Abstract
More than 1 billion cattle are raised annually for meat and milk production. Dairy cows are repeatedly impregnated and separated from their calves, usually within the first 24 h after birth. Here, I suggest that dairy cows undergo a procedure comparable to the ‘Maternal separation combined with unpredictable maternal stress’ paradigm (MSUS), which is used to study the non-genetic inheritance (NGI) of phenotypes in rodents. I discuss what research on dairy cows may bring to the research field of NGI. The resulting research findings are likely to have benefits to our understanding of MSUS, NGI and consumer safety.
Keywords: non-genetic inheritance, epigenetics, maternal separation and unpredictable maternal stress, early life stress, dairy cows
Introduction
In 2017, 30.3 billion livestock animals were raised [1]. The most abundant mammal on this list is cattle with an estimated 1–1.5 billion animals [1]. This prevalence of cattle underlines the importance of establishing the best possible guidelines for their living conditions. Furthermore, the well-being of cattle can have an impact on the quality of resulting food products [2, 3]. While there are national and international guidelines and laws in place to secure living conditions that allow for cattle to express ‘natural’ or ‘innate behaviour’ [4, 5], definitions for what constitutes such behaviour can vary substantially.
One practice of dairy farming, which restricts natural behaviours, is maternal separation of calves shortly after birth. This procedure is conducted to maximize milk yield from the dam. Since dairy cows spend the majority of their adult lives being pregnant and/or lactating, they are in parallel exposed to stressors such as exhaustion due to frequent milking and mastitis. Therefore, maternal separation of calves combined with maternal stress appear analogous to the maternal separation and unpredictable maternal stress (MSUS) model, which is well established in rodents. MSUS has been shown to not only induce molecular and behavioural phenotypes in the maternally separated offspring, but to ripple its effects across future generations as well. The underlying mechanisms involve non-genetic inheritance (NGI), a rapid form of phenotypic transmission across generations independent of genotypic changes.
While the molecular and behavioural consequences of MSUS are beginning to be understood in rodent models, the consequences on maternal separation in humans and other large mammals are much less well documented. There is evidence that maternal separation, maternal stress and early life trauma have long-lasting effects on human health later in life and potentially across generations [6–8]. However, for obvious ethical reasons, these studies are purely correlative.
Box 1: dairy cows as models for non-genetic inheritance?
Dairy farms as model for maternal separation have multiple benefits:
They are widely used for other purposes, so that no extra costs arise due to housing.
More than 99% of livestock are housed in large industrial farms under controlled conditions [94].
Dairy cows have been selectively bred for centuries, thereby reducing genotypic variability that may increase phenotypic variation.
Breeding, diet and other parameters are neatly documented in a longitudinal and multigenerational fashion.
Protocols for artificial insemination are established.
Cows are intelligent mammals and amenable to behavioural observation.
They provide large quantities of research material such as milk, blood semen and faeces over a lifetime, facilitating longitudinal studies.
Maternal separation is a common practice, which has gained ethical approval.
Protocols for measuring the quality of milk and meat as well as certain behavioural tests have been established.
There are also drawbacks:
Compared with other animal models, cows have a long life span.
They are predominantly monotocous.
Intergenerational studies require that renewed exposure to maternal separation is excluded in offspring, which may result in milk consumption by the calf and thereby reduced milk yield.
Composition of milk varies during milking, across milkings and is dependent on a variety of factors including season, food intake, previous pregnancies and duration after birth.
The composition of body fluids including sperm can be highly dependent on external variables, which need to be tightly controlled for.
In this perspective article, I discuss whether dairy cows may be adequate model organisms for the study of MSUS and NGI (Box 1). This neglected aspect of cattle research is likely to open new perspectives for our understanding of the consequences of maternal separation in large mammals and may offer new insight into the biological mechanisms underlying NGI. Furthermore, since milk, a main transmitter of environmental cues between dam and calf in natural settings, may be affected by MSUS, these studies may be of interest to the dairy industry as well as to the consumer.
What Is NGI?
One conundrum in the field of biology is how environmentally induced phenotypes can be transmitted across generations without altering the genomic sequence. This concept, called NGI, is thought to prepare the offspring for an environment as experienced by previous generations [9]. Since environmental changes often occur on a faster scale than Darwinian evolution, NGI acts complementary to the slower form of genetic inheritance. The molecular basis of NGI and its implications on health and behaviour are comparatively understudied. Heritable environmental cues with an impact across generations can comprise diet, trauma and toxins including endocrine disrupting chemicals [10].
There are different processes by which NGI can occur: Social transmission allows for environmental cues to be communicated from parents to the offspring, either by language or other behaviours. Germline-transmission refers to alterations that are carried across generations via sperm or oocytes. Since germ cells, in particular those of male mammals, undergo heavy chromatin remodelling, mechanisms underlying the persistence of environmentally induced marks in the germline are a heavily researched subject. Non-germline transmission relies on the intergenerational transfer of environmentally induced molecular alterations via body fluids such as milk or semen. While the importance of breast milk to offspring health is widely acknowledged (see below), mechanisms underlying the transfer of phenotypes via milk remain largely unexplored.
NGI has been demonstrated in many laboratory species including Caenorhabditis elegans [11], Drosophila [12] and mice [13] and has been suggested to occur in humans [14]. However, because of the relatively long human life span and difficulties that research on cohorts for longitudinal studies entails, data on NGI in humans and other large mammals are scarce.
One of the main goals of the NGI research field is to identify the biological substrate, which enables the intergenerational transmission of phenotypes. Several mechanisms have been suggested as biological mediators of NGI across generations. The most promising concept to date is epigenetics, ‘the branch of biology which studies the causal interactions between genes and their products, which bring the phenotype into being’ [15]. Epigenetics comprises a group of mechanisms by which environmentally regulated molecular cascades affects chromatin structure and binding factors, resulting in changed transcription and genome architecture [16].
