Abstract
Glycosylation is a major form of enzymatic modification of organic molecules responsible for multiple biological processes in an organism. The biosynthesis of glycans is controlled by a series of glycosyltransferases, glycosidases and glycan-modifying enzymes that collectively assemble and process monosaccharide moieties into a diverse array of structures. A number of studies have provided insight into various pathways of glycosylation at the ocular surface, such as those related to the biosynthesis of mucin-type O-glycans and N-glycans on proteins, but many others still remain largely unknown. This review provides an overview of the different classes of glycans described at the ocular surface focusing on their biosynthetic pathways and biological relevance. A precise understanding of these pathways under physiological and pathological conditions could help identify biomarkers and novel targets for therapeutic intervention.
Introduction
Glycosylation is a common and highly conserved cellular process that involves the modification of cellular and secreted macromolecules to ultimately influence mechanisms critical to the development and function of unicellular and multicellular organisms. Monosaccharides are the basic structural units of glycans and are unique in that they can be attached to each other in many more ways than amino acids or nucleotides, resulting in the generation of compounds with high degree of structural complexity [1]. In eukaryotes, glycosylation starts with the import of activated monosaccharides from either the cytoplasm or the nucleus into the lumen of the endoplasmic reticulum and the Golgi apparatus, a process that requires the assistance of nucleotide sugar transporters. Such transport provides a wide range of substrates required for the action of glycosyltransferases, a group of enzymes that assemble monosaccharide moieties into acceptor substrates such as oligosaccharides, monosaccharides, polypeptides, lipids and small organic molecules [2]. The glycosyltransferases act in conjunction with several other enzymes, such as glycosidases, sulfotransferases, and O-acetyl-transferases, responsible for the processing and turnover of glycans.
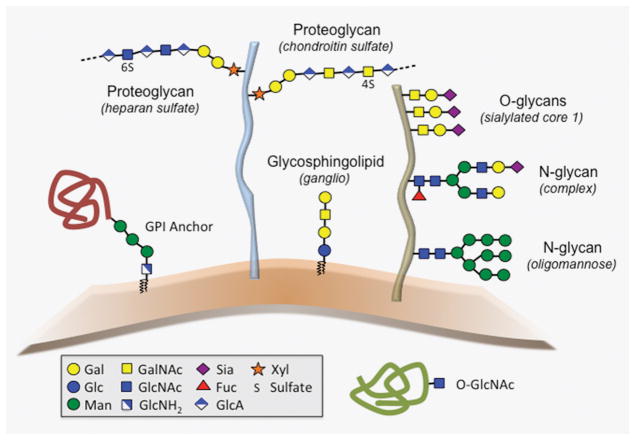
The nature of the linkage between the glycan moiety and the backbone (or aglycone) defines how glycans are classified. As shown in Figure 1, the major classes of glycans found in eukaryotes include O-glycans, N-glycans, glycosaminoglycans, glycosphingolipids, and glycosylphosphatidylinositol (GPI) anchors [3,4]. O-glycans are commonly attached to the hydroxyl groups of serine (Ser) or threonine (Thr) via N-acetylgalactosamine (GalNAc) and have been extensively studied on mucins, a group of highly glycosylated proteins characterized by the presence of tandem repeat domains with abundant clustered O-glycans. There are other classes of O-glycans in eukaryotes, which include N-acetylglucosamine (GlcNAc) linked to serine or threonine on nuclear, mitochondrial, and cytoplasmic proteins. N-glycans consist of GlcNAc attached to the amide group of asparagine (Asn) in the consensus peptide sequence Asn-X-Ser/Thr, where X denotes any amino acid except proline. Glycosaminoglycan chains are large linear polysaccharides containing repeating disaccharide units that can be covalently attached to core proteins to form proteoglycans. Finally, glycosphingolipids and glycosylphosphatidylinositols are components of the plasma membrane and consist of a hydrophobic lipid tail attached to a glycan moiety. In the case of glycosylphosphatidylinositols, the highly conserved glycan moiety is covalently linked to the C-terminus of a variety of proteins. In this review we summarize progress made toward understanding the different glycosylation pathways at the ocular surface with a focus on their biological relevance and alteration in disease.
Figure 1.
Schematic diagram of common glycans found in eukaryotes (adapted from [1]: Varki A, Kornfeld S. Historical background and overview. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, et al., editors. Essentials of glycobiology. Cold Spring Harbor (NY) 2015. p. 1–18). Examples of structures found at the ocular surface are shown in parenthesis.
