Abstract
Ne-(2-furoylmethyl)-l-lysine (furosine) is well-known indicator of early stage of Maillard reaction in processed food. Yet the toxicological aspects associated with its exposure remain rarely studied. Here, we investigated the effects of furosine exposure on cell viability, DNA damage, and its mutagenic potential by using MTT assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide), TUNEL assay (Terminal deoxynucleotidyl transferase mediated dUTP Nick End Labeling assay), and Ames assay techniques on human cell lines, i.e., liver HepG-2, kidney Hek-293, neuronal SK-N-SH, and intestinal Caco-2, respectively. Our results showed that kidney Hek-293 cell line was the most sensitive to furosine exposure as significant reduction in cell viability and induction of DNA damage were observed at 50 mg/L concentration. In contrast, intestinal Caco-2 cell lines showed resistance to furosine exposure as DNA damage was only observed at 800 mg/L concentration of furosine. Ames assay indicated that furosine has no mutagenic effects on TA 100 and TA 1535 strains. Hence, this study suggests that furosine is a strong toxicant for kidney cells.
Keywords: Cytotoxicity, DNA damage, Furosine, TUNEL assay, Genotoxicity, Risk assessment
Introduction
Advancement in heat treatment technology carries the advantage of ensured microbiological safety and enhanced shelf life of food products. However, thermal treatment and long-term storage result in Maillard reaction, which is a crucial index of food quality and leads to the formation of anti-nutritional or toxic Amadori compounds [1]. Thus, health-safety issues concerning to the consumption of Maillard reaction products have excited considerable research attention regarding to the role of MRPs in the pathogenesis of various degenerative diseases, such as diabetic complications [2], chronic renal failure [3], atherosclerosis [4], Alzheimer’s, and neurodegenerative diseases [5, 6]. Although a wide range of MRPs has been reported to be produced during the heat treatment of food, the most commonly studied Amadori products are Nε-(carboxymethyl) lysine (CML), acrylamide, furosine (ε-N-2-furoylmethyl-l-lysine), 5-hydroxymethyl furfural (5-HMF), pyrraline (5-hydroxymethyl-1-neopentylpyrole-2-carbaldehyde), and pentosidine [7–9]. Moreover, accumulating studies have demonstrated the toxicity of MRPs; for example, hydroxymethyl furfural (Amadori product) has been reported to cause genotoxic effects in human heptoma HepG-2 cell lines [10] and CML (N (epsilon)-carboxymethyllysine) and FL (N (epsilon)-fructoselysine) has been reported to affect kidney cells [11]. Acrylamide intake is known to be associated with tumor formation [12]. However, there is scarcity of data for determining the toxicity of furosine.
Nevertheless, N-(2-furoylmethyl)-l-lysine (furosine) is a stable derivative of the Amadori compound [13–15] and acknowledged as the most sensitive and reliable marker of heat-induced lysine damage in the early stage of Maillard reaction [16, 17]. Heyns et al. [18] and Finot et al. [19] were the first to report the structure of compound “x”, which possesses close similarity with e-N-(2 furoylmethyl)-l-lysine, and named it furosine [9]. Although Finot et al. also identified another Maillard reaction product (MRP) named pyridosine [20], it did not reach the same importance as an indicator of the nutritional value of a heat-treated protein as furosine did [9]. Since then, furosine has been extensively applied for technological process control and nutritional evaluation, particularly for dairy products [20–22], as it allows the estimation of unavailable lysine (blocked lysine), which further determines the available lysine and/or percent of lysine loss [16]. Furosine is also an indicator of the loss of nutritional value of food products as it causes the destruction of vitamins, loss of essential amino acids, and reduction in digestibility of protein, which can be quantified by estimating the ratio of reactive and blocked lysine concentration [23].
The presence of furosine has been detected in various food items, such as dairy products, infant formulas, cereals, honey, baby cereal, tomato products, and bakery products [21–27]. Infant formula and honey have been reported to contain the highest furosine concentration ranges of 471.9–639.5 mg/100 g and 10.0 g/kg, respectively [1, 27]. Despite these evidences, no experimental data have yet been reported to determine the cytotoxic or genotoxic potential of furosine.
