Abstract
Polysaccharides from Auricularia auricula-judae (AP) have unique functionalities. The influence of AP-blending ratios (0.1–0.8%), temperature, pH, and ionic strength on the rheological interactions of yam starch (YS)–AP blends (YA, 6%) was investigated. YA gels showed shear-thinning behavior with flow-behavior indices of 0.28–0.37 and greater pseudoplasticity in the presence of 0.8% AP. With incremental AP addition, the viscosity and consistency indices significantly increased, whereas the activation energy decreased from 7.55 to 5.12 kJ/mol (p < 0.05). YA gels exhibited excellent thickening and heat tolerance and behaved as weak gels over an AP concentration range of 0.1–0.4% but as true gels at higher concentrations (e.g. 0.8%). The viscosity/elasticity of the combinations weakened with NaCl addition or extreme pH values. A complex 3-dimensional network structure of the YA system was confirmed by scanning electron microscopy. These results indicated that AP has great potential for functional applications in the starchy food industry.
Keywords: Polysaccharide, Auricularia auricula-judae, Yam starch, Synergistic interaction, Rheological properties
Introduction
Starch/hydrocolloid combinations have been extensively used, especially in the food industry, to enhance the tolerance to processing conditions and storage stability, control moisture or water mobility, supply nutrition and healthcare functions, improve the texture and overall product quality, and provide other attributes desired by food manufacturers and consumers [1]. Recently, increasing attention has been paid to the effects of synergistic interactions between a particular starch (e.g. high-amylose cornstarch, pea starch, or yam starch) and a unique hydrocolloid (e.g. guar gum, xanthan, chitosan, or xyloglucan) on the pasting, gelatinization, retrogradation, and digestibility of composite systems [2–4]. In addition to their physicochemical characteristics, detailed knowledge concerning the roles of hydrocolloid interactions on rheological properties associated with specific functionalities of starch paste is also crucial for developing their potential for industrial applications [5–8].
Most previous findings have shown that polysaccharides from Auricularia auricula-judae (AP) offer multiple health benefits for humans because of their anti-tumor, anti-diabetic, and hypolipidemic properties [9–11]. Glucose, mannose, and galactose are the major monosaccharide components in water-extracted AP, which is characterized as a β-(1 → 3)-d-glucan with 2 β-(1 → 6)-d-glucosyl residues for every 3 main chain glucose residues, showing a comb-branched structure [12]. Previously, we also found that AP exhibited high viscosity, strong shear-thinning characteristics, and excellent thermal stability, indicating its great potential for applications in the food and pharmaceutical industries [13]. Starch is one of the most important food components in human diets and contributes to approximately 60–70% of the total energy consumed [14]. Thus, it is of great significance to explore interactions between AP and starch granules during food processing. Moreover, predicting the outcome of such interactions is impossible because of the inherent degrees of complexity and variability.
Yam tubers are widely consumed as staple foods by millions of people in Asia, Africa, South America, and the Pacific countries, and they play a key role in food security. A major component of yam is starch, which can account for up to 85% of the dry matter [15]. Compared to maize starch, yam starch (YS) has more amylose, higher viscosity, excellent heat stability, a more regular structure with amylopectin, fewer branch points, and shorter average chain lengths, which contribute to enhanced intermolecular interactions [16, 17]. Moreover, YS has a higher resistant starch content and, thus, hinders digestion more than common starchy plants such as maize, wheat, rice, cassava, and sweet potato [18]. Most importantly, Huang et al. [19] demonstrated that YS had important anti-constipation and hypolipidemic effects, which can be useful in the functional food industry [19]. It is well known that the overall quality of starchy food products is largely determined by the physicochemical and nutritional properties of starch, its interactions with other ingredients, as well as practical industrial processing conditions including high temperatures, acid–base concentrations, and ionic strengths [15]. However, to the best of our knowledge, the synergistic interactions of the mixed YS–AP system have not been examined under different processing conditions. Tailoring these interactions is also effective for optimizing food formulation and designing products [20].
Therefore, the objective of this study was to systematically investigate the effects of the AP-blending ratio, temperature, pH, and ionic strength on the rheological properties of YS/AP combinations, based on our previous research on AP [13]. Both steady and dynamic shear rheological experiments were performed to characterize the rheological interactions, while scanning electron microscopy was used to evaluate the microstructures of composite gels. The present work sheds insight into regulating the rheological properties of YS-based products to meet consumer demands, as well as into the potential use of the mixed YS/AP system as a new hydrocolloid source (AP) and a healthy ingredient in the starch-based food industry.
Materials and methods
Materials
Commercial yam (Dioscorea aimadoimo) originating from Andong, Gyeongsangbuk-do, Korea, was purchased from the North Andong National Agricultural Cooperative Federation. Water-soluble polysaccharide from the fruiting bodies of A. auricula-judae was prepared as we previously reported [13], with the chemical composition mostly consisting of carbohydrate (72.1%), protein (8.6%), water (9.6%), uronic acid (5.3%), and ash (4.4%). All chemicals and reagents used were of analytical grade.
Starch preparation
YS was isolated using water according to a modified method of Jiang et al. [18]. The fresh yam tuber was washed, hand-peeled, sliced, mixed with distilled water (4 times the tuber volume), and then ground in a laboratory food blender for 2 min at high speed. The homogenate was consecutively sieved and washed through 200-mesh screens using distilled water until the wash water was free from impurities. After standing for 4 h, the filtrate supernatant was removed and the precipitate was resuspended in distilled water (5 times the precipitate volume) until the supernatant became transparent. The slurry was then centrifuged (MF-80; Hanil Science Industrial Co., Ltd., Seoul, Korea) at 3000×g for 10 min. The upper nonwhite layer was scraped, and the white layer was resuspended in distilled water (5 times the precipitate volume) and re-centrifuged 5–6 times until the top starch layer displayed a firm, dense, and snow-white deposit. The starch layer was then collected and dried at 40 °C in an air-oven for 24 h. The resulting YS powder was ground to pass through a 100-mesh (<150 μm) sieve and desiccated at 25 °C until use. The extracted starch had moisture, ash, crude fat, and protein contents of 9.28 ± 0.16%, 0.12 ± 0.02%, 0.10 ± 0.01%, and 0.21 ± 0.03%, respectively, as determined by a previously described method [21], and the amylose content (31.23 ± 0.57%) was determined using the iodine colorimetric method [22].