The ocular surface
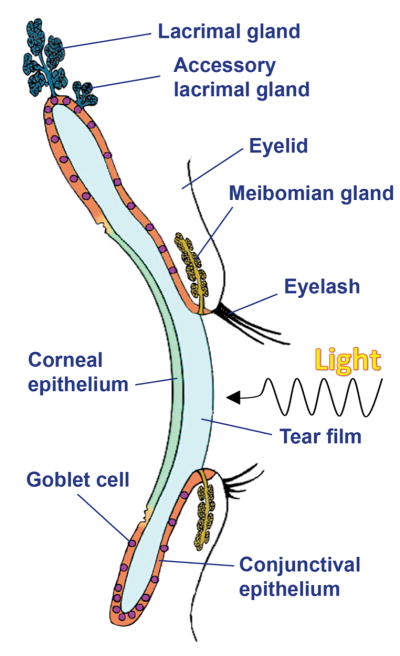
Understanding the mechanisms that lead to an effective epithelial barrier on mucosal surfaces is the subject of intense scientific study given its extraordinary relevance to health and disease. The maintenance and protection of the mucosal surface of the eye is ascribed to numerous structures. These are primarily formed by the surface and glandular epithelia of the cornea, conjunctiva, lacrimal gland, accessory lacrimal glands, and meibomian gland, all of which contribute to the production of tear fluid (Figure 2). All the epithelia of the ocular surface are continuous and are linked functionally to preserve corneal transparency and refractive power and to protect the eye against desiccation, injury and infection. The functions of the various regions of the ocular surface are supported by connective tissue and are integrated by the nervous, endocrine, circulatory and immune systems in what is known collectively as the ocular surface system [5].
Figure 2.
Schematic diagram of the ocular surface (adapted from [66]: Gipson IK, Argueso P. Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol. 2003;231:1–49).
Mucin-type O-glycosylation
Similarly to the lining of other mucosal tissues, the ocular surface epithelia produce abundant amounts of high molecular weight, heavily glycosylated mucins. Apical cells on the stratified epithelia of the cornea and conjunctiva produce the transmembrane mucins MUC1, MUC4 and MUC16, whereas secretory goblet cells intercalated within the conjunctival epithelium produce primarily the gel-forming mucin MUC5AC [6]. Interestingly, the transmembrane mucin MUC16 has also been localized to mucin granules within the goblet cells [7,8] and, together with MUC1, MUC4 and MUC5AC, can be found in the tear fluid [9]. MUC20 is one of the most highly expressed mucins at the ocular surface and is predominantly present along the plasma membranes of intermediate cell layers but not in tears [10]. The mucins MUC1, MUC5AC, MUC5B, MUC7 and MUC16 have been detected in the lacrimal gland [11].
The biosynthesis of mucin-type O-glycans is initiated in the Golgi apparatus by a large family of up to 20 homologous genes encoding UDP-GalNAc:polypeptide GalNAc-transferases (GalNAc-Ts) [12]. Studies at the ocular surface have demonstrated the expression of multiple GalNAc-Ts in cornea, conjunctiva and lacrimal gland [13,14]. Immunohistochemical analyses have revealed that these enzymes are distributed within the epithelia in a cell-layer- and cell-type-specific manner, with GalNAc-T4 and GalNAc-T2 present in the apical and basal cell layers, respectively, and GalNAc-T6 being restricted exclusively to conjunctival goblet cells [14]. Structural analyses have further demonstrated that mucin-type O-glycans at the ocular surface are relatively small compared to O-glycans found in other mucosal secretions, consisting predominantly of mono-sialylated core 1 structures [15–17]. These structural analyses have provided the basis for delineating the potential biosynthetic pathways of mucin-type O-glycosylation at the ocular surface (reviewed in [18]). In this context, the use of glycogene microarrays has demonstrated expression of core1 β1,3-galactosyltransferase (T-synthase) responsible for the biosynthesis of core 1 and a number of α2-3 and α2-6-sialyltransferases that could potentially catalyze the addition of sialic acid to core 1 [15,19].
Multiple functions have been ascribed to mucin-type O-glycans at the ocular surface. Biosynthesis of T-synthase promotes the binding of transmembrane mucins to galectin-3 and the establishment of barrier function in corneal epithelial cells [20–22]. In addition, mucin-type O-glycan biosynthesis has been shown to promote boundary lubrication at the apical portion of the ocular surface [23] and to reduce the risk of ocular infection by limiting Staphylococcus aureus adherence to human epithelial cells [24]. Interestingly, recent findings indicate that Pseudomonas aeruginosa preferentially binds mouse corneal epithelium containing chemically modified mucin-type O-glycans [25], suggesting that the protective effect of these glycans is pathogen/species-specific or, alternatively, influenced by the diverse experimental conditions. There is evidence indicating that the local cellular distribution of mucin-type O-glycan epitopes within the ocular surface epithelia, other than secretion into the tear film [15], is an early hallmark of dry eye, an ocular surface disease affecting millions of people worldwide. Research has shown that the distribution of O-acetyl sialic acids present on MUC16, as determined by H185 antibody binding, is significantly altered in the apical conjunctival epithelium of patients with dry eye and decreases in keratinized cells from patients with superior limbic keratoconjunctivitis [26–28]. This concept is further supported by evidence indicating that GalNAc-T localization, but not overall expression, is altered at early stages in ocular cicatricial pemphigoid and decreases as the disease progresses and the epithelium becomes keratinized [13,14]. Interestingly, the number of sulfated O-glycans appears to increase in the tear fluid of patients with rosacea, a chronic skin disorder frequently associated with inflammation of the ocular surface and eyelids [29,30]. Other glycoproteins carrying mucin-type O-glycans at the ocular surface include Deleted In Malignant Brain Tumors 1 [31], which has been recently found to protect against Pseudomonas aeruginosa infection by suppressing twitching motility and virulence [32].
