Abstract
This study investigated the effect of pressure-roasted dried radish (PRDR) against oxidative stress. To prepare PRDR extract, dried radish (DR) was pressure-roasted, boiled, and then freeze-dried. Mice fed a chow diet with oral administration of distilled water (DW) (normal group) or a high-fat diet with DW (control, CON group), DR (DR group, 237 mg/kg bw/day), or PRDR (PRDR group, 237 mg/kg bw/day) (n = 8 each group) for 12 weeks. Hepatic lipid peroxidation level in the DR and PRDR groups was lower than that in the CON group, whereas hepatic glutathione level in these groups was higher (p < 0.05). Hepatic expression of nuclear factor (erythroid-derived 2)-like 2 and its related antioxidant enzymes such as catalase, glutathione S-transferase, and peroxidases was the highest in the PRDR group (p < 0.05). It is apparent that radish attenuate oxidative stress and the process of pressure roasting might contribute positively to this effect.
Keywords: Radish, Pressure-roasting, Hepatic oxidative stress, Nrf2, Antioxidant enzyme
Introduction
Antioxidative systems in the body are the principle mechanism to protect against oxidative stress [1]. Oxidative stress is a known contributing factor in DNA damage, lipid peroxidation, and protein modification, all of which can lead to the development of diseases, including cancer, diabetes, cardiovascular disease, and neurological disorders such as Alzheimer’s disease and Parkinson’s disease [2]. Consequently, the antioxidative systems in aerobic organisms are primary defense mechanism to protect the body from the oxidative damage. Antioxidant enzymes such as catalase (CAT), glutathione S-transferase (GST), glutathione peroxidases (GPx), and superoxide dismutase (SOD) are regulated by nuclear factor (erythroid-derived 2)-like 2 (Nrf2) [3]. In public practice, people are consuming tea to increase antioxidant status in the body. Antioxidant properties of various teas have been well established, in particular, with their polyphenols that contributes to the pharmacological effects of tea [4]. Tea polyphenols are flavanols, commonly known as catechins including epicatechin, epicatechin-3-gallate, epigallocatechin, and epigallocatecin-3-gallate [5].
Radish (Raphanus sativus L.) is a root vegetable that belongs to the Brassicaceae family. In folk medicine, radish has been used as a laxative, a stimulant, and a digestive aid in the treatment of stomach disorders [6], as well as for urinary complications [7]. Radish has demonstrated antimicrobial [7], lipid-lowering [8], anticancer [9], and antioxidative effects [10, 11]. The health benefits of radish are due to its antioxidant compounds, such as glucosinolates, isothiocyanates, flavonoids, and phenolics [4, 12]. In Asian countries, radish has been used for tea preparation and people consume radish tea to prevent coughing. In our previous study [13], the radical scavenging activity of hot water extracts of pressure-roasted dried radish (PRDR) was higher than that of the unheated dried radish (DR), in vitro and LLC-PK1 cell study. The Maillard reaction intermediate 5-hydroxyl methyl furfural (5-HMF) was found in PRDR but not in DR. The Maillard reaction is considered undesirable because it causes a color change in the final product, however, health benefits of Maillard reaction products such as antioxidative [14], antimicrobial [15], and probiotic activities [16] have been established.
In this study, we investigated the effects of PRDR tea consumption against the oxidative stress induced by a high-fat diet (HFD) in mice. The free radical scavenging activity of hot water extracts of PRDR and the hepatic expression levels of Nrf2 and its regulated antioxidant enzymes, including CAT, GST, and GPx were studied.
Materials and methods
Preparation of pressure-roasted dried radish
Radish (Rapharus sativus L.) harvested in a rural area of Korea (Unduryeong, Gangwon-do, Korea) was washed to remove impurities/dirt and sliced into 3 × 3 × 0.5 cm pieces. The sliced pieces were left to air dry for 1 day and then were placed in a product dryer (USD-6533F; Kyung Dong Navien, Seoul, Korea) at 50 °C for 24 h. Following this, the DR was roasted under a pressure of 4.5 kg/cm2 for 2 min. The resultant PRDR was stored in a dark place.
Preparation of hot water extracts of radish samples
The DR and PRDR were separately boiled at 100 °C for 1 h in 20 volumes of water (w/v). The hot water extracts of the radishes were filtered (No. 2 filter paper; Whatman, Springfield Mill, UK), concentrated in a rotary evaporator (R-200; Bΰchi, Flawil, Switzerland), and then freeze-dried (SFDSM06; Samwon, Busan, Korea). The freeze-dried samples were stored at −80 °C for further use.
Free radical scavenging activities
DPPH [17] and nitrite [18] radical scavenging activities were determined with 2,2-diphenyl-1-picrylhydrazyl solution and a Griess reagent, respectively. Superoxide anion [19] scavenging activity was determined using a nitrobluetetrazolium and xanthine solution.