The present study aims to investigate the toxicity of furosine and the health risk associated with its exposure. Here, toxicity of furosine was determined by estimating the cell viability, DNA damage, and mutagenic responses in cultured human cell lines such as kidney (HEK-293), neuronal (SK-N-SH), hepatic (Hep-G2), and intestinal (Caco-2) cell lines. Hence, this study is not only a major contribution to assess the risk associated with furosine exposure but it also appeals to improve the existing heat treatment standards for development of safe food products.
Materials and methods
Chemicals
Furosine (CAS 19746-33-9) of analytical standards was purchased from Polypeptide laboratories (Strasbourg, France). 3-(4,5-Dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) (CAS 298-93-1), dimethyl sulfoxide (DMSO) (CAS-67-68-5), and positive control 4-nitroquinoline-N-oxide (CAS-56-57-5) were obtained from Sigma-Aldrich Chemical Corp. (St. Louis, MO, USA).
Cell culture media and its supplements such as glutamine, penicillin/streptomycin, and fetal bovine serum were purchased from Gibco Life Technologies (Grand Island, New York, USA).
One Step TUNEL Apoptosis Assay Kit (C-1088) was purchased from Beyotime, Shanghai, China. Culture flasks were purchased from Corning Costar (Cambridge, MA, USA).
Cell culture
Human epithelial kidney HEK-293 cell
HEK293 (human epithelial kidney cell line) with adherent pattern was purchased from American-Type Culture Collection Cells (ATCC). Cells were cultured in DMEM with 8% FBS, 1.8% Pen/Strep, and 0.9% l-glutamine at 37 °C with 5% CO2. Cells in passage 10–14 were used in the experiments.
Human hepato-cellular HepG2 cells
Hepato-cellular HepG2 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with Gluta MAX, 10% heat-inactivated fetal bovine serum, and 100 U/mL penicillin/100 mg/mL streptomycin, and the cells were maintained and cultured in a humidified environment at 37 °C with 5% CO2.
Human neuroblastoma SK-N-SH cell line
A human neuroblastoma cell line (SK-N-SH) was maintained in DMEM supplemented with 10% heat-inactivated fetal bovine serum, 1% antibiotics (penicillin/streptomycin/neomycin), and 1% non-essential amino acids. Cells were grown at 37 °C with 5% CO2 in a humidified atmosphere.
Human intestinal epithelial (Caco-2) cells
Caco-2 (human colon carcinoma-derived cell line) was obtained from ATCC. Cells were maintained in DMEM supplemented with 8% heat-inactivated fetal bovine serum (GIBCO; Sweden), 1.8% penicillin/streptomycin (Pen/Strep), and 0.9% l-glutamine. Cells in passage 86–91 were used in the following experiments.
Sample preparation
Furosine-spiked culture medium was used in this study as this method has been applied previously by Delgado-Andrade [28]. All cell lines were grown with their respective standard medium at 37 °C (5% CO2, 95% air). After achieving 80% confluence, cells were washed with 1× PBS and exposed to DMEM spiked with different concentrations of furosine, i.e., 50, 100, 150, 200, 300, 400, 600, 800, and 1000 mg/L, for 24 h. Finally, cells were maintained by incubating at 37 °C with 5% CO2 in 95% air.
Cytotoxic evaluation of furosine
Colorimetric MTT assay was applied to investigate the cytotoxicity of furosine. Stock solution of MTT (Sigma-Aldrich) was prepared in PBS buffer (5 mg/mL) and stored at 4 °C, and further diluted by serum-free DMEM from the stock solution to 1 mg/mL before use. Cell lines were maintained in a 96-well plate at an initial density of 4 × 104 cells with 100 μL of medium in each well. After 24 h, cells were exposed to the medium with different concentrations of furosine ranging from 0 to 2000 mg/L in a total volume of 200 μL for further 24 h. After furosine treatment, the medium was removed and cells were exposed to 200 μL MTT solution in each well and incubated at 37 °C for 4 h. Then, 200 μL of dimethyl sulfoxide was added to each well and the plates were shaken for 5 min to dissolve the crystals. The final optical density was determined using a microplate reader at a wavelength of 450 nm.