Chromatin function can be altered by modifying DNA structure itself without affecting DNA sequence, for instance through DNA methylation [17]. Chromatin also contains a multitude of binding proteins, including histones or protamines as well as transcription and structural factors, which may be potential carriers of environmentally induced epigenetic changes in germ cells [18]. Additionally, chromatin can be regulated by RNA [18]. Such modifications in chromatin states can explain how environmental stimuli can interplay with the genome to permanently alter phenotypes, such as the pre-disposition for certain disease risks.
The dairy industry has become interested in epigenetics to explain variations in milk yield [19] and to identify potential ways by which productivity and quality can be causally manipulated [20, 21]. In consequence, protocols for epigenetic analysis in cattle are beginning to be established [20]. Compared with rodent models, there is still little information on epigenetic mechanisms underlying cattle health and behaviour, in particular in relation to stress [20]. However, since the rodent MSUS-model has shown that NGI is accompanied with a variety of epigenetic modifications across generations [22, 18], this is likely to be the case in cattle as well.
It is likely that behaviourally communicated information about environmental states finds epigenetic analogues in the brain as well. However, since this aspect of neuroscience is still little understood, the term NGI is used instead of epigenetic inheritance to include the transfer of information about environmental states via social communication as well.
A better understanding of NGI in dairy cows will benefit our understanding of animal well-being, food quality and the biological basis of transmission of phenotypes across generations.
Evidence for NGI of Early Life Trauma and Stress from Human and Rodent Studies
Humans
Early life trauma and early life stress have multiple consequences on the exposed individual’s mental state and risk for psychiatric conditions [6]. Stressors include war trauma, physical and verbal abuse, heavy disease, as well as the loss of a caretaker.
For instance, children living in institutions that are characterized by social and maternal deprivation often show substantial psychiatric morbidity [23, 24]. Furthermore, it has been shown that losing a parent during childhood significantly increases the likelihood of developing major depression later in life [25]. In the year following death of a loved one, grieving adults have an elevated risk of illness and death themselves [26] and it is well established that the effects of stressors in general have more long-lasting and profound effects in children [27].
Importantly, studies suggest that the effects of traumatic experiences can be transmitted, at least in part, across generations. For example, racial discrimination across generations may contribute to a variety of health risks in African Americans [28]. There is a higher prevalence of depression and anxiety disorders in descendants of Holocaust survivors [8]. Offspring from the Tutsi tribe, whose parents suffered from the 1994 Rwandan genocide, show a spectrum of symptoms for post-traumatic stress disorder as well as hormonal and epigenetic markers [29]. It is important to note that these phenotypes may be transmitted via a combination of social communication and molecular mechanisms in bodily tissues.
Mother’s milk may transfer, at least in part, biological stress markers to the infant, including epidermal growth factor [30], immunoglobulins [31] and TGF-β [32]. Because studies on humans are by nature correlative, they need to be complemented with animal studies to untangle underlying causalities.
Rodents
Because humans represent a diverse genetic population with a large variability in life styles, diet and living conditions, it is crucial to perform studies on animals, which can take place in more controlled environments and furthermore allow a causal manipulation of molecular underlying pathways. Understanding the risk for mental illness as well as the fundamental basis of NGI are the main motivation for these studies.
In rodents, early life trauma can be approximated with the model of MSUS. Here, pups are separated for 3 h daily from the dam and remain as a group in the nest, while the dam undergoes a variety of unpredictable moderate stressors such as tube restraint and swimming in a water-filled beaker [13]. Mice that have undergone MSUS during the first 14 days after birth develop depressive-like behaviours and abnormal social behaviours as adults. They also show alterations in risk assessment and novel object recognition behaviours [13, 33–35].
Strikingly, some of these phenotypes can persist across generations and are not prevented by cross-fostering [35], suggesting a transgenerational inheritance independent of social transmission. Transgenerational phenotypes are accompanied with epigenetic and transcriptional changes [13], including altered expression and methylation of Crfr2 and Nr3c1 genes in mice, which are implicated in the hormonal stress response [13, 35].
Although the mechanisms underlying NGI are mostly studied in male mice, MSUS induces long-lasting changes across generations in both sexes [13, 34, 35]. While the bulk of research has focused on germline transmission, this is not the only route.
Transmission of milk may be a possible route as well. In this case both the amount of milk consumption as well as milk composition may be relevant to transgenerational effects [36]. For instance, pups from stressed rat dams consume more milk in a test for independent milk-digestion, while the dam’s milk contains higher insulin levels [36].
Since rodents are pluriparous have a short life span and less complex social interactions than large mammals, it is essential to look for additional animal models that allow for a more sophisticated investigation of mother–infant bonding.
Natural Maternal Behaviour in Dairy Cows
In order to study the effects of maternal separation in dairy cows, it is necessary to understand the natural maternal behaviour of cattle: After a pregnancy of nine months, cows give birth to a single calf in an isolated location. There, the dam keeps the calf hidden for two to ten days to protect it from predators and to form a dam–calf bond, before rejoining the herd [37]. Cows lick their calves for several hours post-partum to stimulate calf breathing, circulation, urination, defecation and drying [38]. This behaviour also increases the calf’s absorption of colostrum immunoglobulins [37], which are crucial for calf health. Cows are natural hider species and are accustomed to periods of separation. Therefore, distress due to separation is most strongly displayed at 12–24 h after separation [37, 39].
Cows naturally wean their calves after nine to eleven months [40]. During this time, they show extensive maternal care, including licking, nursing and protective behaviour and help integrating the calf into the herd [38]. The close proximity between dam and calf also provides opportunities for the social transmission of information about the environment [41]. When separated, the dam displays increased vocalization towards the calf, restlessness and sniffing while the calf is suckling on random objects and vocalizing to communicate with the dam [37, 42]. Therefore, the duration of pregnancy, weaning and integration of the offspring into a social network represent strong similarities to humans.
Maternal Separation in Dairy Farming
A common procedure in dairy farming is to repeatedly breed, fertilize or artificially inseminate cows to ensure lactation after a dry period of approximately three months [43]. Calves are typically removed from the dam within the first 24 h after birth in order to avoid the formation of a dam–calf bond [37]. The calf is fed the colostrum for the first eight milkings to meet crucial nutritional needs. Severing dam and calf also allows the cow to return to the oestrous faster so that she can be re-impregnated. Calves are housed either in single pens, pair-housed or remain in a herd of calves until they are either used for beef production if male, are raised to be dairy cows themselves.