N-Glycosylation
N-glycan biosynthesis in eukaryotic cells starts with the transfer of a dolichol phosphate oligosaccharide precursor into secretory and membrane proteins during their translocation into the endoplasmic reticulum. Glycosidases and glycosyltransferases subsequently modify this precursor in the lumen of the endoplasmic reticulum and Golgi to generate three different types of N-glycans based on the content of mannose and the presence of antennae with GlcNAc, i.e., oligomannose, complex, and hybrid. The early N-glycosylation steps in the endoplasmic reticulum ensure proper protein folding via interaction with chaperones, whereas N-glycan number and degree of branching in mature glycoproteins regulate biological activities such as those mediated by the interaction with glycan-binding proteins [33].
Most efforts to characterize N-glycan structures at the ocular surface have been performed using tear fluid since it is easily accessible and the sampling procedure is non-invasive. Use of mass spectrometry has allowed the identification of at least 43 proteins carrying N-glycans in human tears, which included lacritin, galectin-3 binding protein, clusterin, and lactoferrin—glycoproteins known to play important roles in maintaining corneal homeostasis [34]. Glycomic analysis of the carbohydrate composition of tear fluid has also revealed the presence of 50 major N-glycans [17]. The vast majority of these were complex N-glycans, and half of them contained a bisecting GlcNAc residue. Consistent with these results, glycogene microarray analysis has indicated that one of the most highly expressed glycogenes in the human conjunctival epithelium is B4GALT5 [19], a galactosyltransferase responsible for the synthesis of complex N-glycans [35]. An additional and highly expressed gene identified by microarray analysis in human conjunctiva included MGAT4B, which has also been detected in corneal epithelial cells along with other N-acetylglucosaminyltransferases involved in the formation of antennary branching structures in the medial Golgi compartment [36].
N-glycosylation is essential for the many functions ascribed to the cornea. Treatment of wounded corneas with tunicamycin, an inhibitor of N-glycosylation in the endoplasmic reticulum, impairs the continued migration of epithelial sheets during healing [37]. More recently, it has been shown that MGAT5-modified complex N-glycans play a key role in promoting corneal epithelial cell migration by regulating the interaction between α3β1 integrin and galectin-3 [38]. Structural analysis of transmembrane mucins has also revealed an important role for N-glycans in the formation of a protective glycocalyx in corneal epithelial cells. Most of the N-glycans on corneal mucins have compositions consistent with complex multi-antennary structures and, contrary to findings in tear fluid, contain limited bisecting GlcNAc structures [36]. In these experiments, MGAT1 was found to be involved in maintaining the stability and barrier function of the MUC16 mucin and in retaining galectin-3 on the epithelial glycocalyx. Lastly, evidence has emerged indicating that N-glycans on tear glycoproteins bind Pseudomonas aeruginosa to facilitate its removal from the ocular surface epithelia [39]. Indeed, two of the major components in tears, secretory immunoglobulin A and lactoferrin, contain N-glycan epitopes that can be recognized by bacteria [39,40].
Both N-glycosylation gene expression and N-glycan structure can be altered in ocular surface disease. The expression of ALG11, a mannosyltransferase involved in the biosynthesis of the dolichol phosphate precursor in the endoplasmic reticulum, is significantly reduced in the conjunctival epithelium of patients with dry eye [19]. Mass spectrometry analyses have also revealed that the amount of fucosylated N-glycans is reduced in the tear fluid of rosacea patients [29], and that lacritin N-glycosylation decreases in tears of patients with climatic droplet keratopathy, a degenerative corneal disease [34]. Finally, changes to the N-linked profile have been observed in tears of diabetic patients, although only among the relatively low abundance glycans [17].
Glycosaminoglycans
Early investigations into the distribution of proteoglycans in human corneas using immunogold electron microscopy revealed that heparan sulfate and chondroitin sulfate, but not keratan sulfate, are the major glycosaminoglycans found in epithelium [41]. The biosynthesis of heparan sulfate or chondroitin sulfate involves copolymerization of glucuronic acid with GlcNAc or GalNAc respectively, followed by a series of modification reactions. Both polysaccharide chains are linked to serine residues in core proteins although, compared to O- and N-glycans, relatively few proteins carry glycosaminoglycans—approximately 17 contain heparan sulfate and approximately 20 contain chondroitin sulfate [42].