DNA damage analysis by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) method
DNA damage was investigated by the TUNEL method by labeling the 3′-end of fragmented DNA of the apoptotic cells after treatment with furosine. For this purpose, “One Step TUNEL Apoptosis Assay Kit” (Millipore, USA) was used. All cell lines (used in present study) were treated with different concentrations of furosine for 24 h and fixed with 4% paraformaldehyde in PBS, and then, rinsed with PBS; 0.1% Triton X-100 was used to permeabilize cells according to the manufacturer’s instructions. The FITC-labeled (fluorescein isothiocyanate) TUNEL-positive cells were observed under a fluorescent microscope by using 488- and 530-nm emission.
Mutagenic assessment by Ames assay
The Ames test was performed by the plate incorporation method (by pre-incubation) with or without metabolic activation by using two histidine-dependent auxotrophic mutants of S. typhimurium strains: TA-100 and TA-1535 [29]. Additionally, tryptophan-dependent auxotrophic mutant Escherichia coli WP2uvrapKM-101 was used in this assay [30]. Selected strains were culture in a liquid broth medium at 37 °C under constant agitation for 10 h. After incubation, all bacterial strains were exposed to 0.5 mL of S9 mix (the presence of metabolic activation) or 0.5 mL of 0.1 M sodium phosphate buffer (pH 7.4) (the absence of metabolic activation) and to 50–1000 mg/L furosine solutions in a test tube and pre-incubated for 1 h at 37 °C. After that, 3 mL of semi-liquid agar was added to the mixture, and then, transferred to a minimal glucose agar plate. For S. typhimurium, 10 mL of 0.5 mM histidine/biotin solution per 100 mL agar was added to the top agar and mutations to histidine independence were scored on minimal glucose agar plates. In contrast, for E. coli strain, mutants to tryptophan independence were counted on minimal glucose agar plates, which were supplemented with 10 mL of 0.5 mM tryptophan per 100 mL agar. After this treatment, the plates were incubated at 37 °C for 48 h and the number of revertant colonies was scored. We repeated this experiment three times by using seven concentrations of furosine while using 4-nitroquinoline-N-oxide as positive control. Here, induction factors (IFs) represent the mutagenic potential and are expressed as multiples of the background levels. According to Ames assay, the test compound is considered as mutagenic if the numbers of His+ revertant colonies reach at least twice the value of the corresponding control (induction factor >2).
Statistical analysis
To determine the statistical significance, each measurement was replicated at least three times. Values are expressed as mean ± SD; one-way (ANOVA) analysis of variance was applied by the using Tukey test for comparison of mean values to exhibit significant variation (p < 0.05) for Fig. 1. For Fig. 3, multiple means comparisons were performed with the Duncan’s multiple range test. Statistically significant differences were identified when p < 0.05. Analyses were performed using Graph Pad Prism 5 software.
Fig. 1.
Cell survival rates detected by the MTT assay. Cell survival rates of HepG-2 (A), Hek293 (B), SK-N-SH (C), and Caco-2 (D) cells assayed at 24 h after exposure to furosine at various concentrations, i.e., 0, 50, 100, 200, 300, 400, 600, 800, and 1000 mg/L for Hek293, HepG-2, and SK-N-SH cells. Concentration ranges from 200 to 2000 mg/L of furosine were exposed to Caco-2 cells. Data obtained are the mean ± SD of triplicate determinations by the Tukey test for comparison of mean value to exhibit significant variation (p < 0.05). Values with symbols (*) within samples are significantly different at *p < 0.05; **p < 0.01, and ***p < 0.001 with respective negative control, while ns non-significant value
Fig. 3.
