Abstract
To improve the quality of modified atmosphere (60% CO2/15% O2/25% N2)-packaged or vacuum-packaged bigeye tuna (Thunnus obesus) chunks, an edible film containing whey protein isolates (WPI) were added. During storage at 2 °C, the samples coated with WPI prior to packaging exhibited slower microbial growth, thiobarbituric acid (TBA), and total volatile basic-nitrogen (TVB-N) values than did those without films. On comparing the two formulations of WPI with 4 and 8% (v/v) glycerol, it was observed that WPI containing 8% glycerol induced more severe weight loss but retarded the lipid oxidation more effectively. The usage of WPI films with 8% glycerol is proved to be helpful to enhance the effect of modified atmosphere packaging on the quality of tuna, as the samples (MAP-2) displayed the lowest bacterial counts (3.63 log CFU/g) and TBA (0.349 mg malondialdehyde (MDA)/kg) and TVB-N (12.94 mg N/100 g) contents.
Keywords: Tuna, Modified atmosphere packaging, Vacuum packaging, Whey protein isolates, Moisture
Introduction
Bigeye tuna (Thunnus obesus) is a commercially important fish and is widely consumed due to its high level of nutrients and meat quality. This fish is usually harvested for sashimi (fresh muscle) productions. Nevertheless, raw fish are highly perishable and apt to deteriorate easily due to microbial contamination and the autolytic enzymes in the tissues [1]. During this process, some mesophilic and psychrotolerant bacteria, including Morganella psychrotolerans and Photobacterium spp., can produce histamine by decarboxylating abundant free histidine in the fish muscle, which may lead to histamine fish poisoning (HFP) [2, 3]. Another problem with tuna is the discoloration that is caused by the autoxidation of myoglobin, which may lead to sensory unacceptability [4]. To maintain the freshness of tuna, the fish is usually kept at an ultralow temperature (<−55 °C) in large blocks for long-term storage. However, during the retail process, tuna cut into smaller chunks can more easily be displayed, sold, and delivered to customers. These chunks are usually kept at −2 to 4 °C, which cannot fully preserve its quality and safety because of a high HFP risk [5]. Investigations into the characteristics of aquatic products subjected to modified atmosphere packaging (MAP) and vacuum packaging (VP) have shown promising results [1, 4, 5]. However, these packaging treatments unexpectedly lead to more exudates and drip loss, thereby decreasing the sensory acceptability [6].
Edible coating films, which are primarily composed of polysaccharides, protein, and lipids, are beneficial for the preservation of aquatic products [7, 8]. The whey protein isolates (WPI) are most commonly used as protein-based films [9], which can not only work as desirable barriers but also serve as carriers of antimicrobials, antioxidants, or other nutriceuticals and have been successfully used for the preservation of seafood and other agricultural products [10, 11]. In this sense, the application of WPI films may provide new approaches to remove some of the deficiencies of MAP and VP. However, there is little information about how WPI films work with MAP or VP to promote the quality of tuna.
Hence, this study was performed to investigate the effect of WPI-based edible films incorporated with MAP or VP on the deterioration of tuna. Quality indicators, including microbial (total viable counts), physical (color, weight loss and texture), biochemical (met-myoglobin (met-Mb), thiobarbituric acid (TBA), and (total volatile basic-nitrogen) TVB-N), and sensory scores, were used to monitor the quality changes of tuna.
Materials and methods
Preparation of edible films
WPI (protein content > 92.0%, lactose content < 1.0%, fat content < 1.8%, and ash content < 3.5%) from Hilmar Ingredients (Hilmar, CA, USA) was dissolved in deionized water to a final concentration of 8% (w/v). The pH value of the base solution was adjusted to 8.0 using 1.0 mol/L NaOH, and the solution was incubated at 90 °C for 30 min. In order to investigate the effect of glycerol concentrations on the characteristics of the edible films, two formulations of film solution were prepared: (1) base solution containing 4% (v/v) glycerol and (2) base solution containing 8% (v/v) glycerol. The film solutions were filtered through a layer of gauze. The film formed after the solutions (15 mL) were poured into flat Plexiglas plates (9 cm diameter, SCRC, Shanghai, P. R. China) and dried at 22 ± 2 °C with a relative humidity of 56 ± 2% for 48 h. The thicknesses of dried films containing 4 and 8% (v/v) glycerol for use were measured as 0.169 ± 0.011 and 0.173 ± 0.015 mm (± standard error, n = 10), respectively, by a Digimatic Mitutoyo Micrometer (Mitutoyo Corporation, Kawasaki, Japan) to the nearest 0.001 mm.
Sample preparations
Tuna samples (T. obesus) from Zhejiang Fenghui Pelagic Fishery Co. (Hangzhou, China) were packaged in vacuum and stored at −55 °C. Upon arrival at the laboratory, the tuna block was cut into several small blocks (5 cm × 4 cm × 3 cm) with an average weight of 60 ± 5 g. Then, the samples were placed into polythene bags and stored at −55 °C until use.
