Abstract
Background
Despite the medical importance of trichomoniasis, little is known about the genetic relatedness of Trichomonas vaginalis strains with similar biological characteristics. Furthermore, the distribution of endobionts such as mycoplasmas or Trichomonas vaginalis virus (TVV) in the T. vaginalis metapopulation is poorly characterised.
Results
We assayed the relationship between 20 strains of T. vaginalis from 8 countries using the Random Amplified Polymorphic DNA (RAPD) analysis with 27 random primers. The genealogical tree was constructed and its bootstrap values were computed using the program FreeTree. Using the permutation tail probability tests we found that the topology of the tree reflected both the pattern of resistance to metronidazole (the major anti-trichomonal drug) (p < 0.01) and the pattern of infection of strains by mycoplasmas (p < 0.05). However, the tree did not reflect pattern of virulence, geographic origin or infection by TVV. Despite low bootstrap support for many branches, the significant clustering of strains with similar drug susceptibility suggests that the tree approaches the true genealogy of strains. The clustering of mycoplasma positive strains may be an experimental artifact, caused by shared RAPD characters which are dependent on the presence of mycoplasma DNA.
Conclusions
Our results confirmed both the suitability of the RAPD technique for genealogical studies in T. vaginalis and previous conclusions on the relatedness of metronidazol resistant strains. However, our studies indicate that testing analysed strains for the presence of endobionts and assessment of the robustness of tree topologies by bootstrap analysis seem to be obligatory steps in such analyses.
Background
Trichomonads are anaerobic flagellated protists often considered to represent one of the most ancient branches of eukaryotes [1]. Except a few pathogenic species, trichomonads are harmless commensals living in the alimentary tract of wide range of hosts. Trichomonas vaginalis is a human pathogen causing a sexually transmitted infection of the urogenital tract. Clinical manifestations of the infections occur mainly in women and vary from mild to severe vaginitis accompanied with profuse inflammatory discharge. Colonisation of vagina and ectocervical epithelium by adherent parasites induces inflammatory changes leading to focal erosions and proliferation of granulation tissue [2,3]. However, many infections are asymptomatic. Different clinical manifestations can be ascribed in part to differences in virulence controlling biological properties of strains. Strains of T. vaginalis differ also in susceptibility to metronidazole, the major antitrichomonal drug. Clinical reports [4-6] on infections refractory to standard treatment with metronidazole were published soon after its introduction in 1959. However, the existence of T. vaginalis strains with convincingly proven drug resistance phenotype was not confirmed until 1979 [7]. Strains of T. vaginalis vary also in other biological traits like the presence of endobionts. Some strains are infected with mycoplasmas, the representatives of the class Mollicutes [8,9], others harbour TVV, the first dsRNA virus described in protists [10,11].
Although T. vaginalis is a medically important parasite, little effort was invested in the study of the genetic relatedness of T. vaginalis strains, especially with respect to its correlation with their phenotypic similarities. Vohra et al.[12] found that isolates from symptomatic and asymptomatic patients could not be grouped on the basis of their zymodeme pattern. Similar results were obtained also by Proctor et al.[13]. Krieger et al.[14] demonstrated differences in the antigenic composition of trichomonads isolated from various regions of the United States. Stiles et al.[15] used for molecular typing of T. vaginalis isolates restriction fragment length polymorphism (RFLP) analysis with HSP 70 gene probe hybridising to EcoR1 digested genomic DNA. The authors did not find any concordance between RFLP subtype and metronidazole resistance or geographic origin of isolates. Snipes et al.[16] and our group [17] employed the RAPD technique in the study of the DNA polymorphism among strains of T. vaginalis. Both analyses showed a correlation of the genetic relatedness of strains and the similarity in their susceptibility to metronidazole in vitro. Our results also suggested that the relatedness of strains correlated with their geographic origin, clinical manifestation of infection in patients and their response to standard metronidazole treatment. Contrary to our results Snipes et al.[16] found a correlation between the presence of TVV and relatedness of strains.
RAPD is currently widely used in phylogenetic analyses at both species and intraspecies levels [18-21]. An important advantage of this method is that it compares polymorphisms in multiple genomic loci instead of the polymorphism in only one locus. The results of single locus studies can be misleading, because the phylogeny of one gene can differ from the phylogeny of the species. Other advantages of RAPD method are its high capacity and low cost. The main disadvantage of the RAPD method is generally low reproducibility of electrophoretic patterns. The analyses are also complicated by the fact that for comparison of RAPD patterns all samples must be analysed in a single experiment. Therefore it is not possible to add new samples in a completed study. Until recently a substantial disadvantage of the RAPD method was the absence of software for performing resampling tests (bootstrapping, jackknifing), and therefore the impossibility to assess the reliability of obtained trees. However, since 1998 the program FreeTree, which is primarily intended for such kind of analyses, is available [22].
In this study we try to elaborate our previous results [17] on the correlation between genetic relatedness and phenotypic similarities of T. vaginalis strains using a three times higher amount of RAPD characters and employing FreeTree program for evaluation of the robustness of phylogenetic trees.
Results
RAPD analysis with 19 random primers was used to reveal the genealogical relationship of 20 Trichomonas vaginalis strains. For all strains 249 new characters were obtained from this analysis and 134 characters were used from the previous analysis [17]. On the basis of all these characters a phylogenetic tree was constructed by the Neighbor-joining method (NJ) (Fig. 1). The tree was rooted with representatives of six other genera of trichomonads.
Figure 1.