Preparation of sample pastes
YA mixtures (6%, w/w) were prepared by mixing YS with distilled water and AP to obtain 0, 0.1, 0.2, 0.4, and 0.8% (by weight) hydrocolloid levels. YS-alone (5.9, 5.8, 5.6, and 5.2%, w/w) and AP-alone (0.1, 0.2, 0.4, and 0.8%, w/w) solutions were prepared to elucidate the impact of AP on the rheological and morphological properties of YA combinations. The mixture was allowed to evenly disperse and thoroughly hydrate by vortexing for 1 min before constantly stirring at 600 rpm on a Cimarec ceramic stirring hot plate (Thermo Scientific, SP131320-33Q) for 1 h at room temperature. The mixture was then heated in a boiling water bath for 30 min with stirring for full gelatinization (600 rpm; Thermo Scientific, Cimarechot plate). After heating, the hot pastes were immediately transferred to a cold-water bath (4 °C), cooled for 20 min to minimize potential experiment errors, and subsequently used for rheological measurements. To prevent water evaporation from influencing the concentration of the blended materials, all samples were prepared in a closed system using a vial (WH.225142, Wheaton, Millville, NJ, USA) with a deep-skirted screw cap.
Steady flow behavior
The effects of the mixing rate, temperature, pH, and NaCl on YA gels were studied using a rheometer (AR 2000, TA Instruments Inc., New Castle, DE, USA), equipped with a cone and geometry plate (40-mm diameter with a gap of 0.053 mm, angle 2°). During the experiment, the edge of the gap was covered with a thin layer of low-density silicon oil (dimethylpolysiloxane, 50-cPa viscosity) to minimize evaporation.
The power-law model [23] was used to fit the experimental flow curves with the following formula:
| 1 |
where η s is the apparent viscosity (Pa s), γ is the shear rate (s−1), K is the consistency index (Pa sn), and n is the flow-behavior index. The apparent viscosity (η a,100) at 100 s−1 was calculated from the magnitudes of K and n.
To determine the flow properties at different mixing rates, YA-composite pastes with different mixing ratios (6/0, 5.9/0.1, 5.8/0.2, 5.6/0.4, and 5.2/0.8) were continuously sheared from 0.01 to 100 s−1, and then from 100 to 0.01 s−1 at 25 °C. To determine the flow properties at different temperatures, samples were equilibrated to a set-point temperature (25, 55, or 85 °C) for 30 min in a water bath and loaded onto the base plate of a rheometer, and the shear rate was measured from 0.01 to 100 s−1. To determine flow properties at different NaCl concentrations at 25 °C, NaCl was added to the mixed YA solution, vortexed for 2 min to obtain final concentrations of 0.001–1 M, and then the dispersions were pasted. To determine flow properties at different pH values at 25 °C, mixed YA solutions at different pH values (~2, 4, 6, 8 or 10) were attained by adding 4 M NaOH or HCl before pasting.
Dynamic oscillatory behavior (DOB)
DOB measurements included frequency and temperature sweeps. A 40-mm parallel plate and a 1-mm gap were used. Dynamic oscillatory frequency sweeps were performed at 25 °C over the range of 0.1–10 Hz at a constant strain of 2% for YA-composite pastes with different mixing ratios, pH values, and NaCl concentrations. The 2% strain was within the linear viscoelastic region and was determined beforehand through a strain-sweep measurement at 1 Hz and 25 °C. The storage modulus (G′, a measure of elastic response) and loss modulus (G″, a measure of viscous response) were determined, and the corresponding complex viscosity (η * = [(G′)2 + (G″)2]1/2/ω, a measure of overall resistance to flow, where ω is the angular frequency) and loss-tangent (tan δ = G″/G′) values were calculated from the experimental data [4]. Logarithmic plots of frequency versus G′ and G″ were obtained. Temperature sweeping was performed from 25 to 85 °C, with a heating rate of 2 °C/min. The oscillation frequency was 1 Hz and the strain was 2%. G′ and G″ were recorded as functions of frequency. All samples were held on the plate for 3 min at the initial temperature to equilibrate the temperature and relax the pastes before steady and dynamic shear rheological measurements were taken. All rheological measurements were taken in triplicate.
Morphological changes
Morphological changes in samples were observed using a field-emission scanning electron microscope (FE-SEM) (JSM-7500F; JEOL, Ltd., Tokyo, Japan). YS (5.2%, w/w), AP (0.8%, w/w), and YA (6%, w/w) gels with an AP concentration of 0.8% were immediately freeze-dried to a moisture content below 5% and cut with a razor blade to expose the cut-surfaces, which were attached on an SEM stub using double-sided cellophane tape. Stubs and samples were sputter-coated with a thin layer of gold–palladium alloy in a vacuum, and the cross-sectioned area was examined and photographed [24]. The accelerating voltage and magnification were 5 kV and 400, respectively.
Statistical analysis
All experiments were run in triplicate. The data are presented as mean values and standard deviations. All statistical computations and analyses were achieved using Statistical Analysis System for windows, version 9.1 (SAS Institute, Cary, NC, USA) and the significance level was p < 0.05. Statistical differences were assessed by Student’s t test, one-way analysis of variance, and Duncan’s multiple-range test.
Results and discussion
Steady shear flow behavior of YA mixtures
Apparent-viscosity (η) versus shear-rate (γ) data for both upward and downward flow curves at 25 °C fit well to the power-law model (Eq. 1), with high determination coefficients (R 2 ≥ 0.99; Table 1). Adding AP at certain levels significantly increased the apparent viscosity (η) and consistency index (K) and decreased the flow-behavior index (n) for both upward and downward shearing (Table 1). This result agreed with those found in YS-mucilage [25] and YS-xanthan gum mixtures [26]. The η a,100 of the YA-composite gels with 0% AP (control) was 1.332 Pa s for upward shearing and 1.165 Pa s for downward shearing, which significantly increased to 1.844 and 1.618 Pa s, respectively, as the AP concentration increased to 0.8%. A similar trend in K values (a viscosity indicator) revealed that the upward- and downward-shearing values increased by ~67 and 82%, respectively. Notably, for the YA combination (6%, w/w) with 0.8% AP, the viscosities were 2.8-, 2.2-, and 1.5-fold higher than the sum of the viscosities for the YS gel (5.2%) and AP solution (0.8%) at shear rates of 1, 10, and 100 s−1, respectively, indicating a synergistic thickening effect of AP on the YS paste. The enhanced viscosity in composite gels can be attributed to the formation of 3-dimensional (3-D) and cross-linking network structures. The cross-linked network structures result from intra/intermolecular interactions between leached starch polymer molecules (primarily amylose) and AP molecules through hydrogen binding and/or random entanglement initiation as well as phase separation, which results from mutual exclusion or depletion flocculation and/or crystalline aggregation, based on the thermodynamic incompatibility of dissimilar polysaccharides [27]. The viscosities of all YA combinations decreased significantly with the shear rate, and respective n values were as low as 0.33–0.28 and 0.37–0.30 (n < 1), respectively, during upward and downward shearing, indicating that these were typical pseudoplastic fluids exhibiting shear-thinning flow behaviors. Compared with the n values for upward shearing, the rate of change of those for downward shearing dropped rapidly from 11.28 to 5.65% as the AP concentration increased from 0 to 0.8%.
