Abstract
BACKGROUND
Obesity is a public health problem, associated with salt sensitive hypertension, kidney inflammation, and fibrosis. N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) is a tetra peptide with anti-inflammatory and anti-fibrotic properties. However, its effect on preventing kidney damage in obesity is unknown. We hypothesized that Zucker obese (ZO) rats on a high-salt (HS) diet develop renal damage, inflammation, fibrosis, and this is prevented with Ac-SDKP treatment.
METHODS
Zucker lean (ZL) and ZO rats (8 weeks old) were treated with Ac-SDKP (1.6 mg/kg/day) while maintained on either a normal-salt (NS; 0.4%) or HS (4%) diet for 8 weeks. Systolic blood pressure (SBP), albuminuria, renal inflammation, and fibrosis were evaluated.
RESULTS
HS diet increased macrophage infiltration in the kidneys of both ZL and ZO rats but was significantly higher in ZO rats receiving the HS diet (ZL + NS, 13.9 ± 1.3 vs. ZL + HS, 19.14 ± 1.5 and ZO + NS, 25.5 ± 1.4 vs. ZO + HS, 87.8 ± 10.8 cells/mm2; P < 0.05). Ac-SDKP prevented macrophage infiltration in ZO rats (ZO + HS + Ac-SDKP, 32.18 ± 2.4 cells/mm2; P < 0.05). Similarly, glomerulosclerosis, cortical, and medullary interstitial fibrosis were increased in ZO rats fed the HS diet, and Ac-SDKP attenuated these alterations (P < 0.05). SBP was increased in ZO rats fed the HS diet (ZO + NS, 121.3 ± 8.9 vs. ZO + HS, 164 ± 6.9 mm Hg; P < 0.05), and it was significantly decreased with Ac-SDKP treatment (ZO + HS + Ac-SDKP, 144.05 ± 14.1 mm Hg; P = 0.004). Albuminuria was higher in ZO rats than in ZL rats; however, neither HS nor Ac-SDKP treatment affected it.
CONCLUSIONS
Ac-SDKP treatment in ZO rats fed a HS diet prevented renal damage by reducing inflammation, fibrosis, and SBP.
Keywords: Ac-SKDP, albuminuria, blood pressure, fibrosis, high-salt diet, hypertension, inflammation, Zucker obese rat
Obesity is a public health problem, in the United States almost 70% of the population is overweight; among them, approximately 35% are obese, with a body mass index above 30 kg/m2.1 Obesity is an important risk factor for end-stage renal disease due to its strong association with diabetes and hypertension.2 The incidence of kidney damage associated to obesity has increased 10-fold in the last 15 years and is expected to rise further in the coming years.3 Obesity is also linked to salt-sensitive hypertension in both human and animals.4,5 In the obese population, salt-sensitive hypertension is strongly associated with the progression of target-organ damage, including end-stage renal disease.6 The underlying mechanism of obesity-related salt sensitivity and its association with renal injury remains unclear. However, inflammation plays a key role in the development of hypertension and kidney damage associated with obesity.7,8 Previous studies have shown that, in obesity, renal injury is associated with glomerulosclerosis, tubule-interstitial damage, inflammation, and albuminuria. High-salt (HS) intake further aggravated these renal changes.9N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) is a tetra-peptide, naturally present in many tissues including kidney.10 Ac-SDKP is released from its precursor thymosin β4 by 2 enzymatic steps mediated by meprin-α and prolyl oligopeptidase enzymes.11,12 Ac-SDKP is hydrolyzed by angiotensin-converting enzyme (ACE), and its concentration in plasma and tissue, are increased by ACE inhibitors (ACEi).13 Studies using several experimental animal models have demonstrated that Ac-SDKP has anti-inflammatory and anti-fibrotic properties14,15 and that a decrease in endogenous Ac-SDKP levels promoted fibrosis in the kidney and heart.16 Recently, we have also shown that Ac-SDKP can delay the onset of hypertension in systemic lupus erythematous.17 However, the effect of Ac-SDKP on obesity-related kidney damage and hypertension is still unknown.
Zucker obese (ZO) rats exhibit many phenotypic traits that are common in the obesity observed in humans such us hyperinsulinemia with normoglycemia and is associated with albuminuria and a progressive decline of renal function.18,19
Therefore, we hypothesized that ZO rats on a HS diet develop hypertension and renal damage, and this is prevented with Ac-SDKP treatment.
METHODS
Animals
Male ZL and ZO rats at 5 weeks of age (Charles River Laboratories, Wilmington, MA) were housed in an air-conditioned room with a 12-hour light/dark cycle and received standard laboratory rat chow and tap water. Rats were allowed 7 days to acclimatize to the new environment before the experiments were performed. All surgical procedures were performed under anesthesia (50 mg/kg of sodium pentobarbital, intraperitoneal). All protocols performed in this study were approved by the Henry Ford Hospital Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Experimental protocols
ZL and ZO rats (8 weeks old) were placed on either a normal-salt (NS; 0.4% NaCl) or HS (4% NaCl) diet (Teklad diets, Harlan, Madison, WI) and were subcutaneously infused with vehicle (0.01 N acetic acid 0.9% saline solution) or Ac-SDKP (1.6 mg/kg/day) for 8 weeks using osmotic mini-pumps (Alzet, Cupertino, CA). Each strain of rat (ZL and ZO) was divided into three groups: (i) NS infused with vehicle (NS + vehicle, n = 6); (ii) HS infused with vehicle (HS + vehicle, n = 6); and (iii) HS infused with Ac-SDKP (HS + Ac-SDKP, n = 6). Blood pressure was measured weekly with a tail-cuff method; 24-hour urine collection was carried out for urinary Ac-SDKP, albumin, and sodium excretion. At the end of the experiment, the animals were sacrificed, and tissues were weighed and collected for biochemical and histological studies.
