Respiratory syncytial virus (RSV) causes significant illness in older adults. In this first-in-humans study a small hydrophobic glycoprotein (SHe) of RSV A formulated with the lipid and oil-based platform DepoVaxTM was safe and highly immunogenic with sustained antibody responses.
Keywords: Respiratory syncytial virus vaccines, aged, adult, immunization, vaccine immunogenicity, vaccines, inactivated vaccines, adjuvant
Abstract
Background
Respiratory syncytial virus infection can cause lower respiratory tract infection in older adults comparable to influenza, but no vaccines are available.
Methods
This was a randomized, observer-blinded, first-in-humans study of a novel synthetic RSV antigen based on the ectodomain of the small hydrophobic glycoprotein (SHe) of RSV subgroup A, formulated with either the lipid and oil–based vaccine platform DepoVax (DPX-RSV[A]) or alum (RSV[A]-Alum), in healthy, 50–64-year-old individuals. Two dose levels (10 or 25 µg) of SHe with each formulation were compared to placebo. A booster dose was administered on day 56.
Results
There was no indication that the vaccine was unsafe. Mild pain, drowsiness, and muscles aches were the most common solicited adverse events (AEs), and the frequencies of the AEs did not increase after dose 2. Robust anti-SHe–specific immune responses were demonstrated in the DPX-RSV(A) 10-μg and 25-μg groups (geometric mean titer, approximately 10-fold and 100-fold greater than that of placebo at days 56 and 236, respectively), and responses were sustained in the DPX-RSV(A) 25-μg group at day 421. Responses to the RSV(A)-Alum vaccines were very low.
Conclusions
A novel antigen from the SH protein of RSV, formulated in a lipid and oil–based vaccine platform, was highly immunogenic, with sustained antigen-specific antibody responses, and had an acceptable safety profile.
Respiratory syncytial virus (RSV) is increasingly recognized as an acute lower respiratory tract pathogen that causes significant illness throughout life. Bronchiolitis and pneumonia are the most common cause of childhood respiratory tract infection worldwide [1]. In immunocompromised adults, RSV may cause life-threatening pneumonia [2], and in healthy older adults or those with cardiac or pulmonary disease, RSV infection is associated with use of health services at a level similar to that for seasonal influenza [3]. The mean rate of RSV-associated hospitalization in older adults was 55.3 events/100000 person-years (95% confidence interval [CI], 44.4–107) between 1993 and 2008, compared with 63.5 events/100000 person-years (95% CI, 37.5–237) for influenza [4]. In a retrospective cohort of 607 RSV-infected hospitalized adults, supplemental oxygen and ventilatory support were required in 67.9% and 11.1%, respectively, and lower respiratory tract complications occurred in 71.9% [5].
No prophylactic antivirals or vaccines are currently available to prevent RSV infection in adults. At least 6 RSV vaccine candidates directed at older adults are in development, predominantly based on the fusion transmembrane RSV protein, including a nanoparticle vaccine [6], a subunit nasally administered vaccine, and vector-delivered and live attenuated vaccines [7], all of which are presumed to act through antibody-mediated virus neutralization. We previously reported that a vaccine targeting the ectodomain of the RSV subgroup A surface small hydrophobic glycoprotein (SHe) can induce protection against intranasal RSV challenge in mice and cotton rats [8]. SHe-vaccinated animals had reduced pulmonary replication of RSV as compared to controls. Further, SHe-specific antibodies were detected bound to the surface of RSV-infected cells. Protection in these models was demonstrated to be dependent on Fcγ receptor activation and resident alveolar macrophages. Thus, it was proposed that SHe-specific immunoglobulin G (IgG) control RSV replication by instructing alveolar macrophages to clear RSV-infected cells by phagocytosis. RSV vaccines for the older adult population must be sufficiently immunogenic to overcome age-related changes in the innate and adaptive immune systems [9]. Vaccine adjuvants copresented with the RSV antigen could improve the immunogenicity of RSV vaccine in older persons. In this first-in-humans study, we evaluated the safety and immunogenicity of a depot-based lipid-in-oil delivery platform containing a novel antigenic target, SHe, in adults 50–64 years of age.
METHODS
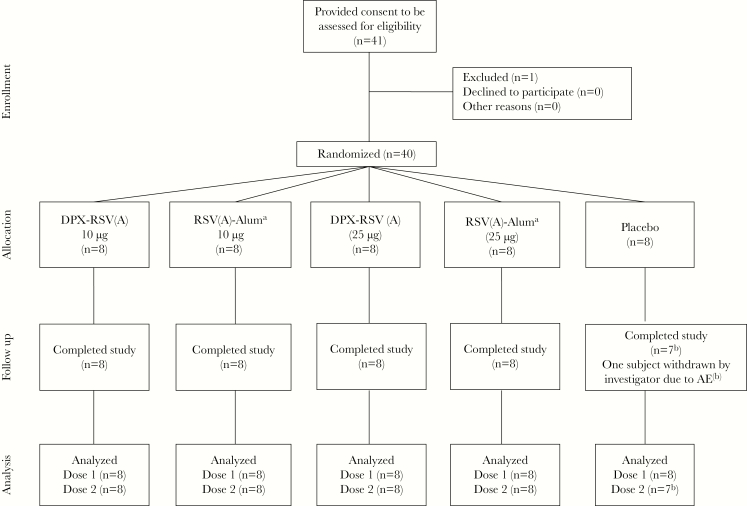
This was a randomized, placebo-controlled, observer-blinded, first-in-humans, phase 1 clinical trial to evaluate the safety and reactogenicity of a 2-dose schedule of 4 formulations of an adjuvanted RSV vaccine, compared with placebo, in 50–64-year-old healthy persons at 1 site in Canada (Figure 1). The study was conducted in 2 sequential steps to permit dose escalation, with participants randomized at ratios of 2:2:1 in both steps. The study was initiated on 30 June 2015, and the final (day 421) visit was 13 March 2017.