Table 1.
Effect of the AP concentration on the flow rheological properties of YA1-composite gels at 25 °C
| AP1 concentration (%) | Upward | Downward | ||||||
|---|---|---|---|---|---|---|---|---|
| K | n | R 2 | η a,100 | K | n | R 2 | η a,100 | |
| Control | 30.057 ± 1.139d2 | 0.328 ± 0.016a | 0.990 | 1.332 ± 0.014e | 22.032 ± 0.721d | 0.365 ± 0.014a | 0.997 | 1.165 ± 0.018d |
| 0.1 | 33.596 ± 1.308cd | 0.323 ± 0.012a | 0.991 | 1.483 ± 0.026d | 22.705 ± 0.887d | 0.360 ± 0.013ab | 0.998 | 1.197 ± 0.016d |
| 0.2 | 37.014 ± 0.913c | 0.318 ± 0.010a | 0.992 | 1.600 ± 0.023c | 26.486 ± 1.170c | 0.348 ± 0.016ab | 0.999 | 1.312 ± 0.011c |
| 0.4 | 42.974 ± 1.590b | 0.305 ± 0.012ab | 0.991 | 1.751 ± 0.019b | 31.996 ± 1.308b | 0.328 ± 0.011bc | 0.998 | 1.447 ± 0.018b |
| 0.8 | 50.024 ± 1.698a | 0.283 ± 0.007b | 0.993 | 1.844 ± 0.026a | 40.797 ± 1.884a | 0.299 ± 0.009c | 0.999 | 1.618 ± 0.022a |
1 AP Auricularia auricula-judae polysaccharide, K consistency index, n flow behavior index, R 2 determination coefficient, η a,100 apparent viscosity at 100 s−1, YA yam starch and A auricula-judae polysaccharide composite
2 Mean ± standard deviation values of flow rheological parameters within the same column for each sample followed by different lower case superscript letters (a–e) for different AP concentrations are significantly different at p < 0.05 by Duncan’s multiple-range test
Effects of temperature, pH, and salt on viscosity
Food processing often involves exposure to high temperatures, acid–base concentrations, and ionic strengths. Therefore, it is necessary to investigate how the rheological characteristics of YA gels are influenced by these conditions. Figure 1(A) shows the apparent viscosities of YA-composite gels with 0 or 8% AP (w/w) versus the shear rate at temperatures of 25, 55, and 85 °C. The effect of temperature on the apparent paste viscosity can be determined using an Arrhenius model (Eq. 2) [5], where the apparent viscosity (η a,100) decreases exponentially with temperature:
| 2 |
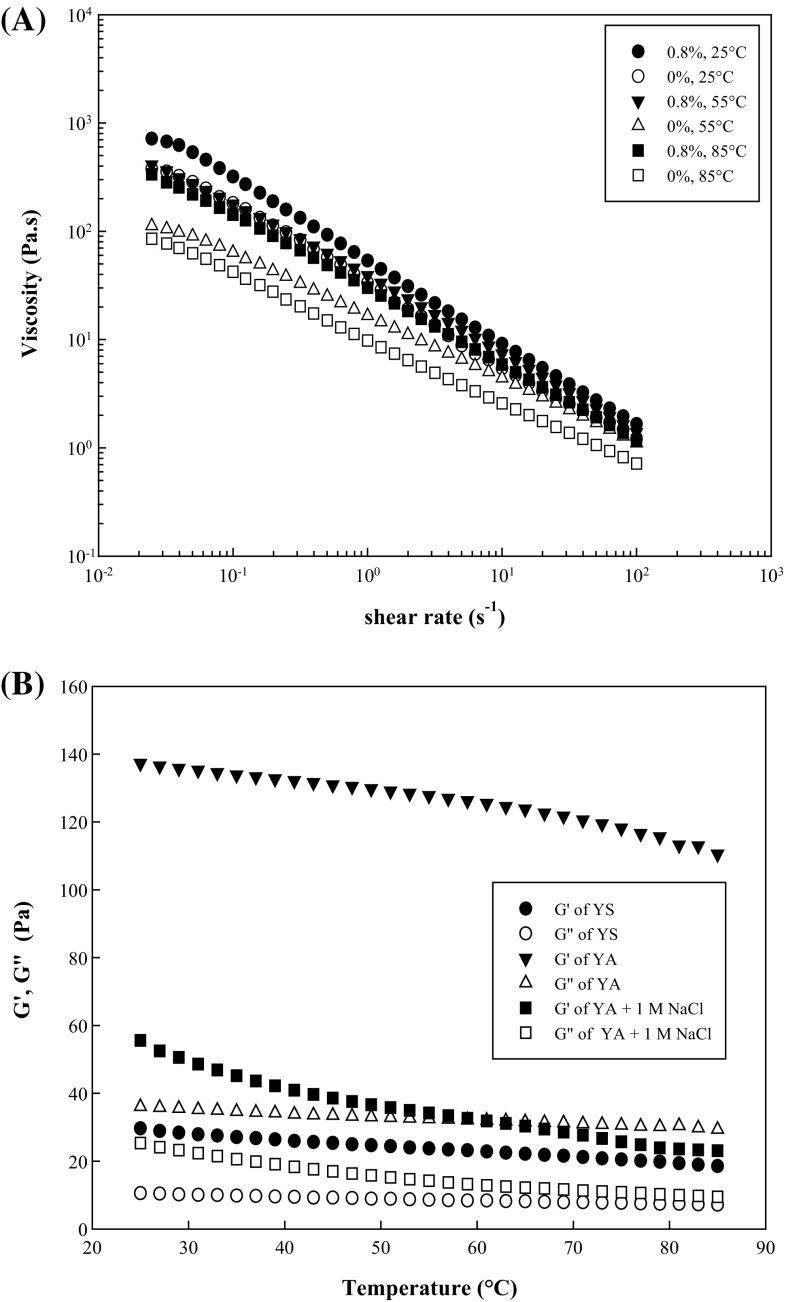
where η a,100 is the apparent viscosity (Pa s) at 100 s−1, A is a constant (Pa s), T is the absolute temperature (K), R is the gas constant (8.3144 J mol−1 K −1), and E a is the activation energy (kJ/mol). The viscosity of YA with 0% AP (control) decreased faster than that of YA with 0.8% AP during the temperature increment. For example, at a shear rate of 10 s−1, the viscosity for the control was 5.42 Pa s at 25 °C, which significantly decreased by 18.9 and 52.6% at 55 and 85 °C, respectively, while it slightly decreased by 18.6 and 35.6% for a mixed YA gel with 0.8% AP at these temperatures. Thus, the thermal stability of YS gel likely improved because of its excellent temperature tolerance to AP dispersions [12, 13]. In addition, the E a decreased from 7.55 to 5.12 kJ/mol as the AP concentration increased from 0 to 0.8%. The E a values were relatively lower than those of sweet potato starch–xanthan gum mixtures (8.29–12.6 kJ/mol) [5], indicating that the viscosity of YA gels is less responsive to temperature changes. The effect of temperature on the viscosity of YA gels significantly weakened with increasing AP concentrations, which may be related to its lower flow behavior index (n) (Table 1) and greater pseudoplastic characteristics [28].