Systolic blood pressure
Systolic blood pressure (SBP) was measured in conscious rats with a noninvasive computerized tail-cuff system (CODA, Kent Scientific, Torrington, CT), as described previously.20
Urinary Ac-SDKP, sodium excretion, and albuminuria
Animals were placed in metabolic cages for a 24-hour period for acclimatization before 24 hours urine collection. The ACEi captopril was applied to the collecting funnels and tubes at the final concentration of 10−5 M to prevent Ac-SDKP degradation by urinary ACE. The total volume of collected urine was measured; aliquots were prepared and centrifuged twice at 4 °C and 1,200 g for 10 min (Eppendorf centrifuge 5415R). The supernatants were filtered and stored at −80 °C until further analysis. Urinary Ac-SDKP was measured using competitive enzyme linked immunosorbent assay (ELISA) kit according to the manufacturer’s protocol (SPI Biolaboratories, France) as previously described.21 Urinary albumin was determined with an ELISA kit according to the manufacturer’s protocol (GenWay Biotech, San Diego, CA). The 24-hour sodium excretion values were calculated from 24-hour urine volumes and the sodium concentrations measured with a Nova Biomedical 1 electrolyte analyzer (Waltham, MA). Urine albumin excretion was calculated as the urine albumin concentration multiplied by 24-hour urine volume output.
Renal macrophage infiltration
Frozen kidney sections (6 µm) were fixed with acetone for 20 minutes. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide. Nonspecific antibody binding was blocked with 1% bovine serum albumin. Primary antibody mouse anti-rat CD68, a marker for macrophages (clone: ED-1, 1:200, Millipore), was applied, and samples were incubated overnight at 4°C. The following day, sections were incubated with biotinylated secondary horse anti-mouse immunoglobulin G antibodies. Immunoreactivity was detected with ABC peroxidase kit (Vectastain Elite, Vector Laboratories, Burlingame, CA) and visualized with 3-amino-9-ethylcarbazole (Zymed Laboratories, San Francisco, CA). Reddish-brown staining was considered positive. Sections were counterstained with hematoxylin to see the nucleus of the cell. Twenty consecutive regions of the section were examined at 200× magnification using a Nikon’s Eclipse E600 microscope and evaluated with a computerized image analysis system (Microsuite Biological Imaging, Olympus America, Center Valley, PA). Positive cells with clearly visible nuclei were counted at high power for each section and expressed as cells per square millimeter. All histological studies have been performed with blinded analysis.
Renal fibrosis
Picrosirius red staining (PSR) was used to quantify the renal cortical and medullary interstitial collagen deposition as described previously.14
Additionally, total collagen content of the renal cortex was determined using the hydroxyproline assay, as described previously.22 Briefly, samples were dried and weighed, homogenized, and hydrolyzed with 6 N HCI for 16 hours at 110 °C. A standard curve of 0–5 μg of hydroxyproline was used. Data were expressed as micrograms of collagen per milligram of dry weight, assuming that collagen contains an average of 13.5% hydroxyproline.
Glomerular injury
The glomerular matrix was evaluated by periodic acid-Schiff (PAS) staining (Sigma–Aldrich, SL), according to the manufacturer’s protocol. A trans-mural section was taken from the upper mid-kidney section. Sequential 4 μm paraffin-embedded sections were stained with PAS. Glomeruli (30–50) within the 20 consecutive fields of the renal cortex were photographed at 200× magnification. The dark purple color within the glomeruli was considered a positive signal representing the extracellular matrix. The degree of glomerulosclerosis was determined as a percentage of the glomerular tuft area using Microsuite Biological Imaging Software.
Intraperitoneal glucose tolerance test (ipGTT)
On week 8 of the treatment, rats were fasted overnight, blood samples were taken from the tail vein, and glucose was measured using a glucometer (Bayer Contour Blood glucose meter) at 0 (fasting), 15, 30, 60, 90, and 120 minutes after giving an intraperitoneal injection of glucose (2 g/kg). The total area under the curve (AUC) for glucose during the ipGTT (2-hour glucose area under curve) was calculated using the Graph pad Prism software version 5.01.
Statistical analysis.
A nonparametric two-sample Wilcoxon test was used to compare contrasts of interest in all the data. To adjust for multiple testing, Hochberg’s method was used to determine the significance. The adjustment was made on groups of similar tests. A P value less than 0.05 were considered evidence of significant differences.
