described previously [13].
RESULTS
Case Report
An 8-year-old girl of Turkish ancestry, offspring of a first-cousin union, presented with a 3-month history of abdominal pain and bloody diarrhea. She was born at full term with no complications and had no noteworthy past medical or family history. Patient’s exam was remarkable for failure to thrive (height: 110 cm, <5th percentile; weight: 17 kg, <5th percentile). Her laboratory tests showed anemia, eosinophilia, elevated immunoglobin E level (Supplementary Table 1), and hypoalbuminemia. Colonoscopy revealed diffuse erythema, ulcerations, and friable colonic mucosa consistent with pancolitis. Patient was initially treated with mesalamine, corticosteroids, and several antibiotic therapies with no clinical improvement.
Bloody Diarrhea Due to Prototheca zopfii Colitis
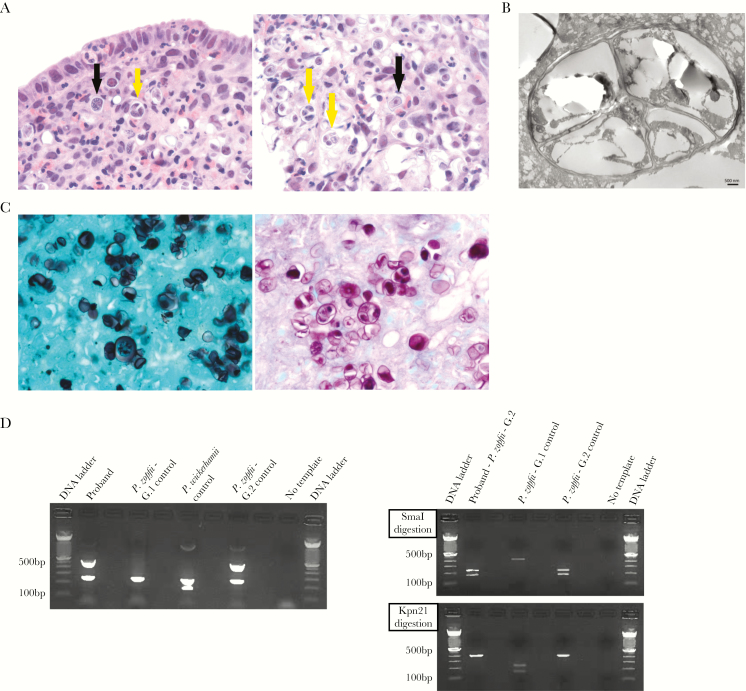
Multiple fragments of colonic tissue, including mucosa and submucosa, were markedly expanded by numerous small clusters of organisms that were round to oval, 8–20 micron in diameter and had a 2–4-micron thick cell wall, central nucleus, and large round nucleolus that was surrounded by a florid inflammatory response, which was comprised of numerous eosinophils and macrophages and fewer neutrophils, lymphocytes, and plasma cells. There were multiple areas of erosion/ulceration. The differential diagnosis included fungal infection, acanthamebiasis, and microalgae infection. Pathological analysis showed many organisms undergoing endosporulation (Figure 1A and B), with multiple wedge-shaped endospores occasionally arranged in a radial tripartite configuration (“Mercedes-Benz” endospores), favoring algae. Moreover, these microorganisms stained strongly with Grocott’s methenamine silver and periodic acid–Schiff stains (Figure 1C), consistent with Prototheca rather than Acanthamoeba. For molecular confirmation and speciation, a genotype-specific multiplex PCR assay [14] was adapted and validated against positive culture controls. Polymerase chain reaction on DNA extracts taken from the formalin-fixed paraffin-embedded blocks resulted in an amplicon that had 100% sequence homology to Prototheca zopfii. A PCR-RFLP assay confirmed Prototheca zopfii genotype 2 [13] (Figure 1D). The patient was treated with amphotericin B [14] for 28 days. At day 5 after treatment, bloody diarrhea resolved. At days 14 and 28 after treatment, repeat colonoscopy showed normal- appearing mucosa, except for localized hyperemic mucosa in the sigmoid colon and rectum. Colonic biopsies showed persistent inflammation and presence of organisms with vague outlines and no internal details or nuclei (Supplementary Figure 1). Hence, considering patient’s clinical and endoscopic responses, no evidence of clear data on the drug of choice and duration of treatment, and patient’s family wishes (as proband had an anaphylactic reaction to amphotericin B for which she had to undergo desensitization), the treatment was discontinued after 28 days.
