Abstract
The objective of this study was to select and identify yeasts and molds isolated from traditional nuruk and to investigate their brewing characteristics for Cheongju production. The yeast strains Y190 (Accession ID-KACC 93251P), Y263 (Accession ID-KACC 93252P), and Y270 (Accession ID-KACC 93253P) showing high alcohol and flavor productivity were isolated and identified by phylogenetic inference based on an internal transcribed spacer 2 region sequence analysis. In addition, Aspergillus oryzae 83-10 (Accession ID-KACC 93254P) showing the highest enzyme activity was isolated. This study provides basic data for the production of Korean Cheongju and assesses the applicability of these three yeast strains and A. oryzae 83-10 isolated from traditional nuruk.
Keywords: Brewing microorganisms, Nuruk, ITS 2 sequence, Cheongju, Aroma
Introduction
Korean traditional alcoholic beverages are classified as Makgeolli (Takju), Jakju (Cheongju), and distilled Soju using nuruk. Cheongju is a Korean traditional rice beer brewed by a simultaneous two step fermentation process using fermenting agents [1, 2]. Therefore, Koji, used as a fermenting agent, influences the quality characteristics of Cheongju such as taste, aroma, and color [3]. There are two types of nuruk, namely, the Korean traditional nuruk prepared with whole flour in which various microorganisms grow naturally and the improved nuruk prepared with sterile cereal in which pure culture mold is artificially propagated [4, 5]. The traditional nuruk results in lack of uniformity during fermentation and a deficiency of saccharification power (SP) but provides variety in quality because of various microorganisms [6, 7]. So et al. [8] reported the characteristics of modified nuruk prepared by inoculation and cultivation of Rhizopus japonicus T2, Aspergillus oryzae L2, and Hansenula sp. BC26. Kwon et al. [9] noted the quality properties of Takju brewed with various types of rice and nuruk. For the properties of Koji made with 19 types of rice, the acidity was over 5.0 and the saccharogenic power was more than 60 SP.
Other studies have isolated, identified, and optimized the production conditions of Takju using R. japonicus and A. oryzae [10, 11]. Meanwhile, Jung et al. [12] reported the characteristics of Hahyangju (a traditional Korean liquor) prepared with the Saccharomyces cerevisiae strain HA3 isolated from traditional nuruk. Kim et al. [13] noted the characterization and volatile flavor components (phenylethyl alcohol, isoamyl alcohol, ethyl oleate, ethyl linoleate, and tetradecanoic acid ethyl ester) in glutinous rice wines prepared with different yeasts of nuruk. Shin et al. [14] reported the volatile components (4 alcohols, 2 esters, and 7 acids) and characterization of Yakju prepared with yeasts from fruits. Seo et al. [15] reported the changes in microflora (Saccharomyces cerevisiae, Candida magnoliae) during the fermentation of Takju and Yakju. Lee et al. [16] reported the volatile flavor components (14 alcohols, 13 esters, 5 acids, 3 aldehydes, 7 amines, and 2 other components) in Takju mashes prepared using different yeasts (Saccharomyces coreanus, S. ellipsoideus, S. carlsbergensis, S. cerevisiae, and S. rouxii). Park et al. [17] noted the isolation and identification of Takju yeasts. Previous studies regarding microorganisms related to mash or improving fermentation conditions using traditional or improved nuruk have focused on Takju and Yakju. Studies focused on selecting compatible brewing yeast and mold for preparing Cheongju have been sporadic. Korean Cheongju was produced using nuruk prepared with whole meals containing various molds and yeasts, which produced sweet and organoacidic fruit flavor. Nowadays, Cheongju (Jakju) is mainly brewed with Japanese yeasts and rice Koji. Therefore, this study aimed to select and identify yeasts and molds isolated from Korean traditional nuruk and to investigate their brewing characteristics for Cheongju production.
Materials and methods
Materials
For the isolation and selection of microorganisms used in the brewing industry, we collected 30 nuruk from commercial and traditional nuruk in Korea and 120 strains of molds were isolated from different nuruk. Saccharomyces sp. (Y190, Y263, and Y270) indicated excellent alcohol producing ability and mold (A. oryzae 83-10) showed a better productivity of saccharogenic and proteolytic enzymes. Songcheon yeast (Songcheon fermentation, Chungnam, Korea, 2015, Saccharomyces sp.) was used as a control sample.