Box 2: potential evidence for non-genetic inheritance in dairy cows
A calf’s prenatal environment influences the milk production of the calf itself as well as grand calf [54]. This effect may be either due to NGI or direct effects on germ cells in utero.
Prenatal lactation has intergenerational effects on the offspring [95].
Prenatal mastitis reduces the reproductive lifespan of the calf [95].
Persistent organic pollutants (POP) that occur in paints and sealants can be transmitted from dam to calf [96].
Very few studies have been performed on NGI in farming animals [90]. Importantly, these studies suggest that NGI does occur in cows. However, the routes of transmission and molecular underpinnings are unclear. Given their abundance, controlled environments, and large yields of research material, I encourage the scientific community to expand the research of dairy cows in this field.
Artificial Weaning and Calf Housing after Maternal Separation
Maternally separated calves are raised under specific living conditions to keep them apart from the herd of the dam. Housing conditions have a documented impact on calf stress and behaviour and need to be taken into consideration when designing NGI experiments:
Single versus Group Housing of Calves
Single housing represents a stressor for animals that naturally live in herds. While single housing is discouraged by animal welfare regulations [44], it is still practice to separate calves in single pens after weaning, under the rationale of reducing the spreading of infections. In Europe ∼60% of calves are single-housed [45]. This leads to measurable cognitive differences, such as impaired novel object recognition and reversal learning in 7-week old single housed calves [46]. Furthermore, single housed calves show social anxiety when introduced to an unfamiliar peer [47]. To ameliorate animal wellbeing, pair housing is conducted as an alternative approach. Paired housing additionally benefits the physiological performance of calves including milk consumption from teats before weaning, weight gain and feeding after weaning, as well as the sociability of calves [48, 49].
Environmental Enrichment and Maternal Separation
Enriched environments refer to living conditions that contain adequate space, sensory stimulation and opportunities to freely interact with objects as well as other individuals. Animals reared in enriched conditions show lower levels of aggression, fewer anti-social behaviours, higher maternal care towards their own offspring and better coping with pre-mature weaning [50–52].
Rodent studies indicate that phenotypes in the F1-offspring of parents that had undergone MSUS can be ameliorated by housing in an enriched environment [53]. Hence, enriched environments are not only beneficial for the exposed animal, but they may also help to reverse or overcome phenotypic effects due to stress and maternal separation in previous generations. There is evidence that a calf’s prenatal environment influences both the calf’s (F0) and its offspring’s (F1) subsequent milk production [54]. Hence, the benefits of environmental enrichment can be transmitted either via NGI or in utero (Box 2). The authors demonstrated this effect in Jersey as well as Holstein breeds across generations and suggest epigenetics as an underlying mechanism.
Abrupt versus Gradual Weaning
Abrupt weaning refers to a complete exchange of milk against solid food, whereas gradual weaning provides a transitory phase in which both types of food are available. Milk can stem from the dam, a foster dam or an artificial teat.
Early weaning at <24 h reduces stress in calves compared with weaning after a few days [37]. However, in beef cattle, gradually weaned calves are less stressed and vocalize less [55]. In dairy calves, stereotypical sucking behaviour can be reduced by allowing brief suckling from a cow post-milking [56, 57]. This suggests that gradual weaning may be a more healthy process than abrupt weaning. On the other hand, male beef calves that were temporarily separated from their dams for ‘processing’ (ear-marking, vaccination, branding, dehorning and castration) six weeks prior to weaning showed poorer weight gains after weaning than those that were ‘processed’ at the time of weaning [58]. However, it is unclear whether this effect is due to maternal separation during processing or due to the stress inflicted to the dam during these procedures.
Consequences of Maternal Separation
Despite the positive economical aspects, maternal separation has pre-dominantly negative consequences for dam and calf at any stage [37, 42].
Dam
Dam and calf live in a relationship of mutual benefit. While the dam is vital to the calf’s health, the presence of the calf after birth also benefits the dam’s health: For instance, suckling by the calf for several days accelerates the involution of the uterus after birth and reduces the incidence of retained foetal membranes [59]. In contrast, early weaning increases the risk of post-calving disease and mastitis due to remaining milk in the dam’s udder [37]. Furthermore, repeated stress such as separation from the calf may reduce future milk production in the dam [60]. However, few studies investigate the consequences of maternal separation to the dam. It is likely to have effects comparable to other chronic adult stressors (see paragraph 8).
Calf
Productivity
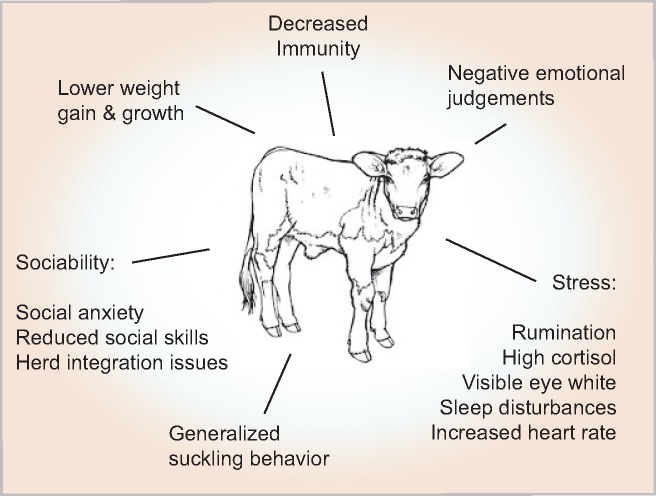
Maternally separated calves show reduced daily weight gain and future productivity [37, 61, 62]. Additionally, they have a delayed onset of fertility and reduced future milk production [62], indicating that early maternal separation has chronic consequences (Fig. 1).
Figure 1:
consequences of maternal separation in calves
Health Status
Calves that are pre-maturely separated from the dam show growth impediments [63], reduced immunity [63] and an increased risk of diarrhoea for up to 3 weeks [37]. Female calves later show a higher propensity for mastitis [64].