Figure 1.
Participant flow through the study. See “Methods” for a description of vaccine formulations. DPX, DepoVax; RSV(A), respiratory syncytial virus subgroup A. aParticipants in the RSV(A)-Alum group received placebo on day 56, rather than RSV(A)-Alum. bOne subject was withdrawn by the investigators because of an adverse event.
The study (clinical trials registration NCT02472548) was undertaken in compliance with Good clinical practice guidelines, the Declaration of Helsinki, and national regulatory requirements and was approved by the local institutional review board. An independent data safety monitoring committee reviewed safety data 28 days after each vaccine dose and immunogenicity results after step 1.
Participants
Participants were 50–64 years of age; were healthy, based on medical history, clinical examination, and hematological and biochemical values; and had given informed written consent. Exclusion criteria included human immunodeficiency virus infection; hepatitis B or C virus infection; personal or family history of immunocompromise; autoimmune disease or malignancy; receipt of RSV vaccine, immunoglobulins, or blood products within the past 3 months; or hypersensitivity to any vaccine component. No vaccines were permitted within 28 days of study vaccine receipt, except for influenza vaccine (which was permitted ≥15 days before study vaccination). Women of childbearing potential had to have a negative result of a pregnancy test on the day of immunization and had to practice adequate contraception for 30 days before and 180 days after vaccine receipt.
Vaccines
The RSV(A) antigen is a 23–amino acid synthetic peptide (NKLCEYNVFHNKTFELPRARVNT) representing the SH ectodomain of RSV(A) and was synthesized by PolyPeptide Group (San Diego, CA). The obtained peptide powder was dimerized before vaccine formulation by dissolution in a solution of water, dimethyl sulfoxide, and acetic acid (89.5:10:1 w/w/w) and heating at 37°C overnight. Dimer formation was confirmed by high-pressure liquid chromatography and liquid chromatography–mass spectrometry analysis.
The SHe peptide (10 or 25 μg) was formulated in DPX, a synthetic lipopeptide adjuvant, which is a lipid mixture of cholesterol and phosphatidylcholine, and Montanide ISA 51 VG (Seppic, France). The DPX-RSV(A) vaccine was supplied in 2 vials. Vial 1 contained the antigen and adjuvant system lyophilized to a dry cake and was stored at −20°C. It was thawed to room temperature on the day of vaccination and then mixed with vial 2, containing Montanide. The reconstituted vaccine appeared as a clear suspension.
The RSV(A)-Alum vaccine consisted of the SHe dimer and the mineral adjuvant aluminum hydroxide (Alhydrogel, 2% colloidal suspension; Brenntag Biosector, Denmark) and was supplied in 2 vials. Vial 1, containing the SHe peptide in sodium acetate buffer, was stored at −20°C and thawed to room temperature on the day of vaccination. It was then mixed with 50 μg of alum from vial 2. The reconstituted RSV(A)-Alum vaccine was hazy in appearance. The placebo was normal saline (0.9% sodium chloride) and was clear in appearance.
An unblinded pharmacist prepared vaccines at room temperature on the day of immunization. An obscuring label with the study number was affixed to the prepared vial. An unblinded nurse administered study vaccines (dose volume, 0.05 mL [50 µL]) in the deltoid. The first dose was given in the nondominant arm and the second dose was given in the contralateral arm.
Study Procedures
After individuals provided consent to participate, screening- eligible participants attended visit 1 within 30 days. A step-specific randomization list was computer generated in 3 stages, corresponding to the staggered injection of participants over time. The first 3 participants in each step were randomly assigned in a ratio of 1:1:1 to receive 10 µg of DPX-RSV(A), 10 µg of RSV(A)-Alum, or placebo, and the remaining 17 subjects were randomly assigned in a ratio of 7:7:3 to receive DPX-RSV(A) vaccine, RSV(A)-Alum, or placebo, to achieve an overall randomization ratio of 2:2:1. The same process occurred for step 2, with the RSV(A) antigen dose increased to 25 μg.
Participants stayed in their assigned group for the booster dose. Per protocol, the second dose in the RSV(A)-Alum groups was homologous vaccine or placebo, depending on the blinded day 28 serologic test results for the group. If no anti-SHe antibodies were detected in the day 28 serologic analysis of any participant in any vaccine group, then participants received a homologous booster dose on day 56. If anti-SHe antibodies were detected in any participant in any group in day 28 serologic tests, then the RSV(A)-Alum group would receive placebo on day 56. This precautionary measure was taken because a preclinical study showed that some RSV(A)-Alum recipients had adverse reactions in the first hour after receiving the booster dose [10]; this reaction did not occur in DPX-RSV(A) recipients.
Participants attended the study site at the screening visit and 0, 7, 28, 56, 63, 84, and 236 days later (8 visits). Participants in step 2 attended a ninth visit on day 421.
Outcome Measurement
Safety and Reactogenicity
The primary objective was to measure safety and reactogenicity from vaccination to day 28 after the first injection. A secondary objective was to determine safety and reactogenicity up to 180 days after the second injection. The adverse event (AE) outcomes and severity-grading scheme are presented in Supplementary Table 1. Hematologic, biochemical, and screening serologic analyses were performed at the IWK Health Centre, using standard methods and local reference ranges.