Quantitative analysis of TUNEL-positive cell content in representative cell lines. Data are mean ± SD of triplicate determinations. Values with different letters within samples are significantly different at p < 0.05 by Duncan’s multiple range test. Values with symbols (*) within samples are significantly different at *p < 0.05; **p < 0.01; ***p < 0.001 with respective negative control, while ns non-significant value
Results and discussion
Susceptibility of various cell lines to furosine
In this part of the study, Hek-293, HepG-2, and SK-N-SH cell lines were exposed to furosine in concentrations ranging from 50 to 1000 mg/L for 24 h. Caco-2 cells were exposed to furosine concentration ranging from 200 to 2000 mg/L. We found that reduction in viability of Hek-293 and HepG-2 cells was observed after exposure to furosine at 50 mg/L (p < 0.05) (Fig. 1A, B). However, significant reduction in cell survival of Hep-G2 and Hek-293 was observed at a dose range of 50–200 mg/L of furosine (p < 0.05 for 50 mg/L, p < 0.01 for 150 mg/L, and p < 0.001 for 200–1000 mg/L). Susceptibility of SK-N-SH to furosine exposure began to appear at 100 mg/L (p < 0.05), which significantly increased (p < 0.01) at 150 mg/L and (p < 0.001) at 200–1000 mg/L of furosine concentration (Fig. 1C). Nevertheless, Caco-2 cells exhibited resistance to furosine exposure from 200 to 400 mg/L. However, significant reduction in the survival of Caco-2 cells was detected for concentrations ranging from 600 to 1200 mg/L (p < 0.05 for 600 mg/L, p < 0.01 for 800 mg/L, and p < 0.001 for 1000–2000 mg/L) (Fig. 1D). Hence, these data reveal that furosine leads to cell death in a dose-dependent manner in cultured human cell lines. Noticeably, liver HepG-2 and kidney Hek-293 cells lines appear to be the potential targets of furosine toxicity as reduction in cell viability appears at a very low dose range of 50–100 mg/L of furosine (Fig. 1A, B). Accordingly, these results report the susceptibility of kidney cells to furosine exposure (Figs. 1, 3), which may support the previous studies which reported that furosine exposure results in renal dysfunction and hyperglycemia [5, 14, 31]. Moreover, reduction in the survival of neuronal SK-N-SH (Figs. 1C, 3C) suggests the association between furosine exposure and neuro-damaging effects. Unfortunately, due to uncertainties and lack of experimental data on genotoxicity of furosine, the standards for tolerable daily intake for furosine and for other heat-induced byproducts in food products have not been established [32].
Furosine exposure induced DNA damage in cell culture
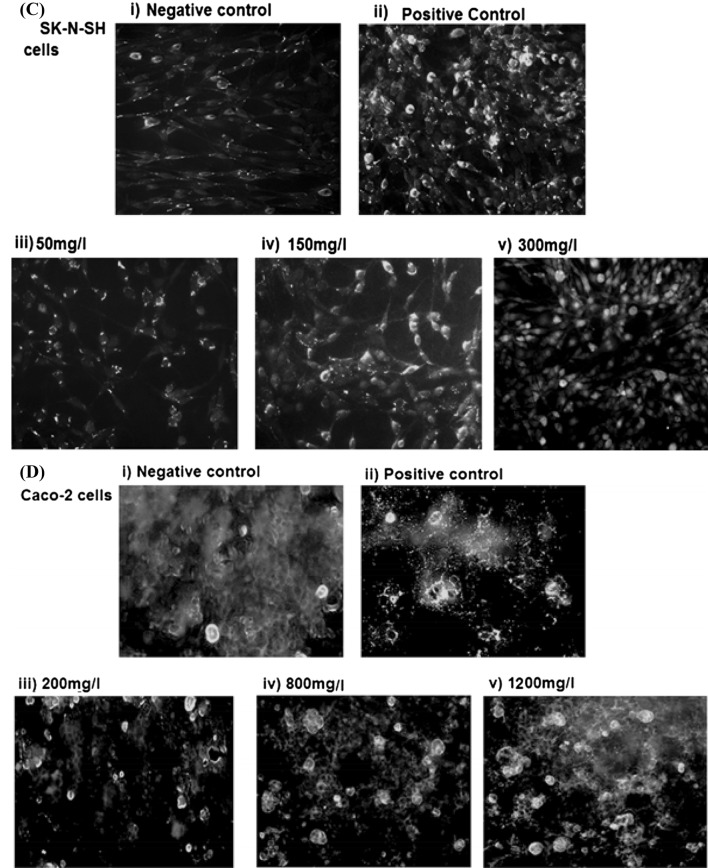
Furosine-induced DNA damage was investigated by TUNEL assay in all cells lines. Three cytotoxic concentrations of furosine were selected for each cell line: 50, 100, and 200 mg/L were selected for HEK-293 and HepG-2, while 50, 150, and 300 mg/L were selected for SK-N-SH cell lines and 200, 800, and 1200 mg/L for Caco-2 cells (Fig. 2). Results in Fig. 3 were calculated by counting approximately 1000 cells for TUNEL-positive cells in each experiment. Scored data depict that the number of TUNEL-positive cells increased with the dose of furosine (Fig. 3), i.e., Hek-293 and HepG-2 cells showed statistically significant (p < 0.001) increase in TUNEL-positive cells at 100 and 200 mg/L of furosine