Several studies on the role of glycosaminoglycans at the ocular surface have been carried out in the context of infection. The stratified epithelium of the cornea is continuously exposed to pathogens and adhesion of microorganisms to the most apical epithelial cell layer is regarded as an essential first step in infectious disease. Staphylococcus aureus, a common bacterial pathogen causing keratitis, has the ability to exploit the cell surface syndecan-1 proteoglycan to infect the cornea in a heparan sulfate-dependent manner [43]. Moreover, recent evidence indicates that when bacterial pathogens adhere, it appears to occur preferentially through sulfated residues along the chains, particularly to those containing glucosamine residues [44]. Additional studies have also implicated heparan sulfate and syndecan-1 in the pathogenesis of herpes simplex virus type-1 infection in corneal epithelium [45,46].
The net biological effects of glycosaminoglycan biosynthesis in corneal epithelium include those related to wound healing and cell differentiation. Lumican, a keratan sulfate proteoglycan, is expressed by stromal keratocytes in normal unwounded cornea, but can be upregulated by corneal epithelium during the early phase of wound healing to modulate cell adhesion and migration [47,48]. Similarly, biosynthesis of corneal epithelial heparan sulfate is of paramount importance to maintaining corneal homeostasis, and both wound healing and corneal cell stratification can be impaired following abrogation of the enzymes responsible for its elongation and modification [49]. The pattern of heparan sulfation also appears to play an important role in the migration of corneal epithelial cells during wound repair [50]. Deficiency in perlecan, a basement membrane-specific heparan sulfate proteoglycan, has been linked to a thinner corneal epithelium and the decreased expression of epithelial differentiation markers [51]. Finally, recent evidence has demonstrated alterations in the morphology of intraepithelial nerve terminals in corneas lacking syndecan-1 [52].
Decreased expression of EXTL2, a glycosyltransferase required for the biosynthesis of heparan sulfate, and reduced levels of heparan sulfotransferases HS2ST1 and HS3ST6, have been reported in dry eye disease [19]. Interestingly, administration of lubricin in these patients appears to improve both signs and symptoms of dry eye [53]. Lubricin is a proteoglycan with a chondroitin sulfate-attachment site and mucin-like repeats [54]. It is synthesized by the ocular surface epithelia, where it functions as a boundary lubricant and appears to play a protective role in preventing corneal epithelial damage [55].
Glycosphingolipids
Nearly all glycolipids in vertebrates are glycosphingolipids, a subclass of glycolipids with ceramide as the lipid core structure. The ceramide moiety can be attached to multiple glycans to produce a vast array of structures, being the ganglio, globo, and neolacto tetrasaccharides the most common cores in vertebrates. Glycosphingolipids are expressed in a tissue-specific manner and constitute <5% to >20% of the total membrane lipids in plasma membranes [56]. At the ocular surface epithelia, the few studies evaluating glycosphingolipids have focused primarily on their role in mediating infection. The available data indicates that Ad37, an adenovirus causing epidemic keratoconjunctivitis, uses a sialylated GD1a ganglioside as a cellular receptor [57], whereas the asialo-GM1 ganglioside serves as a receptor for Pseudomonas aeruginosa and Staphylococcus aureus in cornea [58,59].
A recent mass spectrometric analysis has demonstrated that glucosylceramide and the GM3 ganglioside are present in tears, where they correlate positively with the amount of fluid [60], but the cellular origin of these glycolipids, however, remains unknown [61]. Glycogene microarray analysis has further evidenced that hexosaminidase B, a lysosomal glycosidase involved in the degradation of the GM2 ganglioside, is highly expressed in the human conjunctiva [19]. In this study, the ceramide glucosyltransferase UGCGL2 and the galactosylceramidase GALC were found to be downregulated in patients with dry eye.
Conclusion and future prospects
Glycosylation has long been considered an important factor in regulating ocular surface homeostasis. However, we currently only have a superficial understanding of the multiple mechanisms regulating glycosylation at the ocular surface. Pathways that remain understudied include those related to the biosynthesis of glycosylphosphatidylinositol, which can prove relevant to the proper differentiation of the corneal epithelium [62], and the O-GlcNAc modification, which appears to be altered in dry eye and diabetic corneas [19,63]. Some findings remain observational and are in need of subsequent interventional studies to determine biological relevance. For instance, the role of carbohydrate modifications such as acetylation and sulfation, which occur on mucins and glycosaminoglycans [64,65], are not completely understood. Nevertheless, the introduction of new technologies in glycomics and the development of novel chemical and genetic approaches to alter the different glycosylation pathways will likely prove rewarding in the field of ocular surface research.