RESULTS
Body weight and urinary Ac-SDKP
ZO rats showed a significantly higher body weight than ZL rats (Table 1). Neither HS diet nor Ac-SDKP treatment showed any effect on body weight in ZL or ZO rats. Compared to ZL rats, ZO rats showed significant glucose intolerance (Table 1). We observed that the high-salt diet further increased the glucose intolerance in ZO rats, but it had no effect on ZL rats. Ac-SDKP treatment showed no effect on glucose intolerance. As we expected, 24-hour urinary Ac-SDKP excretion was significantly higher (10- to 20-fold) in ZL and ZO rats receiving Ac-SDKP treatment than in the vehicle-treated groups. No effects of high salt were observed in Ac-SDKP excretion. Upon high salt diet, ZO rats have significantly higher urine volume compared to ZL rats but Ac-SDKP treatment did not affect it.
Table 1.
Effect of high salt and Ac-SDKP in Zucker rats at 8 weeks of HS diet and Ac-SDKP treatment
| Parameters | Zucker lean rats | Zucker obese rats | ||||
|---|---|---|---|---|---|---|
| Normal salt | High salt | High salt + Ac-SDKP | Normal salt | High salt | High salt + Ac-SDKP | |
| Body weight (g) | 357.8 ± 12.46 | 354.67 ± 10.2 | 351.5 ± 10.46 | 637.4 ± 14.9ǂ | 587.3 ± 17.1 | 576 ± 16.64 |
| Renal collagen content (µg/mg dry weight) | 17.7 ± 0.65 | 25.2 ± 2.6§ | 17.1 ± 1.14¶ | 20.4 ± 1.2 | 33.87 ± 6.19* | 21.46 ± 2.5† |
| Albuminuria (mg/24 h) | 0.53 ± 0.23 | 0.97 ± 0.43 | 2.25 ± 1.34 | 62.3 ± 22.97ǂ | 85.73 ± 15.1 | 43.47 ± 15.65 |
| Urinary Ac-SDKP (nmol/24 h) | 1.35 ± 0.06 | 1.73 ± 0.21 | 73.56 ± 7.3ǁ,¶ | 3.56 ± 1.38 | 2.6 ± 0.37 | 117.63 ± 23.75#,† |
| Urinary sodium (mmol) | 1.76 ± 0.08 | 8.14 ± 1.3§ | 7.2 ± 0.6ǁ | 4.1 ± 1.2 | 13.5 ± 0.8* | 16.4 ± 1.5# |
| 24 h urine excretion (ml) | 12.3 ± 1.43 | 19.2 ± 1.9§ | 18.3 ± 1.8 | 24.3 ± 8.6 | 49.8 ± 4.6* | 47 ± 5.8 |
| Fasting glucose (mg/dl) | 74.2 ± 3.3 | 85.8 ± 3.7 | 81.6 ± 4.6 | 94.8 ± 2.5 | 96.6 ± 4.5 | 98.3 ± 6.0 |
| GTT (area under curve) | 1,516 ± 125.6 | 1,349 ± 74.36 | 1276.75 ± 111.6 | 2188.5 ± 126.5ǂ | 2537.8 ± 71.9* | 2383.67 ± 78.9 |
Abbreviations: Ac-SDKP, N-acetyl-seryl-aspartyl-lysyl-proline; GTT, glucose tolerance test; HS, high-salt; NS, normal-salt; ZL, Zucker lean; ZO, Zucker obese.
*P < 0.05 (n = 6) ZO + NS vs. ZO + HS; †P < 0.05 ZO + HS vs. ZO + HS + Ac-SDKP; ǂP < 0.05 ZL + NS vs. ZO + NS; §P < 0.05 ZL + NS vs. ZL + HS; ǁP < 0.05 ZL + NS vs. ZL + HS + Ac-SDKP; ¶P < 0.05 ZL + HS vs. ZL + HS + Ac-SDKP; #P < 0.05 ZO + NS vs. ZO + HS + Ac-SDKP.
Renal inflammation
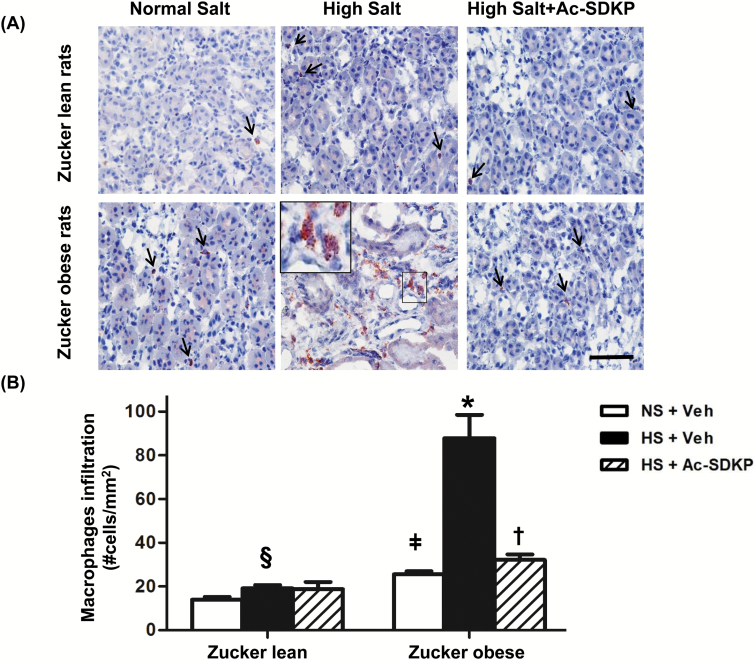
Macrophage infiltration was examined by immunohistochemistry. Compared to ZL rats, ZO rats showed increased numbers of CD68+ positive cells (macrophages) infiltrating the renal parenchyma (Figure 1). The HS diet significantly increased the number of infiltrating macrophages in both ZL and ZO rats, but this increase was markedly higher in ZO rats fed HS. Ac-SDKP treatment significantly decreased the infiltrating renal macrophages in ZO rats but not in ZL rats. These data indicated that HS diet exaggerated the renal inflammation, markedly in obese animals, and that is prevented by Ac-SDKP treatment.