Behaviour
Maternally separated calves display multiple signs of stress [37], such as an increased heart rate [42], reduced rumination [37], sleep disturbances [65] and visible eye white [66]. Calves also display abnormal licking behaviour [64] and suck on random objects. When integrated into the main herd, they exhibit symptoms of anxiety, reduced social behaviour, increased social anxiety, reduced social skills, less time feeding and grooming, as well as increased cortisol levels [37, 67]. When isolated again later in life, maternally separated cows exhibit decreased sociability and behavioural activity [68].
Interestingly, maternally separated calves display increased negative emotional judgement during a go/no-go task, which is in its magnitude comparable to the effects of hot-iron dehorning, a procedure that is currently under scrutiny due to an infliction of high levels of pain [69]. The authors of this study suggest that this behaviour relates to negative interpretations of ambiguous stimuli in judgement tasks, as seen in depressed patients [69, 70].
These data show that maternal separation in calves has widespread physiological and behavioural consequences, which are quantifiable and reproducible. Importantly, maternal separation affects a female calf’s ability to nurse her own offspring later in life [37], suggesting that transmission of phenotypes across generations may occur.
The variety of phenotypes strongly suggests that underlying biological pathways are implicated as well.
Unpredictable Maternal Stress
Since dairy cows are either pregnant or lactating during most of their adult lives, stressors may have direct intergenerational effects on the offspring when the dam’s milk is fed to the calves. Additionally, they may impact the germline of the cow and thereby affect future offspring.
The most common stressors of adult dairy cows include branding, transport stress, limited living space, social stress, unnatural food sources, sickness (e.g. mastitis), lameness, metabolic exhaustion due to constant milking, repeated impregnation and heat-stress [71, 72]. Recommendations are in place to reduce some of these stressors [4]; however, they can be at times inevitable.
The best studied adult stressor in dairy cows is heat stress [73] and its effects on milk are well characterized: Heat stress increases total protein, albumin, glucose, pO2 and lowers lactate levels [2, 3]. LC–MS mass spectrometry on milk from heat stressed cows also reveals altered profiles of triacylglycerol (TAG) and polar lipid profiles [74]. These effects may be in part due to reduced food intake, and in part attributed to direct metabolic effects on the mammary glands [75, 76]. Studies on heat stress demonstrate manifold effects on milk. They therefore underline that environmental stressors can indeed affect milk composition, which as the calves’ main food source is likely to affect their health. Furthermore, research on heat stress offers potential biomarkers that are responsive to environmental impact and may be measures in studies of MSUS and NGI as well.
Potential Impact of Early Life Separation on Food Quality
The main deliverables from the dairy industry are dairy products and beef from male calves. Here, dairy is produced, at least in part, by dams that are currently undergoing separation from the calf, have experienced this process themselves and across generations. Hence, it is important to know the consequences of these practices on food quality and safety in current and future generations of cattle.
Since humans and cows represent different species, it needs to be addressed, whether factors in bovine products may be active in the human body. This is the case for at least a percentage of compounds. For instance, the first dose of insulin for human use was extracted from cattle’s pancreas, suggesting that some bovine hormones can function well when injected into the human body. Furthermore, ingested hormones may have indirect effects on human health by altering the balance of the gut microbiome [77]. Other routes include digestion and absorption of molecules of bovine origin, however studies on the bioavailability of those molecules to the human body via this route are scarce.
Milk and Dairy
Milk is designed by nature to pass on nutrients, immunity and non-genetic information about environmental states to the offspring. For this reason, breastfeeding mothers are encouraged to pay attention to their nutrition and stress levels to ensure optimal infant health. Milk contains immunoactive components including cytokines, hormones, anti-bodies, micro-RNA containing exosomes and viable immune cells [78–82]. These same components may be ingested by human consumers.
Most of the studies on hormone levels in dairy cows refer to acute pre-slaughter and transport stress, which is why the effects of chronic and intergenerational stress deserve further investigation. Since milk is collected from the dam during or after maternal separation, the milked animal is exposed to a combination of chronic and acute stressors. In mice, milk insulin levels are increased in dams that underwent a combination of high-fat diet and unpredictable stress (compared with either condition alone this effect appeared to be cumulative) [36]. Oestrogen levels are affected by stress in rodent models [83] and luteinizing hormone is affected by stress in beef cows [84]. Furthermore, a variety of hormones is altered in a stress-dependent manner in human breast-milk (see above). Hence, there is a variety of hormones within milk that can in principle be affected by stressful events and further studies are necessary to understand to what extend they are altered by MSUS.
It is unclear in how far altered milk composition may affect the health status of calves. Furthermore, there is an increasing dispute in how far hormones present in bovine milk may be connected to consumer health. For instance, hormones in cow’s milk may be a risk factor for human cancer [78, 85] and diseases associated with a Western life style [86]. While some of them may be inactive in the human body or destroyed by pasteurization [87], this is certainly not the case for all hormones. For instance, oestrogens are contained in pasteurized, commercially available dairy products. While the concentration is substantially lower than in human tissues, oestrogens are concentrated in fat-rich foods such as butter [88]. Glucocorticoid hormones, which are key mediators of the response in the body, are not affected by pasteurization or skimming [89].
While hormones and other potentially active molecules are present in bovine milk, it needs to be assessed, whether the changes induced by stress would (i) increase hormone levels in milk in a reproducible fashion and (ii) whether consumption of this milk would affect and predict biomarkers in the consumer in relevant amounts.
Taken together, it is crucial to obtain a more complete picture about the effect of stressors such as maternal separation on dairy products, and by extension on calves and the human body.
Discussion
Currently, no studies explore the intergenerational effects of maternal separation stress in cattle [20, 90]. This is a missed opportunity for the field of NGI.
Firstly, cattle and humans have a similar duration of pregnancy and weaning, they uniparous and highly social. Therefore cows represent valuable models to causally link the impact of mother–infant bonding on molecular and behavioural phenotypes that may benefit our understanding of health in large mammals. Secondly, there is an increasing interest of the consumer to understand the welfare of livestock and any health benefits or concerns associated with it. Thirdly, such studies would help to fine tune welfare parameters recommendations for the housing for dairy cows, especially for calves that are bread from stressed dams and bulls. While stress should generally be minimized, breeding from non-stressed parents may potentially have an impact as well.