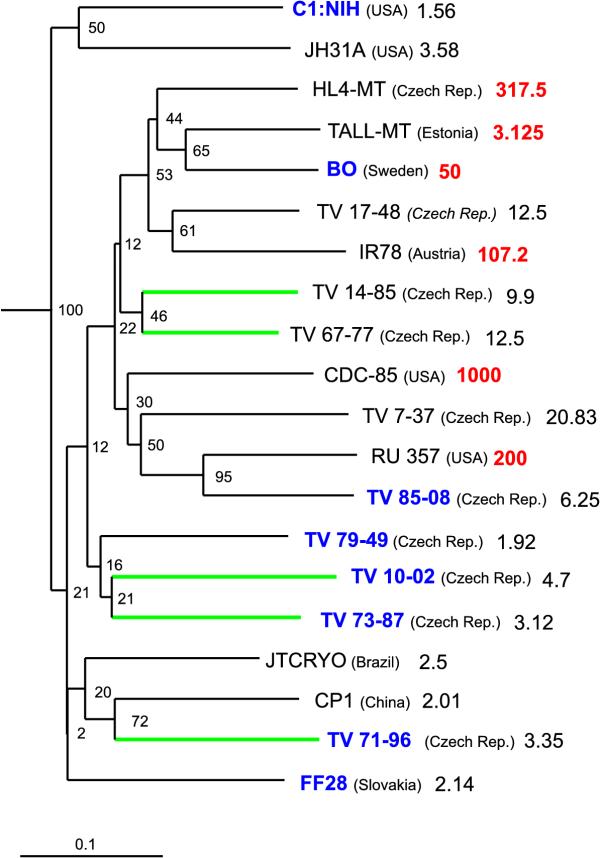
The genealogical tree for 20 strains of Trichomonas vaginalis. The tree was constructed by Neighbor-joining method and rooted with representatives of other trichomonadid genera. The branch lengths reflect the genetic distances between the strains. The numbers show the bootstrap values (in percent) for every branch of the tree. The geographical origins and the values of minimal lethal concentration (MLC) for metronidazole (μg.ml-1) are listed for every strain. MLC values printed in red designate the strains, which were refractory to standard metronidazole treatment. The names of strains printed in blue designate strains infected with TVV. The apical branches printed in green designate strains infected with mycoplasmas.
Correlations and concordances between the similarity of biological characteristics of strains and their positions in the genealogical tree (Fig. 1), i.e., their genetic relatedness, were tested by permutation tail probability tests. The proximity of minimal lethal concentration (MLC) values (the measure of susceptibility to metronidazole) (p < 0.01) and the presence of mycoplasmas (p < 0.05) appeared to correlate with positions of strains in the tree. Near statistical significance was also a concordance between positions of strains in the tree and patient response to metronidazole treatment (p = 0.056). We did not find such concordances neither for the presence/absence of TVV (p = 0.361) nor for the geographical origin of strains (p=0.438). Similarly, we did not find any correlation or concordance for five indices of virulence. Values of statistical significance for correlations or concordances between the relatedness of strains and their virulence assessed on the basis of mice mortality, Cavier index [23], volume of abscess after subcutaneous inoculation in mice or symptoms and histopathological changes in human patients were p = 0.622, p = 0.388, p = 0.67, p = 0.137 and p = 0.647, respectively.
Discussion
Concordance between genetic and phenotypic similarities of Trichomonas vaginalis strains
Strains of Trichomonas vaginalis formed a distinct branch in the tree indicating that all belonged to the same species. We found that the distribution of the strains in the tree (Fig. 1) reflected the level of their susceptibility to metronidazole in in vitro tests, infection with mycoplasmas and perhaps also patient responsiveness to metronidazole treatment. On the other hand, we did not find a significant association of the strains with similar virulence, with presence of TVV and with common geographical origin.
The strongest correlation revealed by our analysis was the correlation between the similarity of the values of MLC for metronidazole and the relatedness of T. vaginalis strains. Also the significance of the clustering of strains isolated from patients refractory to standard treatment with metronidazole was near 5% level. Both findings are apparently interrelated because refractory strains are usually the strains with high MLC values. The existence of these correlations was also evident from the tree topology, where the strains with lower susceptibility to the drug clustered in a distinct branch. This branch consisted of all treatment refractory strains (HL-4MT, CDC-85, IR78, BO, TALL – MT) and 5 strains (TV 67–77, TV 14–85, TV 85–08, TV 7–37, TV 17–48) that did not cause refractory infections but showed MLC values higher than those of the remaining strains. The presence in this branch of the strain TALL – MT displaying low MLC level in vitro is noteworthy. This strain was isolated from a refractory infection in Estonia and claimed to be drug resistant in vitro (Teras, personal communication). In subsequent in vitro tests performed in our laboratory this strain was found susceptible to metronidazole. Results of present analysis, however, indicate its genetic relatedness to other metronidazole-resistant strains. Hypothetically, the resistance of this strain might be secondarily lost due to its prolonged in vitro cultivation. Different geographical origin of strains that cluster in a common branch of strains with increased MLCs suggests, that perhaps only one lineage of genetically related strains able to develop resistance to metronidazole was spread around the world. Our data do not allow deciding whether this lineage had spread due to the selective pressure of metronidazole. A lineage already preadaptated to resistance might be actually present in the population of T. vaginalis before the advent of this drug and resistant strains could arise under the metronidazole pressure repeatedly and independently within this line. The correlation of genetic relatedness of T. vaginalis strains with the similarity in levels of their in vitro metronidazole susceptibility has been shown in our previous study [17] and was reported also by Snipes et al.[16]. These authors found association between metronidazole susceptibility and RAPD strains genealogy as well as between metronidazole susceptibility and a point mutation in ITS 1 (internal transcribed spacer 1 between 16S and 5.8S rRNA). On the other hand Stiles et al.[15] did not find an association between RFLP subtype and metronidazole susceptibility of T. vaginalis isolates. These authors divided 36 isolates into 10 subtypes according to the autoradiographic band pattern after EcoR1 digestion of genomic DNA and subsequent hybridising with probe from cytoplasmic HSP70 gene family. The oligolocus character of their method, however, preclude to construct phylograms and therefore to study the concordance between phenetic similarity and genetic relatedness of strains.