Immunogenicity
Other secondary objectives of the study were to evaluate the immunogenicity of study vaccines 7 and 28 days after receipt of 1 and 2 doses of vaccine and the persistence of immune responses up to 180 days after the second vaccination. In step 2 participants (ie, those who received a higher antigen dose), immunogenicity at day 421 was measured.
End point enzyme-linked immunosorbent assays (ELISAs) were done at the Immunovaccine laboratories (Halifax, Canada) to measure anti–SHe antibody levels, using SHe antigen. Each subject’s serum sample was evaluated in 5 replicates on the same plate as the matched day 0 serum sample, itself in 3 replicates, to establish a cutoff, using the day 0 absorbance. The ELISA results are expressed as end point titrations calculated using the method described by Frey et al [11].
Binding of Human IgG to Cells Expressing RSV SH
Human embryonic kidney HEK293T cells (a gift from Dr M. Hall) were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated fetal calf serum, 1% penicillin, 1% streptomycin, 2 mM glutamine, and 1 mM sodium pyruvate at 37°C in the presence of 5% carbon dioxide.
The SH expression plasmid was constructed by amplifying SHe complementary DNA from complementary DNA obtained from total RNA derived from Hep-2 cells (CCL-23; ATCC, Rockville, MD) that had been infected with RSV A2 (VR-1540; ATCC). The primers were ATAAGAAAGCGGGCCGCTATGGAAAATACATCCATAACAATAG and GAAGATCTCTA TGTGTTGACTCGAGCTCTTGGTAACTCAAA. The resulting polymerase chain reaction fragment was cloned as a NotI/BglII fragment in the pCAGGS expression vector.
For flow cytometry, HEK293T cells were transfected with the RSV A2 SH expression vector in combination with a promoter-driven enhanced green fluorescent protein (GFP) transfection vector, using the Fugene transfection reagent (Promega). Forty-eight hours after transfection, the cells were detached using ice-cold ethylenediaminetetraacetic acid–trypsin, washed twice with phosphate-buffered saline, and subsequently fixed with 1% paraformaldehyde for 40 minutes. After blocking with 1% bovine serum albumin for 1 hour, the cells were stained with a 100-fold dilution of participant sera or with phosphate-buffered saline for 2 hours. Binding of human IgG to cells was detected with Alexa Fluor 633–conjugated goat anti-human IgG (Invitrogen). The median fluorescence intensity of the cells was determined with an LSRII HTS flow cytometer (BD). The specific binding of human IgG to cells that express RSV SH was calculated as the ratio of the Alexa Fluor 633 median fluorescence intensity (MFI) of GFP-positive cells and the Alexa Fluor 633 MFI of GFP-negative cells.
Sample Size and Statistical Analysis
This was a first-in-humans study, and therefore there were no previous estimates of the frequency of AEs or of immune responses. As the primary outcome was safety, the sample size was based on the likely precision around estimates of the percentage of participants in each study vaccine group with symptoms following vaccination. For example, AEs occurring in DPX-RSV(A) group G1 (n = 16) at a rate of ≥15% were detectable with a probability >.93, while AEs occurring in one of the other comparison groups (8 participants each) at a rate of ≥15% will be detectable with a probability >.73. No formal power calculation was conducted. The precision of AE frequency was estimated on the basis of standard deviations of estimated proportions for combined sample sizes of 8 or 16, with the true AE rate probability between .05 and .50. The expected width of the 95% CI on the true rate is approximately twice the tabulated standard deviation. The frequency of demographic characteristics of participants was described. The safety analysis presented here was conducted on the total vaccinated cohort, defined as all participants who received at least 1 dose of study vaccine.
The percentages of participants with at least 1 AE, grade 3 AE, or serious AE following vaccine receipt were tabulated, each with an exact 95% CI, and the number and percentage of participants with a hematologic or biochemistry result below or above the local laboratory range was tabulated. Unsolicited AEs were classified and coded according to the Medical Dictionary for Regulatory Activities (MedDRA), version 19.0.
Fisher exact tests were used to assess differences in rates of solicited AEs. All statistical tests performed were 2-sided with a type I error of 5%. No adjustments were made for performing multiple tests.
For the analysis of proportions, binomial point estimates and exact binomial confidence intervals (CIs) were calculated for each group, and differences between groups were compared using Fisher exact tests. The analysis of continuous variables consisted of point estimates and interval estimates for means, and differences between groups were assessed using t tests and analysis of variance.
Geometric mean antibody titers (GMTs) and their 95% CIs were calculated by group for each time point after vaccination. CIs for the difference of geometric means were calculated for the pair-wise comparison of groups. For each participant, the antibody titer was defined as the inverse of the highest dilution (starting at 1:100) of postvaccination serum above the cutoff, which was determined for each subject on the basis of the day 0 titer. Serum samples below the detection limit were assumed to have an antibody titer of 50. These analyses were performed using statistical and summarization procedures in SAS, version 9.4 (Cary, NC).
For the repeated measurement analysis, log-transformed ratios of GFP-positive cells to GFP-negative cells were analyzed as repeated measurements, using the residual maximum likelihood approach as implemented in Genstat, version 18 [12]. Briefly, a linear mixed model with treatment, time, and the treatment × time interaction as fixed terms and subject time as a residual term was fitted to data. Times of measurement were set at unequal intervals, and the unstructured correlation structure was selected as the best model fit, based on the Akaike information coefficient. Significances of the fixed terms and significances of changes in differences between treatment effects over time were assessed by an F test.