The small blocks of tuna sample were tightly wrapped with precooled WPI films (2 °C) while squeezing air. Then, the filmed samples and un-filmed samples were randomly distributed in polyethylene/polyamide (PE/PA, Eno-Packaging Ltd., Shanghai, P. R. China) barrier pouches (13 cm × 19 cm) with 90 μm thickness and an O2 transmission of 10–20 mL/(m2 d) at 75% relative humidity (RH), 23–25 °C, and 1 atm pressure. The samples were then packaged in a modified atmosphere or vacuum. Six different groups were settled: (1) vacuum (VP-T), (2) vacuum plus WPI films containing 4% (v/v) glycerol (VP-1), (3) vacuum plus WPI films containing 8% (v/v) glycerol (VP-2), (4) MAP (MAP-T), (5) MAP plus WPI films containing 4% (v/v) glycerol (MAP-1), and (6) MAP plus WPI films containing 8% (v/v) glycerol (MAP-2).
For VP, air was exhausted and the pouches were sealed by a thermal sealing machine model DQB-360 W (Packing Machine factory of Qingpu, Shanghai, P. R. China). For MAP, the mixed gas was flushed into the pouches by a Densensor-MAP mix9000 (PBI Densor Co., Denmark) with a product to gas ratio of 1:2 after evacuating air. The gas composition was set as 60% CO2/15% O2/25% N2, which was reported by Ruiz-Capillas et al. [12] to be optimal for the preservation of tuna.
All the tuna samples were packaged and stored at 2 °C. Sampling was conducted every 12 h.
Determination of the moisture absorption of WPI film
The moisture absorption (MA) was determined according to the modified version of the method suggested by Lei et al. [13] and Zolfi et al. [14]. Film specimens (20 mm × 20 mm) were thoroughly dried in an air blow oven at 50 °C for 24 h. After weighing, the dried sheets of film were placed in a desiccator containing calcium nitrite saturated solution at 25 °C to ensure a RH of 55%. The samples were weighed at intervals until the equilibrium state was reached. The MA was calculated as follows:
| 1 |
where m t and m 0 are the final and initial weights of the sample, respectively. Measurements were performed in triplicate.
Microbiological analysis
The total viable counts (TVC) of the tuna fillets were determined according to the Chinese national standard (2010) (GB/T 4789.2-2010). Samples with a weight of 25 g were homogenized with 225 mL of sterilized saline water [NaCl, 0.85% (m/v)] at room temperature by a stomacher (Seward Medical, London, England). Other decimal dilutions were prepared using sterilized saline water, and 1 mL of these dilutions was added to Petri dishes, along with 15 mL of plate count agar containing 0.5 g/100 mL NaCl. The plates were incubated at 30 °C for 48 h.
Chemical quality analysis
The content of met-Mb was determined following the method of Thiansilakul et al. [15]. Ground sample (2 g) was homogenized in 20 mL of cold 40 mmol/L Na-phosphate buffer (pH 6.8) at 13,500 g for 10 s. Then, the mixture was centrifuged 3000 g for 30 min at 4 °C. The supernatant was filtered through a Whatman No. 1 filter paper, and its absorbances at 503, 525, 557 and 582 nm were measured and recorded. The met-Mb content was calculated as in Eq. (2):
| 2 |
where R 1 = A 582/A 525, R 2 = A 557/A 525, and R 3 = A 503/A 525.
The TBA value was used to quantify the lipid oxidation degree of the sample by measuring the MDA concentration [16] using a spectrophotometer (Unico (Shanghai) Instrument Co., Ltd., Shanghai, China) at a 532 nm wave length. The TBA value was expressed as milligrams of MDA per kilogram of fish sample.
The TVB-N content was determined using the FOSS method [17] by direct steam distillation. The results were expressed as milligrams N per 100 g of fish sample.
Physical quality analysis
The surface color of the tuna samples was determined using a digital Konica colorimeter CR-10 (Tokyo, Japan) according to Thiansilakul et al. [15]. The instrument was calibrated using a black-and-white standard, and measurements were conducted on each side of the sample. The a*-value (redness) was recorded, and the mean value was calculated (n = 6).
Tuna fillets from each package were weighed, and the percentage of weight loss (W t loss) was calculated as follows (Eq. 3) [6]:
| 3 |
where W t and W 0 are the weight of a tuna slice after t days and the initial weight, respectively.
Texture profile analysis (TPA) was conducted using a texture analyzer (TA.XT Plus, Stable Micro Systems Ltd., Surrey, England). The fish sample was cut into small squares (20 mm × 20 mm × 10 mm) and then compressed using a cylindrical probe of 6 mm in diameter (P/6) on the platform. TPA was performed under the following conditions: constant test speed, 1.0 mm/s; sample deformation, 50%; and holding time between cycles, 5 s. The test was conducted immediately after the samples were moved out of the packages. Six measurements were made for each sample in the same batch. From the force–time curve of the texture profile, the textural parameters, including hardness and chewiness, were obtained [18].
Sensory evaluation
The attributes of tuna chunks were evaluated by eight panelists using a Quality Index Method scheme that was developed for raw-consumed tuna (Table 1). The scheme was based on significant and well-defined characteristics of general appearance, mucus, color, odor, and texture [19]. The total scores of each attributes were recorded.