Phylogenetic analysis
Amplification and sequencing of the internal transcribed spacer (ITS) 2 region of strain Y190, Y263, and Y270 were conducted as described by Kim et al. [18]. Briefly, a single colony grown on YPD agar was suspended in 100 µL 5% (w/v) Chelex®100 solution (Sigma) and boiled for 10 min to prepare crude genomic DNA lysates. PCR amplification of the ITS region from the crude lysates was performed using the universal primer ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′), as described previously [19]. The resulting ITS2 region sequence was checked manually for quality and gaps and compared with the sequence from Genbank using the BLAST program (http://www.ncbi.nlm.nih.gov/BLAST/) to determine an approximate phylogenetic affiliation. It was then aligned with sequences of closely related members using the CLUSTAL W software [20]. Phylogenetic trees were constructed by using the neighbor-joining (NJ) algorithms available in the PHYLIP software, version 3.6 [21]. The resulting tree topologies were evaluated using bootstrap analysis based on 1000 resampled datasets with the PHYLIP package.
Activity of α-amylase
0.5% sodium chloride (50 mL) was added to 10 g of Guk and the solution was shaken occasionally overnight in a cold room temperature. The leached and filtered solution was diluted to produce a crude enzyme extract. After 2 mL of 1% starch solution was added to a test tube and the mixture pre-heated for 5 min at 40 °C, 0.1 mL of the enzyme extract was added to initiate the reaction. Afterwards, 0.1 mL of the reaction solution was added to each test tube containing 10 mL of iodine solution every 0.5 or 1 min with a pipette. The product was kept at 25 °C until it was evaluated at 670 nm with 10 mm colorimetric tubes and the transmittance (T%) was measured. The enzyme activity was calculated using the formula mentioned below, which follows the Wohlgemuth value.
Activity of glucoamylase
0.2 mL of 0.2 M acetic acid buffer was added to a starch solution and the solution was pre-heated for 5 min. Afterwards, 0.1 mL of enzyme extract was added and reacted for 20 min at 40 °C. 0.1 mL of 1 N sodium hydroxide was added to stop the reaction and the solution was left alone for 30 min. Then, it was neutralized with 0.1 mL of 1 N hydrochloric acid. The enzyme activity was expressed as the amount of glucose produced when reducing sugar was measured using the dinitrosalicylic acid method. The glucoamylase activity of 1 g of sample was calculated using the following formula.
Activity of acidic protease
50 mL of 0.5% sodium chloride solution was added to 10 g of the sample and the mixture was shaken occasionally overnight in a cold room (4 °C) temperature. Afterwards, it was leached and filtered. 10 mL of the filtered extract was added to a semi-permeable membrane and dialyzed in a 2 M acetic acid buffer solution overnight at 4 °C. Then, the enzyme extract was diluted and used as a crude enzyme extract. 1.0 mL of Mcllvaine’s buffer (pH 3.0) and 0.5 mL of crude enzyme extract were added to 1.5 mL of casein solution and reacted for 60 min at 40 °C. Afterwards, 3 mL of trichloroacetic acid solution and 1 mL of phenol reagent were added, colorized for 30 min at 40 °C, and the absorbance was measured. The enzyme activity was calculated using the following formula.
Manufacturing of rice Guk and culture condition
Raw rice (Cheorwon, Odaesan, Korea) was washed and soaked for 2 h and then drained in water for 1 h. The rice was then steamed using a steamer (MS-30, Yaegaki Food & System Inc. Himeji, Japan) and the temperature was reduced to 36 °C. The steamed rice was inoculated (0.1% w/v) as steamed rice base with A. oryzae 83-10 (1.0 × 106 spores/mL) and cultured with potato dextrose agar at 38 °C for 48 h in an incubator. The yeast strains were cultivated at 30 °C on a horizontal shaker at 150 rpm for 48 h.
Fermentation condition
Four mashes containing 0.05% of each yeast, 1 kg rice Guk, and 150% brewing water were prepared. The fermentations were performed at 15, 20, 25, and 30 °C for 10 days.
Chemical analysis and cell counting
The pH during fermentation was measured using a pH meter (Model Orion 720A, Beverly, MA, USA). The soluble solid concentrations (Brix) were measured using a refractometer (Atago, Torrance, CA, USA). For the determination of acidity, the samples (100 mL) were titrated with 1–2 drops of phenolphthalein to pH 8.3 with 0.1 N NaOH. Alcohol in the samples was analyzed using the Anton Paar beer analyzer with an auto sampler (Model SP-1, Beverly MA, USA). Total titratable acidity was calculated with the following equation using the consumed amounts (mL) of 0.1 N NaOH at the end-point (pink color). . The cell concentration was determined by direct microscopic (OS-THPL, Suwon, Korea) counting (cell/mL) using a hemocytometer (DHC-N01-5C-chip, Baverly, MA, USA). The analysis of volatile compounds, sensory evaluation and aroma using electron nose was conducted with samples that were stored at 2 °C for 4 weeks.