RESULTS
Forty participants received the first dose of vaccine (Figure 1). The investigator withdrew 1 participant at the time of the second dose because of new-onset cutaneous herpes zoster. After data lock, this subject was noted to be in the placebo group. On preordained review of immunogenicity results for participants in step 1 by the data safety monitoring committee, the second dose of vaccine at day 56 in the RSV(A)-Alum group was placebo, rather than RSV(A)-Alum. Characteristics of study participants are seen in Table 1.
Table 1.
Characteristics of Participants
| Characteristic | DPX-RSV(A) (10 µg) (n = 8) | RSV(A)-Alum (10 µg) (n = 8) | DPX-RSV(A) (25 µg) (n = 8) | RSV(A)-Alum (25 µg) (n = 8) | Placebo (n = 8) | Total (n = 40) |
|---|---|---|---|---|---|---|
| Age, y | ||||||
| Mean ± SD | 56.4 ± 4.84 | 53.4 ± 3.54 | 56.3 ± 3.99 | 56.1 ± 3.64 | 54.3 ± 3.77 | 55.3 ± 3.97 |
| Range | 50–63 | (50,60) | (51,62) | (53,63) | (50,61) | |
| Sex, no. (%) | ||||||
| Male | 1 (12.5) | 5 (62.5) | 2 (25.0) | 1 (12.5) | 2 (25.0) | 11 (27.5) |
| Female | 7 (87.5) | 3 (37.5) | 6 (75.0) | 7 (87.5) | 6 (75.0) | 29 (72.5) |
| Race, no. (%) | ||||||
| Black/African Canadian | 0 (0) | 0 (0) | 0 (0) | 1 (12.5) | 0 (0) | 1 (2.5) |
| White | 8 (100) | 8 (100) | 8 (100) | 7 (87.5) | 7 (87.5) | 38 (95.0) |
| Other | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (12.5) | 1 (2.5) |
Data are no. (%) of participants, unless otherwise indicated. See “Methods” for a description of vaccine formulations.
Abbreviations: DPX, DepoVax; RSV(A), respiratory syncytial virus subgroup A.
Safety and Reactogenicity
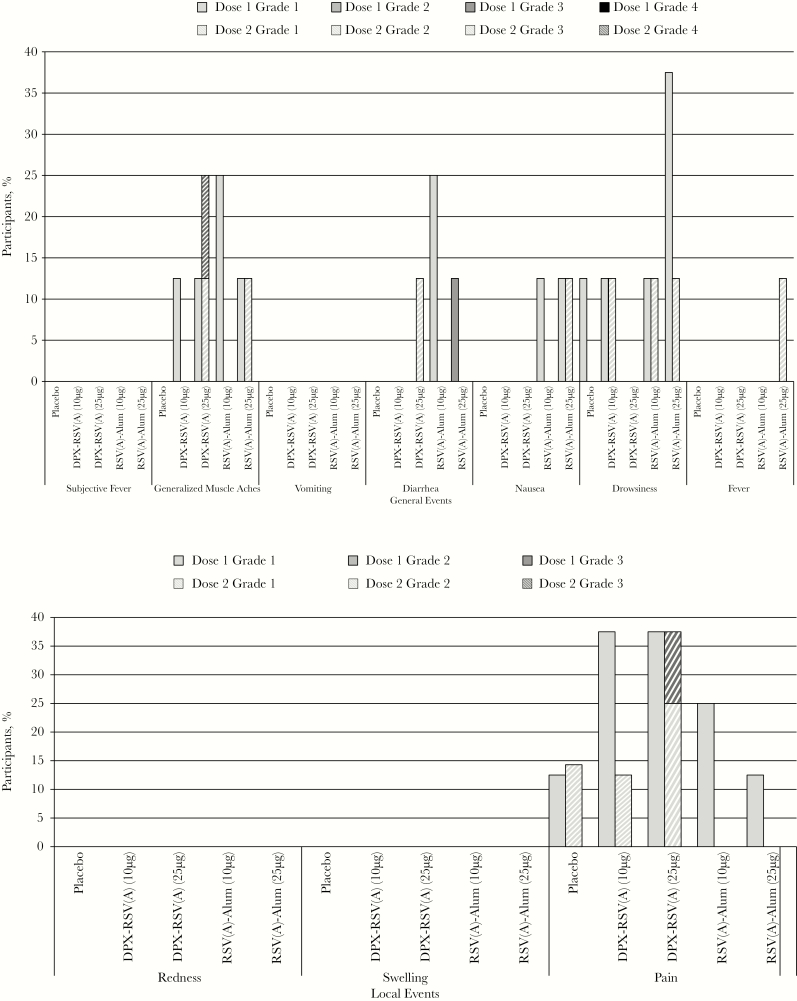
There were no serious AEs or potential immune-mediated diseases, and no holding rule was met during the study. The most common local AE was pain (Figure 2); no redness or swelling was observed in any study group. All but 1 participant who reported pain had mild (grade 1) pain. One participant in the 25-µg DPX-RSV(A) group reported having moderate (grade 2) pain for 1 day after the second dose. Pain was less common after the second dose.
Figure 2.
Percentage of participants with solicited injection site and general adverse events on days 0–6 after vaccination (doses 1 and 2 at days 0 and 56). Grade 3 (severe) pain was defined as pain that is significant at rest and prevents normal everyday activities. Redness and swelling were considered present if the greatest surface diameter of each was >100 mm. Grade 3 (severe) systemic adverse events were defined as those that prevent normal everyday activities. aParticipants in the RSV(A)-Alum group received placebo on day 56, rather than RSV(A)-Alum. See “Methods” for a description of vaccine formulations. DPX, DepoVax; RSV(A), respiratory syncytial virus subgroup A.