Table 1.
Sensory evaluation data sheet for bigeye tuna chunks
| Feature | Score | Description |
|---|---|---|
| General appearance | 0 | Bag is vague; chunk is out of shape; a large amount of exudates |
| 1 | Bag is vague; chunk is slightly out of shape; much exudates | |
| 2 | Package is apparent; chunk is in good condition; no drip loss | |
| Mucus | 0 | Stronger slimy |
| 1 | Clammy | |
| 2 | Slight clammy | |
| 3 | Fresh shiny texture, no mucus | |
| Color | 0 | Light brown |
| 1 | Red brown, lighter color | |
| 2 | Dark red, red brown | |
| 3 | Bright red | |
| Odor | 0 | Strong putrid, urea, acetic, fecal, strong ammoniacal |
| 1 | Offensive, silage | |
| 2 | Neutral to bland | |
| 3 | Seaweedy, characteristic of the species | |
| Texture | 0 | Mushy, sponge-like, sunken |
| 1 | Soft body | |
| 2 | Slightly soft body | |
| 3 | Firm, elastic | |
| Quality index (0–14) | ||
Statistical analysis
The results were expressed as the mean and standard deviation. The data were subjected to Duncan’s multiple range test using SPSS (SPSS Version 19.0, Inc, Chicago, IL, USA). The level of significance was set at P < 0.05. Curves were plotted using Origin Pro V8.6 (OriginLab Cor., Northampton, MA, USA).
Results and discussion
MA of the WPI film
The MAs of the two WPI films with different concentrations of glycerol (WPI-1: 4% glycerol; WPI-2: 8% glycerol) were 2.55 ± 0.29 and 5.14 ± 0.72%, respectively. The MA of the WPI films with 8% glycerol was more than twice that of the WPI films with 4% glycerol. Glycerol was added to the WPI films as a hydrophilic plasticizer usually to increase the elongation and flexibility of the films but also to stimulate an increase in MA, which verified the results of Yoshida et al. [20] and Lei et al. [13].
Microbial growth
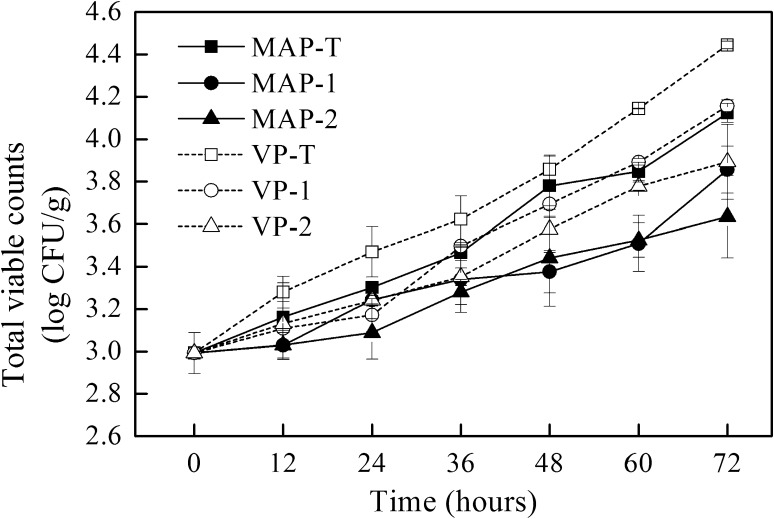
Figure 1 provides the changes in the TVC of tuna chunks during cold storage after frozen storage. The initial count of tuna chunks was 2.99 ± 0.10 log CFU/g, which was lower than that of yellowfin tuna [21]. The bacterial counts of the tuna chunks increased with storage time. The counts of sample VP-T grew much faster than did those of the other groups, reaching 4.44 ± 0.02 log CFU/g after 72 h of storage. The bacterial counts in samples that were kept in vacuum were relatively higher than those that kept in MAP with the same filming treatments (P < 0.05), in accordance with the previous research on MAP- and vacuum-packaged rainbow trout [22]. The antibacterial activity of MAP should be due to both CO2 and O2 [23]. Compared to the results of samples with or without the WPI-filming treatment, the bacterial counts were lower in samples that were coated by WPI films (P < 0.05). Moreover, the samples that were coated with films containing 8% glycerol had lower bacterial counts than did those that were coated with films containing 4% glycerol (P < 0.05). The absorption of the exudates of tuna chunks by the films might be responsible for this phenomenon, as the exudates are favored for spoilage bacteria to grow. After 72 h of storage, the bacterial counts of MAP-1, MAP-2, and VP-2 were 3.86, 3.63 and 3.89 log CFU/g, respectively, which were still lower than the Chinese Industrial Standard “SC/T 3117-2006” limits of sashimi-grade tuna (≤104 CFU/g).
Fig. 1.