Volatile compounds analysis
The volatile compounds were isolated using a gas chromatography–mass spectrometer (HP 6890 N, Hewlett-Packard, PA, USA) and a head-space auto sampler (Agilent 7694E, Agilent Technologies, CA, USA). Isolation of the volatile substances was performed for each vial at 80 °C for 30 min, with the injection loop at 90 °C and the temperature transfer line at 100 °C. The carrier gas was ultrapure helium with a flow rate of 1 mL/min and the pressure was at 7.5 kPa. The oven temperature of the gas chromatography was held at 50 °C for 5 min and then set to 150 °C at a rate of 3 °C/min. The injector and transfer line were heated at 250 and 280 °C, respectively. The ionization voltage applied was 70 eV and the mass spectra were obtained in a scan range from 40 to 350 m/z.
Sensory evaluation
The sensory characteristics of samples were evaluated by 30-membered panels. The preferences for appearance, smell, taste, quality, and overall acceptability were determined with a 5-point scale (1: dislike very much; 2: dislike moderately; 3: like slightly; 4: like moderately; 5: like very much).
Analysis of aroma using an electron nose
The aroma patterns of various Cheongju, produced by fermenting with different types of yeast, were analyzed using an electronic nose system (α-Moss Fox 3000, Alpha M.O.S, Toulouse, France). 1 mL of each sample produced with each type of yeast was added to a 20 mL vial and sealed with a cap. Five vials were prepared for each type of sample, and an auto sampler (32 samples, Alpha M.O.S, Toulouse, France) was used to vaporize the aromatic substances for 120 s at 45 °C. Afterwards, 500 µL of injection volume was injected into the electronic nose system. Data are represented according to the principle component analysis method.
Statistical analysis
Significant differences among samples for each of the parameters analyzed were assessed with a one-way analysis of variance using the SPSS Version 12.0 K statistical package for Windows (SPSS, Chicago, Illinois, USA).
Results and discussion
Yeast strain identification
The nearly complete ITS2 region sequences of strain Y190, Y263, and Y270 were obtained. Phylogenetic tree was derived from the ITS2 region sequences of seven types of Saccharomyces species and other members in the fungal species. Phylogenetic analysis based on ITS2 region sequences using the NJ algorithm showed that strain Y190, Y263, and Y270 were closely related to the S. cerevisiae (GU256758) in the family Saccharomycetaceae, indicating that the three yeast strains belong to S. cerevisiae (Fig. 1).
Fig. 1.
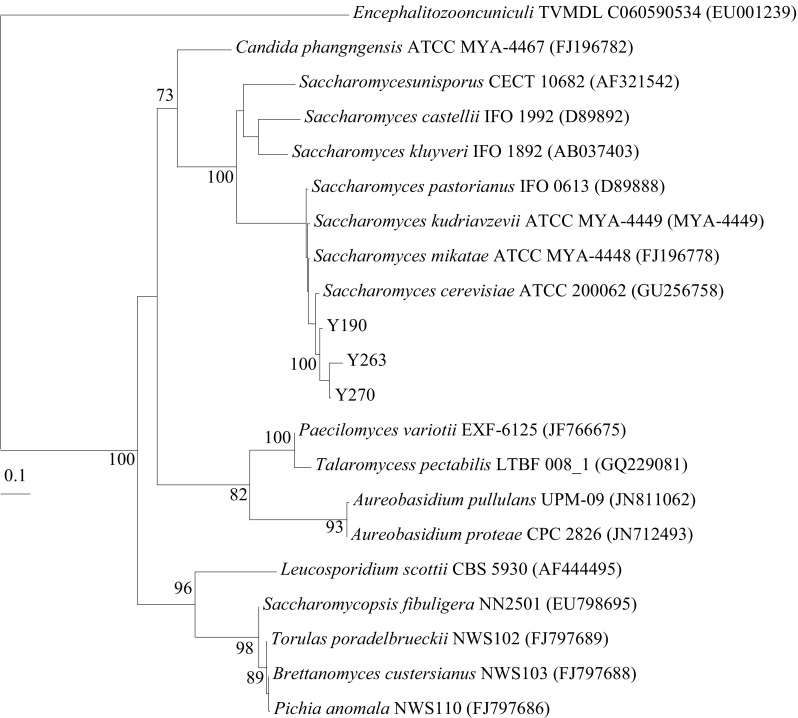
Neighbor-joining tree based on the ITS2 region sequences showing the phylogenetic relationships of strain Y190, Y263, Y270, and related taxa. Bootstrap values greater than 70% are shown on nodes in percentages of 1000 replicates. Encephalitozoon cuniculi TVMDL C060590534 (EU001239) was used as an out-group. The scale bar equals 0.1 changes per nucleotide position
Enzyme activity of molds and rice Guk
The enzyme activity of molds is shown in Table 1 wherein the α-amylase, glucoamylase, and acidic protease activity are denoted. Different molds from different Korean traditional nuruk in domestic were isolated of which A. oryzae 83-10 showed the strongest enzyme activity among the molds. In addition, the α-amylase, glucoamylase, and acidic protease content of rice Guk prepared by A. oryzae 83-10 were 87.2, 301.4, and 1617.5 (units/g), respectively. These results indicated that the isolated A. oryzae 83-10 was proper strain for making good quality rice Guk.