Fig. 1.
Apparent viscosity-shear rate curve of the YA gels at AP concentrations of 0 and 0.8% (A), and G′ and G″ at 1 Hz and 2% strain for the YS, YA, and YA gels with 1 M NaCl while heating at 2.0 °C/min (B). YS yam starch, AP Auricularia auricula-judae polysaccharide, YA yam starch and A. auricula-judae polysaccharide composite
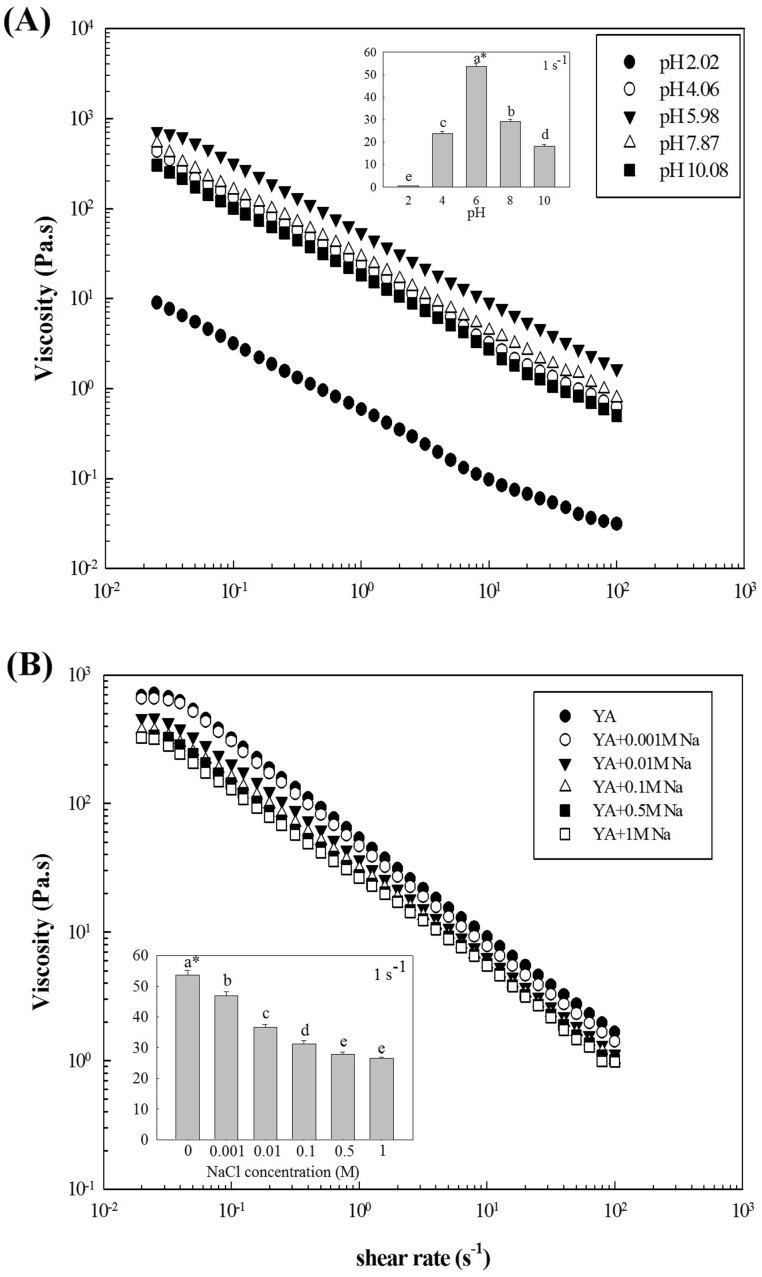
The apparent viscosities of YA-combination gels with 0.8% AP at differing pH are shown in Fig. 2(A). The results showed that both acidic (pH 2–6) and alkaline (pH 6–10) conditions changed the rheological behavior of the YA paste. For instance, the viscosity of the YA gel was 53.61 Pa s at pH 6, which sharply changed to 0.59 or 18.31 Pa s at pH 2 or 10, respectively. Acidic conditions might cause starch and polysaccharide hydrolysis, whereas the restrained swelling power of starch granules and conformational or compositional alterations of polymer systems by alkaline and heat treatment have been attributed to pH variations [29]. We previously observed this trend with polysaccharide solutions from A. auricula-judae [13] and YS pastes [26], in which the viscosity decreased when the neutral pH became alkaline or acidic.
Fig. 2.