Despite the relevance of NGI research on cattle, there is a variety of reasons why such studies are scarce:
When designing experiments with cattle, variability needs to be kept to a minimum. For instance, the breed and genetic line of cattle should be specified to improve the reproducibility of findings. The quality of bovine milk and semen is known to vary with a plethora of factors, including season, temperature, food, social status of the dam, disease of the dam due to chronically increased energy expenditure via milking, freezing protocol of the material and the relationship to caretakers (e.g. [91, 92]). To circumvent these problems, studies would either have to be performed on an experimental herd or very large cohorts would need to be investigated, so that data could be controlled for those variables.
Another challenge is to tear apart the impact of social and biological factors of maternal separation. For instance, separated calves are fed with formula (a combination from dried milk of various dams) at fixed times of the day, while suckling calves can drink ad libitum from milk that may vary in composition. As a result, the food intake is altered. One approach would therefore be to cover the udder of the dam in the non-separated cohort, to milk both cohorts of separated and non-separated dams and to feed all offspring with a bottle. While this is in principle possible, this setup can induce certain effects of distress as well [93] and therefore complicates the interpretation of data.
Cattle has been bred for centuries to maintain a docile nature. Hence, it would need to be determined to what extend environmental factors such as maternal separation can affect cattle health and productivity across generations.
An additional concern is the generation time of cows, which is substantially shorter than that of humans but considerably longer than that of smaller mammals including rodents. Hence, NGI experiments on cattle would require long-term planning and generous funding.
The gold standard to prove germ-line transmission is in vitro fertilization. This approach limits the route of transfer to the germ-cells and excludes factors such as seminal fluids. Cattle can be routinely artificially inseminated. However, bovine sperm quality can be highly variable, depending on factors such as caretakers, time of last ejaculation and food intake.
In conclusion, given the long life span and phenotypic variation in cows, NGI experiments are unlikely to be implemented by a single team of scientists and, while potentially relevant for our understanding of NGI in large mammals as well as consumer health, will take considerable time and funding to be realized.
While the studies on NGI in cattle are likely to be relevant to help determine adequate living conditions for dairy cows and their offspring, research findings will take years to be implemented on a larger scale. The following strategies may be applied to ameliorate the effects of maternal separation at the present time or in the near future:
Whenever maternal separation is conducted, calves should not be single housed and should be exposed to enriched environments.
Breeder animals may be selected in such way that predicts the healthiest and most stress resilient phenotypes in the offspring.
Since the negative physiological and behavioural effects of maternal separation to the calf have been widely documented, the demand for products from maternally separated cows such as dairy products and beef may be reduced by the responsible consumer.
Given the number of >1 billion cows currently being kept for food production, the proposed studies potentially have global consequences for food quality as well as the health of dairy cows.
Acknowledgements
Work on this publication was supported by a UZH Forschungskredit. I thank Prof. Heinrich Bollwein, Prof. Martin Kaske and Dr Eleni Malama of Vetsuisse, UZH for detailed discussions on this manuscript. Furthermore, I thank Prof. Isabelle Mansuy (ETH Zurich, UZH) for her general support, Theodor Riley and Andrew McDonald (ETH Zurich, UZH) for proofreading.
Conflict of interest statement. None declared.
References
- 1. United States Department of Agriculture Foreign. Livestock and Poultry: World Markets and Trade, 2017. [Google Scholar]
- 2. Garcia AB, Angeli N, Machado L, de Cardoso FC, Gonzalez F.. Relationships between heat stress and metabolic and milk parameters in dairy cows in southern Brazil. Trop Anim Health Prod 2015;47:889–94. [DOI] [PubMed] [Google Scholar]
- 3. Calamari L, Abeni F, Calegari F, Stefanini L.. Metabolic conditions of lactating Friesian cows during the hot season in the Po valley. 1. Blood indicators of heat stress. Int J Biometeorol 2007;52:97–6. [DOI] [PubMed] [Google Scholar]
- 4. World Organisation for Animal Health. Terrestrial Animal Health Code. 1, 2017. [DOI] [PubMed]
- 5. UK Department for Environment Food & Rural Affairs. Animal Welfare Act. Chapter 45, 2006.