Any systemic or general AE was reported in 25.0% of participants after the first dose and in 21.0% after the second dose, with all but 2 of these events being mild in severity. Muscle aches and drowsiness were the most commonly reported systemic AEs (Figure 2). The severe AEs were diarrhea (duration, 24 hours) on day 4 after the first 25-µg dose of RSV(A)-Alum and muscle aches (duration, 48 hours) within 24 hours after the second 25-µg dose of DPX-RSV(A).
Unsolicited AEs were reported by 37.5%–75.0% of participants after dose 1 and by 25%–57% after dose 2; the incidence was similar across groups, by MedDRA category. Five unsolicited AEs in four participants were graded as severe (ie, grade 3) in intensity and were as follows: pneumonia and anemia post dose 2 (placebo recipient), migraine post dose 1 (RSV(A)-Alum 25 µg); diarrhea post dose 1 (DPX-RSV(A) 25 µg) and exacerbation of shoulder osteoarthritis post dose 2 (DPX-RSV(A) 25 mg).
Immunogenicity
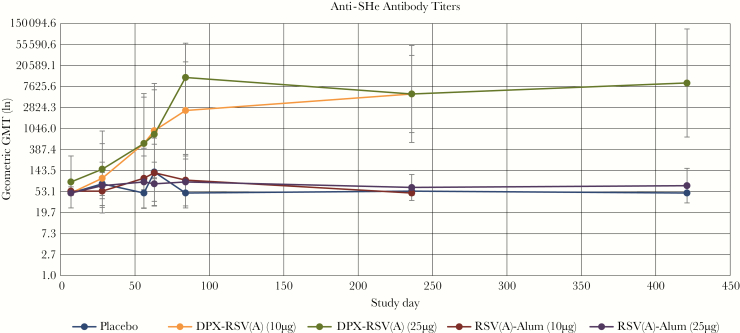
DPX-RSV(A) vaccines elicited robust anti-SHe immune responses by day 56, which continued to increase to day 236 (Table 2 and Figure 3). DPX-RSV(A) vaccines had 10-fold higher geometric mean antibody titer ratios (GMTRs), compared with placebo, at day 56, and almost 100-fold higher GMTRs at day 236 (day 236 GMT, 5381.74 [95% CI, 866.82–33413.1; GMTR, 98.69] in the 10-µg DPX-RSV[A] group and 5381.74 [95% CI, 543.94–53 246.8; GMTR, 98.69] in the 25-µg DPX-RSV[A] group).
Table 2.
Geometric Mean Antibody (Ab) Titers to Respiratory Syncytial Virus Subgroup A (RSV[A]) Small Hydrophobic Protein Ectodomain (SHe) Antigen Among Recipients of Alum- or DepoVax (DPX)–Adjuvanted RSV(A) SHe Vaccine
| Study Day, Group | Participants, No. | Ab Titer | GMTR (Specified Group: Placebo Group) | P a | ||||
|---|---|---|---|---|---|---|---|---|
| GM | Median (95% CI) | RSV(A)-Alum (10 µg) | DPX-RSV(A) (25 µg) | RSV(A)-Alum (25 µg) | Placebo | |||
| Day 7 | ||||||||
| DPX-RSV(A) (10 µg) | 8 | 50 | 50 (50–50) | 1.00 | .796 | .128 | 1 | 1 |
| RSV(A)-Alum (10 µg) | 8 | 54.53 | 50 (44.42–50) | 1.09 | .202 | .796 | .796 | |
| DPX-RSV(A) (25 µg) | 8 | 84.09 | 50 (24.6–66.92) | 1.68 | .128 | .128 | ||
| RSV(A)-Alum (25 µg) | 8 | 50 | 50 (50–287.48) | 1.00 | 1 | |||
| Placebo | 8 | 50 | 50 (50–50) | NA | ||||
| Day 28 | ||||||||
| DPX-RSV(A) (10 µg) | 8 | 100 | 50 (19.42–515.02) | 1.30 | .411 | .556 | .637 | .723 |
| RSV(A)-Alum (10 µg) | 8 | 54.53 | 50 (44.42–66.92) | 0.71 | .162 | .723 | .637 | |
| DPX-RSV(A) (25 µg) | 8 | 154.22 | 50 (25.38–937.16) | 2.00 | .292 | .348 | ||
| RSV(A)-Alum (25 µg) | 8 | 70.71 | 50 (38.06–131.38) | 0.92 | .906 | |||
| Placebo | 8 | 77.11 | 50 (27.68–214.78) | NA | ||||
| Day 56b | ||||||||
| DPX-RSV(A) (10 µg) | 8 | 518.74 | 282.84 (49.09–5481.28) | 10.37 | .107 | 1 | .076 | .025 |
| RSV(A)-Alum (10 µg) | 8 | 100 | 50 (24.18–413.48) | 2.00 | .107 | .863 | .491 | |
| DPX-RSV(A) (25 µg) | 8 | 518.74 | 200 (58.12–4629.83) | 10.37 | .076 | .025 | ||
| RSV(A)-Alum (25 µg) | 8 | 84.09 | 50 (24.6–287.48) | 1.68 | .605 | |||
| Placebo | 8 | 50 | 50 (50–50) | NA | ||||
| Day 63 | ||||||||
| DPX-RSV(A) (10 µg) | 8 | 951.37 | 1131.37 (102.48–8832.1) | 7.07 | .058 | .866 | .019 | .072 |
| RSV(A)-Alum (10 µg) | 8 | 129.68 | 50 (33.69–499.22) | 0.96 | .083 | .613 | .972 | |
| DPX-RSV(A) (25 µg) | 8 | 800 | 565.69 (97.89–6538.25) | 5.94 | .028 | .1 | ||
| RSV(A)-Alum (25 µg) | 8 | 77.11 | 50 (27.68–214.78) | 0.57 | .6 | |||
| Placebo | 7 | 134.59 | 50 (26.98–671.45) | NA | ||||
| Day 84 | ||||||||
| DPX-RSV(A) (10 µg) | 8 | 2467.54 | 4525.48 (248.42–24 509.7) | 49.35 | <.001 | .086 | <.001 | <.001 |
| RSV(A)-Alum (10 µg)c | 8 | 91.7 | 50 (27.15–309.68) | 1.83 | <.001 | .922 | .497 | |
| DPX-RSV(A) (25 µg) | 8 | 11 737.7 | 12 800 (2317.1–59 459) | 234.75 | <.001 | <.001 | ||