concentrations (Figs. 2A, B and 3), while DNA damage was significant (p < 0.001) for SK-N-SH cells at 150 and 300 mg/L (Figs. 2C and 3). We found that significant increase (p < 0.001) in the numbers of TUNEL-posit ive cells was observed in Caco-2 cells at 800 and 1200 mg/L of furosine concentrations (Figs. 2D, 3). Our data of TUNEL assay showed that (furosine induced) DNA damage was dose dependent in all cell lines (used in the present study). In accordance with the cell viability results, TUNEL assay results also demonstrated that DNA damage was more prominent in kidney HEK-293 cells after furosine exposure, even at low concentrations such 50 mg/L. Thus, the present study is the pioneer evidence for sensitivity of kidney cells to furosine, which can be explained by previously reported studies which determined that the kidney plays central role in the regulation of the metabolism and disposal of Maillard reaction products, which make it the most sensitive organ to MRP exposure and ultimately lead to direct and indirect toxicity in renal failure [33]. Here, we may infer that kidney is the potential target for furosine exposure, which appeals an urgent need to evaluate the role of furosine in renal function. Moreover, young children can be at maximum risk for exposure to furosine due to the presence of the highest furosine level in infant formula, which ranges from 471.9 to 639.5 mg/100 g [1].
Fig. 2.
(A–D) (i–v) Representative images of TUNEL staining of (A) Hek-293, (B) HepG-2, (C) SK-N-SH, (D) Caco-2 cells after exposure to different concentrations of furosine for 24 h, after which TUNEL staining and fluorescent imaging were performed. The percentage of TUNEL-positive cells (bright green) was calculated as the ratio of the number of TUNEL-positive cells to the total number of (A) Hek-293, (B) HepG-2, (C) SK-N-SH, (D) Caco-2 cells. (Color figure online)
DNA damage in neuronal SK-N-SH (Figs. 1C, 3C) suggests the possibility of furosine as a neurotoxic agent, which may confirm the preceding studies that demonstrated the association between MRPs and neurodegenerative disorders [5, 34, 35]. Yet more comprehensive studies are required for a complete understanding of the underlying mechanism for cytotoxic effects of furosine.
Furosine exhibits no mutagenic activity by Ames test
Mutagenic potential of furosine was investigated by Ames test (with/without exogenous metabolic activation) after exposure to the different concentrations of furosine presented in Table 1. According to classically set standard for Ames assay, the test compound was considered positive if the number of His+ revertant colonies increased by at least two times the number of colonies in respective control (induction factor >2) and its quotient ranges for induction factor were approximately 1.7 and 1.9 (depends on exposure of dose). However, in our study, no mutagenic effects of furosine were observed, as the quotient range did not exceed 1.6 and remained “1” or lower for all exposed dose ranges of furosine (used in present study). Our data showed that furosine did not induce mutation in S. typhimurium TA 100 and TA 1535 strains under both condition, i.e., with or without exogenous activation as none of the results of the Ames test (+S9 or −S9) surpass the standard value of 2.0 in comparison to respective control (Table 1).
Table 1.