Changes in TVC of tuna chunks during storage. Filled square: MAP-T (60% CO2/15% O2/25% N2); filled circle MAP-1 (MAP plus WPI films containing 4% (v/v) glycerol); filled triangle MAP-2 (MAP plus WPI films containing 8% (v/v) glycerol); open square: VP-T (vacuum); open circle: VP-1 (vacuum plus WPI films containing 4% (v/v) glycerol); open triangle: VP-2 (vacuum plus WPI films containing 8% (v/v) glycerol). Data were taken as the mean ± standard deviation (n = 6)
Chemical changes
Myoglobin (Mb) is the most important pigment for tuna chunks. However, ferrous Mb is easily autoxidized into ferric met-Mb post-mortem, which is a major negative contributor to the meat color and is closely related to lipid oxidation [4, 24, 25]. Changes in the met-Mb concentration of tuna chunks that are treated with or without WPI films and kept in vacuum or MAP are presented in Fig. 2(A). The initial percentage of met-Mb in tuna chunks was approximately 20.1% and increased in all samples during storage. A remarkable increase in the percent met-Mb was observed in the first 24 h, suggesting that a rapid oxidation of Mb occurred in the early stage of storage. Regardless of the WPI films, the percent met-Mb in the MAP samples increased more significantly after 24 h of storage and was greater than 41.9% after 72 h of storage. However, the percent met-Mb in the VP samples remained approximately 26.2–28.9% from 24 to 48 h and was lower than 51% at the end of storage. Our results agree with those of a previous study [15] that suggested that MAP containing O2 induces Mb oxidation. After 36 h of storage, the samples that were coated with WPI films had a lower percent met-Mb compared to the samples without WPI films that were kept under the same conditions (P < 0.05). This phenomenon should be contributed to the WPI film, which is an O2 barrier [10, 11]. These results suggest that the WPI films decreased the autoxidation of Mb in tuna chunks when packaged in MAP.
Fig. 2.
Changes in the met-Mb (A), TBA (B) and TVB-N (C) of tuna chunks during storage. Filled square: MAP-T (60% CO2/15% O2/25% N2); filled circle: MAP-1 (MAP plus WPI films containing 4% (v/v) glycerol); filled triangle MAP-2 (MAP plus WPI films containing 8% (v/v) glycerol); open square: VP-T (vacuum); open circle: VP-1 (vacuum plus WPI films containing 4% (v/v) glycerol); open triangle: VP-2 (vacuum plus WPI films containing 8% (v/v) glycerol). Data were taken as the mean ± standard deviation (n = 6)
Figure 2(B) shows the changes of TBA in tuna chunks during storage. The initial TBA value of tuna chunks was 0.091 mg MDA/kg and increased gradually in all the samples in the first 36 h. Thereafter, sharp increases in TBA were observed, and the highest value (0.511 mg MDA/kg) was found in VP-T at the end of storage. The TBA of the samples that were kept in MAP was relatively lower than that of the samples that were kept in vacuum, supporting the hypothesis that MAP, with a high level of CO2 and a low level of O2, can inhibit lipid oxidation [15]. Regardless of the packaging conditions, the samples that were coated with WPI films had a lower TBA than did those that were not coated, suggesting that the WPI films inhibited lipid oxidation. The low oxygen permeability of WPI films should be responsible for this phenomenon [26]. After 72 h of storage, the TBA values of MAP-2 and VP-2 were 0.349 and 0.368 mg MDA/kg, respectively. The WPI films with a higher concentration of glycerol were more effective in the inhibition of lipid oxidation.
The TVB-N index is used to evaluate food decay, as volatile nitrogen is the product of the degradation of protein and non-protein nitrogen compounds; this index is mainly associated with microbial activity [27]. The TVB-N index increased in all the samples during storage (Fig. 2(C)). The VP-T and MAP-T samples showed a greater rate of increase than that of the other samples, reaching 14.43 and 13.92 mg N/100 g after 72 h of storage, respectively. The TVB-N values of the samples that were kept in MAP were relatively lower than that of the same samples with the same filming treatments but that were kept in vacuum, which should contribute to the antibacterial activity of CO2 [28]. Regardless of the packaging conditions, the samples that were coated with WPI films also had lower TVB-N values, in agreement with the study of rohu fillets [11]. The lowest TVB-N values were found in MAP-2. The water-absorbing capacity of WPI films containing glycerol also should be responsible for the inhibition of TVB-N value increase by retarding the growth of spoilage bacteria.
Physical changes
As shown in Table 2, the changes in the redness (a*-value) of the tuna chunks with or without WPI films during storage indicated that the redness value of all the samples decreased with the increased storage time. The redness of all the samples decreased sharply in the first 12 h and that of the samples that were kept in vacuum was much lower compared to that of the samples with the same filming treatments but that were kept in MAP (P < 0.05). The lower redness of the samples in vacuum was possibly due to the formation of deoxymyoglobin because of the low concentrations of oxygen, as a purplish color of deoxymyoglobin in the muscle was observed [29]. In addition, the samples that were coated with the WPI films (MAP-1, MAP-2, VP-1, and VP-2) had higher a* compared to that of the samples without films (MAP-T and VP-T). WPI films have a low oxygen permeability [30], which might maintain the state of oxymyoglobin. Among these treatments, the highest redness was observed in MAP-2, ca. 8.46 ± 0.33 after 72 h of storage, which is significantly higher than that of the samples without WPI films and that of the samples that were kept in vacuum.