A conspicuous clustering of strains infected by mycoplasmas was found in the genealogical tree of T. vaginalis strains. This clustering appeared to be statistically significant on the 5% level. The high relatedness of infected strains might be explained to be the result of one or a few ancient infections of some T. vaginalis strains and persistence of this intracellular parasite in their descendant lineages due to the vertical transfer. However, a more probable explanation is that infected strains artificially appeared to be more related to each other in our analysis just because of their contamination with DNA of mycoplasmas. It has been already reported [24], that the presence of foreign DNA can influence the presence/absence of particular fragments in the RAPD pattern. Moreover, some bands can even originate by amplification of mycoplasmal DNA. Because PCR reactions of all mycoplasma-positive samples were biased in the same way, they could be artificially grouped in the RAPD-based tree. Because the tree topology reflected the strains susceptibility to metronidazole more than presence or absence of mycoplasmas, the influence of mycoplasmal contamination on the tree topology was probably weaker than the influence of the real genetic relatedness. However, in the course of the bootstrap analysis we found that the pair of mycoplasma-positive strains TV 67–77 and TV 14–85 often clustered with other positive strains TV 10–02 and TV 73–87. This tendency could strongly deteriorate the bootstrap values of T. vaginalis tree, which was confirmed by excluding mycoplasma-positive strains from the analysis, see below.
We did not find a statistically significant concordance between the presence of TVV and the position of strains in the tree. In this respect, our results were consistent with the results of the previous analysis [17] and supported the view that the virus could horizontally spread among different strains of T. vaginalis. On the other hand Snipes et al.[16] obtained completely opposite result from their RAPD analysis of 109 T. vaginalis isolates. The resulting genealogical tree showed that among their isolates existed a branch, in which all but one strain were positive for TVV. The discrepancy between our results and those of Snipes could be due to differences in composition of the sets of analysed strains. While our set included 20 strains from various continents, the set of Snipes consisted of a larger amount of strains, however, all of them were isolated in the USA.
The topology of our tree did not reflect the geographic origin of strains. This could be a consequence of low viscosity of the T. vaginalis population resulting probably from intensive migration of their human hosts. Snipes et al.[16] obtained the same results. These findings disagree with results of our previous study [17], which indicated association of the strains of similar geographic origin. However the results presented here based on roughly three times more RAPD characters should more reliably reflect the real genealogy of these strains.
The topology of the tree did not reflect the similarity in the virulence of strains measured by five different methods. This confirmed the results based on isoenzyme and RAPD analyses [12,13,16], but partly disagreed with our previous results [17] that indicated the existence of a concordance between the tree topology and two of five indices of virulence (symptoms and histopathological findings in patients). Again, we consider present results to be more reliable because they are based on more data. The absence of this concordance can result from the fact that higher virulence of a T. vaginalis strain prevents successful spreading in population of T. vaginalis. Virulent strains are selectively eliminated by treatment, as the patients with severe symptoms are more likely to visit the physician. Moreover, the discomfort caused by the infection with a virulent strain could decrease the sexual activity of the patient and consequently the efficiency of the transmission. For this reason virulent strains cannot spread and survive for a long time in the population. They probably arose and went extinct repeatedly and independently in various lineages so there could not be found any concordance between the virulence and the strain position in the tree.
The suitability of RAPD method for phylogenetic analyses of trichomonads on intraspecies level
The bootstrap values in the genealogical tree of T. vaginalis strains were low (average 38.7%). Interestingly, the bootstrap values in the tree of T. foetus and T. suis strains acquired from the RAPD analysis with the same set of primers were considerably higher (average 65.7%) than bootstrap values for strains of T. vaginalis[25]. It is evident that the suitability of RAPD method depends to a great extent on the analysed taxa. The RAPD method as any other method of molecular taxonomy could successfully resolve the relatedness of taxa or strains only for a particular range of their genetic distances [26]. Under and above this resolution window the method could not give reliable results. Lower bootstrap values in the present tree could be caused by higher genetic distances among T. vaginalis strains than among strains of T. foetus and T. suis[25], which could result either from earlier radiation of T. vaginalis strains or from a higher rate of molecular clock for the RAPD characters in this species. Another reason for low bootstrap values could be a possible presence of some kind of sexual process in this species. Only indirect indications for sexual processes in trichomonads exist [27], but some karyological studies suggest occurrence of meiosis in a fraction of T. vaginalis cells in cultures [28]. We would expect that even a very rare occurrence of sex in the population of this species, i.e., the existence of a weak gene flow among different T. vaginalis strains, could obscure the phylogenetic signal in the molecular data. Also the contamination of some strains with mycoplasmas and the artificial clustering of infected strains could bring further noise to our analysis causing the decrease in bootstrap values. After removing five mycoplasma-positive strains, the average bootstrap value in our tree increased from 38.7% to 46.5% and that of the branch with strains with higher MLC values increased from 22% to 41%. Although the bootstrap values in the tree obtained from our analysis were low, we suppose that the tree approaches the real genealogy of these strains. This conclusion is supported firstly by the existence of positive correlation between the amount of RAPD data and the magnitude of average bootstrap values in the resulting tree (results not shown) and secondly by the presence of significant correlations between some biological traits of the strains and their distribution in the tree. The probability of an appearance of such correlations in a random tree or in a tree with a wrong topology is very low.
Conclusions
The present study demonstrated the existence of concordance between the genetic relatedness and level of metronidazole susceptibility of T. vaginalis strains. No concordance between genetic relatedness and virulence, geographic origin or presence of TVV was found. The study also proved the suitability of RAPD technique for genealogical studies in trichomonads on the intraspecies level. The number of data sufficient for such analysis, however, varies among species. The testing of analysed strains for the presence of mycoplasmas and other intracellular parasites in order to avoid the contamination with foreign DNA as well as assessing the reliability of the tree by bootstrap analysis seem to be the obligatory steps in such analyses.
Material and methods
Organisms
20 strains of Trichomonas vaginalis and 6 strains of other trichomonad species were included in this study. The information on the origin of strains is summarised in table 1. All organisms are deposited in the culture collection of the Department of Parasitology, Charles University in Prague.
