Abstract
Nonalcoholic fatty liver disease (NAFLD) is considered the hepatic manifestation of insulin resistance, which is the hallmark of type 2 diabetes (T2D). NAFLD is a known risk factor for developing T2D and has a very high prevalence in those with existing T2D. The diabetes spectrum includes several conditions from prediabetes to T2D to insulin‐dependent diabetes leading to macrovascular and microvascular complications. Similarly, NAFLD has a histologic spectrum that ranges from the relatively benign nonalcoholic fatty liver to the aggressive form of nonalcoholic steatohepatitis with or without liver fibrosis to nonalcoholic steatohepatitis‐cirrhosis leading to end‐stage liver disease. The management of T2D has witnessed significant changes over the past few decades with multiple new drug classes entering the treatment algorithm. Unfortunately, there are no U.S. Food and Drug Administration‐approved medications to treat NAFLD, and guidelines for the management of NAFLD are less established. However, the field of drug development in NAFLD has witnessed a revolution over the past 5 years with the establishment of a regulatory pathway for Food and Drug Administration approval; this has generated substantial interest from pharmaceutical companies. Several diabetes medications have been studied as potential treatments for NAFLD with promising results; moreover, drugs that target specific pathways that play a role in NAFLD development and progression are being developed at a rapid pace. Given the similarities between NAFLD and T2D in terms of pathogenesis, underlying risk factors, and disease spectrum, lessons learned from optimizing treatment for T2D can be extrapolated to the management of NAFLD. The aim of this review is to use the founding principles of the comprehensive type 2 diabetes management algorithm to optimize the management of NAFLD. (Hepatology Communications 2018;2:778‐785)
Abbreviations
- A1C
hemoglobin A1c
- ALT
alanine aminotransferase
- CVC
cenicriviroc
- FDA
U.S. Food and Drug Administration
- HCC
hepatocellular carcinoma
- NAFL
nonalcoholic fatty liver
- NAFLD
nonalcoholic fatty liver disease
- NAS
nonalcoholic fatty liver disease activity score
- NASH
nonalcoholic steatohepatitis
- OCA
obeticholic acid
- PPAR
peroxisome proliferator‐activated receptor
- RCT
randomized controlled trial
- T2D algorithm
comprehensive type 2 diabetes management algorithm
- T2D
type 2 diabetes
Nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes (T2D) share many similarities in their underlying risk factors, pathogenesis, epidemiology, and the fact that both diseases include a spectrum of conditions. NAFLD is a relatively newer condition, and although the initial description dates back to 1980,1 its full spectrum and impact as a major chronic liver disease have only been recognized for the past 2 decades.2 Today, NAFLD is a leading indication for liver transplantation in the United States3 and contributes as the underlying etiology to a significant percentage of hepatocellular carcinoma (HCC) cases each year.4
NAFLD includes a histologic spectrum of diseases that starts with bland accumulation of fat within the hepatocytes without evidence of significant necro‐inflammation or fibrosis, a condition called nonalcoholic fatty liver (NAFL).5 It is thought that NAFL is a relatively benign condition in terms of liver disease progression but is considered a risk factor for developing the aggressive form of the disease that is called nonalcoholic steatohepatitis (NASH). Therefore, we compare NAFL to prediabetes, which is not a disease per se but a risk factor for developing T2D. As with prediabetes, NAFL is associated with an increased risk for atherosclerotic cardiovascular disease.6
With NASH, there is evidence of liver inflammation and hepatocyte injury in the form of ballooning and the presence of Mallory‐Denk bodies. The severity of NASH can be assessed with a score called the NAFLD activity score (NAS), which gives grades to the severity of the individual histologic features of NASH, namely, steatosis, inflammation, and hepatocyte ballooning.7 The score ranges from 0‐8, and a score of 4 or higher is considered as moderate to severe NASH. Although the utility of this score in predicting clinical outcomes is limited,8 it has been used as a primary endpoint in short‐term clinical trials. NASH is considered the driving force behind the development of liver fibrosis and eventually cirrhosis, which occurs in 10%‐25% of patients.9 Therefore, we consider NASH a serious illness that warrants aggressive medical management with different pharmacologic agents similar to what is required for patients with T2D.