Table 2.
Changes of a* value of tuna during storage
| Time (h) | MAP-T | MAP-1 | MAP-2 | VP-T | VP-1 | VP-2 |
|---|---|---|---|---|---|---|
| 0 | 16.71 ± 0.31a | 16.71 ± 0.31a | 16.71 ± 0.31a | 16.71 ± 0.31a | 16.71 ± 0.31a | 16.71 ± 0.31a |
| 12 | 11.11 ± 0.35b | 11.38 ± 0.52ab | 11.70 ± 0.40a | 8.79 ± 0.54cd | 8.56 ± 0.38d | 9.17 ± 0.13c |
| 24 | 10.21 ± 0.28c | 10.66 ± 0.37b | 11.09 ± 0.20a | 8.23 ± 0.23d | 8.21 ± 0.42d | 8.49 ± 0.38d |
| 36 | 9.81 ± 0.31a | 10.14 ± 0.51a | 10.29 ± 0.42a | 7.67 ± 0.31c | 8.03 ± 0.83bc | 8.29 ± 0.33b |
| 48 | 8.79 ± 0.61b | 9.17 ± 0.41b | 9.76 ± 0.28a | 7.30 ± 0.21d | 7.67 ± 0.69cd | 7.94 ± 0.25c |
| 60 | 8.37 ± 0.31b | 8.84 ± 0.32a | 9.27 ± 0.45a | 7.26 ± 0.29d | 7.61 ± 0.51cd | 7.89 ± 0.32c |
| 72 | 7.56 ± 0.23c | 8.10 ± 0.23ab | 8.46 ± 0.33a | 6.80 ± 0.43d | 7.37 ± 0.32c | 7.72 ± 0.59bc |
MAP-T (60% CO2/15% O2/25% N2); MAP-1 (MAP plus WPI films containing 4% (v/v) glycerol); MAP-2 (MAP plus WPI films containing 8% (v/v) glycerol); VP-T (vacuum); VP-1 (vacuum plus WPI films containing 4% (v/v) glycerol); VP-2 (vacuum plus WPI films containing 8% (v/v) glycerol)
Data were taken as a mean ± standard deviation (n = 6). Different lower case in the same row indicated significant differences (P < 0.05)
Many changes, including moisture loss, occur in fish tissue after death, and frozen fish and animal meats are more likely to produce exudates and undergo weight loss because of cell membrane disruption by ice crystals during freezing and thawing [31]. Exudates from fish containing water-soluble nutrients seem to be harmless to consumers, but they reduce the sensory acceptability. The changes in the weight loss in tuna chunks with or without a WPI film during storage are shown in Fig. 3(A). Drip loss was observed in all the samples after 12 h of storage. Compared to the VP with MAP, the drip losses of samples that were kept in vacuum were significantly greater than were those of samples in MAP, ca. 4.5% for VP-T and 1.9% for MAP-T after 72 h of storage. This phenomenon validates the results of a previous study of sausages in vacuum or MAP [32]. The greater weight loss in the vacuum-packaged samples should result from the effect of low pressure on products [33], whereas the lower weight loss of MAP samples might contribute to the high content of CO2, which might lead to a loss of the water-holding capacity of protein [34]. VP-2 and MAP-2 had a greater weight loss than did VP-1 and MAP-1, respectively. This phenomenon might be due to the water-absorbing capacity of glycerol, as there were higher concentrations of glycerol in the WPI films of VP-2 and MAP-2 samples. Interestingly, MAP-1 and MAP-2 had a greater weight loss than did MAP-T, whereas VP-1 and VP-2 had a lower weight loss than did VP-T. It was presumed that the WPI films containing glycerol served as humidity-stabilizing sheets [31]. The films decreased the weight loss of samples in vacuum by holding in the extra exudates while they absorbed the moisture from tuna chunks that were kept in MAP-1 and MAP-2, leading to greater weight loss.
Fig. 3.
Changes in the weight loss percentage (A), hardness (B), and chewiness (C) of tuna chunks during storage. MAP-T (60% CO2/15% O2/25% N2); MAP-1 (MAP plus WPI films containing 4% (v/v) glycerol); MAP-2 (MAP plus WPI films containing 8% (v/v) glycerol); VP-T (vacuum); VP-1 (vacuum plus WPI films containing 4% (v/v) glycerol); VP-2 (vacuum plus WPI films containing 8% (v/v) glycerol). Data were taken as the mean ± standard deviation (n = 6)
The changes in the hardness and chewiness of the tuna chunks during storage are shown in Fig. 3(B), (C), respectively. A decreasing tendency in hardness and chewiness was observed in all the samples. Enzymatic degradation induces gaping, which contributes to muscle tenderization [35]. The VP-T samples had the lowest hardness and chewiness values, indicating more severe enzymatic degradation. The samples that were coated with WPI films (MAP-1, MAP-2, VP-1, and VP-2) had higher hardness and chewiness values than did the uncoated samples (MAP-T and VP-T), which should contribute to the water-holding capacity of WPI films as demonstrated above. These results demonstrate the beneficial effects of WPI films on inhibiting muscle softening.