Table 1.
Enzyme activity of different molds from Korean traditional nuruk
| Strains | α-amylase (units/g) | Glucoamylase (units/mg) | Acidic protease (units/μg) |
|---|---|---|---|
| Aspergillus oryzae KACC 44967 | 84.2 ± 1.2 | 92.8 ± 2.3 | 429.3 ± 4.1 |
| Aspergillus oryzae Suwon | 64.6 ± 1.0 | 74.8 ± 2.6 | 445.9 ± 6.3 |
| Aspergillus oryzae 44-5 | 48.2 ± 1.1 | 39.3 ± 1.1 | 1560.8 ± 10.1 |
| Aspergillus oryzae 46-8 | 51.7 ± 1.3 | 24.2 ± 1.1 | 250.0 ± 3.7 |
| Aspergillus oryzae 74.4 | 71.1 ± 1.4 | 183.1 ± 3.4 | 752.5 ± 6.7 |
| Aspergillus oryzae 74.5 | 23.8 ± 1.1 | 403.9 ± 2.4 | 1549.3 ± 8.2 |
| Aspergillus oryzae sp. 25-1 | 58.5 ± 1.5 | 148.2 ± 4.1 | 306.9 ± 3.6 |
| Aspergillus oryzae sp. 34-1 | 30.2 ± 2.1 | 98.3 ± 3.2 | 1518.0 ± 8.2 |
| Aspergillus oryzae sp. 40-2 | 59.1 ± 1.7 | 196.6 ± 4.6 | 233.8 ± 2.3 |
| Aspergillus oryzae 47-2 | 47.3 ± 1.6 | 37.9 ± 1.1 | 1560.8 ± 7.8 |
| Rhizopus oryzae KACC 42736 | 22.6 ± 1.1 | 220.1 ± 5.3 | 118.2 ± 1.4 |
| Rhizopus oryzae 82-7 | 15.5 ± 1.0 | 310.7 ± 2.9 | 885.3 ± 4.1 |
| Rhizopus sp. 26-4 | 30.0 ± 2.1 | 255.2 ± 2.8 | 206.6 ± 1.2 |
| Aspergillus oryzae 83-10 | 87.2 ± 1.5 | 301.4 ± 3.7 | 1615.7 ± 7.9 |
Results are expressed as mean ± SD (n = 3)
Chemical properties and cell growth during fermentation prepared with different yeast strains with A. oryzae
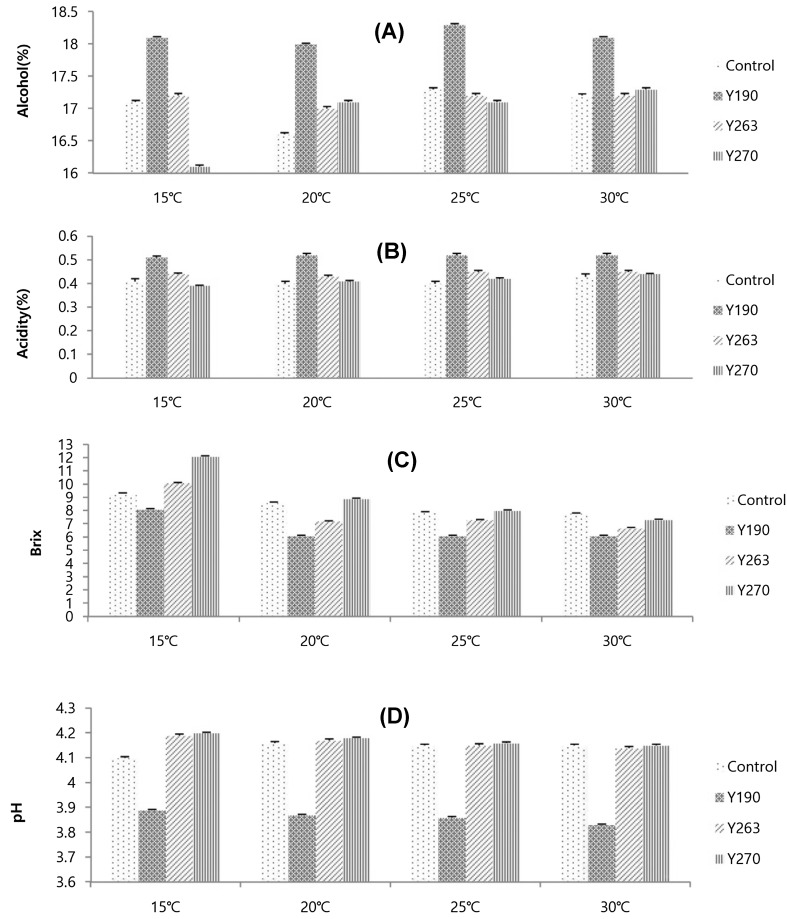
The chemical properties of mashes prepared with different yeast strains with A. oryzae are shown in Fig. 2 wherein alcohol concentration (%), titratable acidity (%), pH, and soluble solid concentration (Brix) analysis results are denoted. In particular, pH and acidity were key indicators for the fermentation conditions of Cheongju [22, 23]. The mashes made with different yeast strains exhibited normal fermentation patterns for 10 days with regard to the chemical properties; therefore, it was assumed that the yeast strains used were suitable for preparing Cheongju. The pattern of fermentation by mash was similar to previous studies [24]. However, the mash prepared with Y190 showed the highest alcohol concentration (18.0–18.1%) at all fermentation temperatures tested. This indicated that the mash prepared with Y190 had higher fermentative and alcohol tolerance than the other mashes (control, Y263, and Y270), as reported by Lee et al. [7]. The acidity content increased during alcoholic fermentation in all mashes, which was the same as the results of Park et al. [22]. However, the highest acidity was obtained in the mash prepared with Y190 (0.51–0.52%) at all the fermentation temperatures tested, whereas the mash made with Y270 had the lowest acidity (0.39–0.47%). This range of acidity was consistent with those of Jeong et al. [25] and Cho et al. [26]. The soluble solid concentration (brix) showed a decrease during fermentation at all temperatures tested and the mash prepared with Y190 had the lowest content at the final days of fermentation. This result coincided with the results of alcohol changes. The pH levels among the mashes changed during the fermentation, similar to that reported by Lee et al. [27], from 5.50 to 4.20 in the mash prepared with control, from 5.52 to 3.89 in the mash prepared with Y190, 5.19 to 4.19 in the mash made with Y263, and 5.20 to 4.15 in the mash made with Y270. The pH level in this study was similar to that reported by Kim et al. [28]. The lower the pH level, the more stable the mash would be against microbial contamination; therefore, it was assumed that the mash made with Y190 was more suitable for the preparation of Cheongju than the other mashes. In addition, considerable differences were observed in cell growth among the mashes at various fermentation temperatures. Thus, the mash made with Y190 showed the fastest cell growth during fermentation at 15 °C. At this temperature, the maximum cell growth duration