Finally, the development of NASH cirrhosis with portal hypertension complications indicates end‐organ damage to the liver. This is similar to the development of insulin‐dependent diabetes and its macrovascular and microvascular complications.
In this review, we evaluate the utility of certain founding principles of the comprehensive type 2 diabetes management algorithm (T2D Algorithm)10 that were developed by the American Association of Clinical Endocrinologists and American College of Endocrinology in optimizing the management of patients with NAFLD. Lessons learned from developing comprehensive care plans for those with T2D should guide clinicians taking care of patients with NAFLD to obtain the best possible outcomes.
Lifestyle Modifications/Weight Loss in NAFLD
T2D Algorithm “Lifestyle optimization is essential for all patients with diabetes. Weight loss should be considered in all patients with prediabetes and T2D who also have overweight or obesity. The need for medical therapy should not be interpreted as a failure of lifestyle management, but as an adjunct to it.”
Similar to diabetic individuals, patients with NAFLD tend to consume more calories and participate less in physical activities than age‐matched controls. In fact, data from the National Health and Nutrition Examination Survey demonstrated that when patients with NAFLD have concomitant diabetes, they perform at the lowest quartile of physical activity and moderate–vigorous physical activity.11 Also similar to the management of diabetes, the first tool in the management of NAFLD should be sustained weight loss through lifestyle optimization with diet and exercise. Although a modest weight loss of 3%‐5% of total body weight may help reduce hepatic steatosis, weight loss of 7%‐9% is typically required to reduce inflammation/ hepatocyte injury, and weight loss of 10% or more is needed to induce fibrosis regression.12 A large prospective study from Cuba included 293 patients with histologically proven NASH who were encouraged to adopt recommended lifestyle changes to reduce their weight. Liver biopsies were collected at baseline and at week 52 of the diet. Ninety percent of patients who lost 10% or more of their weight (n = 29) had resolution of NASH and 45% had fibrosis regression. We recommend a Mediterranean diet to our patients with NAFLD given its known benefits in terms of improving glycemic control and preventing cardiovascular disease, malignancy, and all‐cause mortality.13 In addition to diet, lifestyle optimization should emphasize physical activity. The T2D Algorithm recommends at least 150 minutes per week of moderate‐intensity exercise and strength training, and these goals may be applied to patients with NAFLD. Unfortunately, lifestyle modifications to induce sustained weight loss are often unsuccessful, with only 10% of individuals losing more than 10% of body weight. Other approaches to weight loss, such as pharmacologic agents, endoscopic bariatric therapies, and bariatric surgery, should be considered as part of the management algorithm for NAFLD, although more data are needed to establish their efficacy on histologic severity and disease progression. The failure to induce meaningful weight loss in patients with NAFLD should not exclude them from being considered for NASH‐specific therapies to delay disease progression and potentially induce resolution of NASH or regression of fibrosis.
NAFLD/NASH‐Specific Therapies
T2D Algorithm “The choice of diabetes therapies must be individualized based on attributes specific to both patients and the medications themselves. The treatment target should be individualized based on numerous factors, such as age, life expectancy, comorbid conditions, and risk of adverse consequences.”
Unlike diabetes where the U.S. Food and Drug Administration (FDA) has approved several classes of medications, we do not currently have any FDA‐approved medications for NAFLD. However, several major pharmaceutical companies have programs focused on drug discovery for NAFLD that will ultimately result in the approval of some of these agents by the FDA. Once these medications are approved for the indication of NAFLD/ NASH, we anticipate that physician caring for this patient population will have to make choices similar to those that are made by diabetologists in terms of selecting the most appropriate medication for each individual patient. Drugs to treat NAFLD can be divided into diabetic medication with potential efficacy for NAFLD and NASH‐specific therapies.