Ames test with the strains TA 100, TA 1535, and E. coli WP2 uvrapKM101 exposed to seven concentrations of furosine tested with or without the exogenous activation system
| Sample | TA1535 | TA100 | E. coli W2, uvra, pKM 101 | |||
|---|---|---|---|---|---|---|
| −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | |
| Mean ± SD of revertants in negative control | 37 | 45 | 90 | 110 | 210 | 230 |
| Induction ratio | ||||||
| Negative control | 1 | 1 | 1 | 1 | 1 | 1 |
| 50 mg/L | 0.6 | 0.69 | 0.7 | 0.8 | 1.1 | 1.0 |
| 100 mg/L | 0.71 | 0.78 | 1 | 1.1 | 0.9 | 1.0 |
| 200 mg/L | 0.74 | 0.88 | 0.9 | 1.1 | 0.9 | 1.0 |
| 400 mg/L | 0.76 | 1.33 | 0.8 | 0.9 | 1.1 | 1.1 |
| 600 mg/L | 0.88 | 1.49 | 0.8 | 1.2 | 1.0 | 1.1 |
| 800 mg/L | 0.77 | 0.99 | 0.7 | 1.3 | 1.0 | 1.1 |
| 1000 mg/L | 0.99 | 1.52 | 1.2 | 1.5 | 1.0 | 1.0 |
| Positive control (NQNO) | 2.61 | 2.72 | 3.54 | 4.1 | 3.81 | 4.5 |
Note: Results are expressed as induction factors (i.e., multiple of negative control) [the whole test is considered valid if the positive controls reach an induction ratio (IR) of ≥2]. A sample dilution is considered genotoxic if the IR ≥ 1.5 and the growth factor ≥0.5
The present study reveals that furosine poses no mutagenic effect on TA1535 and TA1002 strains with and without metabolic activation as compared to positive control (Table 1). One possible interpretation for these findings is that exposure to a toxic agent results in DNA damage, which either leads to apoptotic cell death (removal of damaged cells) or may cause mutation, which leads to carcinogenesis [36]. These results reveal that the major mechanism of furosine toxicity is DNA damage leading to cell death (by apoptosis) rather than mutagenicity.
Finally, to the best of our knowledge, the present study comprises the first approach to determine the dose response relationships and toxic effects of furosine in in vitro cell lines. Our data depict that furosine is a strong genotoxic agent to kidney Hek-293 and liver HepG-2 cell lines, where it induces significant DNA damage and cell death even at low concentrations. However, there is an urgent need to evaluate the role of furosine exposure in vivo, particularly on renal function, to determine whether these toxic effects continue after furosine is absorbed into the circulation.
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (31501399), special fund for Agro-Scientific research in the public interest (201403071), Project of Risk Assessment on raw milk (GJFP2016008), and Agriculture Science and Technology Innovation Program (ASTIP-IAS12).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Troise AD, Fiore A, Wiltafsky M, Fogliano V. Quantification of Nε- (2- Furoylmethyl)- L- lysine (furosine), Nε- (Carboxymethyl)- L- lysine (CML), Nε- (Carboxyethyl)- L- lysine (CEL) and total lysine through stable isotope dilution assay and tandem mass spectrometry. Food Chem. 2015;188:357–364. doi: 10.1016/j.foodchem.2015.04.137. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed N. Advanced glycation endproducts role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Hartog JW, Smit AJ, van Son WJ, Navis G, Gans RO, Wolffenbuttel BH, de Jong PE. Advanced glycaion end products in kidney transplant dysfunction. Am. J. Kidney Dis. 2004;43:966–975. doi: 10.1053/j.ajkd.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardio vasc. Res. 2004;63:582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi M, Yamagishi SI. Possible involvement of advanced glycation end products (AGEs) in the pathogenesis of Alzheimer’s disease. Curr. Pharm. Des. 2008;14:973–978. doi: 10.2174/138161208784139693. [DOI] [PubMed] [Google Scholar]