Steady shear flow curves of YA-composite gels with an AP concentration of 0.8% at different pHs (A) or NaCl concentrations (B). AP Auricularia auricula-judae polysaccharide, YA yam starch, and A. auricula-judae polysaccharide composite, *Values of apparent viscosity at 1 s−1 followed by different letters (a–e) above each histogram for different pHs or different NaCl concentrations are significantly different at p < 0.05 by Duncan’s multiple-range test
The effect of NaCl at different concentrations on the apparent viscosity at 25 °C also influences rheological properties. The experimental results fit well to the simple power-law model (Eq. 1) with high determination coefficients (R 2 > 0.99). The apparent viscosity decreased as the NaCl concentration increased from 0 to 1 M [Fig. 2(B)]. At a shear rate of 1 s−1, the viscosity of a YA gel with 0.8% AP was 53.61 Pa s, which decreased significantly from 46.84 to 26.47 Pa s as the NaCl concentration increased from 0.001 to1 M. All pastes had high shear-thinning behavior with flow-behavior index values of 0.23–0.30. Although the addition of 0.001 M NaCl affected the flow-behavior index slightly, n values significantly increased (p < 0.05) as the NaCl concentration rose to 1 M, indicating that the YA gels became less pseudoplastic. Similar tendencies were observed in studies of sweet potato starch and xanthan mixtures [6]. The data indicated the synergistic interaction between AP and YS could be controlled by added NaCl, probably because of the more compact conformation of hydrocolloid molecular chains caused by charge-screening effects, resulting in a reduction in hydrodynamic size and a difficulty in forming intermolecular associations [30]. Such synergy could also have been due to inhibited gelatinization and allomorph structural loss of YS granules, weakened electrostatic interactions between starch-OH groups and Na+ ions, and reinforcement of the water structure-making effect of Na+ resulting from the formation of 2 strong hydration complexes (Na+[H2O]5 and Na+[H2O]6) [31, 32].
Dynamic–viscoelasticity spectrum
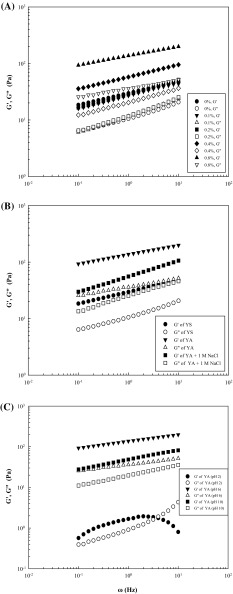
Figure 3(A) depicts the variation of storage modulus (G′) and loss modulus (G″) as a function of the frequency (ω) at 25 °C for YA gels with different AP concentrations (0.1–0.8%). It can be seen that the magnitudes of G′ and G″ increased with the increase of ω, and G′ values were higher than the G″ values at all ω values for all samples. Additionally, both G′ and G″ values increased significantly as the AP concentration increased from 0.2 to 0.8%, reflecting a typical gel-like system with frequency- and concentration-dependence. To evaluate the viscoelasticity of YA combinations, the dynamic rheological data of log (G′, G″) versus log ω were subjected to linear curve fitting with the magnitudes of the slopes (n′ and n″), intercepts (K′ and K″), and determination coefficients (R 2). Rinaudo [31] showed that polymers exhibit viscous gel behavior when the slope approaches or equals 1, whereas gels display an elastic property when the slope approaches 0. The n′ value for the YA gel increased from 0.207 to 0.245 as the AP addition rose to 0.2% and then rapidly decreased to 0.165 with the AP concentration increasing to 0.8%. Similarly, the n″ value also increased first to 0.302 at 0.1% AP addition, but it continuously dropped to 0.151 afterward as the AP concentration increased to 0.8%. Although the AP concentration fluctuated slightly in the range of 0.1–0.2%, both K′ and K″ significantly increased with increasing AP concentrations. In addition, K′ values (26.89–136.58) increased twofold to fourfold compared to those of K″ (10.85–36.04). This trend was also found with YS-mucilage [24] and sweet potato starch–xanthan mixtures [5]. These results indicated that the elastic properties of the YS paste may be enhanced by AP addition potentially because mutual exclusion between leached starch and polysaccharide molecules (based on thermodynamic incompatibility) strengthened the 3-D and cross-linked polymer network structure [27].
Fig. 3.
Plots of log G′ and log G″ versus log ω of YA-composite gels with different AP concentrations (A), of YS, YA and YA gels with 1 M NaCl (B), and of YA-composite gels at different pHs at 25 °C (C). YS yam starch, AP Auricularia auricula-judae polysaccharide, YA yam starch and A. auricula-judae polysaccharide composite
The modulus (G′ and G″), tan δ (G″/G′), and complex-viscosity (η*) values for YA-composite gels at 1 Hz are shown in Table 2. The G′ value slightly decreased from 29.65 Pa (control) to 28.29 Pa at 0.2% AP, but when the AP concentration was higher (0.4–0.8%), the figures approximately increased by onefold to fourfold. Similar to G′, the η* gradually reduced by 3–7% relative to the control (5.01 Pa s) at lower AP concentration (0.1–0.2%) and then significantly increased by ~1- to 3.5-fold (p < 0.05). In contrast, the G″ value rose slowly and continuously from 10.53 Pa for the control to 11.65 Pa at 0.2% AP followed by a fast increase to 36.06 Pa for 0.8% AP, suggesting that AP improved the elasticity. Similar results were reported for tapioca starch–arabic gum [33]. The tan δ is a good indicator of the solid- or liquid-like behavior of polymer solutions, and a strong gel was formed when the G″/G′ ratio was <0.33 [34]. At lower AP concentrations (0.1–0.2%) in YA-combination gels, the tan δ slowly increased by 16–20% relative to the control (0.355). Surprisingly, when the AP concentration increased to 0.8%, the tan δ value significantly decreased to 0.263, suggesting that a strong elastic gel formed at 0.8% AP. This observation is in good agreement with a report [4] showing that tan δ values decreased by the addition of 1% xyloglucan (XG) in YS/XG-combination gels (6%, w/w), compared to those of YS paste alone (6%, w/w) or the XG solution alone (1%, w/w).
Table 2.