Sensory quality changes
Table 3 shows the changes in the sensory quality of raw tuna chunks during storage. The sensory scores decreased with increased storage time. The sensory scores of VP-T were lower than those of other samples (P < 0.05) due to the exudates after the thawing and deformation of the chunk (Fig. 4). In the first 48 h, the tuna chunks that were kept in MAP had a better appearance than that of the other samples. After 60 h of storage, exudates were found in MAP-T, whereas no apparent exudates or droplets were found in MAP-1 or MAP-2 bags (Fig. 4), indicating that WPI films in combination with MAP could effectively inhibit the production of exudates. The MAP-2 sample became more slimy than MAP-1. The higher concentration of glycerol in the WPI films of MAP-2 should be responsible for this phenomenon.
Table 3.
Changes in sensory quality of tuna chunks during storage
| Storage time (h) | MAP-T | MAP-1 | MAP-2 | VP-T | VP-1 | VP-2 |
|---|---|---|---|---|---|---|
| 0 | 12.32 ± 0.18a | 12.32 ± 0.18a | 12.32 ± 0.18a | 12.32 ± 0.18a | 12.32 ± 0.18a | 12.32 ± 0.18a |
| 12 | 10.37 ± 0.12b | 10.88 ± 0.12a | 10.92 ± 0.33a | 9.30 ± 0.20d | 9.93 ± 0.12c | 10.15 ± 0.11bc |
| 24 | 9.08 ± 0.21d | 9.97 ± 0.20b | 10.22 ± 0.25a | 8.63 ± 0.12e | 9.60 ± 0.14c | 9.75 ± 0.19bc |
| 36 | 8.97 ± 0.22c | 9.47 ± 0.14b | 9.83 ± 0.16a | 8.60 ± 1.14d | 9.00 ± 0.14c | 8.88 ± 0.15c |
| 48 | 8.62 ± 0.26d | 9.28 ± 0.19b | 9.53 ± 0.29a | 8.35 ± 0.11e | 8.87 ± 0.10c | 8.73 ± 0.15cd |
| 60 | 7.68 ± 0.15b | 8.40 ± 0.13a | 8.23 ± 0.20a | 6.63 ± 0.21d | 7.12 ± 0.17c | 7.85 ± 0.11b |
| 72 | 6.65 ± 0.19b | 6.92 ± 0.15a | 7.03 ± 0.18a | 5.17 ± 0.14d | 6.05 ± 0.16c | 6.70 ± 0.14b |
MAP-T (60% CO2/15% O2/25% N2); MAP-1 (MAP plus WPI films containing 4% (v/v) glycerol); MAP-2 (MAP plus WPI films containing 8% (v/v) glycerol); VP-T (vacuum); VP-1 (vacuum plus WPI films containing 4% (v/v) glycerol); VP-2 (vacuum plus WPI films containing 8% (v/v) glycerol)
Data were taken as a mean ± standard deviation (n = 6). Different lower case in the same row indicated significant differences (P < 0.05)
Fig. 4.

Photographs of tuna chunks after storage. MAP-T (60% CO2/15% O2/25% N2); MAP-1 (MAP plus WPI films containing 4% (v/v) glycerol); MAP-2 (MAP plus WPI films containing 8% (v/v) glycerol); VP-T (vacuum); VP-1 (vacuum plus WPI films containing 4% (v/v) glycerol); VP-2 (vacuum plus WPI films containing 8% (v/v) glycerol)
In conclusion, tuna chunks that were treated with a WPI film prior to MAP (60% CO2/15% O2/25% N2) or VP had slower microbial growth, TBA values, TVB-N values, and met-Mb concentrations and higher sensory scores. In general, the WPI film containing 8% glycerol showed higher MA ability than that of the film containing 4% glycerol, which could absorb the exudates of the tuna. This study highlights the effectiveness of WPI films for enhancing the microbiological, physical, and biochemical quality of modified atmosphere- or vacuum-packaged tuna chunks.