Figure 1.
Pathological and molecular characterization of Prototheca zopfii genotype 2 in proband’s colonic tissue. A, Hematoxylin-and-eosin stain shows numerous eosinophils and macrophages surrounding numerous round to oval algae (black arrows), with many of them undergoing endosporulation with multiple wedge-shaped endospores (yellow arrows) (left panel, 400×; right panel, 630×). B, Prototheca algae show characteristic endosporulation by transmission electron microscopy. Scale bar, 500 nm. C, Prototheca algae stain strongly with Grocott’s methenamine silver (left panel, 630×) and with periodic acid–Schiff stain (right panel, 630×). D, Prototheca multiplex polymerase chain reaction (PCR) assay (left panel) shows Prototheca zopfii genotype 2 at 508 base pairs (bp), Prototheca zopfii genotype 1 at 216 bp, and Prototheca wickerhamii at 115 bp. Prototheca PCR–restriction fragment length polymorphism (RFLP) assay distinguishes between P. zopfii genotype 1 and 2. The 450-bp PCR product was cut by the P. zopfii genotype 2–specific restriction enzyme SmaI, depicted in the right top panel, and not with the P .zopfii genotype 1–specific restriction enzyme Kpn21, shown in the right bottom panel. Abbreviations: bp, base pair; G.1, genotype 1; G.2, genotype 2; P. zopfii, Protheca zopfii; P. wickerhamii, Prototheca wickerhamii.
Recessive Loss-of-Function Mutation in CARD9
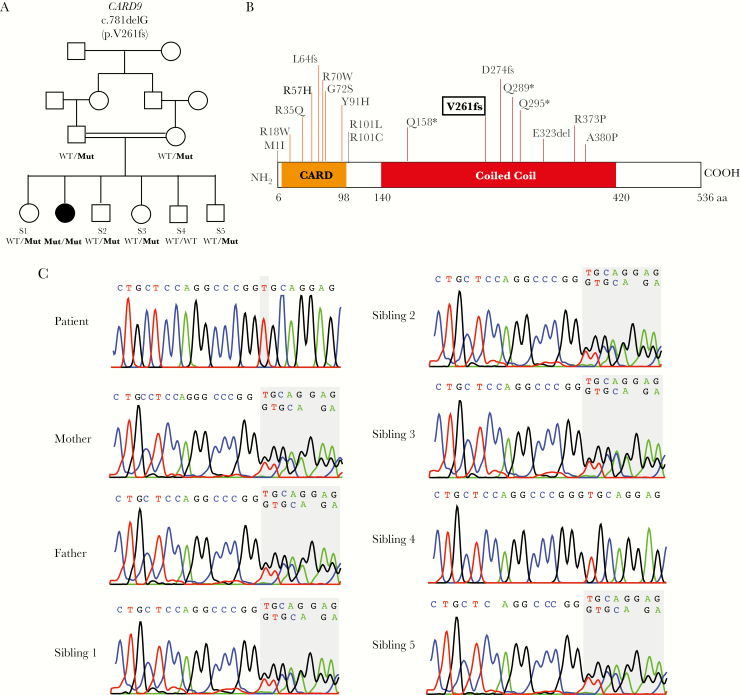
In the absence of evidence for an immune defect in the proband (Supplementary Table 1) that could account for susceptibility to development of visceral protothecosis, the proband and her parents’ exomes were sequenced to a mean depth of 44 independent reads per targeted base, with 97% of targeted bases having >8 independent reads, providing high confidence calling of homozygous and heterozygous coding variants (Supplementary Table 2). Because the affected child is the offspring of a first-cousin union (Figure 2A), we searched for rare (allele frequency ≤0.1% in aforementioned databases), nonsynonymous variants that were homozygous in the proband and heterozygous in each of her parents. Consistent with consanguinity, the proband harbors 12 rare homozygous genotypes. However, only 1 of these variants is damaging (ie, premature termination, frameshift, or splice-site mutations), resulting in 1 single nucleotide deletion at codon 261 (chr9:13964999, AC>A, NM_052813, c.781delG) in the coiled coil domain of CARD9, leading to a premature termination at codon 361 (p.V261fs or p.V261Rfs*100) (Figure 2B). This variant allele was absent among >100000 alleles in the ExAC database as well as all other databases examined (Supplementary Table 3). Sanger sequencing confirmed the homozygous variant in the proband and showed that both parents and 4 of her healthy siblings were heterozygous for this variant, whereas 1 unaffected sibling is homozygous for the reference genotype (Figure 2C).