Figure 1.
Effect of Ac-SDKP on renal macrophages induced by HS diet in obesity. (A) Representative images of renal macrophages infiltration in ZL and ZO rats fed a HS diet and receiving Ac-SDKP (Scale bar = 50 µm). Red staining in the cytoplasm indicates a positive immunohistochemistry staining for macrophages (anti-CD68 antibody). A HS diet increases the CD68+ positive cells in the kidney, and this increase was markedly important in obese rats. Ac-SDKP prevents macrophages infiltration in obese rats. Inset is showing a group of macrophages at higher magnification in the interstitial renal space. (B) Quantitative analysis of macrophages infiltration. Both in ZO and ZL rats, the HS diet increased macrophages infiltration in the kidney. Ac-SDKP significantly reduced HS-induced renal macrophage infiltration in ZO rats but not in ZL rats. Data were calculated as the number of cells per mm2 and expressed as the mean ± standard error of measurement. N = 6 in each group. §P < 0.05 ZL + NS vs. ZL + HS, ǂP < 0.05 ZL + NS vs. ZO + NS, *P < 0.05 ZO + NS vs. ZO + HS, †P < 0.05 ZO + HS vs. ZO + HS + Ac-SDKP. Abbreviations: Ac-SDKP, N-acetyl-seryl-aspartyl-lysyl-proline; HS, high-salt; ZL, Zucker lean; ZO, Zucker obese.
Renal fibrosis
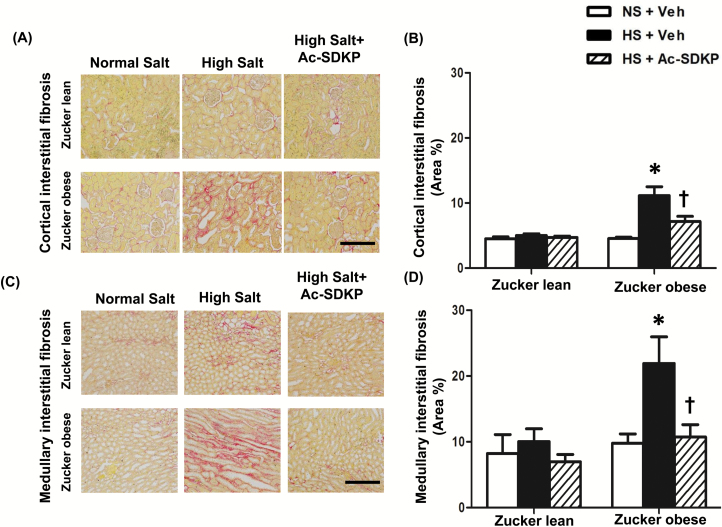
Both the cortical and medullary interstitial fibrosis quantified by PSR staining was similar in ZL and ZO rats fed a normal diet at 16 weeks of age (Figure 2). HS diet in ZO rats showed a significant increase in the cortical and medullary interstitial fibrosis compared to ZL rats and Ac-SDKP treatment attenuated this increase. In addition, analysis of total renal collagen content by a hydroxyproline assay confirmed our finding that a HS diet significantly increased the total renal collagen content in both ZL and ZO rats, which was significantly decreased by Ac-SDKP treatment (Table 1).
Figure 2.
Effect of Ac-SDKP on renal cortical and medullary interstitial fibrosis in obese rats fed a HS diet. (A and C) Representative images of renal cortical and medullary interstitial fibrosis. Red color indicates collagen deposition revealed by Picrosirius Red staining (Scale bar = 100 µm). Interstitial fibrosis was increased in ZO rats fed a HS diet in both the cortex and medulla, and that was prevented by Ac-SDKP. (B and D) Quantitative data analysis. In ZO rats, Ac-SDKP significantly prevented HS-induced renal cortical and medullary collagen deposition. Data are calculated as a percentage of the fibrotic area and expressed as the mean ± standard error of measurement. N = 6 in each group. *P < 0.05 ZO + NS vs. ZO + HS, †P < 0.05 ZO + HS vs. ZO + HS + Ac-SDKP. Abbreviations: Ac-SDKP, N-acetyl-seryl-aspartyl-lysyl-proline; HS, high-salt; ZL, Zucker lean; ZO, Zucker obese.