- 6. Pesonen A-K, Räikkönen K.. The lifespan consequences of early life stress. Physiol Behav 2012;106:722–7. [DOI] [PubMed] [Google Scholar]
- 7. Yehuda R, Engel SM, Brand SR, Seckl J, Marcus SM, Berkowitz GS.. Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy. J Clin Endocrinol Metab 2005;90:4115–8. [DOI] [PubMed] [Google Scholar]
- 8. Yehuda R, Halligan Sl, Bierer LM.. Relationship of parental trauma exposure and PTSD to PTSD, depressive and anxiety disorders in offspring. J Psychiatr Res 2001;35:261–70. [DOI] [PubMed] [Google Scholar]
- 9. Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD, Hanson MA.. Epigenetic mechanisms and the mismatch concept of the developmental origins of health and disease. Pediatr Res 2007;61:5R. [DOI] [PubMed] [Google Scholar]
- 10. Huypens P, Sass S, Wu M, Dyckhoff D, Tschöp M, Theis F, Marschall S, de Angelis MH, Beckers J.. Epigenetic germline inheritance of diet-induced obesity and insulin resistance. Nat Genet 2016;48:497–9. [DOI] [PubMed] [Google Scholar]
- 11. Klosin A, Casas E, Hidalgo-Carcedo C, Vavouri T, Lehner B.. Transgenerational transmission of environmental information in C. elegans. Science 2017;356:320–3. [DOI] [PubMed] [Google Scholar]
- 12. de Vanssay A, Bougé A-L, Boivin A, Hermant C, Teysset L, Delmarre V, Antoniewski C, Ronsseray S.. Paramutation in Drosophila linked to emergence of a piRNA-producing locus. Nature 2012;490:112–5. [DOI] [PubMed] [Google Scholar]
- 13. Franklin TB, Russig H, Weiss IC, Gräff J, Linder N, Michalon A, Vizi S, Mansuy IM.. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry 2010;68:408–15. [DOI] [PubMed] [Google Scholar]
- 14. Pembrey M, Saffery R, Bygren LO.. Human transgenerational responses to early-life experience: potential impact on development, health and biomedical research. J Med Genet 2014;51:563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Waddington CH. The epigenotype. Endeavour 1942;41:10–3. [DOI] [PubMed] [Google Scholar]
- 16. Skinner MK. Environmental epigenetics and a unified theory of the molecular aspects of evolution: a neo-Lamarckian concept that facilitates neo-Darwinian evolution. Genome Biol Evol 2015;7:1296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Engmann O, Cocaine-induced chromatin modifications associate with increased expression and 3D looping of Auts2. Biol Psychiatry 2017;82:794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bohacek J, Mansuy IM.. Molecular insights into transgenerational non-genetic inheritance of acquired behaviours. Nat Rev Genet 2015;16:641–52. [DOI] [PubMed] [Google Scholar]
- 19. Bormann J, Wiggans GR, Druet T, Gengler N.. Within-herd effects of age at test day and lactation stage on test-day yields. J Dairy Sci 2003;86:3765–74. [DOI] [PubMed] [Google Scholar]
- 20. Ibeagha-Awemu EM, Zhao X.. Epigenetic marks: regulators of livestock phenotypes and conceivable sources of missing variation in livestock improvement programs. Front Genet 2015;6:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singh K, Molenaar AJ, Swanson KM, Gudex B, Arias JA, Erdman RA, Stelwagen K.. Epigenetics: a possible role in acute and transgenerational regulation of dairy cow milk production. Animal 2012;6:375–81. [DOI] [PubMed] [Google Scholar]
- 22. Gapp K, Soldado-Magraner S, Alvarez-Sánchez M, Bohacek J, Vernaz G, Shu H, Franklin TB, Wolfer D, Mansuy IM.. Early life stress in fathers improves behavioural flexibility in their offspring. Nat Commun 2014;5:5466. [DOI] [PubMed] [Google Scholar]
- 23. O’Connor TG, Bredenkamp D, Rutter M.. Attachment disturbances and disorders in children exposed to early severe deprivation. Infant Ment Health J 1999;20:10–29. [Google Scholar]
- 24. Zeanah CH, Egger Hl, Smyke AT, Nelson CA, Fox NA, Marshall PJ, Guthrie D.. Institutional rearing and psychiatric disorders in Romanian preschool children. AJP 2009;166:777–85. [DOI] [PubMed] [Google Scholar]
- 25. Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, Troudart T, Bloch M, Heresco-Levy U, Lerer B.. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry 1999;4:163–72. [DOI] [PubMed] [Google Scholar]
- 26. Stroebe W, Stroebe M, Bereavement and Health: The Psychological and Physical Consequences of Partner Loss. Cambridge Univ. Press, 1987. [Google Scholar]
- 27. Gee DG, Casey BJ.. The impact of developmental timing for stress and recovery. Neurobiol Stress (Elsevier) 2015;1:184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goosby BJ, Heidbrink CC. . Transgenerational consequences of racial discrimination for African American Health. Soc Compass 2013;7:630–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perroud N, Rutembesa E, Paoloni-Giacobino A, Mutabaruka J, Mutesa L, Stenz L, Malafosse A, Karege F.. The Tutsi genocide and transgenerational transmission of maternal stress: epigenetics and biology of the HPA axis. World J Biol Psychiatry 2014;15:334–45. [DOI] [PubMed] [Google Scholar]
- 30. Thibeau S, D’Apolito K, Minnick AF, Dietrich MS, Kane B, Cooley S, Groer M.. Relationships of maternal stress with milk immune components in African American mothers of healthy term infants. Breastfeed Med 2016;11:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kawano A, Emori Y.. The relationship between maternal postpartum psychological state and breast milk secretory immunoglobulin A level. J Am Psychiatr Nurses Assoc 2015;21:23–30. [DOI] [PubMed] [Google Scholar]
- 32. Kondo N, Suda Y, Nakao A, Oh-Oka K, Suzuki K, Ishimaru K, Sato M, Tanaka T, Nagai A, Yamagata Z.. Maternal psychosocial factors determining the concentrations of transforming growth factor-beta in breast milk. Pediatr Allergy Immunol 2011;22:853–61. [DOI] [PubMed] [Google Scholar]
- 33. Franklin TB, Linder N, Russig H, Thöny B, Mansuy IM.. Influence of early stress on social abilities and serotonergic functions across generations in mice. PLoS One 2011;6:e21842.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, Farinelli L, Miska E, Mansuy IM.. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci 2014;17:667–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weiss IC, Franklin TB, Vizi S, Mansuy IM.. Inheritable effect of unpredictable maternal separation on behavioral responses in mice. Front Behav Neurosci 2011;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Purcell RH, Sun B, Pass LL, Power ML, Moran TH, Tamashiro KLK.. Maternal stress and high-fat diet effect on maternal behavior, milk composition, and pup ingestive behavior. Physiol Behav 2011;104:474–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Flower FC, Weary DM.. The effects of early separation on the dairy cow and calf. Anim Welfare 2003;12:339–48. [DOI] [PubMed] [Google Scholar]
- 38. von Keyserlingk MAG, Weary DM.. Maternal behavior in cattle. Horm Behav 2007;52:106–13. [DOI] [PubMed] [Google Scholar]
- 39. Marchant-Forde JN, Marchant-Forde RM, Weary DM.. Responses of dairy cows and calves to each other’s vocalisations after early separation. Appl Anim Behav Sci 2002;78:19–28. [Google Scholar]
- 40. Reinhardt V. Artificial weaning of Old World monkeys: benefits and costs. J Appl Anim Welf Sci 2002;5:151–6. [DOI] [PubMed] [Google Scholar]
- 41. Thorhallsdottir AG, Provenza FD, Balph DF.. The role of the mother in the intake of harmful foods by lambs. Appl Anim Behav Sci 1990;25:35–44. [Google Scholar]
- 42. Stěhulová I, Lidfors L, Špinka M.. Response of dairy cows and calves to early separation: effect of calf age and visual and auditory contact after separation. Appl Anim Behav Sci 2008;110:144–65. [Google Scholar]
- 43. Stevenson JS. Impact of reproductive technologies on dairy food production in the dairy industry. Curr Fut Reprod Technol World Food Prod 2014;752. [DOI] [PubMed] [Google Scholar]
- 44.UK, H. O. Code of Practice for the Housing and Care of Animals Bred, Supplied or Used for Scientific Purposes, 2014. https://www.gov.uk/government/publications/code-of-practice-for-the-housing-and-care-of-animals-bred-supplied-or-used-for-scientific-purposes.