Figure 2.
Recessive CARD9 mutation in a child with Prototheca zopfii colitis. A, The affected proband and unaffected subjects are shown with black and white symbols, respectively. Consanguineous union is shown with a double line. CARD9 alleles are denoted wild-type or mutant (p.V261fs). B, Schematic representation of human CARD9 protein (isoform 1) with CARD domain depicted in orange and coiled coil domain shown in red. Proband’s mutation (p.V261fs) is highlighted in bold among other previously reported pathogenic mutations [2–11]. C, Sanger sequencing chromatograms of the proband, her unaffected parents, and 5 siblings. The CARD9 p.V261fs mutation is homozygous in the proband, heterozygous in the unaffected parents and 4 siblings, and wild-type in sibling 4. Abbreviations: MUT, mutant (p.V261fs); S, sibling; WT, wild-type.
The remaining 11 recessive genotypes, which includes genes with potential immune functions, were all missense variants predicted to be tolerated by MetaSVM and therefore unlikely to be pathogenic in this patient (Supplementary Table 3).
Recurrent Prototheca zopfii Colitis
Approximately 12 months after being initially treated for Prototheca colitis, the proband presented with recurrent bloody diarrhea and was found to have pancolitis and terminal ileitis in the setting of numerous viable organisms consistent with Prototheca (Supplementary Figure 2). She was treated once again with amphotericin B with good clinical response.
Discussion
Our findings support a previously undescribed link between visceral human protothecosis and inherited CARD9 deficiency. The evidence implicating CARD9 is strong. First, an unreported frameshift mutation at the coiled coil CARD9 domain that leads to a premature termination of the protein at codon 361 is exclusively detected in homozygozity in the proband. Second, since the first report in 2009 [2], there have been multiple reports of patients with recessive mutations in CARD9 and mucosal and/or systemic infections from a wide spectrum of fungal phyla [3–11], establishing an essential role for CARD9 in human antifungal immunity. Third, Prototheca species have phenotypic similarities with fungi. Specifically, Prototheca zopfii contains β-glucans. Dectin-1, a transmembrane C-type lectin receptor highly expressed in myeloid cells, recognizes β-glucans and, when engaged, induces a downstream CARD9-dependent signaling cascade. Thus, it is plausible that Prototheca β-glucans may elicit this signaling pathway. Furthermore, to our knowledge, the only other case of Prototheca colitis described in the literature is of a 36-year-old man who suffered concomitantly from chronic mucocutaneous candidiasis since the age of 15 years [15].
The identification of such rare human infection led to the investigation and recognition of an underlying primary immunodeficiency, placing the clinical features into context and providing a diagnosis, targeted therapy, and carrier screening. Moreover, because Prototheca species are ubiquitous in nature and can colonize the fingernails, skin, respiratory, and gastrointestinal tracts in humans, this patient’s inherited CARD9 deficiency likely accounts, at least in part, for the recurrence of Prototheca colitis. Consistent with consanguinity, the proband also harbors 11 additional rare recessive genotypes; despite being all missense variants and predicted to be tolerated by MetaSVM, their potential contribution to expressivity of the phenotype cannot be excluded.