Table 1.
Information on the origin of strains included in the analysis
| Species | Strain | Host | Origin | Isolation |
| Trichomonas vaginalis | TV 10–02 | Homo sapiens, vagina | Prague, Czech Rep. | Kulda, 1973 [41] |
| TV 73–87 | Homo sapiens, ditto | Prague, Czech Rep. | Kulda, 1973 | |
| TV 71–96 | Homo sapiens, ditto | Prague, Czech Rep. | Kulda, 1973 | |
| TV 79–49 | Homo sapiens, ditto | Prague, Czech Rep. | Kulda, 1973 | |
| TV 7–37 | Homo sapiens, ditto | Prague, Czech Rep. | Kulda, 1973 | |
| TV 85–08 | Homo sapiens, ditto | Prague, Czech Rep. | Kulda, 1973 | |
| TV 14–85 | Homo sapiens, ditto | Prague, Czech Rep. | Kulda, 1973 [41] | |
| TV 67–77 | Homo sapiens, ditto | Prague, Czech Rep. | Kulda, 1973 [41] | |
| TV 17–48 | Homo sapiens, ditto | Prague, Czech Rep. | Kulda, 1973 [41] | |
| HL-4MT | Homo sapiens, ditto | Liberec, Czech Rep. | Temín, 1986 | |
| FF28 | Homo sapiens, ditto | Bratislava, Slovakia | Demeš, 1987 | |
| C:1-NIH (ATCC 30001) | Homo sapiens, ditto | Washington, D.C., USA | Jacobs, 1956 [42] | |
| JH31A (ATCC 30236) | Homo sapiens, ditto | Baltimore, USA | Hollander, 1963 [43] | |
| CP1 | Homo sapiens, ditto | Peking, China | Tachezy, 1987 | |
| JTCRYO | Homo sapiens, ditto | Rio de Janeiro, Brazil | SilvaFilho, 1982 [44] | |
| CDC-85 (ATCC 50143) | Homo sapiens, ditto | Columbus, USA | Lossick, 1980 [45] | |
| RU357 (ATCC 50139) | Homo sapiens, ditto | Pennsylvania, USA | Sondheimer, 1982 [45] | |
| TALL-MT | Homo sapiens, ditto | Tallin, Estonia | Tompel, 1987 | |
| BO | Homo sapiens, ditto | Gothenburg, Sweden | Forsgren, 1978 [46] | |
| IR78 | Homo sapiens, ditto | Vienna, Austria | Meingassner, 1978 [7] | |
| Trichomonas gallinae | TGK | Columba livid, f.dom., crop | Prague, Czech Rep. | Tachezy, 1994 |
| Tritrichomonas foetus | KVc-1 | Bos taurus, prepucium | Žalmanov, Czech Rep. | Lípová, 1962 [47]* |
| Pentatrichomonas hominis | PHG-2 | Homo sapiens, feces | Košice, Slovak Republic | Giboda, 1981 |
| Trichomitus batrachorum | BUB | Bufo bufo, cloaca | Veselí nad Lužnicí, Czech Rep. | Kulda, 1983 |
| Hypotrichomonas acosta | L3 (ATCC 30069) | Drymarchon corais, cloaca | California, USA | Honigberg, 1948 |
| Tetratrichomonas gallinarum | M3 | Meleagris gallopavo, caecum | Uhlířské Janovice, Czech Rep. | Suchánková, Kulda, 1970 [36] |
* KVc-1 clone obtained from the original stock KV isolated by Lípová 1962 by double serial cloning.
Cultivation of trichomonads
All trichomonads were maintained as axenic cultures in Diamond medium TYM [29] supplemented with 10% heat inactivated horse serum. The pH of the medium was adjusted to pH 6.2 for T. vaginalis strains and to pH 7.2 for strains of other trichomonad species. Trichomonads from birds and mammals were cultivated at 37°C and trichomonads from amphibians and lizards at 26°C. The last three transfers before harvesting were grown in medium without agar.
DNA isolation
DNA was isolated using modified guanidiumhydrochloride method [30]. Cells from 0.5 1 of culture (0.5–5 × 106 cells/ml) were washed with 0.8% NaCl and lysed by adding an equal volume of 8 M guanidiumhydrochloride. The lysate was extracted twice with chloroform-isoamylalcohol (24:1). DNA was precipitated overnight at -20°C by addition of 2 vol 96% ethanol. The precipitate was centrifuged, the pellet was air-dried and dissolved in 0.5 ml of TE buffer. DNA was then treated with 0.2 μg/ml of RNase A (30 mm at 25°C) and 0.4 mg/ml of proteinase K (2 hours at 37°C). After the enzyme treatment, the DNA was reextracted with chloroform-isoamyi alcohol and precipitated with ethanol. The pellet was washed twice with 70% ethanol, air-dried and dissolved in 50 μl of TE buffer.
Presence of TVV and mycoplasmas in strains of T. vaginalis
The presence of TVV in Trichomonas vaginalis strains was detected by 1% agarose gel electrophoresis of raw nucleic acids extract before RNase A treatment.
The presence of mycoplasmas was assayed by a PCR with the specific primers GPO-1 (ACTCCTACGGGAGGCAGCAGTA) and MGSO(TGCACCATCTGTCACTCTGTTAACCTC) [31]. These primers are specific to all members of the class Mollicutes. The PCR was performed in 0.2 ml eppendorf tubes in a thermocycler PT C-200 (MJ Research, Inc.). DNA (5 ng) was amplified in 25 μl of the mix (10 mM Tris-HCl, 50 mM KCl, 1.5 mM MgCl2, 0.1% Triton X-100, 200 μM each dNTP, 12.5 pM of each primer, 0.2 U/μl Taq polymerase). The reaction profile consisted of four phases: 1) 94°C for 5 min and 55°C for 1 min and 45 s. 2) 3 cycles of 72°C for 3 min, 94°C for 45 s. and 55°C for 1 min and 45 s. 3) 40 cycles of 72°C for 3 min, 94°C for 45 s. and 55°C for 45 s. 4) 72°C for 10 min and 27°C for 10 min. The products were separated by electrophoresis in 1% agarose gel. In positive samples a distinct band of 717 bp was detected. Data on the presence of mycoplasmas and TVV are summarised in table 2.