for all mashes was between 5 and 7 days, which was similar to the results from Maemura et al. [29] and Schmidt [30]. However, the maximum number of yeast cells in the mash made with Y190 was reached within 5 days, whereas the maximum number of yeast cells in the mashes prepared with control, Y263 and Y270, was reached within 7 days. This tendency was observed at other fermentation temperatures (20, 25, and 30 °C). This finding was similar to that reported by Cheong et al. [31]. The cell growth of yeast with regard to temperature during fermentation was strongly dependent on the yeast strains similar to that reported by Cheong et al. [32]. The results of the present study also indicated that yeast strain Y190 had more vitality and fermentation ability than those of other yeast strains.
Fig. 2.
Chemical properties in alcohol (A), acidity (B), brix (C), and pH (D) after fermentation with different yeast strains with Aspergillus oryzae. Results are expressed as mean ± SD (n = 3)
Aroma compounds after fermentation at different temperatures
The aroma compounds after fermentation with different yeasts and A. oryzae are indicated in Table 2. Ethyl acetate, phenylethyl acetate, isoamyl acetate, and ethyl formate were detected in the mashes made at different fermentation temperatures. Esters are formed by esterification during yeast fermentation and are important substances in high quality alcoholic beverages, strongly affecting the flavor and taste by adding a sweet, banana-like aroma [33]. Ethyl acetate was the most abundant substance detected, similar to previous results [28, 34]. In the fermentation at 15 °C, the mash prepared with Y190 contained the highest level (45 mg/L) of ethyl acetate compared with the other mashes, whereas the mash made with Y270 had the lowest (24 mg/L). This tendency was also observed at other fermentation temperatures (20, 25, and 30 °C) and the concentration of ethyl acetate increased according to the temperature of fermentation (data not shown for 20 and 25 °C). The concentration of esters was higher than that reported by Kim et al. [27]. In addition, in the fermentation at 15 °C, the mash prepared with Y190 contained the highest level of phenylethyl acetate (2.5 mg/L), isoamyl acetate (2 mg/L), and ethyl formate (3.5 mg/L). Similar results were also obtained at other fermentation temperatures (20, 25, and 30 °C) and the phenylethyl acetate, isoamyl acetate, and ethyl formate contents increased according to the temperature of fermentation (data not shown for 20 and 25 °C). Among the volatile compounds, higher alcohols, such as 3-methyl butanol, 2-methyl butanol, n-propanol, and 2-methyl propanol, were detected in the mashes prepared with different yeast strains, with 3-methyl butanol as the main component, followed by 2-methyl butanol, n-propanol, and 2-methyl propanol. In the fermentation at 15 °C, the mash made with Y190 contained the highest level (45 mg/L) of 3-methyl butanol, whereas the mash prepared with Y270 contained the lowest (26 mg/L). This tendency was also observed at other fermentation temperatures (20, 25, and 30 °C) and the concentration of 3-methyl butanol increased depending on the temperature of fermentation (data not shown for 20 and 25 °C). Similar results were obtained at other temperatures, with the concentrations of 3-methyl butanol, 2-methyl butanol, n-propanol, and 2-methyl propanol increasing with the temperature similar to that of ester. The synthesis of ester and higher alcohols was significantly higher in the mash prepared with Y190 compared with the other mashes. These results strongly suggest that fermentation with the yeast Y190 and A. oryzae 83-10 during the preparation of Cheongju had a positive effect on increasing aroma compounds such as ester and higher alcohols, reported by Cho et al. [26] and Lee et al. [6].