Effect of the AP concentration on the G′, G″, tan δ, and η* parameters of YA-composite gels at 25 °C and 1 Hz
| AP1 concentration (%) | G′ (Pa) | G″ (Pa) | tan δ | η* (Pa s) |
|---|---|---|---|---|
| Control | 29.65 ± 0.76c2 | 10.53 ± 0.08d | 0.355 ± 0.009c | 5.010 ± 0.116c |
| 0.1 | 27.01 ± 0.72d | 11.54 ± 0.36cd | 0.427 ± 0.009a | 4.677 ± 0.132d |
| 0.2 | 28.29 ± 0.73cd | 11.65 ± 0.44c | 0.412 ± 0.008b | 4.872 ± 0.111cd |
| 0.4 | 57.38 ± 1.11b | 20.27 ± 0.56b | 0.353 ± 0.010c | 9.690 ± 0.303b |
| 0.8 | 137.3 ± 1.28a | 36.06 ± 0.84a | 0.263 ± 0.008d | 22.605 ± 0.29a |
1 AP Auricularia auricula-judae polysaccharide, G′ storage modulus, G″ loss modulus, tan δ loss tangent, η* complex viscosity, YA yam starch and A auricula-judae polysaccharide composite
2 Mean ± standard deviation values of dynamic rheological parameters within the same column for each sample followed by different lower case superscript letters (a–e) for different AP concentrations are significantly different at p < 0.05 by Duncan’s multiple-range test
Effect of salt and pH on the viscoelastic properties
The viscoelastic behavior of YS (6%) and YA gels with 0.8% AP, in the presence or absence of 1 M NaCl, were determined at 25 °C, over a frequency range of 0.1–10 Hz [Fig. 3(B)]. Compared with the 6% YS gel alone, the YA-composite gel without added salt had higher G′ and G″ values but lower tan δ values, suggesting an increased gel strength. In contrast, regarding YA gel in the presence of NaCl, both G′ and G″ decreased, whereas tan δ nearly doubled, indicating that the synergistic effect was weakened by NaCl. This may be attributed to the charge-shielding effect by hydrocolloid–salt interactions, resulting in conformational changes, reduced effective volumes, and decreased cross-linking junctions. In addition, Na+ acts as a “structure-maker” that is able to order the structure of water molecules in its surroundings to form two prevalent coordinated complexes, Na+(H2O)5 and Na+(H2O)6, which can interconvert with some degree of flexibility, resulting in an increased free-water fraction in the polymer system [32]. These effects could also be observed in rice starch–guar gum mixtures [7] and in the AP solution [13], in which the gel strength decreased by NaCl addition.
The effect of pH on the viscoelastic properties of YA gels with the AP concentration of 0.8% was also studied [Fig. 3(C)]. As expected, changes in pH from 6 to 10 or 2 led to significantly lower G′ values, from 137.3 Pa to 48.6 or 1.7 Pa at 1 Hz, respectively. However, higher tan δ values from 0.26 to 0.41 or 0.54 at 1 Hz were also obtained from the plots of ω versus G′ and G″, which is consistent with the influence of viscosity. Moreover, G′ was more sensitive to the effect of pH than G″. Interestingly, G′ values at pH 2 increased and then decreased with increasing frequency, which might be ascribed to the unstable system resulting from the severe processing conditions used. In general, extreme acid treatment during cooking could result in stronger hydrolysis or macromolecular degradation of polysaccharides to a great extent, a sharper decrease in the number of cross-linking junctions between the biopolymer chains, more deterioration of the network structure, and even an enormous loss of viscoelasticity [13, 26]. Thus, the higher frequency oscillation (1–10 Hz) caused structural deformations and a thorough breakdown because of the large strain amplitude (2%), which probably exceeded the linear viscoelastic range. Correspondingly, the gel became progressively more liquid-like with a decreasing G′, and eventually G″ exceeded G′. These results suggested that both salt [Fig. 3(B)] and pH changes [Fig. 3(C)] could decrease the gel strength of YA-composite gels. These observations agreed with previous results showing that the viscosity of YA gels was significantly reduced by added salt [Fig. 2(B)] and pH variations [Fig. 2(A)] in blended dispersions.
Effect of temperature on viscoelastic properties
Figure 1(B) shows the temperature dependence of G′ and G″ observed for YS (6%) and YA gels with 0.8% AP in the presence or absence of 1 M NaCl while heating from 25 to 85 °C. For all sample gels, both G′ and G″ decreased as the temperature increased. The YS pastes showed the lowest values in G′ and G″ with variation rates of 37.5 and 32.1%, respectively, in the temperature range employed (25–85 °C), displaying weaker viscoelastic characteristics. In contrast, YA gels without added salt had the highest G′ and G″ values with the lowest change ratios (19.5 and 18.6%, respectively) in the temperature range of 25–85 °C, exhibiting strong thermal stability over the temperature course, which might be attributed to excellent heat tolerance of AP dispersions [12]. However, after adding 1 M NaCl, both G′ and G″ for YA gels rapidly dropped by 50–70% and 30–60%, respectively, but remained higher than that of YS paste alone at the corresponding concentration and temperature range, indicating that the synergistic rheological properties of YA-composite gels during heating could be controlled by NaCl. These observations agreed with the results shown in Figs. 1(A) and 3(B).
Morphological changes
Microstructural changes occurring before and after the blending of YS and AP gels were observed by SEM (Fig. 4). YS gels displayed large-sized, fibrous, or honeycomb-like network structures with a thicker matrix [Fig. 4(A)] owing to the contribution of amylose leaching to the formation of a 3-D network during heating [35]. A firmer and more extended and flaky network with some ice or air cavities was observed in YA gels [Fig. 4(B)], which might be attributed to a comb-branched structure and rigid/stiff chain conformation that arose during cooking in water [12]. YA gels exhibited a more complex 3-D network structure as compared with those of the YS and AP gels, including a thinner lamellar membrane, interactive entanglement, and a linking bridge matrix [Fig. 4(C)], indicating synergy between YS and AP, which agreed with the viscoelastic data discussed above.
Fig. 4.