- 6.Vitek MP, Bhattacharya K, Glendening JM, Stopa E, Vlassara H, Bucala R, Manogue K, Cerami A. Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc. Natl. Acad. Sci. 1994;91:4766–4770. doi: 10.1073/pnas.91.11.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delgado Andrade C, Seiquer I, Navarro MP. Comparative effects of glucose-lysine versus glucose methionine Maillard reaction products consumption: in vitro and in vivo calcium availability. Mol. Nutr. Food Res. 2005;49:679–684. doi: 10.1002/mnfr.200400100. [DOI] [PubMed] [Google Scholar]
- 8.Yaylayan VA, Huyghues Despointes A. Chemistry of amadori products: analysis, kinetics, reactions and spectroscopic properties. Crit. Rev. Food Sci. Nutr. 1994;34:321–369. doi: 10.1080/10408399409527667. [DOI] [PubMed] [Google Scholar]
- 9.Erbersdobler HF, Somoza V. Forty years of furosine forty years of using Maillard reaction products as indicators of the nutritional quality of foods. Mol. Nutr. Food Res. 2007;51:423–430. doi: 10.1002/mnfr.200600154. [DOI] [PubMed] [Google Scholar]
- 10.Severin I, Dumont C, Jondeau Cabaton A, Graillot V, Chagnon MC. Genotoxic activities of the food contaminant 5- hydroxymethylfurfural using different in vitro bioassays. Toxicol. Lett. 2010;192:189–194. doi: 10.1016/j.toxlet.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 11.Somoza V, Wenzel E, Weiss C, Clawin-Rädecker I, Grübel N, Erbersdobler HF. Dose-dependent utilisation of casein-linked lysinoalanine, N-(epsilon)-fructoselysine and N(epsilon)-carboxymethyllysine in rats. Mol. Nutr. Food Res. 2006;50:833–841. doi: 10.1002/mnfr.200600021. [DOI] [PubMed] [Google Scholar]
- 12.Capuano E, Fogliano V. Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT - food science and technology. 2011;44:793–810. doi: 10.1016/j.lwt.2010.11.002. [DOI] [Google Scholar]
- 13.Hartkopf J, Erbersdobler HF. Stability of furosine during ion-exchange chromatography in comparison with reversephase HPLC. J. Chromatogr. 1993;635:151–154. doi: 10.1016/0021-9673(93)83126-D. [DOI] [Google Scholar]
- 14.Henle T, Zehtner G, Klostermeyer H. Fast and sensitive determination of furosine in food. Z. Lebensm. Unters. Forsch. 1995;200:235–237. doi: 10.1007/BF01190503. [DOI] [PubMed] [Google Scholar]
- 15.Resmini P, Pellegrino L, Battelli G. Accurate quantification of furosine in milk and dairy products by a direct HPLC method. Ital. J. Food Sci. 1990;3:173–183. [Google Scholar]
- 16.Erbersdobler HF. Protein reactions during food processing and storage- their relevance to human nutrition. Bibl. Nutr. Dieta. 1989;43:140–150. doi: 10.1159/000416698. [DOI] [PubMed] [Google Scholar]
- 17.Arnoldi A, Resta D, Brambilla F, Boschin G, D’Agostina A, Sirtori E, O’Kane F. Parameters for the evaluation of the thermal damage and nutraceutical potential of lupin based ingredients and food products. Mol. Nutr. Food Res. 2007;51:431–436. doi: 10.1002/mnfr.200600246. [DOI] [PubMed] [Google Scholar]
- 18.Heyns K, Heukeshoven J, Brose KH. Degradation of fructose amino acids to N-(2-furoylmethyle) amino acids- intermediates in browning reaction. Angew Chem. Int. Ed. Engl. 1968;7:628–629. doi: 10.1002/anie.196806281. [DOI] [PubMed] [Google Scholar]
- 19.Finot PA, Bricout J, Viani R, Mauron J. identification of a new lysine derivative obtained upon acid hydrolysis of heated milk. Experientia. 1968;24:1097–1099. doi: 10.1007/BF02147778. [DOI] [PubMed] [Google Scholar]
- 20.Finot PA, Viani R, Bricout J, Mauron J. Detection and identification of pyridosine, a second derivative obtained upon acid hydrolysis of heated milk. Experientia. 1969;25:134–135. doi: 10.1007/BF01899081. [DOI] [PubMed] [Google Scholar]