Table 2.
Aroma compounds after fermentation with different yeasts and A. oryzae 83-10
| Aroma compounds | 15 °C | 30 °C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Y190 | Y263 | Y270 | Control | Y190 | Y263 | Y270 | ||
| Ester (mg/L) | Ethyl acetate | 30 ± 2.1 | 45 ± 2.3 | 29 ± 2.4 | 24 ± 1.4 | 33 ± 4.5 | 50 ± 5.6 | 35 ± 5.1 | 30 ± 5.3 |
| Phenylethyl acetate | 0.5 ± 0.0 | 2.5 ± 0.1 | 0.7 ± 0.0 | 0.4 ± 0.0 | 2.9 ± 0.1 | 4.2 ± 0.1 | 3.0 ± 0.1 | 3.1 ± 0.6 | |
| Isoamyl acetate | 0.6 ± 0.1 | 2 ± 0.0 | 0.6 ± 0.0 | 0.3 ± 0.0 | 2.7 ± 0.0 | 3.2 ± 0.2 | 2.1 ± 0.2 | 2.5 ± 0.3 | |
| Ethyl formate | 1.5 ± 1.0 | 3.5 ± 1.1 | 2.0 ± 1.3 | 1.4 ± 2.4 | 2.4 ± 1.4 | 4.0 ± 2.7 | 3.8 ± 2.6 | 3.7 ± 3.2 | |
| Total ester | 32.6 ± 2.42 | 53 ± 1.51 | 32.3 ± 1.22 | 26.1 ± 1.13 | 41 ± 3.22 | 61.4 ± 4.11 | 43.9 ± 4.32 | 39.3 ± 3.72 | |
| Higher alcohol (mg/L) | 3-Methyl butanol | 30 ± 2.0 | 45 ± 2.1 | 32 ± 3.2 | 26 ± 1.2 | 48 ± 2.4 | 67 ± 3.2 | 58 ± 1.4 | 45 ± 1.1 |
| 2-Methyl butanol | 19 ± 1.0 | 32 ± 1.2 | 20 ± 1.2 | 17 ± 2.1 | 34 ± 2.1 | 53 ± 2.4 | 36 ± 2.4 | 34 ± 2.0 | |
| n-Propanol | 13 ± 1.2 | 20 ± 2.1 | 15 ± 1.1 | 12 ± 1.1 | 27 ± 1.4 | 35 ± 2.1 | 30 ± 1.7 | 28 ± 1.4 | |
| 2-Methyl propanol | 9 ± 0.9 | 15 ± 1.0 | 10 ± 1.0 | 9 ± 1.0 | 18 ± 1.1 | 30 ± 2.0 | 20 ± 1.1 | 17 ± 1.1 | |
| Total higher alcohol | 71 ± 1.32 | 112 ± 4.51 | 77 ± 3.22 | 64 ± 3.23 | 127 ± 3.53 | 185 ± 5.61 | 144 ± 5.52 | 124 ± 4.23 | |
Results are expressed as mean ± SD (n = 3)
1–3 Means with the same letter in columns are not significantly different by duncan’s multiple range test (p < 0.05)
Sensory evaluation
The Cheongju prepared with Y190 and fermented at 15 °C received significantly higher preferences for appearance, smell, taste, quality, and overall acceptability compared with the other Cheongju (data not shown). This tendency was also observed at other fermentation temperatures (20, 25, and 30 °C). The Cheongju prepared with Y190 received high sensory scores due to the enhanced ester and higher alcohols concentration as Lee et al. [6] reported. There were no differences with regard to sensory evaluation between the control, Y263, and Y270. A similar tendency was also observed in the other Cheongju fermented at 20, 25, and 30 °C.
The results of the analysis at a fermentation temperature of 15 °C showed that Y190 was located on the right side of the PC1 axis, whereas control, Y262, and Y270 were located on the left or middle side, respectively, indicating that it is different from Y190 (Fig. 3). On observing the aroma patterns, it can be inferred that control and Y263 are yeasts with similar fermentation characteristics and that Y270 has a completely different fermentation pattern. This tendency (data not shown) was also observed at other fermentation temperatures (20, 25, and 30° C).