Table 2.
The presence of TVV and mycoplasmas in analysed strains of Trichomonas vaginalis and their susceptibility to metronidazole.
| Strain | TVV | mycoplasmas | Patient response to treatment | MLC at 48 hours (metronidazole μg.ml-1)* (aerobic conditions) | ||
| Geometric mean | Range | n | ||||
| IR78 | - | - | Refractory | 107.2 | 50–200 | 20 |
| TV 79–49 | + | - | Cured | 1.92 | 1.56–6.5 | 10 |
| TV 71–96 | + | + | Cured | 3.35 | 3.125–6.25 | 10 |
| FF28 | + | - | Cured | 2.14 | 1.56–3.125 | 11 |
| TALL-MT | - | - | Refractory | 3.125 | 3.125 | 10 |
| CP1 | - | - | Cured | 2.01 | 0.78–6.25 | 11 |
| JH31A | - | - | Cured | 3.85 | 1.56–6.25 | 11 |
| C-1:NIH | + | - | Cured | 1.56 | 1.56 | 6 |
| JTCRYO | - | - | Cured | 2.50 | 1.56–3.125 | 6 |
| TV 10–02 | + | + | Cured | 4.7 | 3.125–12.5 | 36 |
| TV 14–85 | - | + | Cured | 9.9 | 6.25–12.5 | 36 |
| TV 73–87 | + | + | Cured | 3.12 | 3.12 | 12 |
| TV 67–77 | - | + | Cured | 12.5 | 12.5 | 12 |
| TV 85–08 | + | - | Cured | 6.25 | 6.25 | 12 |
| BO | + | - | Refractory | 50 | 50 | 12 |
| HL-4MT | - | - | Refractory | 317.5 | 200–400 | 36 |
| TV 7–37 | - | - | Cured | 20.83 | 12.5–25 | 36 |
| CDC-85 | - | - | Refractory† | 1000† | not available | not available |
| TV 17–48 | - | - | Cured | 12.5 | 12.5 | 3 |
| RU357 | - | - | Refractory† | 200 † | not available | not available |
* Minimal lethal concentrations (MLC) for metronidazole were determined by in vitro microtitre plate assay [32] under aerobic conditions. MLC was defined as the lowest concentration of metronidazole at which no motile parasites were detectable by microscopy. † Drug-susceptibility data for strains CDC-85 and RU 357 were obtained from literature [34].
Susceptibility to metronidazole and virulence of T. vaginalis strains
The susceptibility of T. vaginalis strains to metronidazole was determined in vitro using a microtitre plate assay [32]. The trichomonads were exposed to two-fold serial dilutions of metronidazole in the presence of air for 48 h at 37°C. Two strains, which did not survive exposure to air in plates, were tested by a tube assay [33]. The minimal lethal concentration (MLC) was determined microscopically as the lowest dilution of metronidazole in which no motile organisms were observed. Data on susceptibility to metronidazole and response to treatment of strains CDC-85 and RU 357 were obtained from literature [34]. Data are summarised in table 2.
Clinical and laboratory data allowing the assessment of strain virulence were available for ten T. vaginalis strains (Table 3). Pathogenic effects on donor female patients were assessed by clinical and histopathological findings and rated according to increasing severity by four arbitrary units characterized in reference [2] (page 148). Virulence for mice was evaluated by the following methods:
Table 3.
Virulence of analysed Trichomonas vaginalis strains assessed on the basis of clinical findings, histopathological examination of ectocervical biopsies and results of mouse assays after intraperitoneal and subcutaneous inoculation.
| Intraperitoneal mouse test | Subcutaneous mouse test | Patients(d) | |||||
| Strain | Mortality % (a) | Virulence index (b) | N | Abscess volume (mm3) (c) | N | Symptoms | Histopathology |
| TV 85–08 | 50 | 7.7 | 20 | 132 | 38 | 2 | 2 |
| TV 73–87 | 43 | 10.4 | 30 | 66 | 31 | 2 | 2 |
| TV 71–96 | 24 | 6.0 | 37 | 121 | 38 | 2 | 3 |
| TV 79–49 | 73 | 10.7 | 30 | 176 | 65 | 1 | 1 |
| TV 14–85 | 86 | 10.8 | 30 | 127 | 29 | 4 | 3 |
| TV 67–77 | 47 | 8.7 | 30 | 63 | 40 | 2 | 2 |
| TV 10–02 | 0 | 3.7 | 20 | 79 | 37 | 1 | 1 |
| TV 7–37 | 90 | 13.4 | 30 | 230 | 41 | 4 | 4 |
| FF28 | 75 | 7.6 | 12 | 117 | 28 | 0 | nd |
| JH-31A | nd | nd | nd | 78.6 | 49 | 0 | 0 |
| TV 17–48 | 83 | 11.1 | 30 | 183 | 58 | 4 | 4 |
(a) Percent of inoculated mice that died within three weeks after inoculation. (b) Virulence index of Cavier et al.[23] ranges from 0 (avirulent) to 16 (maximum virulence). (c) Mean volumes of subcutaneous abscesses developed six days after inoculation. (d) Clinical and pathological changes found at gynaecological and histopathological examination rated according to increasing severity from 1 to 4 as indicated in reference [2].
(a) Subcutaneous mouse assay [35] based on measurement of 6 day abscesses resulting from subcutaneous administration of 8 × 105 trichomonads to males of inbred C57BL/6 mice (18–20 g) as described in reference [36]. Abscesses were measured on day 6 after inoculation.
(b) Cumulative mortality of mice expressed as percentage of mice that died within three weeks after intraperitoneal inoculation of 106 trichomonads.