Glomerular damage
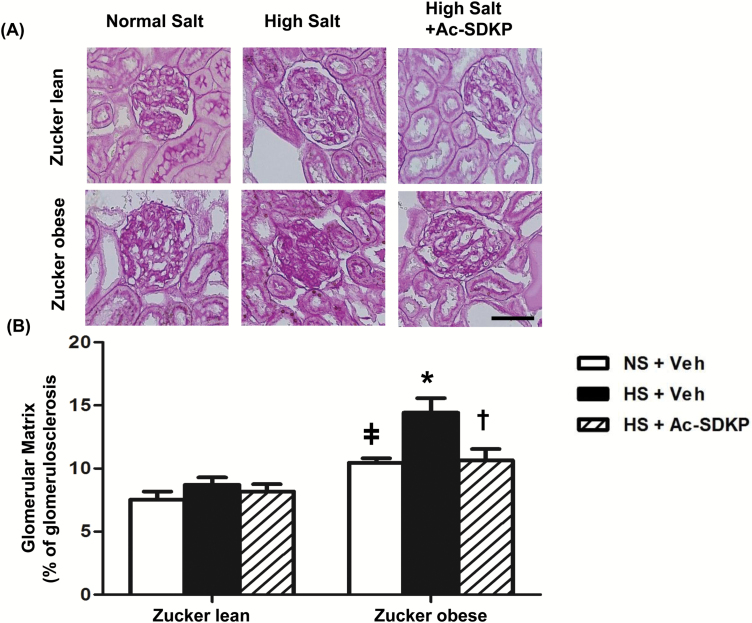
The effect of Ac-SDKP on glomerulosclerosis was assessed by Periodic Acid-Schiff staining (PAS). Compared to ZL rats, ZO rats exhibited markedly glomerulosclerosis on NS diet (Figure 3). HS diet for 8 weeks showed a significant increase in glomerulosclerosis in ZO rats but not in ZL rats, and this increase was significantly attenuated by treatment with Ac-SDKP in ZO rats (Figure 3).
Figure 3.
Effect of Ac-SDKP on glomerular matrix deposition in obese rats fed a HS diet. (A) Representative images of the glomerular matrix. Dark-purple regions indicate the extracellular matrix stained within the glomerular tufts by periodic acid-Schiff staining. Shown are images captured using the ×20 microscope objective. Scale bar = 25 µm. Glomerusclerosis was increased in ZO rats in comparison with the ZL control. A HS diet increased the glomerulosclerosis in ZO rats, and that was prevented by Ac-SDKP. (B) Quantitative data analysis. In ZO but not in ZL rats, glomerulosclerosis was significantly increased by a HS diet compared to a NS diet. Ac-SDKP significantly prevented HS-induced glomerulosclerosis in ZO rats. Data are as expressed as the mean ± standard error of measurement. N = 6 in each group. ǂP < 0.05 ZL + NS vs. ZO + NS, *P< 0.05 ZO + NS vs. ZO + HS, †P < 0.05 ZO + HS vs. ZO + HS + Ac-SDKP. Abbreviations: Ac-SDKP, N-acetyl-seryl-aspartyl-lysyl-proline; HS, high-salt; NS, normal-salt; ZL, Zucker lean; ZO, Zucker obese.
Albuminuria, which is a marker of glomerular damage, was significantly higher in ZO rats compared to ZL rats at 16 weeks of age (Table 1). Interestingly, there was a trend that HS increased albuminuria in ZO rats and Ac-SDKP treatment decreased it but it did not reach the statistical significance.
Systolic blood pressure
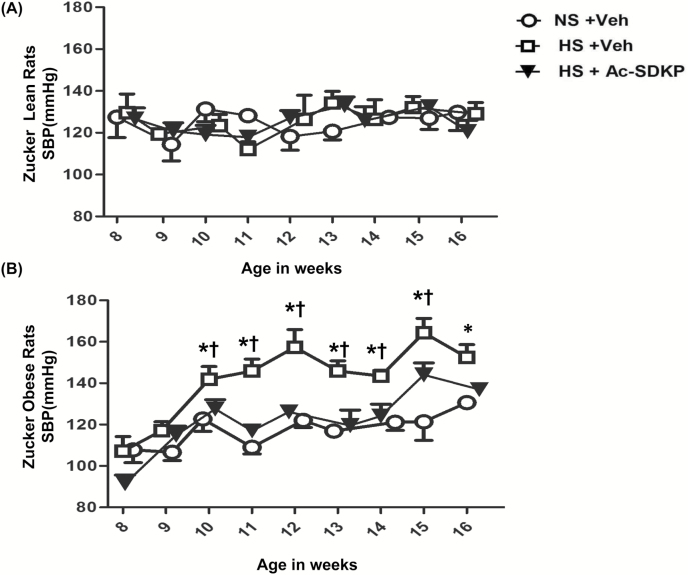
At baseline, no difference was found in SBPs of ZL and ZO rats (Mean ± SD: 127.5 ± 21.85 and 107.8 ± 15.20 mm Hg; P = 0.11) (Figure 4). ZO rats started showing a significant increase in SBP from week 2 of the HS diet (10 weeks of age), and it continued to increase until week 8 (16 weeks of age). In contrast, ZL rats did not show any increase in SBP with the HS diet intake. Ac-SDKP treatment did attenuate the increased blood pressure in ZO rats fed a HS diet, but it did not show any effect on blood pressure in ZL rats. In the last week of the protocol, Ac-SDKP still decreased the blood pressure in ZO rats, but the difference with rats receiving HS was not statistically significant.
Figure 4.