DIABETES MEDICATIONS WITH POTENTIAL EFFICACY IN THE TREATMENT OF NAFLD
Given the fact that NAFLD is tightly associated with both hepatic and peripheral insulin resistance and due to similarities in the pathogenic mechanisms underlying NAFLD and T2D, the effects of almost all diabetes medications have been assessed in patients with NAFLD with variable results. Although metformin is suggested as a first‐line pharmacologic treatment for prediabetes and T2D, several randomized controlled trials (RCTs) reported no effects of metformin on histologic improvement of NAFLD in either children or adults.14, 15 However, metformin may help with weight loss and may lower the risk of HCC in patients with diabetes.16 Both sulfonylureas and insulin are potentially associated with a higher risk of advanced NAFLD and HCC.17 The dipeptidyl peptidase‐4 inhibitor sitagliptin was compared to placebo in a randomized trial that included 50 patients with NAFLD and prediabetes or T2D and showed no beneficial effects on liver steatosis or fibrosis.18
Thiazolidinediones
Thiazolidinediones modulate peroxisome proliferator‐activated receptor γ (PPARγ), a transcription factor with a significant role in adipose tissue biology that influences insulin sensitivity and lipid metabolism. A large RCT of patients with NASH without diabetes demonstrated that, compared to placebo, pioglitazone 30 mg daily for 96 weeks was associated with improvement in histology and higher rates of NASH resolution.19 More recently, the effects of pioglitazone on NASH in patients with prediabetes and T2D were evaluated.20 Fifty‐one percent of patients that received pioglitazone 45 mg daily for 72 weeks had resolution of NASH and improvement in several histologic features, including liver fibrosis. Finally, a meta‐analysis of five clinical trials that evaluated the use of pioglitazone for NASH showed significant improvement in advanced fibrosis even in patients without diabetes.21 Pioglitazone as a treatment for NASH should be used with caution due to several safety concerns, including weight gain and potential risk for congestive heart failure, bone fractures, and bladder cancer.
Glucagon‐Like Peptide‐1 Receptor Agonists
A glucagon‐like peptide‐1 receptor (GLP‐1R) agonist is an incretin hormone that is secreted by the small intestine after coming in contact with nutrients. GLP‐1R agonists increase glucose‐induced insulin secretion, decrease glucagon secretion and hepatic glucose output, and promote satiety leading to weigh loss.22 A recent multicenter, double‐blind, randomized, placebo‐controlled phase 2 study assessed the efficacy of liraglutide 1.8 mg daily for 48 weeks in patients with NASH (the LEAN study).23 NASH resolution was noted in a significantly higher proportion of patients who received liraglutide (39%) than those who were in the placebo arm (9%, P = 0.019). Furthermore, liraglutide was associated with significantly less progression of liver fibrosis and improvement in surrogate biomarkers of fibrosis. These findings need to be confirmed in larger studies. Side effects that were more common in the liraglutide arm were mainly gastrointestinal, including diarrhea, constipation, and loss of appetite.
Indirect evidence from ad hoc analysis of RCTs in diabetic subjects suggests that other GLP‐1R agonists (exenatide, lixisenatide) are associated with improvement in alanine aminotransferase (ALT) and other markers of liver injury, indicating a potential role in the treatment of NAFLD.17
Sodium‐Glucose Cotransporter 2 Inhibitors
These medications represent the newest addition to the armamentarium of T2D medical treatments. By inhibiting glucose reabsorption in the proximal tubule, sodium‐glucose cotransporter 2 (SGLT2) inhibitors lead to significant glucose and calorie loss in the urine, resulting in improved insulin sensitivity, weight reduction, decreased substrate supply to the liver, and potentially a reduction in liver fat content.24 In a post‐hoc analysis of a study that included 336 patients with T2D, remogliflozin for 12 weeks was associated with a 30%‐40% reduction in ALT levels in patients with increased ALT at baseline.25 Given its beneficial effects on liver enzymes, insulin sensitivity, and weight loss, remogliflozin represents an attractive target for drug development in patients with diabetes and NASH. Similar improvements in serum aminotransferases were noted with other SGLT2 inhibitors, including cangliflozin26 and dapagliflozin,27 but the effects of this class of drugs on liver histology remain to be determined in future RCTs.