(c) The mean virulence index of Cavier [23] based on rating lesions in abdominal organs and quantity of ascites fluid after intraperitoneal inoculation of 106 trichomonads. Male outbred "H" mice (18–20 g) with the genetic background of the A strain produced by SEVAC (Prague) were used for intraperitoneal assays.
All mouse assays are described in detail in reference [2].
RAPD analysis
DNA (10 ng) was amplified in 20 μl of the PCR reaction mixture (10 mM Tris-HCl pH 8.8, 50 mM KCl, 0.08% Nonidet P40, 2.5 mM MgCl2, 200 μM of each dNTP, 0.25 μM primer, 0.05 U/μl Taq polymerase). Primers used in our analysis, OPA-08, OPA-09, OPA-11, OPA-12, OPA-14, OPA-15, OPA-17, OPA-19, OPD-19, OPF-01, OPF-03, OPF-04, OPF-05, OPF-06, OPF-09, OPF-10, OPF-12, OPF-14 and OPF-16 were obtained from Operon Technologies, Inc. The PCR was performed in 0.2 ml eppendorf tubes in a thermocycler PT C-200 (MJ Research, Inc.). The thermal cycle used was: 94°C (1 min) for initial denaturation followed by 35 cycles of 94°C (1 min) 38°C(1 min) 72°C (2 min) and ended by the final extension step, 72°C (15 min). The DNA samples from all strains were amplified with the particular primer in the same experiment. The reaction mixture without DNA was prepared for all samples in one tube and then distributed in tubes containing either samples or water (negative control).
10 μl of products was analysed by an agarose gel electrophoresis in a 2% gel. All primers provided a distinct pattern of amplified DNA fragments, in which the number of visible fragments varied from 2 to 17.
Genetic polymorphism analysis
All computations were performed by the program FreeTree (available at http://www.natur.cuni.cz/flegr/programs) [22]. Genetic distances (d) of samples were computed from Nei-Li's coefficient of similarity, as d = 1 - s[37]. The dendrogram was constructed by the Neighbor-joining method (NJ) [38] and bootstrap values (for 250 resamplings) were computed for every node of this dendrogram. The program FreeTree can be used for analysis of composite data sets, i. e., data sets from two or more independent analyses that included non-identical sets of species and strains. This allowed us to put together our data from the analysis of 20 T. vaginalis strains with the 18-strains-data-set from our previous analysis [17] published at http://www.natur.cuni.cz/flegr/manuscript/trich96, in which the data for strains TV 17–48 and RU 357 were missing.
Statistical testing of trees concordance
The correspondence of geographic origin and biological properties of T. vaginalis strains (resistance for metronidazole, virulence, presence of TVV and mycoplasmas) with their position in the tree was estimated by a permutation tail probability test [39]. For any parameter studied, the average distance between sister OTU (i.e., sister strains or sister branches of the tree) was calculated from the genealogical tree obtained from RAPD data by NJ method. Then 100,000 trees were generated by random permutation of apical branches. For every permutated tree the average distance between each sister OTU was calculated and these distances were compared with the average distance of the inferred genealogical tree. If the average distance of the inferred genealogical tree fell among the shortest 5% of lowest distances of permutated trees, we considered the correspondence of a particular biological property with the position of the strain in the genealogical tree to be statistically significant. The testing was carried out by the program Treept (available at http://www.natur.cuni.cz/flegr/programs) [40]. In the aerobic metronidazole susceptibility study, the sensitivities of strains were characterised by minimal lethal concentration (MLC) of the drug (see table 2). These values were transformed before the test as MLCtransf = log (log (MLC)). After this transformation the dependence of the values on their order was linear (Fig. 2). In the TVV and mycoplasmas study the presence of TVV or mycoplasmas was treated as 1 and its absence as 0, in the virulence study five different indices of pathogenicity (see table 3) were used. In the geographical origin study a matrix of nonparametric distances between the sites of origin for every pair of strains was prepared. For strains isolated in the same city, same country, same continent and different continent the distances were considered as 1, 2, 3 and 4, respectively.
Figure 2.
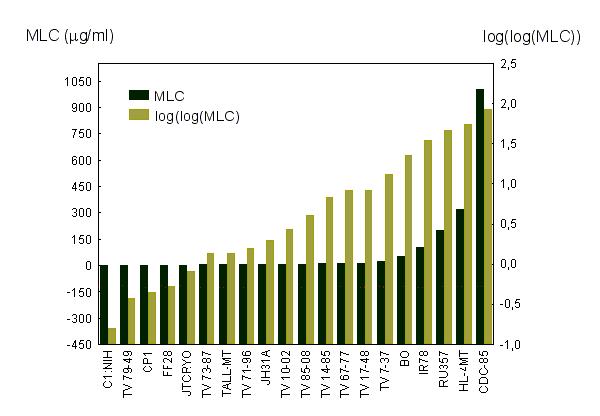
Distribution of MLC values for metronidazole tested under aerobic condition for different strains of T. vaginalis.
List of abbreviations
MLC Minimal lethal concentration
NJ Neighbor-joining
PCR Polymerase chain reaction
RAPD Random amplified polymorphic DNA
TVV Trichomonas vaginalis virus
TYM Trypton, yeast extract, maltose cultivation medium
ITS Internal transcribed spacer
Acknowledgments
Acknowledgements
The work was supported by grant MŠMT 1131-4.
Contributor Information
Vladimír Hampl, Email: vlada@natur.cuni.cz.
Štěpánka Vaňáčová, Email: vanacova@natur.cuni.cz.
Jaroslav Kulda, Email: kulda@natur.cuni.cz.
Jaroslav Flegr, Email: flegr@cesnet.cz.
References
- Sogin ML, Silberman JD. Evolution of the protists and protistan parasites from the perspective of molecular systematics. Int J Parasitol. 1998;28:11–20. doi: 10.1016/S0020-7519(97)00181-1. [DOI] [PubMed] [Google Scholar]
- Kulda J. Employment of experimental animals in studies of Trichomonas vaginalis infection. Trichomonads Parasitic in Humans (Edited by Honigberg BM) New York, Springer-Verlag. 1989. pp. 205–277.