| RSV(A)-Alum (25 µg)c | 8 | 84.09 | 50 (24.6–287.48) | 1.68 | .56 | |||
| Placebo | 8 | 50 | 50 (50–50) | NA | ||||
| Day 236 | ||||||||
| DPX-RSV(A) (10 µg) | 8 | 5381.74 | 6400 (866.82–33 413.1) | 98.69 | <.001 | 1 | <.001 | <.001 |
| RSV(A)-Alum (10 µg)c | 8 | 50 | 50 (50–50) | 0.92 | <.001 | .748 | .915 | |
| DPX-RSV(A) (25 µg) | 8 | 5381.74 | 6400 (543.94–53 246.8) | 98.69 | <.001 | <.001 | ||
| RSV(A)-Alum (25 µg)c | 8 | 64.84 | 50 (35.07–119.89) | 1.19 | .83 | |||
| Placebo | 8 | 54.53 | 50 (44.42–66.92) | NA | ||||
See “Methods” for a description of vaccine formulations. Recipients received 2 doses of vaccine or placebo (one on day 0 and another on day 56).
Abbreviations: CI, confidence interval; GM, geometric mean; GMTR, geometric mean titer ratio; NA, not applicable.
aValues <.05 are statistically significant.
bSerum specimens were procured on day 56, before the second dose of vaccine was given.
cNote that participants in the RSV(A)-Alum group received placebo, rather than RSV(A)-Alum, on day 56.
Figure 3.
Geometric mean antibody titers (GMTs) to respiratory syncytial virus subgroup A (RSV[A]) small hydrophobic protein ectodomain (SHe), by vaccine study group, on days 0–236 among all participants and to day 421 among step 2 participants. Error bars depict 95% confidence intervals. In step 1, SHe-containing vaccines had 10 µg of SHe antigen. In step 2 SHe-containing vaccines had 25 µg of SHe antigen. Two dose levels (10 or 25 µg) of the SHe antigen with each formulation were compared to placebo. A booster dose was administered on day 56. See “Methods” for a description of vaccine formulations. DPX, DepoVax.
In exploratory analysis, a statistically significant difference in immune response to DPX-RSV(A) vaccines as compared to placebo was seen on days 56, 84, and 236. Serum anti-SHe responses to Alum-adjuvanted RSV(A) vaccines were poor, like placebo.
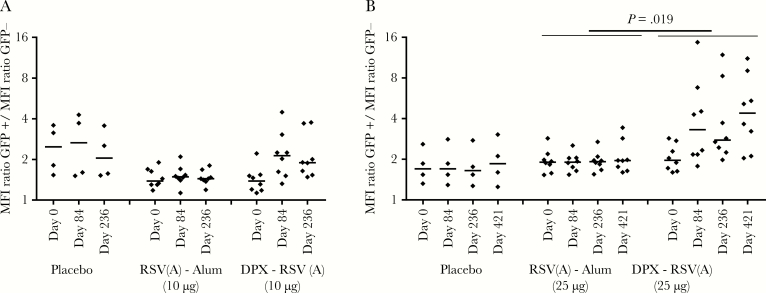
We also analyzed whether the SHe-specific antibodies induced by immunization with DPX-RSV(A) could bind to the antigen in its natural context (ie, at the surface of mammalian cells expressing SHe). Flow cytometry showed that, before vaccination, there was no or very low binding to cells expressing SH (Figure 4 and Supplementary Figure 1). Vaccination with placebo or RSV(A)-Alum also resulted in little binding. In contrast, IgG in serum samples from recipients of DPX-RSV(A) displayed significantly enhanced binding to SH on the surface of SH-transfected cells (Figure 4), and in individuals in the 25-µg DPX-RSV(A) group this persisted for at least 421 days after the first dose. Overall, there was a clear correlation between the level of SHe-specific antibodies as detected by peptide-specific ELISA and the ability of human sera to bind to SH expressed at the surface of cells (Supplementary Figure 1).
Figure 4.
Binding of serum immunoglobulin G (IgG) to respiratory syncytial virus (RSV) small hydrophobic protein (SH) on the surface of cells. DPX-RSV(A) vaccination of human volunteers induces serum IgG that can bind to cells expressing the RSV SH surface protein. HEK293T cells coexpressing RSV SH and green fluorescent protein (GFP; Hek-SH) were analyzed by flow cytometry for human IgG binding, using sera sampled at the indicated time points after the last immunization with placebo, RSV(A)-Alum, or DPX-RSV(A). Data denote the ratio of the mean fluorescence intensity (MFI) of cells expressing SH (ie, GFP-positive cells) to the MFI of control cells (ie, GFP-negative cells) in individual serum samples. Short horizontal lines represent mean values. A, Data from low-dose (step 1) vaccine recipients. B, Data from high-dose (step 2) vaccine recipients. See “Methods” for a description of vaccine formulations. DPX, DepoVax.