NOVEL THERAPEUTIC AGENTS FOR NASH: DRUGS IN PHASE III CLINICAL TRIALS
In addition to lipid accumulation in the liver due to insulin resistance, de novo lipogenesis, and alterations in free fatty acid metabolism, several “secondary” hits are necessary to induce inflammation, hepatocyte injury, and eventually liver fibrosis. They include oxidative stress, mitochondrial dysfunction, hepatocyte apoptosis, inflammatory chemokines/cytokines, and alterations in the bile acid enterohepatic circulation.28, 29 A large number of drugs that target each of these hits are being developed and tested in multiple clinical trials. In fact, as of March 3, 2018, https://www.clinicaltrials.gov/ct2/results?cond=nafld-term=-cntry=-state=-city=-dist= were registered under NAFLD. Four of these NASH drugs are currently in phase III clinical development (Table 1) and will be discussed next.
Table 1.
New Agents in Phase III Randomized Clinical Trials for the Treatment of NASH and NASH Fibrosis
| Phase II Efficacy Data | ||||||
|---|---|---|---|---|---|---|
| Investigational Medication | Mechanism of Action | Effective Dose | Resolution of NASH | Decrease in Fibrosis Stage | Phase III RCT | Planned Interim Analysis Duration |
| Obeticholic acid | FXR agonist | 10‐25 mg/day | No | Yes |
REGENERATE (https://clinicaltrials.gov/ct2/results?cond=NCT02548351-term=-cntry=-state=-city=-dist=) |
72 weeks |
| Elafibranor | PPARα/δ agonist | 120 mg/day | Yes | No |
RESOLVE‐IT (https://clinicaltrials.gov/ct2/results?cond=NCT02704403-term=-cntry=-state=-city=-dist=) |
72 weeks |
| Cenicriviroc | CCR2/CCR5 antagonist | 150 mg/day | No | Yes |
AURORA (https://clinicaltrials.gov/ct2/results?cond=NCT03028740-term=-cntry=-state=-city=-dist=) |
52weeks |
| Selonsertib | ASK1 inhibitor | 6 mg/day and 18 mg/day | No | Yes |
STELLAR 3 (https://clinicaltrials.gov/ct2/results?cond=NCT03053050-term=-cntry=-state=-city=-dist=) STELLAR‐4 (NCT03053063) |
48 weeks |
Abbreviations: ASK1, apoptosis signal‐regulating kinase 1; CCR, C‐C chemokine receptor; FXR, farnesoid X receptor.
Obeticholic Acid
Obeticholic acid (OCA) is a synthetic derivative of the natural bile acid chenodeoxycholic acid with 100‐fold greater agonistic activity against the farnesoid X receptor, a nuclear receptor of bile acids with a major role in regulating glucose and lipid metabolism.30 In a phase IIb, randomized, controlled study of 283 patients with noncirrhotic NASH (the FLINT study), OCA 25 mg daily was more effective than placebo in inducing histologic improvement in the NAS by 2 points or more with no worsening in fibrosis.31 Moreover, 35% of patients treated with OCA had a reduction in their fibrosis score by at least one stage compared to only 19% in the placebo arm. In terms of adverse events, there was an increase in low‐density lipoprotein and a decrease in high‐density lipoprotein in the OCA arm, raising safety concerns about long‐term cardiovascular outcomes. Furthermore, OCA was associated with pruritus in 23% of patients, which could significantly reduce compliance in this typically asymptomatic patient population. REGENERATE is a double‐blind, placebo‐controlled, randomized, multicenter phase III trial that will assess the long‐term efficacy and safety of OCA in patients with NASH and fibrosis (https://clinicaltrials.gov/ct2/results?cond=NCT02548351-term=-cntry=-state=-city=-dist=). Patients will be randomized to OCA 25 mg/day, OCA 10 mg/day, or placebo and followed for 6 years to assess hard outcomes, such as progression to cirrhosis, need for liver transplantation, and death. At 18 months after randomization, patients will undergo a liver biopsy to assess for histologic improvement in fibrosis and resolution of NASH. Other bile acid and nonbile acid farnesoid X receptor agonists are currently in development by several pharmaceutical companies.