- Petrin D, Delgaty K, Bhatt R, Garber G. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300–17. doi: 10.1128/cmr.11.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diddle AW. Trichomonas vaginalis: resistance to metronidazole. Am J Obstet Gynecol. 1967;98:583–585. doi: 10.1016/0002-9378(67)90118-4. [DOI] [PubMed] [Google Scholar]
- Aure JC, Gjonnaess H. Metronidazole treatment of trichomonal vaginitis. A comparison of cure rates in 1961 and 1967. Ada Obstet Gynecol Scand. 1969;48:440–445. doi: 10.3109/00016346909156659. [DOI] [PubMed] [Google Scholar]
- Kurnatowska A. Metronidazole resistance of Trichomonas vaginalis Donne. Wiad Parazytol. 1969;15:399–401. [PubMed] [Google Scholar]
- Meingassner JG, Thurner J. Strain of Trichomonas vaginalis resistant to metronidazole and other 5-metronidazole. Antimicrob Agents Chemother. 1979;15:254–257. doi: 10.1128/aac.15.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MH. The ultrastructure of Trichomonas vaginalis Donne before and after transfer from vaginal secretion to Diamonds medium. Acta Pathol Microbiol Scand Suppl. 1975;83:581–589. doi: 10.1111/j.1699-0463.1975.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Scholtyseck E, Teras J, Kasakova I, Sethi KK. Electron microscope observations on the interaction of Mycoplasma fermentans with Trichomonas vaginalis. Z Parasitenkd. 1985;71:435–442. doi: 10.1007/BF00928346. [DOI] [PubMed] [Google Scholar]
- Wang AL, Wang CC. The double-stranded RNA in Trichomonas vaginalis may originate from virus-like particles. Proc Natl Acad Sci USA. 1986;83:7956–7960. doi: 10.1073/pnas.83.20.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegr J, Čerkasov J, Kulda J, Tachezy J, Štokrová J. The dsRNA of Trichomonas vaginalis is associated with virus like particles and does not correlate with metronidazole resistance. Folia Microbiol. 1987;32:345–348. doi: 10.1007/BF02877224. [DOI] [PubMed] [Google Scholar]
- Vohra H, Sharma P, Sofi BA, Gupta I, Ganguly NK, Mahajan RC, Malla N. Correlation of zymodeme patterns, virulence & drug sensitivity of Trichomonas vaginalis isolates from women. Indian J Med Res. 1991;93:37–39. [PubMed] [Google Scholar]
- Proctor EM, Naaykens W, Wong Q, Bowie WR. Isoenzyme patterns of isolates of Trichomonas vaginalis from Vancouver. Sex Transm Dis. 1988;15:181–185. doi: 10.1097/00007435-198810000-00001. [DOI] [PubMed] [Google Scholar]
- Krieger JN, Holmes KK, Spence MR, Rein MF, McCormack WM, Tam MR. Geographic variation among isolates of Trichomonas vaginalis: demonstration of antigenic heterogeneity by using monoclonal antibodies and the indirect immunofluorescence technique. J Infect Dis. 1985;152:979–984. doi: 10.1093/infdis/152.5.979. [DOI] [PubMed] [Google Scholar]
- Stiles JK, Shah PH, Xue L, Meade JC, Lushbaugh WB, Cleary JD, Finley RW. Molecular typing of Trichomonas vaginalis isolates by HSP70 restriction fragment length polymorphism. American Journal Of Tropical Medicine And Hygiene. 2000;62:441–445. doi: 10.4269/ajtmh.2000.62.441. [DOI] [PubMed] [Google Scholar]
- Snipes LJ, Gamard PM, Narcisi EM, Ben Beard C, Lehmann T, Secor WE. Molecular epidemiology of metronidazole resistance in a population of Trichomonas vaginalis clinical isolates. Journal Of Clinical Microbiology. 2000;38:3004–3009. doi: 10.1128/jcm.38.8.3004-3009.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaňáčová Š, Tachezy J, Kulda J, Flegr J. Characterization of trichomonad species and strains by PCR fingerprinting. J Eukaryotic Microbiol. 1997;44:545–552. doi: 10.1111/j.1550-7408.1997.tb05960.x. [DOI] [PubMed] [Google Scholar]
- Bellinvia E, Munclinger P, Flegr J. Application of the RAPD technique for a study of the phylogenetic relationships among eight species of the genus Apodemus. Folia Zoologica. 1999;48:241–248. [Google Scholar]
- Tibayrenc M, Neubauer K, Barnabe C, Guerrini F, Skarecky D, Ayala FJ. Genetic characterization of 6 parasitic protozoa – parity between random-primer DNA typing and multilocus enzyme electrophoresis. Proc Natl Acad Sci USA. 1993;90:1335–1339. doi: 10.1073/pnas.90.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan UM, Constantine CC, Greene WK, Thompson RCA. RAPD (random amplified polymorphic DNA) analysis of Giardia DNA and correlation with isoenzyme data. Trans R Soc Trop Med Hyg. 1993;87:702–705. doi: 10.1016/0035-9203(93)90303-8. [DOI] [PubMed] [Google Scholar]
- Felleisen RSJ. Comparative genetic analysis of tritrichomonadid protozoa by the random amplified polymorphic DNA technique. Parasitology Research. 1998;84:153–156. doi: 10.1007/s004360050374. [DOI] [PubMed] [Google Scholar]
- Pavlíček A, Hrdá Š, Flegr J. FreeTree – freeware program for construction of phylogenetic trees on the basis of distance data and for bootstrap/jackknife analysis of the trees robustness. Application in the RAPD analysis of genus Frenkelia. Folia biol (Prague) 1999;45:97–99. [PubMed] [Google Scholar]
- Cavier RE, Gobert JG, Savel J. Application D'une methode d'infestation intraperitoneale de la souris par Trichomonas vaginalis a l'etude pharmacologique des trichomonacides. Ann Pharm Fr. 1972;30:637–642. [PubMed] [Google Scholar]