The recognition and study of single patients afflicted by rare infectious diseases had led to the identification of molecular pathways important both in primary immunodeficiencies and in more common infectious diseases. We hope that this single patient report will pave the way for the confirmation of the link between CARD9 deficiency and human protothecosis in other patients, providing further insight into anti-Prototheca immunity and additional knowledge regarding optimal treatment of this rare microalgae infection in a CARD9-deficient host.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the patient and her family for their invaluable contribution to this work and the staff of the Yale Center for Genome Analysis for exome sequencing. S.V. is the recipient of the 2015 AASLDF Sheila Sherlock Clinical and Translational Research Award in Liver Diseases. S. S., B. D., A. M., M. D. C., C. S. G., O. E., D. J., M. K. K., and S. V. performed research, analyzed and interpreted data; S. V. drafted the manuscript; and S. S., B. D., A. M., M. D. C., C. S. G., O. E., D. J., and M. K. K. performed critical revision of the manuscript for important intellectual and/or technical content.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was supported in part by the National Institutes of Health (Yale Liver Center P30DK034989), including Yale Liver Center Research Cores.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Carey WP, Kaykova Y, Bandres JC, Sidhu GS, Bräu N. Cutaneous protothecosis in a patient with AIDS and a severe functional neutrophil defect: successful therapy with amphotericin B. Clin Infect Dis 1997; 25:1265–6. [DOI] [PubMed] [Google Scholar]
- 2. Glocker EO, Hennigs A, Nabavi M et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med 2009; 361:1727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rieber N, Gazendam RP, Freeman AF et al. Extrapulmonary Aspergillus infection in patients with CARD9 deficiency. JCI Insight 2016; 1:e89890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lanternier F, Barbati E, Meinzer U et al. Inherited CARD9 deficiency in 2 unrelated patients with invasive Exophiala infection. J Infect Dis 2015; 211:1241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lanternier F, Mahdaviani SA, Barbati E et al. Inherited CARD9 deficiency in otherwise healthy children and adults with Candida species–induced meningoencephalitis, colitis, or both. J Allergy Clin Immunol 2015; 135:1558–68 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang X, Wang W, Lin Z et al. CARD9 mutations linked to subcutaneous phaeohyphomycosis and TH17 cell deficiencies. J Allergy Clin Immunol 2014; 133:905–8 e3. [DOI] [PubMed] [Google Scholar]
- 7. Lanternier F, Pathan S, Vincent QB et al. Deep dermatophytosis and inherited CARD9 deficiency. N Engl J Med 2013; 369:1704–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drewniak A, Gazendam RP, Tool AT et al. Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood 2013; 121:2385–92. [DOI] [PubMed] [Google Scholar]
- 9. Drummond RA, Collar AL, Swamydas M et al. CARD9-dependent neutrophil recruitment protects against fungal invasion of the central nervous system. PLoS Pathog 2015; 11:e1005293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gavino C, Cotter A, Lichtenstein D et al. CARD9 deficiency and spontaneous central nervous system candidiasis: complete clinical remission with GM-CSF therapy. Clin Infect Dis 2014; 59:81–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones N, Garcez T, Newman W, Denning D. Endogenous Candida endophthalmitis and osteomyelitis associated with CARD9 deficiency. BMJ Case Rep 2016; doi:10.1136/bcr-2015-214117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hayat M. Principles and techniques of electron microscopy: biological applications. 4th ed Cambridge, UK: Cambridge University Press, 2000. [Google Scholar]
- 13. Aouay A, Coppée F, Cloet S, et al. Molecular characterization of prototheca strains isolated from bovine mastitis. J Mycol Med 2008:224–7. [Google Scholar]
- 14. Lass-Flörl C, Mayr A. Human protothecosis. Clin Microbiol Rev 2007; 20:230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raz R, Rottem M, Bisharat N et al. Intestinal protothecosis in a patient with chronic mucocutaneous candidiasis. Clin Infect Dis 1998; 27:399–400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.