Elafibranor
Elafibranor is an agonist of PPARα and PPARδ, which play a role in hepatic lipid oxidation, insulin sensitivity, and may reduce liver inflammation and fibrogenesis.32 In the recently published phase IIb placebo‐controlled RCT (GOLDEN) that included 274 patients with NASH, elafibranor 120 mg daily for 52 weeks resolved moderate to severe NASH in a significantly higher percentage of patients compared to placebo.33 Furthermore, patients that had NASH resolution had reduced liver fibrosis stages compared with those without NASH resolution. Importantly, elafibranor improved lipid and glucose profiles and did not cause weight gain or cardiovascular events, although a mild increase in serum creatinine was noted. The efficacy of this compound will be tested in the RESOLVE IT trial (https://clinicaltrials.gov/ct2/results?cond=NCT02704403-term=-cntry=-state=-city=-dist=), a randomized, placebo‐controlled, double‐blind, multicenter phase III study that will include patients with NASH and fibrosis (stage 1‐3). The primary endpoints are the following: 1) histologic improvement defined as resolution of NASH without worsening of fibrosis at 72 weeks; 2) composite long‐term outcome composed of all‐cause mortality, cirrhosis, and liver‐related outcomes at approximately 4 years.
Cenicriviroc
Cenicriviroc (CVC) is a dual inhibitor of the C‐C chemokine receptors type 2 and 5, which are expressed on Kupffer cells and hepatic stellate cells and play an important role in inducing liver inflammation and fibrosis.34 The results of the phase IIb multicenter CENTAUR trial were recently published.35 In that trial, 289 patients received CVC 150 mg daily or placebo for 52 weeks. There was no difference between the two groups in terms of improvement in NAS (primary endpoint) or resolution of NASH; however, twice as many subjects receiving CVC achieved one stage or greater improvement in fibrosis and no worsening of steatohepatitis compared to placebo (20% versus 10%, respectively; P = 0.023). Importantly, interleukin‐6, high‐sensitivity C‐reactive protein, and fibrinogen levels, all markers associated with systemic inflammation, were significantly decreased with CVC compared to placebo. The most common drug‐related adverse events of grade 2 or higher severity were fatigue (2.8%) and diarrhea (2.1%) for CVC. The AURORA trial is a multicenter phase 3 study that will evaluate the efficacy and safety of CVC for the treatment of liver fibrosis in adults with NASH (https://clinicaltrials.gov/ct2/results?cond=NCT03028740-term=-cntry=-state=-city=-dist=). The study will be conducted in two phases: phase 1 will examine the surrogate endpoint of improvement in fibrosis of at least one stage and no worsening of NASH at month 12; phase 2 will determine long‐term clinical outcomes composed of progression to cirrhosis, liver‐related clinical outcomes, and all‐cause mortality.