Acknowledgments
Funding
This work was supported by the National Institutes of Health, NEI Grants R01EY026147 and R01EY024031.
Abbreviations
- Asn
asparagine
- Fuc
fucose
- Gal
galactose
- GalNAc
N-acetylgalactosamine
- Glc
glucose
- GlcA
glucuronic acid
- GlcNAc
N-acetylglucosamine
- GlcNH2
glucosamine
- T-synthase
core1 β1,3-galactosyltransferase
- GalNAc-Ts
UDP-GalNAc-polypeptide GalNAc-transferases
- Man
mannose
- Ser
serine
- Sia
sialic acid
- Thr
threonine
- Xyl
xylose
Footnotes
Author Contribution
M.C.R.B. and P.A. reviewed the literature and wrote the article.
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Varki A, Kornfeld S. Historical background and overview. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, et al., editors. Essentials of glycobiology. Cold Spring Harbor (NY): 2015. pp. 1–18. [Google Scholar]
- 2.Rini JM, Esko JD. Glycosyltransferases and glycan-processing enzymes. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, et al., editors. Essentials of glycobiology. Cold Spring Harbor (NY): 2015. pp. 65–75. [PubMed] [Google Scholar]
- 3.Dalziel M, Crispin M, Scanlan CN, Zitzmann N, Dwek RA. Emerging principles for the therapeutic exploitation of glycosylation. Science. 2014;343(6166):1235681. doi: 10.1126/science.1235681. [DOI] [PubMed] [Google Scholar]
- 4.Corfield A. Eukaryotic protein glycosylation: a primer for histochemists and cell biologists. Histochem Cell Biol. 2017;147(2):119–147. doi: 10.1007/s00418-016-1526-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gipson IK. The ocular surface: the challenge to enable and protect vision: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2007;48(10):4390, 4391–4398. doi: 10.1167/iovs.07-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mantelli F, Argueso P. Functions of ocular surface mucins in health and disease. Curr Opin Allergy Clin Immunol. 2008;8(5):477–483. doi: 10.1097/ACI.0b013e32830e6b04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gipson IK, Spurr-Michaud S, Tisdale A. Human conjunctival goblet cells express the membrane associated mucin MUC16: Localization to mucin granules. Exp Eye Res. 2016;145:230–234. doi: 10.1016/j.exer.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Argueso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest Ophthalmol Vis Sci. 2003;44(6):2487–2495. doi: 10.1167/iovs.02-0862. [DOI] [PubMed] [Google Scholar]
- 9.Spurr-Michaud S, Argueso P, Gipson IK. Assay of mucins in human tear fluid. Exp Eye Res. 2007;84(5):939–950. doi: 10.1016/j.exer.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodward AM, Argueso P. Expression analysis of the transmembrane mucin MUC20 in human corneal and conjunctival epithelia. Invest Ophthalmol Vis Sci. 2014;55(10):6132–6138. doi: 10.1167/iovs.14-15269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulsen FP, Berry MS. Mucins and TFF peptides of the tear film and lacrimal apparatus. Prog Histochem Cytochem. 2006;41(1):1–53. doi: 10.1016/j.proghi.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, Tabak LA. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology. 2012;22(6):736–756. doi: 10.1093/glycob/cwr182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imbert Y, Jumblatt MM, Foulks GN, Couzin EG, Steele PS, Young WW., Jr Expression in human ocular surface tissues of the GalNAc-transferases that initiate mucin-type O-glycosylation. Cornea. 2006;25(10):1193–1199. doi: 10.1097/01.ico.0000240099.16420.17. [DOI] [PubMed] [Google Scholar]
- 14.Argueso P, Tisdale A, Mandel U, Letko E, Foster CS, Gipson IK. The cell-layer- and cell-type-specific distribution of GalNAc-transferases in the ocular surface epithelia is altered during keratinization. Invest Ophthalmol Vis Sci. 2003;44(1):86–92. doi: 10.1167/iovs.02-0181. [DOI] [PubMed] [Google Scholar]
- 15.Guzman-Aranguez A, Mantelli F, Argueso P. Mucin-type O-glycans in tears of normal subjects and patients with non-Sjogren’s dry eye. Invest Ophthalmol Vis Sci. 2009;50(10):4581–4587. doi: 10.1167/iovs.09-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Royle L, Matthews E, Corfield A, Berry M, Rudd PM, Dwek RA, et al. Glycan structures of ocular surface mucins in man, rabbit and dog display species differences. Glycoconj J. 2008;25(8):763–773. doi: 10.1007/s10719-008-9136-6. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen-Khuong T, Everest-Dass AV, Kautto L, Zhao Z, Willcox MD, Packer NH. Glycomic characterization of basal tears and changes with diabetes and diabetic retinopathy. Glycobiology. 2015;25(3):269–283. doi: 10.1093/glycob/cwu108. [DOI] [PubMed] [Google Scholar]