- 21.Erbersdobler HF, Zucker H. Untersuchungen zum Gehalt an Lysin und verf_gbarem Lysin in Trockenmagermilch. Milchwiss. 1966;21:564–568. [Google Scholar]
- 22.Br-ggemann J, Erbersdobler HF. Fructoselysin als wichtigstes Reaktionsprodukt von Lysin mit Glucose bei Hitzesch digung von Lebens und Futtermitteln. Z. Lebensm. Unters. Forsch. 1968;137:137–143. doi: 10.1007/BF01460693. [DOI] [Google Scholar]
- 23.Nicoletti I, Cogliandro E, Corradini C, Corradini D. Analysis of epsilon-N-2-furoylmethyl-l-lysine (furosine in concentrated milk by reversed phase chromatography with a microbore column. J. Liq. Chromatogr. Relat. Technol. 1997;20:719–729. doi: 10.1080/10826079708014137. [DOI] [Google Scholar]
- 24.Villamiel M, del Castillo MD, Corzo N, Olano A. Presence of furosine in honeys. J. Sci. Food Agric. 2001;81(8):790–793. doi: 10.1002/jsfa.874. [DOI] [Google Scholar]
- 25.Rada-Mendoza M, Olano A, Rada Villamiel M, Mendoza M, Olano A, Villamiel M. Furosine as indicator of maillard reaction in jams and fruit-based infant foods. J. Agric. Food Chem. 2002;50:4141–4145. doi: 10.1021/jf0201024. [DOI] [PubMed] [Google Scholar]
- 26.Rajchl A, Cizkova H, Voldrich M, Jiruskova M, Sevcik R. Evaluation of shelf life and heat treatment of tomato products. Czech j. food sci. 2009;27:130–133. [Google Scholar]
- 27.Seiquer I, Diaz Alguacil J, Delgado-Andrade C, Lopez-Frias M, Munoz Hoyos A, Galdo G, Navarro MP. Diets rich in maillard reaction products affect protein digestibility in adolescent males aged 11-14 y. Am. J. Clin. Nutr. 2006;83(5):1082–1088. doi: 10.1093/ajcn/83.5.1082. [DOI] [PubMed] [Google Scholar]
- 28.Delgado-Andrade C, Seiquer I, Navarro MP, Morales FJ. Estimation of hydroxyethylfurfural availability in breakfast cereals. Studies in Caco-2 cells. Food Chem. Toxicol. 2008;46(5):1600–1607. doi: 10.1016/j.fct.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Maron DM, Ames BM. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 30.Brusick DJ, Simmon VF, Rosenkranz HS, Ray VA, Stafford RS. An evaluation of the Escherichia coli WP2 and WP2 uvrA reverse mutation assay. Mutat. Res. 1980;7:169–190. doi: 10.1016/0165-1110(80)90009-3. [DOI] [PubMed] [Google Scholar]
- 31.Kumano K, Yokota S, Sakai T, Kobayashi N, Yoshida A, Yoshihara T, Shibata K, Izumi G, Wang H. Kinetic analysis of furosine and pentosidine in CAPD patients. Adv. Perit. Dial. 1999;13:53–57. [PubMed] [Google Scholar]
- 32.Abraham K, Gürtler R, Berg K, Heinemeyer G, Lampen A, Appel KE. Toxicology and risk assessment of 5-Hydroxymethyl furfural in food. Mol. Nutr. Food Res. 2011;55:667–678. doi: 10.1002/mnfr.201000564. [DOI] [PubMed] [Google Scholar]
- 33.Gugliucci A, Bendayan M. Renal fate of circulating advanced glycation end products (AGEs): evidence for re-absorption and catabolism of AGE-peptides by the proximal tubular cells. Diabetologia. 1996;39:149–160. doi: 10.1007/BF00403957. [DOI] [PubMed] [Google Scholar]
- 34.Grillo MA, Colombatto S. Advanced glycation end products (AGEs): involvement in aging and in neurodegenerative diseases. Amino Acids. 2008;35:29–36. doi: 10.1007/s00726-007-0606-0. [DOI] [PubMed] [Google Scholar]
- 35.Yan SD, Chen X, Schmidt AM, Brett J, Godman G, Zou YS, Scott CW, Caputo C, Frappier T, Smith MA, Perry G, YENtt SH, Stern D. Glycated tau protein in Alzheimer disease: a mechanism for induction of oxidant stress. Proc. Natl. Acad. Sci. 1994;91:7787–7791. doi: 10.1073/pnas.91.16.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwak H, Lee M, Cho M. Interrelationship of apoptosis mutation and cell proliferation in N- methyl- N’- nitro-N- nitrosoguanidine (MNNG) induced medaka carcinogenesis model. Aquat. Toxicol. 2000;50:317–329. doi: 10.1016/S0166-445X(00)00093-X. [DOI] [PubMed] [Google Scholar]