DISCUSSION
In this first-in-humans study, a depot-based lipid-in-oil platform formulated with a novel RSV antigenic target was highly immunogenic in healthy adults 50–64 years of age, with sustained antigen-specific antibody responses up to 180 days after the second vaccine dose and to 421 days in the higher-dose group. Alum-adjuvanted vaccines with the RSV SHe antigen performed poorly and were not different to placebo. Antibody titers continued to increase from day 28 to day 56 in both DPX-RSV(A) groups, suggesting ongoing antigen presentation at the injection site that continued to stimulate the immune response. In preclinical studies in which this adjuvant platform and test antigen were visualized with magnetic resonance imaging, the injected material could be found in muscle and draining lymph nodes for weeks to months after administration [13]. The response to the booster dose was robust in both the 10-μg and 25-μg dose groups. At day 84, the anamnestic response to the 25-μg dose was higher than that to the 10-μg dose, but at day 236 they were both about 100-fold higher than in placebo recipients.
The RSV genome encodes 3 surface glycoproteins: F (fusion), G (attachment glycoprotein), and SH. RSV vaccine development to date has predominately focused on generating an immune response to F [14] because it is essential for infection, it is the primary target for serum neutralizing antibodies, and titers of anti-F RSV antibody correlate in many circumstances with protection from infection. Unlike G, F is conserved between RSV isolates. Further, prophylactic use of the humanized mouse monoclonal antibody palivizumab, which is directed at F, reduced the incidence of RSV-associated hospitalization in infants [15]. SH is a novel target for a RSV vaccine, and this study is the first to report results of a single-subunit SHe-based vaccine study in humans. SH is a small protein that comprises 64 and 65 amino acids in subgroup A and B RSV, respectively [16]. Recent evidence suggests that SH can function as a viroporin [17, 18], a small viral membrane protein forming oligomers that act as proton or ion channels in the host cell and, thus, enhance membrane permeability. In vitro studies showed that SH inhibits apoptosis by blocking a tumor necrosis factor–mediated signaling pathway [19]. SH could thus promote viral replication by interfering with the host immune response and prolong viral replication in the host cell [20]. In preclinical studies in mice and cotton rats, SHe-vaccinated and intranasally challenged animals had reduced RSV replication, and SHe-specific antibodies were detected bound to the surface of RSV-infected cells [8]. Further, SHe-specific immune serum reduced replication in the lung in a dose-dependent manner. In the mouse model, this protection was strictly dependent on activating Fcγ receptors and on resident alveolar macrophages. As such, it was hypothesized that SHe-specific IgG can control RSV replication by inducing resident alveolar macrophages to clear RSV-infected cells by phagocytosis. The very high anti-SHe antibody titers in recipients of the lipid-in-oil adjuvanted RSV vaccine suggest that this may be a valid vaccination strategy targeting this viral protein.
The study lipid-in-oil adjuvant has been shown in preclinical studies to produce a strong, specific, and sustained immune response [21–23] and has been evaluated in phase 1 studies of 2 DepoVax-formulated therapeutic cancer vaccines, DPX-0907 [24] and DPX-Survivac [25]. In these studies of patients with advanced ovarian, breast, and prostate cancers, in which a considerably higher dose volume of the platform is used, an acceptable safety profile and specific immune responses have been observed. The DPX-RSV(A) formulation was also administered in a very small volume (0.05 mL [50 µL]), which to our knowledge is the lowest volume ever reported for an oil-based vaccine in humans. Injecting this small volume may reduce local adverse events.
This study has several limitations. It is a first-in-humans study designed with a small sample size to minimize exposure of participants to a novel vaccine. Infrequent AEs may not have been detected and might occur at time points later than the follow-up period of this study. Given that our vaccine is targeted at older adults, it was conducted in individuals aged 50–64 years. Although our study population was older than that in most phase 1 trials, which are often conducted in individuals aged 18–40 years, the vaccine was not evaluated in the target age group of ≥65 years. Accordingly, results may not be applicable to that population. Further study in persons >65 years of age is therefore needed. Finally, our vaccine antigen contained a synthetic RSV-A antigen, SHe. Since there is variability in the sequence homology of SHe across RSV A and B subgroups [26], a SHe-containing RSV vaccine would likely need to contain both subgroup antigens.
In summary, in this first-in-humans study a depot-based lipid-in-oil adjuvant platform copresented with a novel RSV antigenic target was safe and highly immunogenic in adults 50–64 years of age, with sustained humoral responses up to 180 days after vaccination.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments We thank the study participants for their contributions to the study.
Financial support. This work was supported by the Canadian Institutes of Health Industry-Partnered Collaborative Research Operating Grant program and Immunovaccine.
Potential conflict of interest. J. M. L., S. A. H., and S. A. M. have received research funding from GlaxoSmithKline, Immunovaccine, Sanofi Pasteur, Pfizer, Pan Provincial Vaccine Enterprise (Prevent), Novavax, the Public Health Agency of Canada, and the Canadian Institutes of Health Research. L. D. M., G. W., and M. S. are employees of Immunovaccine and own stock/share options or restricted shares of Immunovaccine. S. A. H. is a member of the Immunovaccine Advisory Board. VIB and UGent hold patent rights on SHe-based vaccines and treatment options for RSV (patent application WO2012/065997 [24.05.2012]). B. S. and X. S. are named as inventors on this patent, which is licensed to Immunovaccine.