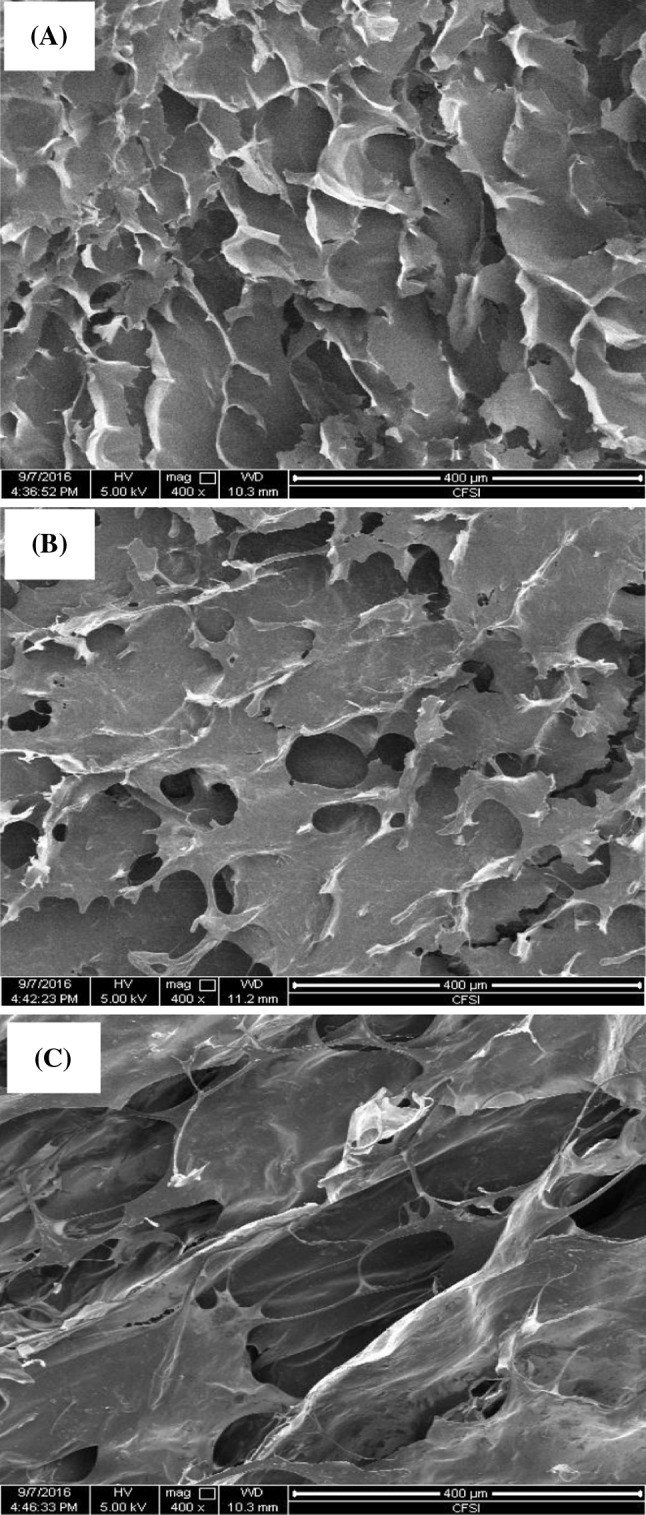
Scanning electron microscope images taken at the cut-surface of a YS gel (5.2%, w/w) (A), an AP gel (0.8%, w/w) (B), and a YA-composite gel (5.2%/0.8%, w/w) (C) respectively, ×400. YS yam starch, AP Auricularia auricula-judae polysaccharide, YA yam starch, and A. auricula-judae polysaccharide composite
Acknowledgements
This research was supported by grants from the open technology program of GWNU Leaders in Industry-university Cooperation (GWNU LINC) of Ministry of Education of Korea, the project of Overseas Talent of Education Department of Heilongjiang Province of China (1253HQ010), and Fostering Research of Heilongjiang Bayi Agricultural University (XZR2016-08).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.BeMiller JN. Pasting, paste, and gel properties of starch–hydrocolloid combinations. Carbohyd. Polym. 2011;86:386–423. doi: 10.1016/j.carbpol.2011.05.064. [DOI] [Google Scholar]
- 2.Kim HS, BeMiller JN. Effects of hydrocolloids on the pasting and paste properties of commercial pea starch. Carbohyd. Polym. 2012;88:1164–1171. doi: 10.1016/j.carbpol.2012.01.060. [DOI] [Google Scholar]
- 3.Raguzzoni JC, Delgadillo I, Silva JAL. Influence of a cationic polysaccharide on starch functionality. Carbohyd. Polym. 2016;150:369–377. doi: 10.1016/j.carbpol.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Freitas RA, Woehl MA, Kaminski GAT, Silva R, Sierakowski MR. Rheological description of the interaction of xyloglucan and starches: effect of the amylose content in starches. CYTA-J. Food. 2015;13:235–242. doi: 10.1080/19476337.2014.946093. [DOI] [Google Scholar]
- 5.Choi HM, Yoo BS. Steady and dynamic shear rheology of sweet potato starch–xanthan gum mixtures. Food Chem. 2009;116:638–643. doi: 10.1016/j.foodchem.2009.02.076. [DOI] [Google Scholar]
- 6.Gil BJ, Yoo BS. Effect of salt addition on gelatinization and rheological properties of sweet potato starch–xanthan gum mixture. Starch/Stärke. 2015;67:117–123. doi: 10.1002/star.201400103. [DOI] [Google Scholar]
- 7.Samutsri W, Suphantharika M. Effect of salts on pasting, thermal, and rheological properties of rice starch in the presence of non-ionic and ionic hydrocolloids. Carbohyd. Polym. 2012;87:1559–1568. doi: 10.1016/j.carbpol.2011.09.055. [DOI] [Google Scholar]
- 8.Liang Q, Zhang SQ, Zhang JS. Rheological behavior and microstructure of Oviductus ranae hydrogels. Food Sci. Biotechnol. 2012;21:467–474. doi: 10.1007/s10068-012-0059-4. [DOI] [Google Scholar]
- 9.Li X, Wang Z, Wang L, Walid E, Zhang H. In vitro antioxidant and anti-proliferation activities of polysaccharides from various extracts of different mushrooms. Int. J. Mol. Sci. 2012;13:5801–5817. doi: 10.3390/ijms13055801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Z, Wang J, Zhang L, Zhang Y, Ding K. Evaluation of water soluble β-D-glucan from Auricularia auricular-judae as potential anti-tumor agent. Carbohyd. Polym. 2010;80:977–983. doi: 10.1016/j.carbpol.2010.01.015. [DOI] [Google Scholar]
- 11.Yuan Z, He P, Cui J, Takeuchi H. Hypoglycemic effect of water-soluble polysaccharide from Auricularia auricula-judae Quel. on genetically diabetic KK-Ay mice. Biosci. Biotechnol. Biochem. 1998;62:1898–1903. doi: 10.1271/bbb.62.1898. [DOI] [PubMed] [Google Scholar]
- 12.Xu S, Xu X, Zhang L. Branching structure and chain conformation of water-soluble glucan extracted from Auricularia auricula-judae. J. Agric. Food Chem. 2012;60:3498–3506. doi: 10.1021/jf300423z. [DOI] [PubMed] [Google Scholar]
- 13.Bao H, You SG, Cao L, Zhou R, Wang Q, Cui SW. Chemical and rheological properties of polysaccharides from fruit body of Auricularia auricula-judae. Food Hydrocolloid. 2016;57:30–37. doi: 10.1016/j.foodhyd.2015.12.031. [DOI] [Google Scholar]
- 14.Asp NG. Classification and methodology of food carbohydrates as related to nutritional effects. Am. J. Clin. Nutr. 1995;61:930s–937s. doi: 10.1093/ajcn/61.4.930S. [DOI] [PubMed] [Google Scholar]
- 15.Zhu F. Isolation, composition, structure, properties, modifications, and uses of yam starch. Compr. Rev. Food Sci. Food Saf. 2015;14:357–386. doi: 10.1111/1541-4337.12134. [DOI] [PubMed] [Google Scholar]