Effect of Ac-SDKP on systolic blood pressure (SBP) in obese rats fed a HS diet. SBP was measured weekly in conscious rats with a tail cuff method. In ZO rats, but not in ZL rats, the HS diet increased SBP significantly compared to the NS diet. Ac-SDKP significantly decreased the HS- induced high blood pressure in ZO rats. Data are expressed as the mean ± standard error of measurement. N = 6 in each group. *P < 0.05 ZO + NS vs. ZO + HS, †P < 0.05 ZO+HS vs. ZO + HS + Ac-SDKP. Abbreviations: Ac-SDKP, N-acetyl-seryl-aspartyl-lysyl-proline; HS, high-salt; NS, normal-salt; ZL, Zucker lean; ZO, Zucker obese.
As we expected, the quantification of sodium excretion shows that a HS diet increases sodium excretion in both ZL and ZO rats and that Ac-SDKP treatment did not affect it, indicating that there was no difference in salt intake in these groups of animals upon Ac-SDKP treatment (Table 1).
DISCUSSION
In the current study, we examined the protective effects of Ac-SDKP on HS-induced kidney damage in obesity. Our results showed that HS diet aggravates renal damage in ZO rats, inducing renal macrophage infiltration, interstitial fibrosis, glomerulosclerosis along with hypertension and that Ac-SDKP prevented all these effects. Additionally, Ac-SDKP reduced both renal cortical and medullary fibrosis but failed to have any beneficial effect on albuminuria. These findings point out the participation of inflammation in the obesity induced salt sensitivity and renal damage. Thus, Ac-SDKP or its analog, resistant to enzymatic degradation, could be used as a novel and specific therapeutic strategy for renal damage in obesity.
Obese individuals are predisposed to develop salt sensitive hypertension and renal damage. In obesity, the kidneys initially become inflamed and eventually develop fibrosis; this effect is further aggravated with HS intake. Ac-SDKP is a naturally occurring tetra-peptide that has anti-inflammatory and anti-fibrotic properties in several models of cardiovascular and renal diseases14,23 but its effect on obesity-related kidney damage are currently unknown. Many studies have provided the evidence that infiltrating macrophages play a vital role in mediating obesity related kidney damage.24,25 Our data indicated macrophage infiltration was markedly increased in the kidney of ZO rats compared to the ZL rats at 16 weeks of age, similar to previous studies.26,27 HS diet further exaggerated macrophage infiltration in both ZL and ZO rats. This is in line with other studies, wherein HS intake induced macrophage infiltration.14,28 Macrophages infiltration leads to the release of proinflammatory cytokines and chemokines like tumor necrosis factor α, Interleukin (IL)-6, IL-1β, Monocyte attractant protein (MCP)-1.29 We report here that Ac-SDKP treatment significantly reduced macrophage infiltration in ZL and ZO rats fed on HS. The beneficial effect exerted by Ac-SDKP on the reduction of macrophage infiltration in ZO rats is similar to our previously reported study, wherein Ac-SDKP prevented macrophage infiltration in both the Dahl salt-sensitive and resistant rats fed a HS diet.14
Generally renal fibrosis is the end result of inflammation, and the same is evident in our current study. Renal fibrosis (total renal collagen content) was increased in both ZL and ZO rats fed a HS diet, and Ac-SDKP treatment prevented this increase. Several mechanisms may be mediating the anti-fibrotic effects of Ac-SDKP. Along with the anti-inflammatory effects, It is known that Ac-SDKP decreases transforming growth factor-beta/Smad signaling, which could be the underlying mechanisms associated with the decreased fibrosis.23 Interestingly, we also noticed increased total renal collagen content in HS-fed ZL rats, indicating high salt, independent of obesity and hypertension per se, can exert mild renal damage. In ZO rats, increases in the glomerulosclerosis are attributed to the high glomerular capillary pressure followed by the infiltration of immune cells.27,30 In our study, glomerulosclerosis was also significantly increased in the ZO rats, and it was further aggravated by a HS diet. Since Ac-SDKP has been shown to reduce glomerulosclerosis in numerous studies, including db/db mice and Dahl salt-sensitive rats,14,31 we investigated whether Ac-SDKP reduced glomerulosclerosis in ZO rats and found that indeed Ac-SDKP treatment significantly reduced glomerulosclerosis in ZO rats.
Parallel to glomerulosclerosis, ZO rats also develop albuminuria, but the HS diet did not worsen it in either ZO rats or lean controls. Although Ac-SDKP treatment decreased glomerulosclerosis, it failed to ameliorate albuminuria in the ZO rats. Ac-SDKP treatment has been shown to decrease albuminuria in several models of renal diseases, such as Dahl salt-sensitive rats, 5/6 nephrectomy, and deoxycorticosterone acetate-salt induced hypertension.14,15,20 ZO rats are obese, hyperinsulinemic, and glucose-intolerant, thus, the mechanism of albuminuria in this animal model could be different from that observed in previously reported models. Additionally, recent findings have suggested that urinary albumin excretion could result either because of the defect in the glomerular filtration barrier and/or defect in the albumin absorption in the proximal tubule.32,33 Since Ac-SDKP treatment improved the glomerular damage observed in ZO rats but did not ameliorate albuminuria, one can speculate that part of the albuminuria observed in ZO rats may be due to a defect in proximal tubule reabsorption. In line with our present finding, Ac-SDKP did not reduce albuminuria in db/db mice, a mouse model of hyperinsulinemic diabetes with obesity.31 However, a separate study is required to understand the mechanism of albuminuria in these ZO rats.