Presented in part: IDWeek, New Orleans, Louisiana, 26–30 October 2016; 10th International RSV Symposium, Patagonia, Argentina, 28 September–1 October 2016; 17th World Vaccine Congress, Barcelona, Spain, 10–12 October 2016.
References
- 1. Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390:946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boeckh M. The challenge of respiratory virus infections in hematopoietic cell transplant recipients. Br J Haematol 2008; 143:455–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749–59. [DOI] [PubMed] [Google Scholar]
- 4. Zhou H, Thompson WW, Viboud CG, et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin Infect Dis 2012; 54:1427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee N, Lui GC, Wong KT, et al. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin Infect Dis 2013; 57:1069–77. [DOI] [PubMed] [Google Scholar]
- 6. Fries L, Shinde V, Stoddard JJ, et al. Immunogenicity and safety of a respiratory syncytial virus fusion protein (RSV F) nanoparticle vaccine in older adults. Immun Ageing 2017; 14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. PATH. RSV vaccine and mAb snapshot http://www.path.org/publications/files/CVIA_rsv_snapshot_final.pdf. Accessed 14 June 2017.
- 8. Schepens B, Sedeyn K, Vande Ginste L, et al. Protection and mechanism of action of a novel human respiratory syncytial virus vaccine candidate based on the extracellular domain of small hydrophobic protein. EMBO Mol Med 2014; 6:1436–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bandaranayake T, Shaw AC. Host Resistance and Immune Aging. Clin Geriatr Med 2016; 32:415–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. MacDonald LD, MacKay A, Kaliaperumal V, et al. Type III hypersensitivity reactions to a B cell epitope antigen are abrogated using a depot forming vaccine platform. Hum Vaccines Immunother 2017:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frey A, Di Canzio J, Zurakowski D. A statistically defined endpoint titer determination method for immunoassays. J Immunol Methods 1998; 221:35–41. [DOI] [PubMed] [Google Scholar]
- 12. Payne JR, Welham SJ, Harding SA.. Part 3 procedures. GenStat reference manual (Release 18). Hemel Hempstead, U.K: VSN International, 2015. [Google Scholar]
- 13. Brewer KD, Lake K, Pelot N, et al. Clearance of depot vaccine SPIO-labeled antigen and substrate visualized using MRI. Vaccine 2014; 32:6956–62. [DOI] [PubMed] [Google Scholar]
- 14. Graham BS. Vaccine development for respiratory syncytial virus. Curr Opin Virol 2017; 23:107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics 1998; 102:531–7. [PubMed] [Google Scholar]
- 16. Chen MD, Vazquez M, Buonocore L, Kahn JS. Conservation of the respiratory syncytial virus SH gene. J Infect Dis 2000; 182:1228–33. [DOI] [PubMed] [Google Scholar]
- 17. Bukreyev A, Whitehead SS, Murphy BR, Collins PL. Recombinant respiratory syncytial virus from which the entire SH gene has been deleted grows efficiently in cell culture and exhibits site-specific attenuation in the respiratory tract of the mouse. J Virol 1997; 71:8973–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carter SD, Dent KC, Atkins E, et al. Direct visualization of the small hydrophobic protein of human respiratory syncytial virus reveals the structural basis for membrane permeability. FEBS Lett 2010; 584:2786–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fuentes S, Tran KC, Luthra P, Teng MN, He B. Function of the respiratory syncytial virus small hydrophobic protein. J Virol 2007; 81:8361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Araujo GC, Silva RH, Scott LP, Araujo AS, Souza FP, de Oliveira RJ. Structure and functional dynamics characterization of the ion channel of the human respiratory syncytial virus (hRSV) small hydrophobic protein (SH) transmembrane domain by combining molecular dynamics with excited normal modes. J Mol Model 2016; 22:286. [DOI] [PubMed] [Google Scholar]
- 21. Karkada M, Weir GM, Quinton T, Fuentes-Ortega A, Mansour M. A liposome-based platform, VacciMax, and its modified water-free platform DepoVax enhance efficacy of in vivo nucleic acid delivery. Vaccine 2010; 28:6176–82. [DOI] [PubMed] [Google Scholar]
- 22. Karkada M, Weir GM, Quinton T, et al. A novel breast/ovarian cancer peptide vaccine platform that promotes specific type-1 but not Treg/Tr1-type responses. J Immunother 2010; 33:250–61. [DOI] [PubMed] [Google Scholar]
- 23. MacDonald LD, Fuentes-Ortega A, Sammatur L, Mansour M. Efficacy of a single dose hepatitis B depot vaccine. Vaccine 2010; 28:7143–5. [DOI] [PubMed] [Google Scholar]
- 24. Berinstein NL, Karkada M, Morse MA, et al. First-in-man application of a novel therapeutic cancer vaccine formulation with the capacity to induce multi-functional T cell responses in ovarian, breast and prostate cancer patients. J Transl Med 2012; 10:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berinstein NL, Karkada M, Oza AM, et al. Survivin-targeted immunotherapy drives robust polyfunctional T cell generation and differentiation in advanced ovarian cancer patients. Oncoimmunology 2015; 4:e1026529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Johnson PR, Spriggs MK, Olmsted RA, Collins PL. The G glycoprotein of human respiratory syncytial viruses of subgroups A and B: extensive sequence divergence between antigenically related proteins. Proc Natl Acad Sci U S A 1987; 84:5625–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.