- 45. Marce Â, Guatteo R, Bareille N, Fourichon C.. Dairy calf housing systems across Europe and risk for calf infectious diseases. Animal 2010;4:1588–96. [DOI] [PubMed] [Google Scholar]
- 46. Gaillard C, Meagher RK, von Keyserlingk MAG, Weary DM.. Social housing improves dairy calves’ performance in two cognitive tests. PLoS One 2014;9:e90205.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jensen MB, Vestergaard KS, Krohn CC, Munksgaard L.. Effect of single versus group housing and space allowance on responses of calves during open-field tests. Appl Anim Behav Sci 1997;54:109–21. [Google Scholar]
- 48. Chua B, Coenen E, van Delen J, Weary DM.. Effects of pair versus individual housing on the behavior and performance of dairy calves. J Dairy Sci 2002;85:360–4. [DOI] [PubMed] [Google Scholar]
- 49. De Paula Vieira A, von Keyserlingk MAG, Weary DM.. Effects of pair versus single housing on performance and behavior of dairy calves before and after weaning from milk. J. Dairy Sci 2010;93:3079–85. [DOI] [PubMed] [Google Scholar]
- 50. Hötzel MJ, Pinheiro Machado FLC, Wolf FM, Dalla Costa OA.. Behaviour of sows and piglets reared in intensive outdoor or indoor systems. Appl Anim Behav Sci 2004;86:27–39. [Google Scholar]
- 51. Tuyttens FAM. The importance of straw for pig and cattle welfare: a review. Appl Anim Behav Sci 2005;92:261–82. [Google Scholar]
- 52. O’Connell NE. Influence of individual predisposition, maternal experience and lactation environment on the responses of pigs to weaning at two different ages. Appl Anim Behav Sci 2005;90:219–32. [Google Scholar]
- 53. Gapp K. Potential of environmental enrichment to prevent transgenerational effects of paternal trauma. Neuropsychopharmacology 2016;41:2749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gudex B, Johnson D, Singh K.. Prenatal maternal and possible transgenerational epigenetic effects on milk production. PLoS One 2014;9:e98928.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Haley DB, Bailey DW, Stookey JM.. The effects of weaning beef calves in two stages on their behavior and growth rate. J Anim Sci 2005;83:2205–14. [DOI] [PubMed] [Google Scholar]
- 56. Jensen MB. The effects of feeding method, milk allowance and social factors on milk feeding behaviour and cross-sucking in group housed dairy calves. Appl Anim Behav Sci 2003;80:191–206. [Google Scholar]
- 57. Margerison JK, Preston TR, Berry N, Phillips CJC.. Cross-sucking and other oral behaviours in calves, and their relation to cow suckling and food provision. Appl Anim Behav Sci 2003;80:277–86. [Google Scholar]
- 58. Holroyd R, Petherwick C.. The impact of weaning and processing on the health and performance of beef weaners In: Hemsworth PH, Spinka M, Kostal L (eds.), Proc. 31st Int. Congr. ISAE, Inst. Anim. Biochem. Genet. Slovak Acad. Sci. Ivanka pri Dunaji, Vol. 159, 1997. [Google Scholar]
- 59. Krohn C, Jonasen B, Munksgaard L.. Cow–calf relations. 2: The effect of 0 vs. 5 days suckling on behaviour, milk production and udder health of cows in different stabling. Natl Inst Anim Sci 1990;16:385–94. [Google Scholar]
- 60. Metz J. Productivity aspects of keeping dairy cow and calf together in the post-partum period. Livest Prod Sci 1987;16:385–94. [Google Scholar]
- 61. Krohn CC, Foldager J, Mogensen L.. Long-term effect of colostrum feeding methods on behaviour in female dairy calves. Acta Agric Scand A Anim Sci 1999;49:57–64. [Google Scholar]
- 62. de Passillé AM, Marnet P-G, Lapierre H, Rushen J.. Effects of twice-daily nursing on milk ejection and milk yield during nursing and milking in dairy cows. J Dairy Sci 2008;91:1416–22. [DOI] [PubMed] [Google Scholar]
- 63. Wagenaar JPTM, Langhout J.. Practical implications of increasing ‘natural living’ through suckling systems in organic dairy calf rearing. NJAS – Wageningen J Life Sci 2007;54:375–86. [Google Scholar]
- 64. Fröberg S, Gratte E, Svennersten-Sjaunja K, Olsson I, Berg C, Orihuela A, Galina Cs, García B, Lidfors L.. Effect of suckling (‘restricted suckling’) on dairy cows’ udder health and milk let-down and their calves’ weight gain, feed intake and behaviour. Appl Anim Behav Sci 2008;113:1–14. [Google Scholar]
- 65. Ruckebusch Y. Feeding and sleep patterns of cows prior to and post parturition. Appl Anim Ethol 1975;1:283–92. [Google Scholar]
- 66. Sandem AI, Braastad BO.. Effects of cow-calf separation on visible eye white and behaviour in dairy cows – a brief report. Appl Anim Behav Sci 2005;95:233–9. [Google Scholar]
- 67. Wagner K, Barth K, Palme R, Futschik A, Waiblinger S.. Integration into the dairy cow herd: long-term effects of mother contact during the first twelve weeks of life. Appl Anim Behav Sci 2012;141:117–29. [Google Scholar]
- 68. Wagner K, Seitner D, Barth K, Palme R, Futschik A, Waiblinger S.. Effects of mother versus artificial rearing during the first 12 weeks of life on challenge responses of dairy cows. Appl Anim Behav Sci 2015;164:1–11. [Google Scholar]
- 69. Daros RR, Costa JHC, Von Keyserlingk MAG, Hötzel MJ, Weary DM.. Separation from the dam causes negative judgement bias in dairy calves. PLoS One 2014;9:e98429.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mathews A, MacLeod C.. Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol 2005;1:167–95. [DOI] [PubMed] [Google Scholar]