- 18.Guzman-Aranguez A, Argüeso P. Structure and biological roles of mucin-type O-glycans at the ocular surface. Ocul Surf. 2010;8(1):8–17. doi: 10.1016/s1542-0124(12)70213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantelli F, Schaffer L, Dana R, Head SR, Argueso P. Glycogene expression in conjunctiva of patients with dry eye: downregulation of Notch signaling. Invest Ophthalmol Vis Sci. 2009;50(6):2666–2672. doi: 10.1167/iovs.08-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Argueso P, Guzman-Aranguez A, Mantelli F, Cao Z, Ricciuto J, Panjwani N. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J Biol Chem. 2009;284(34):23037–23045. doi: 10.1074/jbc.M109.033332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guzman-Aranguez A, Woodward AM, Pintor J, Argueso P. Targeted disruption of core 1 beta1,3-galactosyltransferase (C1galt1) induces apical endocytic trafficking in human corneal keratinocytes. PLoS One. 2012;7(5):e36628. doi: 10.1371/journal.pone.0036628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Argueso P, Tisdale A, Spurr-Michaud S, Sumiyoshi M, Gipson IK. Mucin characteristics of human corneal-limbal epithelial cells that exclude the rose bengal anionic dye. Invest Ophthalmol Vis Sci. 2006;47(1):113–119. doi: 10.1167/iovs.05-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sumiyoshi M, Ricciuto J, Tisdale A, Gipson IK, Mantelli F, Argueso P. Antiadhesive character of mucin O-glycans at the apical surface of corneal epithelial cells. Invest Ophthalmol Vis Sci. 2008;49(1):197–203. doi: 10.1167/iovs.07-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricciuto J, Heimer SR, Gilmore MS, Argueso P. Cell surface O-glycans limit Staphylococcus aureus adherence to corneal epithelial cells. Infect Immun. 2008;76(11):5215–5220. doi: 10.1128/IAI.00708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jolly AL, Agarwal P, Metruccio MME, Spiciarich DR, Evans DJ, Bertozzi CR, et al. Corneal surface glycosylation is modulated by IL-1R and Pseudomonas aeruginosa challenge but is insufficient for inhibiting bacterial binding. FASEB J. 2017;31(6):2393–2404. doi: 10.1096/fj.201601198R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Argueso P, Sumiyoshi M. Characterization of a carbohydrate epitope defined by the monoclonal antibody H185, sialic acid O-acetylation on epithelial cell surface mucins. Glycobiology. 2006;16(12):1219–1228. doi: 10.1093/glycob/cwl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danjo Y, Watanabe H, Tisdale AS, George M, Tsumura T, Abelson MB, et al. Alteration of mucin in human conjunctival epithelia in dry eye. Invest Ophthalmol Vis Sci. 1998;39(13):2602–2609. [PubMed] [Google Scholar]
- 28.Watanabe H, Maeda N, Kiritoshi A, Hamano T, Shimomura Y, Tano Y. Expression of a mucin-like glycoprotein produced by ocular surface epithelium in normal and keratinized cells. Am J Ophthalmol. 1997;124(6):751–757. doi: 10.1016/s0002-9394(14)71691-5. [DOI] [PubMed] [Google Scholar]
- 29.Vieira AC, An HJ, Ozcan S, Kim JH, Lebrilla CB, Mannis MJ. Glycomic analysis of tear and saliva in ocular rosacea patients: the search for a biomarker. Ocul Surf. 2012;10(3):184–192. doi: 10.1016/j.jtos.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozcan S, An HJ, Vieira AC, Park GW, Kim JH, Mannis MJ, et al. Characterization of novel O-glycans isolated from tear and saliva of ocular rosacea patients. J Proteome Res. 2013;12(3):1090–1100. doi: 10.1021/pr3008013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulz BL, Oxley D, Packer NH, Karlsson NG. Identification of two highly sialylated human tear-fluid DMBT1 isoforms: the major high-molecular-mass glycoproteins in human tears. Biochem J. 2002;366:511–520. doi: 10.1042/BJ20011876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Metruccio MME, Evans DJ, Fleiszig SMJ. Mucosal fluid glycoprotein DMBT1 suppresses twitching motility and virulence of the opportunistic pathogen Pseudomonas aeruginosa. PLoS Pathog. 2017;13(5):e1006392. doi: 10.1371/journal.ppat.1006392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanley P, Taniguchi N, Aebi M. N-Glycans. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, et al., editors. Essentials of glycobiology. Cold Spring Harbor (NY): 2015. pp. 99–111. [PubMed] [Google Scholar]