Selonsertib
Selonsertib is a selective inhibitor of apoptosis signal‐regulating kinase 1, which is a protein that promotes inflammation, apoptosis, and fibrosis in the setting of oxidative stress that is typically increased in patients with NASH. In a small, open‐label, randomized phase II trial of 72 patients with biopsy‐proven NASH, NAS ≥5, and moderate to severe liver fibrosis (stage 2‐3), selonsertib 18 mg daily for 24 weeks was associated with significant improvements in several measures of liver disease severity, including fibrosis stage, progression to cirrhosis, liver stiffness, and liver fat content.36 Additionally, patients with fibrosis improvement demonstrated reductions in hepatic collagen content, ALT, and the apoptosis marker cytokeratin‐18, supporting the biological activity of selonsertib. The most common adverse events in patients receiving selonsertib were headache, nausea, and sinusitis. Two phase III studies, STELLAR 3 and 4, will evaluate the safety and efficacy of selonsertib in patients with NASH with bridging fibrosis (stage 3) and compensated cirrhosis (stage 4), respectively.
Combination Therapy for NASH
T2D Algorithm “Combination therapy is usually required and should involve agents with complementary mechanisms of action.”
The initial management of patients with recent‐onset T2D typically starts with lifestyle modifications and metformin monotherapy unless there are contraindications. When this approach fails to achieve the glycemic target, combination therapy with another agent is usually started (dual therapy). If the hemoglobin A1c (A1C) target is still not achieved after 3 months of dual therapy, a third agent is added as part of triple therapy. Each new class of noninsulin agents added to the initial treatment lowers A1C by approximately 1%.37 Patients that present with an entry A1C >7.5% should be started on dual therapy from the beginning.38 Those presenting with A1C >9.0% and symptoms would derive greater benefit from adding insulin.39
We anticipate that similar concepts will be adapted to the treatment of NAFLD in terms of starting with lifestyle changes to induce weight loss plus one medication with reasonable efficacy and an excellent safety profile (similar to metformin in T2D). When initial therapy fails to achieve the desired outcome (improvements in liver injury serum biomarkers, imaging, or histology), other agents that work in complimentary or synergistic ways should be added. The baseline severity of NAFLD will have a significant impact on the choice of treatment. For example, patients presenting with NASH and mild fibrosis will most likely benefit from a combination of medications that target steatosis and inflammation, whereas those presenting with advanced fibrosis or NASH cirrhosis will require antifibrotics and drugs that may lower portal hypertension. We provide a comparison between the current diabetes management algorithm40 and the anticipated future algorithm for treating NAFLD in Fig. 1. We hope that applying these well‐established concepts in T2D management to the NAFLD population will help contain the NAFLD epidemic in a timely manner that will decrease progression to end‐stage liver disease.
Figure 1.
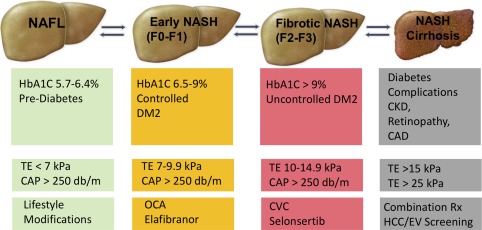
Future management of the NAFLD spectrum compared to current management of the T2D spectrum. Abbreviations: CAD, coronary artery disease, CAP, controlled attenuation parameter; CKD, chronic kidney disease; EV, esophageal varices; HbA1C, hemoglobin A1C; TE, transient elastography.
Future Paradigm of Assessing NAFLD Severity and Its Management
A major issue that hinders effective management and drug development in the NAFLD field is the need for liver histology, obtained through liver biopsy, to determine the severity of disease and assess response to different therapeutic agents. This is in contrast to T2D where a simple blood test, i.e., A1C, is used to make the diagnosis and assess treatment response. However, several novel imaging technologies now allow clinicians to diagnose NAFLD and assess fibrosis severity. The most widely used point‐of‐care imaging modality in clinical practice is Fibroscan (Echosens, Paris, France), which estimates the severity of liver fibrosis based on transient elastography and the severity of steatosis based on the controlled attenuated parameter. We propose to our readers that transient elastography/controlled attenuated parameter scores are the new “hepatologist's A1C” and that the management of NAFLD should follow the same management paradigm for T2D as shown in Fig. 1. We cannot overemphasize that this paradigm is not ready for use in practice because further validation of the utility of noninvasive imaging in predicting clinical outcomes is still necessary and because all the medications listed are not yet FDA approved. However, our intention is to provide the readers with a vision for the future that relies on simple noninvasive tests to determine the severity and optimal management strategy for NAFLD.