- Hallden C, Hansen M, Nilsson NO, Hjerdin A, Sall T. Competition as a source of errors in RAPD analysis. Theor Appl Genet. 1996;93:1185–1192. doi: 10.1007/s00122005035510.1007/s001220050355. [DOI] [PubMed] [Google Scholar]
- Hampl V, Pavlíček A, Flegr J. Construction and bootstrap analysis of DNA fingerprinting-based phylogenetic trees with the freeware program FreeTree: application to trichomonad parasites. Int J Syst Evol Microbiol. 2001;51:731–735. doi: 10.1099/00207713-51-3-731. [DOI] [PubMed] [Google Scholar]
- Tibayrenc M. Genetic epidemiology of parasitic protozoa and other infectious agents: the need for an integrated approach. Int J Parasitol. 1998;28:85–104. doi: 10.1016/S0020-7519(97)00180-X. [DOI] [PubMed] [Google Scholar]
- Kulda J, Nohýnková E, Ludvík J. Basic Structure and Function of the Trichomonad Cell. Acta Universitatis Carolinae – Biologica. 1986;30:181–198. [Google Scholar]
- Drmota T, Král J. Karyotype of Trichomonas vaginalis. Eur J Protistol. 1997;33:131–135. [Google Scholar]
- Diamond LS. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957;43:488–490. [PubMed] [Google Scholar]
- Pramanick D, Forstová J, Pivec L. Guanidine hydrochloride applied to the isolation DNA from different sources. FEBS Lett. 1976;62:81–84. doi: 10.1016/0014-5793(76)80021-X. [DOI] [PubMed] [Google Scholar]
- van Kuppeveld FJ, Johansson KE, Galama JM, Kissing J, Bolske G, van der Logt JT, Melchers WJ. Detection of mycoplasma contamination in cell cultures by a mycoplasma group-specific PCR. Appl Environ Microbiol. 1994;60:149–152. doi: 10.1128/aem.60.1.149-152.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachezy J, Kulda J, Tomková E. Aerobic resistance of Trichomonas vaginalis to metronidazole induced in vitro. Parasitology. 1993;106:31–37. doi: 10.1017/s0031182000074783. [DOI] [PubMed] [Google Scholar]
- Tachezy J, Kulda J. Testing the resistance to metronidazole in vitro in clinical isolates of Trichomonas vaginalis 2. Standard assays proposed. Cs Epid Mikrobiol Immunol. 1991;40:97–104. [PubMed] [Google Scholar]
- Müller M, Lossick JG, Gorrell TE. In vitro susceptibility of Trichomonas vaginalis to metronidazole and treatment outcome in vaginal trichomoniasis. Sex Transm Dis. 1988;15:17–24. doi: 10.1097/00007435-198801000-00004. [DOI] [PubMed] [Google Scholar]
- Honigberg BM. Comparative pathogenicity of Trichomonas vaginalis and Trichomonas gallinae for mice. I. Gross pathology, quantitative evaluation of virulence, and some factors affecting pathogenicity. J Parasitol. 1961;47:545–571. [PubMed] [Google Scholar]
- Kulda J, Suchánková E, Svoboda S. Studies on pathogenicity of Tetratrichomonas gallinarum in mice and turkey poults. Acta Vet (Brno) 1974;43:53–64. [Google Scholar]
- Nei M, Li W-H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA. 1979;76:5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The Neighbor-joining method: a new method for reconstruction of phylogenetics trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Adams DC, Anthony CD. Using randomization techniques to analyse behavioural data. Anim Behav. 1996;51:733–738. doi: 10.1006/anbe.1996.007710.1006/anbe.1996.0077. [DOI] [Google Scholar]
- Flegr J, Záboj P, Vaňáčová Š. Correlation between aerobic and anaerobic resistance to metronidazole in trichomonads: application of a new computer program for permutation tests. Parasitol Research. 1998;84:590–592. doi: 10.1007/s004360050454. [DOI] [PubMed] [Google Scholar]
- Kulda J, Vojtěchovská M, Tachezy J, Demeš P, Kunzová E. Metronidazole resistance of Trichomonas vaginalis as a cause of treatment failure in trichomoniasis-A case. Br J Vener Dis. 1982;58:394–399. doi: 10.1136/sti.58.6.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon LV, Ashburn LL, Jacobs L. Differences in strains of Trichomonas vaginalis as revealed by intraperitoneal injections into mice. J Parasitol. 1961;47:527–532. [PubMed] [Google Scholar]
- Kulda J, Honigberg BM, Frost JK, Hollander DH. Pathogenicity of Trichomonas vaginalis. Am J Obstet Gynecol. 1970;108:908–918. doi: 10.1016/0002-9378(70)90333-9. [DOI] [PubMed] [Google Scholar]
- Silva Filho FC, Elias CA, Desouza W. Further studies on the surface charge of various strains of Trichomonas vaginalis and Tritrichomonas foetus. Cell Biophys. 1986;8:161–176. doi: 10.1007/BF02788492. [DOI] [PubMed] [Google Scholar]
- Lossick JG, Müller M, Gorrell TE. In vitro drug susceptibility and doses of metronidazole required for cure in cases of refractory vaginal trichomoniasis. J Infect Dis. 1986;153:948–955. doi: 10.1093/infdis/153.5.948. [DOI] [PubMed] [Google Scholar]
- Forsgren A, Forssman L. Metronidazole resistant Trichomonas vaginalis. Br J Vener Dis. 1979;55:351–353. doi: 10.1136/sti.55.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulda J, Honigberg BM. Behavior and pathogenicity of Tritrichomonas foetus in chick liver cell cultures. Journal of Protozoology. 1969;16:479–495. doi: 10.1111/j.1550-7408.1969.tb02304.x. [DOI] [PubMed] [Google Scholar]