- 34.Zhou L, Beuerman RW, Chew AP, Kwan Koh S, Cafaro TA, Urrets-Zavalia EA, et al. Quantitative analysis of N-linked glycoproteins in tear fluid of climatic droplet keratopathy by glycopeptide capture and iTRAQ. J Proteome Res. 2009;8:1992–2003. doi: 10.1021/pr800962q. [DOI] [PubMed] [Google Scholar]
- 35.Parker BL, Thaysen-Andersen M, Fazakerley DJ, Holliday M, Packer NH, James DE. Terminal galactosylation and sialylation switching on membrane glycoproteins upon TNF-alpha-induced insulin resistance in adipocytes. Mol Cell Proteomics. 2016;15(1):141–153. doi: 10.1074/mcp.M115.054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taniguchi T, Woodward AM, Magnelli P, McColgan NM, Lehoux S, Jacobo SMP, et al. N-Glycosylation affects the stability and barrier function of the MUC16 mucin. J Biol Chem. 2017;292(26):11079–11090. doi: 10.1074/jbc.M116.770123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gipson IK, Kiorpes TC, Brennan SJ. Epithelial sheet movement: effects of tunicamycin on migration and glycoprotein synthesis. Dev Biol. 1984;101(1):212–220. doi: 10.1016/0012-1606(84)90131-3. [DOI] [PubMed] [Google Scholar]
- 38.Saravanan C, Liu FT, Gipson IK, Panjwani N. Galectin-3 promotes lamellipodia formation in epithelial cells by interacting with complex N-glycans on alpha3beta1 integrin. J Cell Sci. 2009;122(Pt 20):3684–3693. doi: 10.1242/jcs.045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kautto L, Nguyen-Khuong T, Everest-Dass A, Leong A, Zhao Z, Willcox MDP, et al. Glycan involvement in the adhesion of Pseudomonas aeruginosa to tears. Exp Eye Res. 2016;145:278–288. doi: 10.1016/j.exer.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Royle L, Roos A, Harvey DJ, Wormald MR, van Gijlswijk-Janssen D, Redwanel RM, et al. Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J Biol Chem. 2003;278(22):20140–20153. doi: 10.1074/jbc.M301436200. [DOI] [PubMed] [Google Scholar]
- 41.Bairaktaris G, Lewis D, Fullwood NJ, Nieduszynski IA, Marcyniuk B, Quantock AJ, et al. An ultrastructural investigation into proteoglycan distribution in human corneas. Cornea. 1998;17(4):396–402. doi: 10.1097/00003226-199807000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Lindahl U, Couchman J, Kimata K, Esko JD. Proteoglycans and sulfated glycosaminoglycans. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, et al., editors. Essentials of glycobiology. Cold Spring Harbor (NY): 2015. pp. 207–221. [Google Scholar]
- 43.Hayashida A, Amano S, Park PW. Syndecan-1 promotes Staphylococcus aureus corneal infection by counteracting neutrophil-mediated host defense. J Biol Chem. 2011;286(5):3288–3297. doi: 10.1074/jbc.M110.185165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia B, Merayo-Lloves J, Rodriguez D, Alcalde I, Garcia-Suarez O, Alfonso JF, et al. Different use of cell surface glycosaminoglycans as adherence receptors to corneal cells by gram positive and gram negative pathogens. Front Cell Infect Microbiol. 2016;6:173. doi: 10.3389/fcimb.2016.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karasneh GA, Ali M, Shukla D. An important role for syndecan-1 in herpes simplex virus type-1 induced cell-to-cell fusion and virus spread. PLoS One. 2011;6(9):e25252. doi: 10.1371/journal.pone.0025252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agelidis AM, Hadigal SR, Jaishankar D, Shukla D. Activation of heparanase drives pathogenesis of herpes simplex virus-1. Cell Rep. 2017;20(2):439–450. doi: 10.1016/j.celrep.2017.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saika S, Shiraishi A, Liu CY, Funderburgh JL, Kao CW, Converse RL, et al. Role of lumican in the corneal epithelium during wound healing. J Biol Chem. 2000;275(4):2607–2612. doi: 10.1074/jbc.275.4.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeh LK, Chen WL, Li W, Espana EM, Ouyang J, Kawakita T, et al. Soluble lumican glycoprotein purified from human amniotic membrane promotes corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2005;46(2):479–486. doi: 10.1167/iovs.04-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coulson-Thomas VJ, Chang SH, Yeh LK, Coulson-Thomas YM, Yamaguchi Y, Esko J, et al. Loss of corneal epithelial heparan sulfate leads to corneal degeneration and impaired wound healing. Invest Ophthalmol Vis Sci. 2015;56(5):3004–3014. doi: 10.1167/iovs.14-15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maltseva I, Chan M, Kalus I, Dierks T, Rosen SD. The SULFs, extracellular sulfatases for heparan sulfate, promote the migration of corneal epithelial cells during wound repair. PLoS One. 2013;8(8):e69642. doi: 10.1371/journal.pone.0069642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inomata T, Ebihara N, Funaki T, Matsuda A, Watanabe Y, Ning L, et al. Perlecan-deficient mutation impairs corneal epithelial structure. Invest Ophthalmol Vis Sci. 2012;53(3):1277–1284. doi: 10.1167/iovs.11-8742. [DOI] [PubMed] [Google Scholar]