- 71. Kovács L, Kézér Fl, Jurkovich V, Kulcsár-Huszenicza M, Tőzsér J.. Heart rate variability as an indicator of chronic stress caused by lameness in dairy cows. PLOS One 2015;10:e0134792.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bova TL, Chiavaccini L, Cline GF, Hart CG, Matheny K, Muth AM, Voelz BE, Kesler D, Memili E.. Environmental stressors influencing hormones and systems physiology in cattle. Reprod Biol Endocrinol 2014;12:58.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. St-Pierre NR, Cobanov B, Schnitkey G.. Economic losses from heat stress by US livestock industries. J Dairy Sci 2003;86:E52–77. [Google Scholar]
- 74. Liu Z, Ezernieks V, Wang J, Arachchillage NW, Garner JB, Wales WJ, Cocks BG, Rochfort S.. Heat stress in dairy cattle alters lipid composition of milk. Sci Rep 2017;7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rhoads ML, Rhoads RP, VanBaale MJ, Collier RJ, Sanders SR, Weber WJ, Crooker BA, Baumgard LH.. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J Dairy Sci 2009;92:1986–97. [DOI] [PubMed] [Google Scholar]
- 76. Smith D, Smith T, Rude B, Ward S.. Comparison of the effects of heat stress on milk and component yields and somatic cell score in Holstein and Jersey cows. J Dairy Sci 2013;96:3028–33. [DOI] [PubMed] [Google Scholar]
- 77. Tetel MJ, de Vries GJ, Melcangi RC, Panzica G, O’Mahony SM.. Steroids, stress, and the gut microbiome-brain axis. J Neuroendocrinol 2018;30:e12548. doi:10.1111/jne.12548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Malekinejad H, Rezabakhsh A.. Hormones in dairy foods and their impact on public health – a narrative review article. Iran J Public Health 2015;44:742–58. [PMC free article] [PubMed] [Google Scholar]
- 79. Ballard O, Morrow Al.. Human milk composition. Nutrients and bioactive factors . Pediatr Clin N Am 2013;60:49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zempleni J, Aguilar-Lozano A, Sadri M, Sukreet S, Manca S, Wu D, Zhou F, Mutai E.. Biological activities of extracellular vesicles and their cargos from bovine and human milk in humans and implications for infants. J Nutr 2017;147:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Van De Perre P. Transfer of antibody via mother’s milk. Vaccine 2003;21:3374–6. [DOI] [PubMed] [Google Scholar]
- 82. Ghosh MK, Nguyen V, Muller HK, Walker AM.. Maternal milk T cells drive development of transgenerational Th1 immunity in offspring thymus. J Immunol 2016;197:2290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Shors TJ, Pickett J, Wood G, Paczynski M.. Acute stress persistently enhances estrogen levels in the female rat. Stress 1999;3:163–71. [DOI] [PubMed] [Google Scholar]
- 84. Echternkamp SE. Relationship between LH and cortisol in acutely stressed beef cows. Theriogenology 1984;22:305–11. [DOI] [PubMed] [Google Scholar]
- 85. Duarte-Salles T. Dairy products and risk of hepatocellular carcinoma: the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 2014;135:1662–72. [DOI] [PubMed] [Google Scholar]
- 86. Melnik BC, Evidence for acne-promoting effects of milk and other insulinotropic dairy products. Nestle Nutr Workshop Ser: Pediatric Program 2011;67:131–45. [DOI] [PubMed] [Google Scholar]
- 87. Juskevich JC, Guyer CG.. Bovine growth hormone: human food safety evaluation. Science 1990;249:875–84. [DOI] [PubMed] [Google Scholar]
- 88. Pape-Zambito DA, Roberts RF, Kensinger RS.. Estrone and 17beta-estradiol concentrations in pasteurized-homogenized milk and commercial dairy products. J Dairy Sci 2010;93:2533–40. [DOI] [PubMed] [Google Scholar]
- 89. Schwalm J, Kirk J, Secrest S, Tucker H.. Effects of processing milk on concentrations of glucocorticoids in milk. J Dairy Sci 1978;61:1517–8. [DOI] [PubMed] [Google Scholar]
- 90. Feeney A, Nilsson E, Skinner M.. Epigenetics and transgenerational inheritance in domesticated farm animals. J Anim Sci Biotechnol 2014;5:48.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gürler H, Calisici O, Bollwein H.. Inter- and intra-individual variability of total antioxidant capacity of bovine seminal plasma and relationships with sperm quality before and after cryopreservation. Anim Reprod Sci 2015;155:99–105. [DOI] [PubMed] [Google Scholar]
- 92. Bernabucci U, Basiricò L, Morera P, Dipasquale D, Vitali A, Piccioli Cappelli F, Calamari L.. Effect of summer season on milk protein fractions in Holstein cows. J Dairy Sci 2015;98:1815–27. [DOI] [PubMed] [Google Scholar]
- 93. Orihuela A, Suárez E, Vázquez R.. Effect of restricting suckling on the social bond between ewes and their 10-week-old lambs. Livest Prod Sci 2004;87:259–64. [Google Scholar]
- 94. United States Department of Agriculture. 2012 Census Full Report. Census Agric 2012. [Google Scholar]
- 95. González-Recio O, Ugarte E, Bach A.. Trans-generational effect of maternal lactation during pregnancy: a Holstein cow model. PLoS One 2012;7:e51816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bogdal C, Züst S, Schmid P, Gyalpo T, Zeberli A, Hungerbühler K, Zennegg M.. Dynamic transgenerational fate of polychlorinated biphenyls and dioxins/furans in lactating cows and their offspring. Environ Sci Technol 2017;51:10536–45. [DOI] [PubMed] [Google Scholar]