We also showed that a HS diet increases the SBP only in ZO rats but not in ZL rats, confirming previous reports that ZO rats are salt sensitive.4,34 We found that SBP was significantly increased in the ZO rats after 2 weeks of HS and that it remained elevated until the end of the 8 weeks of treatment compared to the ZO rats fed a NS diet. Ac-SDKP treatment significantly reduced systolic blood pressure in the HS-fed ZO rats. In general, Ac-SDKP does not have any beneficial effect in lowering the blood pressure in various models of hypertension.14,15,20 However, recently, we have reported that Ac-SDKP delayed the onset of hypertension in an autoimmune model of systemic lupus erythematous.17 It is known that inflammation plays a role in blood pressure in various hypertension models,35,36 and decreasing the inflammation reduces the elevated blood pressure.35,37 Thus, in our study, the reduction in renal inflammation induced by Ac-SDKP could be a possible explanation for the decreased blood pressure in the HS-fed ZO rats.
We did not observe any change in the 24-hour sodium excretion in HS-fed ZL rats or HS-fed ZO rats with Ac-SDKP treatment. This observation eliminates the potential role of the lower sodium intake in Ac-SDKP-treated animals. At 8th week on HS, the blood pressure still tended to be lower in Ac-SDKP-treated ZO rats than in ZO rats without Ac-SDKP treatment; however, this decrement failed to reach statistical significance. In summary, our study provides evidence that a HS diet increased the renal damage (macrophages infiltration, fibrosis, and glomerulosclerosis) and blood pressure in ZO rats and that Ac-SDKP treatment prevented these changes without reducing albuminuria. Additionally, a HS diet per se was sufficient to exert mild renal inflammation in ZL rats. The HS diet increased glucose intolerance in ZO rats but the mechanism involved in this observation is not clear, however, studies from other labs have also shown similar findings.38,39 On the contrary, Ac-SDKP treatment did not show any effect on glucose intolerance.
We conclude that in HS-fed ZO rats, Ac-SDKP reduced renal inflammation and fibrosis and prevented/delayed the onset of hypertension.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health Grant (P01HL028982 to O.A.C.).
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res 2015; 116:991–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. United States Renal Data System. 2016 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, 2016. [Google Scholar]
- 3. Kovesdy CP, Furth SL, Zoccali C, on behalf of the World Kidney Day Steering C. Obesity and kidney disease: hidden consequences of the epidemic. Clinical Kidney J 2017; 10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ali Q, Patel S, Hussain T. Angiotensin AT2 receptor agonist prevents salt-sensitive hypertension in obese Zucker rats. Am J Physiol Renal Physiol 2015; 308:F1379–F1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DeMarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol 2014; 10: 364–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Quigley JE, Elmarakby AA, Knight SF, Manhiani MM, Stepp DW, Olearzcyk JJ, Imig JD. Obesity induced renal oxidative stress contributes to renal injury in salt-sensitive hypertension. Clin Exp Pharmacol Physiol 2009; 36:724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension 2011; 57:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schiffrin EL. Immune mechanisms in hypertension and vascular injury. Clin Sci (Lond) 2014; 126:267–274. [DOI] [PubMed] [Google Scholar]
- 9. Hall ME, do Carmo JM, da Silva AA, Juncos LA, Wang Z, Hall JE. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis 2014; 7:75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Junot C, Nicolet L, Ezan E, Gonzales MF, Menard J, Azizi M. Effect of angiotensin-converting enzyme inhibition on plasma, urine, and tissue concentrations of hemoregulatory peptide acetyl-Ser-Asp-Lys-Pro in rats. J Pharmacol Exp Ther 1999; 291:982–987. [PubMed] [Google Scholar]
- 11. Cavasin MA, Rhaleb NE, Yang XP, Carretero OA. Prolyl oligopeptidase is involved in release of the antifibrotic peptide Ac-SDKP. Hypertension 2004; 43:1140–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumar N, Nakagawa P, Janic B, Romero CA, Worou ME, Monu SR, Peterson EL, Shaw J, Valeriote F, Ongeri EM, Niyitegeka JM, Rhaleb NE, Carretero OA. The anti-inflammatory peptide Ac-SDKP is released from thymosin-β4 by renal meprin-α and prolyl oligopeptidase. Am J Physiol Renal Physiol 2016; 310:F1026–F1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Azizi M, Rousseau A, Ezan E, Guyene TT, Michelet S, Grognet JM, Lenfant M, Corvol P, Ménard J. Acute angiotensin-converting enzyme inhibition increases the plasma level of the natural stem cell regulator N-acetyl-seryl-aspartyl-lysyl-proline. J Clin Invest 1996; 97: 839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Worou ME, Liao TD, D’Ambrosio M, Nakagawa P, Janic B, Peterson EL, Rhaleb NE, Carretero OA. Renal protective effect of N-acetyl-seryl-aspartyl-lysyl-proline in dahl salt-sensitive rats. Hypertension 2015; 66:816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rhaleb NE, Pokharel S, Sharma U, Carretero OA. Renal protective effects of N-acetyl-Ser-Asp-Lys-Pro in deoxycorticosterone acetate-salt hypertensive mice. J Hypertens 2011; 29:330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cavasin MA, Liao TD, Yang XP, Yang JJ, Carretero OA. Decreased endogenous levels of Ac-SDKP promote organ fibrosis. Hypertension 2007; 50:130–136. [DOI] [PubMed] [Google Scholar]