- 52.Pal-Ghosh S, Tadvalkar G, Stepp MA. Alterations in corneal sensory nerves during homeostasis, aging, and after injury in mice lacking the heparan sulfate proteoglycan syndecan-1. Invest Ophthalmol Vis Sci. 2017;58(12):4959–4975. doi: 10.1167/iovs.17-21531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lambiase A, Sullivan BD, Schmidt TA, Sullivan DA, Jay GD, Truitt ER, 3rd, et al. A two-week, randomized, double-masked study to evaluate safety and efficacy of lubricin (150 mug/mL) eye drops versus sodium hyaluronate (HA) 0.18% eye drops (Vismed(R)) in patients with moderate dry eye disease. Ocul Surf. 2017;15(1):77–87. doi: 10.1016/j.jtos.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 54.Ikegawa S, Sano M, Koshizuka Y, Nakamura Y. Isolation, characterization and mapping of the mouse and human PRG4 (proteoglycan 4) genes. Cytogenet Cell Genet. 2000;90(3–4):291–297. doi: 10.1159/000056791. [DOI] [PubMed] [Google Scholar]
- 55.Schmidt TA, Sullivan DA, Knop E, Richards SM, Knop N, Liu S, et al. Transcription, translation, and function of lubricin, a boundary lubricant, at the ocular surface. JAMA Ophthalmol. 2013;131(6):766–776. doi: 10.1001/jamaophthalmol.2013.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schnaar RL, Kinoshita T. Glycosphingolipids. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, et al., editors. Essentials of glycobiology. Cold Spring Harbor (NY): 2015. pp. 125–135. [PubMed] [Google Scholar]
- 57.Nilsson EC, Storm RJ, Bauer J, Johansson SM, Lookene A, Angstrom J, et al. The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat Med. 2011;17(1):105–109. doi: 10.1038/nm.2267. [DOI] [PubMed] [Google Scholar]
- 58.Hazlett LD, Masinick S, Barrett R, Rosol K. Evidence for asialo GM1 as a corneal glycolipid receptor for Pseudomonas aeruginosa adhesion. Infect Immun. 1993;61(12):5164–5173. doi: 10.1128/iai.61.12.5164-5173.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwab U, Thiel HJ, Steuhl KP, Doering G. Binding of Staphylococcus aureus to fibronectin and glycolipids on corneal surfaces. Ger J Ophthalmol. 1996;5(6):417–421. [PubMed] [Google Scholar]
- 60.Lam SM, Tong L, Reux B, Duan X, Petznick A, Yong SS, et al. Lipidomic analysis of human tear fluid reveals structure-specific lipid alterations in dry eye syndrome. J Lipid Res. 2014;55(2):299–306. doi: 10.1194/jlr.P041780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Butovich IA. Lipidomics of human Meibomian gland secretions: Chemistry, biophysics, and physiological role of Meibomian lipids. Prog Lipid Res. 2011;50(3):278–301. doi: 10.1016/j.plipres.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kriegbaum MC, Jacobsen B, Hald A, Ploug M. Expression of C4.4A, a structural uPAR homolog, reflects squamous epithelial differentiation in the adult mouse and during embryogenesis. J Histochem Cytochem. 2011;59(2):188–201. doi: 10.1369/0022155410394859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akimoto Y, Kawakami H, Yamamoto K, Munetomo E, Hida T, Hirano H. Elevated expression of O-GlcNAc-modified proteins and O-GlcNAc transferase in corneas of diabetic Goto-Kakizaki rats. Invest Ophthalmol Vis Sci. 2003;44(9):3802–3809. doi: 10.1167/iovs.03-0227. [DOI] [PubMed] [Google Scholar]
- 64.Argueso P, Sumiyoshi M. Characterization of a carbohydrate epitope defined by the monoclonal antibody H185: sialic acid O-acetylation on epithelial cell-surface mucins. Glycobiology. 2006;16(12):1219–1228. doi: 10.1093/glycob/cwl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gnadinger MC, Schwager-Hubner ME. Biosynthesis of glycosaminoglycans by mammalian corneal epithelium and fibroblasts in vitro. I. Isolation and fractionation-differences of GAG from the two cell types. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1975;196(1):9–19. doi: 10.1007/BF00410022. [DOI] [PubMed] [Google Scholar]
- 66.Gipson IK, Argueso P. Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol. 2003;231:1–49. doi: 10.1016/s0074-7696(03)31001-0. [DOI] [PubMed] [Google Scholar]