Acknowledgements
The authors acknowledge the financial assistance provided by the National Key Research and Development Program (2016YFD0400106), the Shanghai Science and Technology Key Project on Agriculture from Shanghai Municipal Agricultural Commission [(2016) 1-1], and the Shanghai Municipal Science and technology project to enhance the capabilities of the platform (16DZ2280300).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Silbande A, Adenet S, Smith-Ravin J, Joffraud J-J, Rochefort K, Leroi F. Quality assessment of ice-stored tropical yellowfin tuna (Thunnus albacares) and influence of vacuum and modified atmosphere packaging. Food Microbiol. 2016;60:62–72. doi: 10.1016/j.fm.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Tsironi T, Gogou E, Velliou E, Taoukis PS. Application and validation of the TTI based chill chain management system SMAS (Safety Monitoring and Assurance System) on shelf life optimization of vacuum packed chilled tuna. Int. J. Food Microbiol. 2008;128:108–115. doi: 10.1016/j.ijfoodmicro.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 3.Silva TM, Sabaini PS, Evangelista WP, Gloria MBA. Occurrence of histamine in Brazilian fresh and canned tuna. Food Control. 2011;22:323–327. doi: 10.1016/j.foodcont.2010.07.031. [DOI] [Google Scholar]
- 4.Thiansilakul Y, Benjakul S, Richards MP. The effect of different atmospheric conditions on the changes in myoglobin and colour of refrigerated Eastern little tuna (Euthynnus affinis) muscle. J. Sci. Food Agric. 2011;91:1103–1110. doi: 10.1002/jsfa.4290. [DOI] [PubMed] [Google Scholar]
- 5.Emborg J, Laursen BG, Dalgaard P. Significant histamine formation in tuna (Thunnus albacares) at 2 & #xB0;C-effect of vacuum- and modified atmosphere-packaging on psychrotolerant bacteria. Int. J. Food Microbiol. 2005;101:263–279. doi: 10.1016/j.ijfoodmicro.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Turan H, Kocatepe D. Different MAP conditions to improve the shelf life of Sea bass. Food Sci. Biotechnol. 2013;22:1589–1599. doi: 10.1007/s10068-013-0255-x. [DOI] [Google Scholar]
- 7.Sanchez-Ortega I, Garcia-Almendarez BE, Santos-Lopez EM, Amaro-Reyes A, Barboza-Corona JE, Regalado C. Antimicrobial edible films and coatings for meat and meat products preservation. Sci. World J. 2014;2014:248935. doi: 10.1155/2014/248935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falguera V, Pablo Quintero J, Jimenez A, Aldemar Munoz J, Ibarz A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011;22:292–303. doi: 10.1016/j.tifs.2011.02.004. [DOI] [Google Scholar]
- 9.Ramos OL, Fernandes JC, Silva SI, Pintado ME, Xavier Malcata F. Edible films and coatings from whey Proteins: A review on formulation, and on mechanical and bioactive properties. Crit. Rev. Food Sci. Nutr. 2012;52:533–552. doi: 10.1080/10408398.2010.500528. [DOI] [PubMed] [Google Scholar]
- 10.Ouattara B, Sabato SF, Lacroix M. Combined effect of antimicrobial coating and gamma irradiation on shelf life extension of pre-cooked shrimp (Penaeus spp.) Int. J. Food Microbiol. 2001;68:1–9. doi: 10.1016/S0168-1605(01)00436-6. [DOI] [PubMed] [Google Scholar]
- 11.Khan MI, Adrees MN, Arshad MS, Anjum FM, Jo C, Sameen A. Oxidative stability and quality characteristics of whey protein coated rohu (Labeo rohita) fillets. Lipids Health Dis. 2015;14:58. doi: 10.1186/s12944-015-0060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiz-Capillas C, Moral A. Sensory and biochemical aspects of quality of whole bigeye tuna (Thunnus obesus) during bulk storage in controlled atmospheres. Food Chem. 2005;89:347–354. doi: 10.1016/j.foodchem.2004.02.041. [DOI] [Google Scholar]
- 13.Lei Q, Pan J, Bao J, Huang Z, Zhang Y. Analysis and modeling of moisture sorption behavior for antimicrobial composite protein films. Bio-med. Mater. Eng. 2014;24:1969–1978. doi: 10.3233/BME-141006. [DOI] [PubMed] [Google Scholar]
- 14.Zolfi M, Khodaiyan F, Mousavi M, Hashemi M. Characterization of the new biodegradable WPI/clay nanocomposite films based on kefiran exopolysaccharide. J. Food Sci. Technol. 2015;52:3485–3493. doi: 10.1007/s13197-014-1407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thiansilakul Y, Benjakul S, Richards MP. Effect of phenolic compounds in combination with modified atmospheric packaging on inhibition of quality losses of refrigerated Eastern little tuna slices. LWT-Food Sci. Technol. 2013;50:146–152. doi: 10.1016/j.lwt.2012.06.009. [DOI] [Google Scholar]
- 16.Ibrahim Sallam K. Antimicrobial and antioxidant effects of sodium acetate, sodium lactate, and sodium citrate in refrigerated sliced salmon. Food Control. 2007;18:566–575. doi: 10.1016/j.foodcont.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.FOSS. Determination of total volatile basic nitrogen of fresh fish and frozen fish. Application Sub Note. Hillerød, Denmark. 8. pp. 16 (2002)