- 17. Nakagawa P, Masjoan-Juncos JX, Basha H, Janic B, Worou ME, Liao TD, Romero CA, Peterson EL, Carretero OA. Effects of N-acetyl-seryl-asparyl-lysyl-proline on blood pressure, renal damage, and mortality in systemic lupus erythematosus. Physiol Rep 2017; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kasiske BL, O’Donnell MP, Keane WF. The Zucker rat model of obesity, insulin resistance, hyperlipidemia, and renal injury. Hypertension 1992; 19:I110–I115. [DOI] [PubMed] [Google Scholar]
- 19. Kava R, Greenwood MRC, Johnson PR. Zucker (fa/fa) rat. ILAR J 1990; 32:4–8. [Google Scholar]
- 20. Liao TD, Yang XP, D’Ambrosio M, Zhang Y, Rhaleb NE, Carretero OA. N-acetyl-seryl-aspartyl-lysyl-proline attenuates renal injury and dysfunction in hypertensive rats with reduced renal mass: council for high blood pressure research. Hypertension 2010; 55:459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liao TD, Yang XP, Liu YH, Shesely EG, Cavasin MA, Kuziel WA, Pagano PJ, Carretero OA. Role of inflammation in the development of renal damage and dysfunction in angiotensin II-induced hypertension. Hypertension 2008; 52:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peng H, Carretero OA, Liao TD, Peterson EL, Rhaleb NE. Role of N-acetyl-seryl-aspartyl-lysyl-proline in the antifibrotic and anti-inflammatory effects of the angiotensin-converting enzyme inhibitor captopril in hypertension. Hypertension 2007; 49:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang F, Yang XP, Liu YH, Xu J, Cingolani O, Rhaleb NE, Carretero OA. Ac-SDKP reverses inflammation and fibrosis in rats with heart failure after myocardial infarction. Hypertension 2004; 43:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tang JYH, Zhuang S. Inflammation and oxidative stress in obesity-related glomerulopathy. Int J Nephrol 2012; 2012:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coimbra TM, Janssen U, Gröne HJ, Ostendorf T, Kunter U, Schmidt H, Brabant G, Floege J. Early events leading to renal injury in obese Zucker (fatty) rats with type II diabetes. Kidney Int 2000; 57:167–182. [DOI] [PubMed] [Google Scholar]
- 26. Rodríguez-Iturbe B, Quiroz Y, Shahkarami A, Li Z, Vaziri ND. Mycophenolate mofetil ameliorates nephropathy in the obese Zucker rat. Kidney Int 2005; 68:1041–1047. [DOI] [PubMed] [Google Scholar]
- 27. Lavaud S, Michel O, Sassy-Prigent C, Heudes D, Bazin R, Bariéty J, Chevalier J. Early influx of glomerular macrophages precedes glomerulosclerosis in the obese Zucker rat model. J Am Soc Nephrol 1996; 7:2604–2615. [DOI] [PubMed] [Google Scholar]
- 28. Wei SY, Wang YX, Zhang QF, Zhao SL, Diao TT, Li JS, Qi WR, He YX, Guo XY, Zhang MZ, Chen JY, Wang XT, Wei QJ, Wang Y, Li B. Multiple mechanisms are involved in salt-sensitive hypertension-induced renal injury and interstitial fibrosis. Sci Rep 2017; 7:45952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol 2014; 5:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park SK, Kang SK. Renal function and hemodynamic study in obese Zucker rats. Korean J Intern Med 1995; 10:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shibuya K, Kanasaki K, Isono M, Sato H, Omata M, Sugimoto T, Araki S, Isshiki K, Kashiwagi A, Haneda M, Koya D. N-acetyl-seryl-aspartyl-lysyl-proline prevents renal insufficiency and mesangial matrix expansion in diabetic db/db mice. Diabetes 2005; 54:838–845. [DOI] [PubMed] [Google Scholar]
- 32. Dickson LE, Wagner MC, Sandoval RM, Molitoris BA. The proximal tubule and albuminuria: really!J Am Soc Nephrol 2014; 25:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol Regul Integr Comp Physiol 2011; 300:R1009–R1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reddy SR, Kotchen TA. Dietary sodium chloride increases blood pressure in obese Zucker rats. Hypertension 1992; 20:389–393. [DOI] [PubMed] [Google Scholar]
- 35. Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, Münzel T. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 2011; 124:1370–1381. [DOI] [PubMed] [Google Scholar]
- 36. Harrison DG, Marvar PJ, Titze JM. Vascular inflammatory cells in hypertension. Front Physiol 2012; 3:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 2007; 204:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ogihara T, Asano T, Ando K, Sakoda H, Anai M, Shojima N, Ono H, Onishi Y, Fujishiro M, Abe M, Fukushima Y, Kikuchi M, Fujita T. High-salt diet enhances insulin signaling and induces insulin resistance in Dahl salt-sensitive rats. Hypertension 2002; 40:83–89. [DOI] [PubMed] [Google Scholar]
- 39. Donovan DS, Solomon CG, Seely EW, Williams GH, Simonson DC. Effect of sodium intake on insulin sensitivity. Am J Physiol 1993; 264:E730–E734. [DOI] [PubMed] [Google Scholar]