Fig. 3.
Analysis of the electronic nose system data for aroma pattern of Cheongju prepared with different yeasts with A. oryzae fermented at 15 °C
To date, Cheongju is prepared with Japanese yeast and rice Guk. In this study, three new yeast strains and A. oryzae 83-10 isolated from the Korean traditional nuruk were tested to investigate their brewing characteristics for the preparation of Cheongju. The results revealed that use of the yeast strains and A. oryzae 83-10 selected were very suitable and can be employed for the preparation of Korean Cheongju. In particular, the yeast Y190 with A. oryzae 83-10 showed the best results for the preparation of Cheongju with regard to fermentation pattern, chemical properties, aroma compounds, and sensory evaluation.
Acknowledgements
This study was conducted with the support of the “Cooperative Research Program for Agriculture Science & Technology Development (Establishment of brewing process for production of Korean Cheongju, PJ01007402),” Rural Development Administration, Republic of Korea.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Chang KJ, Yu TJ. Studies on the components of sogokju, and commercial yakju. Korean J. Food Sci. Technol. 1981;13:307–313. [Google Scholar]
- 2.Kim YT, Kim JH, Yeo SH, Lee DH, Im JU, Jeong ST, Choi JH, Choi HS, Hwang HJ. Uri Sul Bomulchang-go. The treasure houses of Korean liquor. The Foundation of Agri. Tech. Commercialization and Transfer, Suwon, Korea (2011)
- 3.Han EH, Lee TS, Noh BS, Lee DS. Volatile flavor components in mash of takju prepared by suing different Guks. Korean J. Food Sci. Technol. 1997;29:563–570. [Google Scholar]
- 4.Lee YJ, Yi HC, Hwang KT, Kim DH, Kim HJ, Jung CM, Choi YH. The qualities of makgeolli (Korean rice wine) made with different rice cultivars, milling degrees of rice, and Guks. Korean Soc. Food Sci. Nutr. 2012;41:1785–1791. doi: 10.3746/jkfn.2012.41.12.1785. [DOI] [Google Scholar]
- 5.Lee SJ, Ahn BH. Sensory profiling of rice wines made with Guks using different ingredients. Korean J. Food Sci. Technol. 2010;42:119–123. [Google Scholar]
- 6.Lee DH, Lee YS, Cho CH, Park IT, Kim JH, Ahn BH. The qualities of liquor distilled from ipguk (Guk) or Guk under reduced or atmospheric pressure. Korean J. Food Sci. Technol. 2014;46:563–570. [Google Scholar]
- 7.Lee TS, Choi JY. Volatile flavor components in mash of takju prepared by using Aspergillus kawachii Guks. Korean J. Food Sci. Technol. 2005;37:944–950. [Google Scholar]
- 8.So MH, Lee YS, Noh WS. Changes in microorganism and main components during takju brewing by a modified Guk. Korean Food Nutr. 1999;12:226–232. [Google Scholar]
- 9.Kwon YH, Lee AR, Kim HR, Kim JH, Ahn BH. Quality properties of Makgeolli brewed with various rice and Guk. Korean J. Food Sci. Technol. 2013;45:70–76. doi: 10.9721/KJFST.2013.45.1.70. [DOI] [Google Scholar]
- 10.Lee TS, Han EH. Volatile flavor components in mash of takju by using Rhizopus japonicus Guks. Korean J. Food Sci. Technol. 2000;32:691–698. [Google Scholar]
- 11.Lee TS, Han EH. Volatile flavor components in mash of takju prepared by using Aspergillus oryzae Guks. Korean J. Food Sci. Technol. 2001;33:366–372. [Google Scholar]
- 12.Jung HK, Park CS, Park HH, Lee GD, Lee IS, Hong JH. Manufacturing and characteristics of Korean traditional liquor, Hahyangju prepared by Saccharomyces cerevisiae HA3 isolated from traditional Guk. Korean J. Food Sci. Technol. 2006;38:659–667. [Google Scholar]
- 13.Kim HR, Kwon YH, Jo SJ, Kim JH, Ahn BH. Characterization and volatile flavor components in glutinous rice wines prepared with different yeasts of Guks. Korean J. Food Sci. Technol. 2009;41:296–301. [Google Scholar]
- 14.Shin KR, Kim BC, Yang JY, Kim YD. Characterization of yakju prepared with yeasts from fruits 1. Volatile components in yakju during fermentation. J. Korean Soc. Food Sci. Nutr. 28: 794–800 (1999)
- 15.Seo MY, Lee JK, Ahn BH, Cha SK. The changes of microflora during the fermentation of takju and yakju. Korean J. Food Sci. Technol. 2005;37:61–66. [Google Scholar]