- 16.Jayakody L, Hoover R, Liu Q, Donner E. Studies on tuber starches. II. Molecular structure, composition and physicochemical properties of yam (Dioscorea sp.) starches grown in Sri Lanka. Carbohyd. Polym. 2007;69:148–163. doi: 10.1016/j.carbpol.2006.09.024. [DOI] [Google Scholar]
- 17.Tischer PCSF, Noseda MD, Freitas RA, Sierakowski MR, Duarte MER. Effects of iota-carrageenan on the rheological properties of starches. Carbohyd. Polym. 2006;65:49–57. doi: 10.1016/j.carbpol.2005.12.027. [DOI] [Google Scholar]
- 18.Jiang Q, Gao W, Shi Y, Li X, Wang H, Huang L, Xiao P. Physicochemical properties and in vitro digestion of starches from different Dioscorea plants. Food Hydrocolloid. 2013;32:432–439. doi: 10.1016/j.foodhyd.2013.02.001. [DOI] [Google Scholar]
- 19.Huang H, Jiang Q, Chen Y, Li X, Mao X, Chen X, Huang L, Gao W. Preparation, Physico-chemical characterization and biological activities of two modified starches from yam (Dioscorea opposita Thunb.) Food Hydrocolloid. 2016;55:244–253. doi: 10.1016/j.foodhyd.2015.11.016. [DOI] [Google Scholar]
- 20.Dickinson E. Colloid science of mixed ingredients. Soft Matter. 2006;2:642–652. doi: 10.1039/b605670a. [DOI] [PubMed] [Google Scholar]
- 21.AACC. Approved method of the AACC. 11th ed. Method 76-13.01. American Association of Cereal Chemist, St. Paul, MN, USA (2010)
- 22.Williams PC, Kuzina FD, Hlynka I. A rapid colorimetric procedure for estimating the amylose content of starches and flours. Cereal Chem. 1970;47:411–421. [Google Scholar]
- 23.Barnes HA, Hutton JF, Walters K. An introduction to rheology. New York: Elsevier Applied Science Press; 1989. pp. 11–35. [Google Scholar]
- 24.Yeh A, Chan TY, Chuang GCC. Effect of water content and mucilage on physico-chemical characteristics of yam (Dioscorea alata Purpurea) starch. J. Food Eng. 2009;95:106–114. doi: 10.1016/j.jfoodeng.2009.04.014. [DOI] [Google Scholar]
- 25.Huang CC, Lai P, Chen IH, Liu YF, Wang CCR. Effects of mucilage on the thermal and pasting properties of yam, taro, and sweet potato starches. LWT-Food Sci. Technol. 2010;43:849–855. doi: 10.1016/j.lwt.2009.11.009. [DOI] [Google Scholar]
- 26.Mali S, Ferrero C, Redigonda V, Beleia AP, Grossmann MVE, Zaritzky NE. Influence of pH and hydrocolloids addition on yam (Dioscorea alata) starch pastes stability. LWT-Food Sci. Technol. 2003;36:475–481. doi: 10.1016/S0023-6438(03)00043-4. [DOI] [Google Scholar]
- 27.Alloncle M, Doublier JL. Viscoelastic properties of maize starch/hydrocolloid pastes and gels. Food Hydrocolloid. 1991;5:455–467. doi: 10.1016/S0268-005X(09)80104-5. [DOI] [Google Scholar]
- 28.Sajjan SU, Rao MRR. Effect of hydrocolloids on the rheological properties of wheat starch. Carbohyd. Polym. 1987;7:395–402. doi: 10.1016/0144-8617(87)90005-1. [DOI] [Google Scholar]
- 29.Nadiha MZN, Fazilah A, Bhat R, Karim AA. Comparative susceptibilities of sago, potato and corn starches to alkali treatment. Food Chem. 2010;121:1053–1059. doi: 10.1016/j.foodchem.2010.01.048. [DOI] [Google Scholar]
- 30.Rochefor WE, Middleman S. Rheology of xanthan gum: salt, temperature, and strain effects in oscillatory and steady shear experiments. J Rheol. 1987;31:337–340. doi: 10.1122/1.549953. [DOI] [Google Scholar]
- 31.Li Q, Zhang L, Ye Y, Gao Q. Effect of salts on the gelatinization process of Chinese yam (Dioscorea opposita) starch with digital image analysis method. Food Hydrocolloid. 2015;51:468–475. doi: 10.1016/j.foodhyd.2015.05.045. [DOI] [Google Scholar]
- 32.Sripa P, Tongraar A, Kerdcharoen T. “Structure-making” ability of Na+ in dilute aqueous solution: an ONIOM-XS MD simulation study. J. Phys. Chem. A. 2013;117:1826–1833. doi: 10.1021/jp312230g. [DOI] [PubMed] [Google Scholar]
- 33.Singh A, Gevekea DJ, Yadav MP. Improvement of rheological, thermal and functional properties of tapioca starch by using gum arabic. LWT-Food Sci. Technol. 2017;80:155–162. doi: 10.1016/j.lwt.2016.07.059. [DOI] [Google Scholar]
- 34.Lapasin R, Lorenzi L, Pricl S, Torriano G. Flow properties of hydroxypropyl guar gum and its long-chain hydrophobic derivatives. Carbohyd. Polym. 1995;28:195–202. doi: 10.1016/0144-8617(95)00134-4. [DOI] [Google Scholar]
- 35.Ring SG. Some studies on starch gelation. Starch/Stärke. 1985;37:80–83. [Google Scholar]