- 18.Tsironi T, Dermesonlouoglou E, Giannakourou M, Taoukis P. Shelf life modelling of frozen shrimp at variable temperature conditions. LWT-Food Sci. Technol. 2009;42:664–671. doi: 10.1016/j.lwt.2008.07.010. [DOI] [Google Scholar]
- 19.Cyprian O, Lauzon HL, Jóhannsson R, Sveinsdóttir K, Arason S, Martinsdóttir E. Shelf life of air and modified atmosphere-packaged fresh tilapia (Oreochromis niloticus) fillets stored under chilled and superchilled conditions. Food Sci. Nutr. 2013;1:130–140. doi: 10.1002/fsn3.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshida CM, Antunes AC, Antunes LJ, Antunes AJ. An analysis of water vapour diffusion in whey protein films. Int. J. Food Sci. Technol. 2003;38:595–601. doi: 10.1046/j.1365-2621.2003.00690.x. [DOI] [Google Scholar]
- 21.Kamalakanth CK, Ginson J, Bindu J, Venkateswarlu R, Das S, Chauhan OP, Gopal TKS. Effect of high pressure on K-value, microbial and sensory characteristics of yellowfin tuna (Thunnus albacares) chunks in EVOH films during chill storage. Innov. Food Sci. Emerg. Technol. 2011;12:451–455. doi: 10.1016/j.ifset.2011.06.001. [DOI] [Google Scholar]
- 22.Arashisar Ş, Hisar O, Kaya M, Yanik T. Effects of modified atmosphere and vacuum packaging on microbiological and chemical properties of rainbow trout (Oncorynchus mykiss) fillets. Int. J. Food Microbiol. 2004;97:209–214. doi: 10.1016/j.ijfoodmicro.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 23.Kostaki M, Giatrakou V, Savvaidis IN, Kontominas MG. Combined effect of MAP and thyme essential oil on the microbiological, chemical and sensory attributes of organically aquacultured sea bass (Dicentrarchus labrax) fillets. Food Microbiol. 2009;26:475–482. doi: 10.1016/j.fm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Thiansilakul Y, Benjakul S, Richards MP. Changes in heme proteins and lipids associated with off-odour of seabass (Lates calcarifer) and red tilapia (Oreochromis mossambicus × O. niloticus) during iced storage. Food Chem. 2010;121:1109–1119. doi: 10.1016/j.foodchem.2010.01.058. [DOI] [Google Scholar]
- 25.Sohn JH, Taki Y, Ushio H, Kohata T, Shioya I, Ohshima T. Lipid oxidations in ordinary and dark muscles of fish: Influences on rancid off-odor development and color darkening of yellowtail flesh during ice storage. J. Food Sci. 2005;70:s490–s496. doi: 10.1111/j.1365-2621.2005.tb11497.x. [DOI] [Google Scholar]
- 26.Bonilla J, Atarés L, Vargas M, Chiralt A. Edible films and coatings to prevent the detrimental effect of oxygen on food quality: Possibilities and limitations. J. Food Eng. 2011;110:208–213. doi: 10.1016/j.jfoodeng.2011.05.034. [DOI] [Google Scholar]
- 27.Mousakhani-Ganjeh A, Hamdami N, Soltanizadeh N. Impact of high voltage electric field thawing on the quality of frozen tuna fish (Thunnus albacares) J. Food Eng. 2015;156:39–44. doi: 10.1016/j.jfoodeng.2015.02.004. [DOI] [Google Scholar]
- 28.Qian YF, Yang SP, Xie J, Xiong Q, Gao ZL. Impact of the O2 concentrations on bacterial communities and quality of modified atmosphere packaged Pacific white shrimp (Litopenaeus Vannamei) J. Food Sci. 2013;78:M1878–M1884. doi: 10.1111/1750-3841.12305. [DOI] [PubMed] [Google Scholar]
- 29.Jeong JY, Claus JR. Color stability of ground beef packaged in a low carbon monoxide atmosphere or vacuum. Meat Sci. 2011;87:1–6. doi: 10.1016/j.meatsci.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Ozdemir M, Floros JD. Optimization of edible whey protein films containing preservatives for water vapor permeability, water solubility and sensory characteristics. J. Food Eng. 2008;86:215–224. doi: 10.1016/j.jfoodeng.2007.09.028. [DOI] [Google Scholar]
- 31.Saito K, Ahhmed AM, Kawahara S, Sugimoto Y, Aoki T, Muguruma M. Effects of humidity-stabilizing sheets on the quality of bigeye tuna meat (Thunnus obesus) during refrigerated storage. Food Sci. Technol. Res. 2009;15:283–292. doi: 10.3136/fstr.15.283. [DOI] [Google Scholar]
- 32.Stasiewicz M, Lipiński K, Cierach M. Quality of meat products packaged and stored under vacuum and modified atmosphere conditions. J. Food Sci. Technol. 2014;51:1982–1989. doi: 10.1007/s13197-012-0682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Sullivan M, Cruz-Romero M, Kerry J. Carbon dioxide flavour taint in modified atmosphere packed beef steaks. LWT-Food Sci. Technol. 2011;44:2193–2198. doi: 10.1016/j.lwt.2011.06.010. [DOI] [Google Scholar]
- 34.Goulas AE, Kontominas MG. Effect of modified atmosphere packaging and vacuum packaging on the shelf-life of refrigerated chub mackerel (Scomber japonicus): Biochemical and sensory attributes. Eur. Food Res. Technol. 2007;224:545–553. doi: 10.1007/s00217-006-0316-y. [DOI] [Google Scholar]
- 35.Delbarre-Ladrat C, Chéret R, Taylor R, Verrez-Bagnis V. Trends in postmortem aging in fish: understanding of proteolysis and disorganization of the myofibrillar structure. Crit. Rev. Food Sci. Nutr. 2006;46:409–421. doi: 10.1080/10408390591000929. [DOI] [PubMed] [Google Scholar]