Potential conflict of interest: Dr. Alkhouri, Dr. Poordad, and Dr. Lawitz are on the speakers' bureau and received grants from Intercept and Gilead; they also received grants from Allergan and Genfit.
REFERENCES
- 1. Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc 1980;55:434‐438. [PubMed] [Google Scholar]
- 2. Caldwell SH, Crespo DM. The spectrum expanded: cryptogenic cirrhosis and the natural history of non‐alcoholic fatty liver disease. J Hepatol 2004;40:578‐584. [DOI] [PubMed] [Google Scholar]
- 3. Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011;141:1249‐1253. [DOI] [PubMed] [Google Scholar]
- 4. White DL, Thrift AP, Kanwal F, Davila J, El‐Serag HB. Incidence of hepatocellular carcinoma in all 50 United States, from 2000 through 2012. Gastroenterology 2017;152:812‐820.e815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999;116:1413‐1419. [DOI] [PubMed] [Google Scholar]
- 6. Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010;363:1341‐1350. [DOI] [PubMed] [Google Scholar]
- 7. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al.; Nonalcoholic Steatohepatitis Clinical Research Network . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313‐1321. [DOI] [PubMed] [Google Scholar]
- 8. Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease‐specific mortality in NAFLD after up to 33 years of follow‐up. Hepatology 2015;61:1547‐1554. [DOI] [PubMed] [Google Scholar]
- 9. Goh GB, McCullough AJ. Natural history of nonalcoholic fatty liver disease. Dig Dis Sci 2016;61:1226‐1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2017 Executive Summary. Endocr Pract 2017;23:207‐238. [DOI] [PubMed] [Google Scholar]
- 11. Gerber L, Otgonsuren M, Mishra A, Escheik C, Birerdinc A, Stepanova M, et al. Non‐alcoholic fatty liver disease (NAFLD) is associated with low level of physical activity: a population‐based study. Aliment Pharmacol Ther 2012;36:772‐781. [DOI] [PubMed] [Google Scholar]
- 12. Lassailly G, Caiazzo R, Pattou F, Mathurin P. Perspectives on treatment for nonalcoholic steatohepatitis. Gastroenterology 2016;150:1835‐1848. [DOI] [PubMed] [Google Scholar]
- 13. Nguyen V, George J. Nonalcoholic fatty liver disease management: dietary and lifestyle modifications. Semin Liver Dis 2015;35:318‐337. [DOI] [PubMed] [Google Scholar]
- 14. Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, et al.; Nonalcoholic Steatohepatitis Clinical Research Network . Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA 2011;305:1659‐1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rakoski MO, Singal AG, Rogers MA, Conjeevaram H. Meta‐analysis: insulin sensitizers for the treatment of non‐alcoholic steatohepatitis. Aliment Pharmacol Ther 2010;32:1211‐1221. [DOI] [PubMed] [Google Scholar]
- 16. Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, et al. Metformin decreases hepatocellular carcinoma risk in a dose‐dependent manner: population‐based and in vitro studies. Gut 2013;62:606‐615. [DOI] [PubMed] [Google Scholar]
- 17. Mazzotti A, Caletti MT, Marchignoli F, Forlani G, Marchesini G. Which treatment for type 2 diabetes associated with non‐alcoholic fatty liver disease? Dig Liver Dis 2017;49:235‐240. [DOI] [PubMed] [Google Scholar]
- 18. Cui J, Philo L, Nguyen P, Hofflich H, Hernandez C, Bettencourt R, et al. Sitagliptin vs. placebo for non‐alcoholic fatty liver disease: a randomized controlled trial. J Hepatol 2016;65:369‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al.; NASH CRN . Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675‐1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz‐Lopez C, et al. Long‐term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med 2016;165:305‐315. [DOI] [PubMed] [Google Scholar]