- 16.Lee HS, Lee TS, Noh BS. Volatile flavor components in the mashes of takju prepared using different yeasts. Korean J. Food Sci. Technol. 2007;39:593–599. [Google Scholar]
- 17.Park YJ, Lee SK, Oh MJ. Studies on takju yeast. Part 1. Isolation and identification of takju yeasts. J. Korean Agric. Chem. Soc. 16: 78–84 (1973)
- 18.Kim JM, Le NT, Chung BS, Park JH, Bae JW, Madsen EL, Jeon CO. Influence of soil components on the biodegradation of benzene, toluene, ethylbenzene, and o-, m-, and p-xylenes by the newly isolated bacterium Pseudoxanthomonasspadix BD-a59. Appl. Environ. Microbiol. 2008;74:7313–7320. doi: 10.1128/AEM.01695-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toju H, Tanabe AS, Yamamoto S, Sato H. High-coverage ITS primers for the DNA-based identification of ascomycetes and basidiomycetes in environmental samples. PLoS One. 2012;7:e40863. doi: 10.1371/journal.pone.0040863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson JD, Higgins DG, Gibson TJ. Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felsenstein J. Phylogeney inference package, version 3.6a. Distributed by the author. Department of Genome Sciences, University of Washington, Seattle, USA (2002)
- 22.Park JH, Ho CH. Characteristics of takju (a cloudy korean rice wine) prepared with Guk (a traditional korean rice wine fermentation starter), and identification of lactic acid bacteria in Guk. Korean J. Food Sci. Technol. 2014;46:153–164. doi: 10.9721/KJFST.2014.46.2.153. [DOI] [Google Scholar]
- 23.Song JC, Park HJ, Shin WC. Change of takju qualities by addition of cyclodextrin during the brewing and aging. Korean J. Food Sci. Technol. 2007;29:895–900. [Google Scholar]
- 24.Lee HS, Park CS, Choi JY. Quality characteristics of the mashes of takju prepared using different yeasts. Korean J. Food Sci. Technol. 2010;42:56–62. [Google Scholar]
- 25.Jeong ST, Kwak HJ, Kim SM. Quality characteristics and biogenic amine production of makgeilli brewed with commercial Guks. Korean J. Food Sci. Technol. 2013;45:727–734. doi: 10.9721/KJFST.2013.45.6.727. [DOI] [Google Scholar]
- 26.Cho HK, Lee JY, Seo WT, Kim MK, Cho KM. Quality characteristics and antioxidant effects during makgeolli fermentation by purple sweet potato-rice Guk. Korean J. Food Sci. Technol. 2012;44:728–735. doi: 10.9721/KJFST.2012.44.6.728. [DOI] [Google Scholar]
- 27.Lee JW, Kang SA, Cheong C. Quality characteristics of distilled alcohols prepared with different fermenting agents. J. Korean Soc. Appl. Biol Chem. 2015;58:275–283. doi: 10.1007/s13765-015-0028-8. [DOI] [Google Scholar]
- 28.Kim HR, Kim JH, Bae DH, Ahn BH. Characteristics of yakju brewed from glutinous rice and wild-type yeast strains isolated from Guks. J. Microbiol. Biotechnol. 2010;20:1702–1710. [PubMed] [Google Scholar]
- 29.Maemura H, Morimura S, Kida K. Effects of aeration during the cultivation of pitching yeast on its characteristics during the subsequent fermentation of wort. J. Inst. Brew. 1998;104:207–211. doi: 10.1002/j.2050-0416.1998.tb00993.x. [DOI] [Google Scholar]
- 30.Schmidt HJ. Beschleunighthefefuehrung von reinzuchthefen. Brauwelt. 1993;44(45):2254–2274. [Google Scholar]
- 31.Cheong C, Wackerbauer K, Kang SA. Influences of aeration during propagation of pitching yeast fermentation and beer flavor. J. Microbiol. Biotechnol. 2007;17:297–304. [PubMed] [Google Scholar]
- 32.Cheong C, Wackerbauer K, Lee SK, Kang SA. Optimal conditions for propagation in bottom and top brewing yeast strains. J. Microbiol. Biotechnol. 2008;17:297–304. [PubMed] [Google Scholar]
- 33.Lee YH, Eom T, Cheong C, Cho HC, Kim IY, Lee YS, Kim MS, Yu SY, Jeong YH. Quality characteristics of spirits by different distillation and filtration. J. Korean Soc. Food Sci. Nutr. 2013;42:2012–2018. doi: 10.3746/jkfn.2013.42.12.2012. [DOI] [Google Scholar]
- 34.In HY, Lee TS, Lee DS, Noh BS. Quality characteristics of soju mashes brewed by Korean traditional method. Korean J. Food Sci. Technol. 1995;27:134–140. [Google Scholar]