- 21. Musso G, Cassader M, Paschetta E, Gambino R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta‐analysis. JAMA Intern Med 2017;177:633‐640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Drucker DJ, Nauck MA. The incretin system: glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors in type 2 diabetes. Lancet 2006;368:1696‐1705. [DOI] [PubMed] [Google Scholar]
- 23. Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non‐alcoholic steatohepatitis (LEAN): a multicentre, double‐blind, randomised, placebo‐controlled phase 2 study. Lancet 2016;387:679‐690. [DOI] [PubMed] [Google Scholar]
- 24. Mudaliar S, Polidori D, Zambrowicz B, Henry RR. Sodium‐glucose cotransporter inhibitors: effects on renal and intestinal glucose transport: from bench to bedside. Diabetes Care 2015;38:2344‐2353. [DOI] [PubMed] [Google Scholar]
- 25. Wilkison W, Cheatham B, Walker S. Remogliflozin etabonate reduces insulin resistance and liver function enzymes: role for treatment of NASH. J Hepatol 2015;62(Suppl. 2):S211‐S212. [Google Scholar]
- 26. Lavalle‐Gonzalez FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia 2013;56:2582‐2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double‐blind, placebo‐controlled trial. Lancet 2010;375:2223‐2233. [DOI] [PubMed] [Google Scholar]
- 28. Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev Gastroenterol Hepatol 2009;3:445‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Noureddin M, Zhang A, Loomba R. Promising therapies for treatment of nonalcoholic steatohepatitis. Expert Opin Emerg Drugs 2016;21:343‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev 2009;89:147‐191. [DOI] [PubMed] [Google Scholar]
- 31. Neuschwander‐Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al.; NASH Clinical Research Network . Farnesoid X nuclear receptor ligand obeticholic acid for non‐cirrhotic, non‐alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo‐controlled trial. Lancet 2015;385:956‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Musso G, Cassader M, Gambino R. Non‐alcoholic steatohepatitis: emerging molecular targets and therapeutic strategies. Nat Rev Drug Discov 2016;15:249‐274. [DOI] [PubMed] [Google Scholar]
- 33. Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, et al.; GOLDEN‐505 Investigator Study Group . Elafibranor, an agonist of the peroxisome proliferator‐activated receptor‐alpha and ‐delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology 2016;150:1147‐1159.e1145. [DOI] [PubMed] [Google Scholar]
- 34. Kufareva I, Salanga CL, Handel TM. Chemokine and chemokine receptor structure and interactions: implications for therapeutic strategies. Immunol Cell Biol 2015;93:372‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Friedman SL, Ratziu V, Harrison SA, Abdelmalek MF, Aithal GP, Caballeria J, et al. A randomized, placebo‐controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology 2018;67:1754‐1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Loomba R, Lawitz E, Mantry PS, Jayakumar S, Caldwell SH, Arnold H, et al.; GS‐US‐384‐1497 Investigators . The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: a randomized, phase 2 trial. Hepatology 2017;67:549‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bennett WL, Maruthur NM, Singh S, Segal JB, Wilson LM, Chatterjee R, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2‐drug combinations. Ann Intern Med 2011;154:602‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Phung OJ, Scholle JM, Talwar M, Coleman CI. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA 2010;303:1410‐1418. [DOI] [PubMed] [Google Scholar]
- 39. Leahy JL. Insulin therapy in type 2 diabetes mellitus. Endocrinol Metab Clin North Am 2012;41:119‐144. [DOI] [PubMed] [Google Scholar]
- 40. Reusch JE, Manson JE. Management of type 2 diabetes in 2017: getting to goal. JAMA 2017;317:1015‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]