Animals and experimental diets
C57BL/6 mice (male, 4 weeks old, n = 32) were purchased from DooYeol Biotech (Seoul, Korea). The mice were housed individually under controlled humidity (55 ± 5%) and temperature (22 ± 1 °C) conditions, with a 12 h light–dark cycle. After acclimation for 1 week, the mice were divided into 4 groups based on body weight (bw). Experimental groups were mice fed a chow diet and orally administrated distilled water (DW) (normal, NOR group), and fed a HFD and administered DW (control, CON group), DR (DR group, 237 mg/kg bw per day), or PRDR (PRDR group, 237 mg/kg bw per day). The concentration for oral administration was determined by assuming that 2 cups (1 teabag is 2 g) of tea are consumed by 60 kg adult [20]. The yield of hot water extraction of freeze-dried radish was about 35.6%. When an adult consumes 1.42 g (4 g × 35.6%) of hot water extracts, the dose concentration was calculated into 23.7 mg/kg bw (1.42 g/60 kg bw) and the metabolic rate between a human and a mouse (×10) were considered. HFD was prepared by mixing 20 g % lard into the chow diet (2018S Teklad Global 18% protein rodent diet; Harlan Teklad, Madison, WI, USA). HFD contains 14.9 g crude protein, 5.0 g fat (ether extract), 20.0 g lard, 35.4 g carbohydrate, 2.8 g crude fiber, 11.8 g neutral detergent fiber, 4.2 g ash per diet 100 g. After 12 weeks, the mice were subjected to a 12 h fasting period and then anesthetized by intraperitoneal injection of zoletil (30 mg/kg bw; Virbac Laboratories, Carros, France) and xylazine (10 mg/kg bw; Bayer Korea, Seoul, Korea). After perfusion with cold phosphate-buffered saline (PBS; 10 mM, pH 7.2) through the hepatic artery, the livers were excised and stored at −80 °C for the further use. The animal study was approved by the Pusan National University-Institutional Animal Care and Use Committee (Approval No. PNU-2015-0824).
Hepatic reactive oxygen species and peroxynitrite levels
Hepatic reactive oxygen species (ROS) and peroxynitrite (ONOO−) levels were measured in the post-mitochondrial fraction of liver tissue that had been homogenized in PBS using a polytron homogenizer (PT-MR 3100; Kinematica Inc., Lucerne, Switzerland). The ROS and ONOO− levels were quantified using 2′,7′-dichlorofluorescein diacetate [21] and dihydrorhodamine 123 buffer [22], respectively. For 30 min, the changes in the fluorescence values were determined at an excitation wavelength of 485 nm and an emission wavelength of 530 nm using a microplate reader (FLUOstar OPTIMA; BMG Labtech, Offenburg, Germany).
Hepatic thiobarbituric acid reactive substances and glutathione levels
Hepatic levels of thiobarbituric acid reactive substances (TBARS) [23] and glutathione (GSH) [24] were determined using malondialdehyde and GSH as a standard, respectively. In brief, the liver tissue homogenates and TBARS solution (0.67% thiobarbituric acid and 0.05 N HCl) were mixed and lipid oxidation was carried out at 95 °C for 30 min. The GSH level was measured using a disulfide reagent comprising 0.1 M sodium phosphate buffer (pH 8) and 0.01 M 5,5′-dithiobis(2-nitrobenzoic acid).
Western blot assay
Western blotting was performed as described in a previous study [25]. In brief, liver tissue was homogenized for 40 s in a lysis buffer (1:9, v/w) (50 mM Tris, pH 8.0, 5 mM ethylenediaminetetraacetic acid, 150 mM NaCl, and 1% nonidet-P40) containing a protease inhibitor cocktail (10 μL/mL protease inhibitor cocktail; Sigma-Aldrich Co., St. Louis, MO, USA), 1 mM dithiothreitol, and 1 mM phenylmethylsulfonyl fluoride. The supernatant of the homogenate was obtained by centrifugation at 18,627×g for 20 min at 4 °C. The protein concentration was measured using a Bio-Rad protein assay kit (500-0002; Bio-Rad Laboratories, Hercules, CA, USA). Hepatic expression of antioxidant enzymes was identified. The primary antibodies used in this study were Nrf2 (H-300) (sc-13032; Santa Cruz Biotechnology, Santa Cruz, CA, USA), CAT (F-17) (sc-34285; Santa Cruz Biotech.), GPx (B-6) (sc-133160; Santa Cruz Biotech.), and GST (B-14) (sc-138; Santa Cruz Biotech.). Protein expression was visualized by the enhanced chemiluminescence method, detected using CAS-400 (Core Bio, Seoul, Korea), and then calculated using ImageJ software (National Institutes of Health, Bethesda, MD, USA). Protein expression was normalized to that of beta-actin (ab8226; Abcam Inc., Cambridge, UK).
Statistical analysis
Values are presented as the mean ± standard deviation (SD). Student’s t-test was performed with data from in vitro analysis. For data from animal study, one-way analysis of variance (ANOVA) followed by Duncan’s multiple-range test for post hoc analysis was performed using SPSS version 20 (SPSS Inc., Chicago, IL, USA). Significance was considered at 0.05 level.
Results and discussion
In vitro radical scavenging activities of DR and PRDR
As shown in Table 1, IC50 values for DPPH, nitrite, and superoxide anion radical scavenging activities in the PRDR group were lower by 73.95, 80.08, and 77.30%, respectively, than those of DR group (p < 0.001). Several studies have suggested that products formed during the process of roasting, such as melanoidins, contribute to the elevation of antioxidant levels [13, 26]. The antioxidative properties of coffee, cereals, meat, juices or nuts against oxidation have been recognized as Maillard reaction product [14]. In addition, our previous study reported 5-HMF in PRDR not in DR, which might be produced during the pressure roasting of DR [13]. As a result, total phenol content in PRDR was higher than that of DR. Besides these antioxidant effects, antimicrobial, and probiotic activities of Maillard reaction product has been reported [15, 16, 27].
Table 1.
IC50 for radical scavenging activity of hot water extracts of DR and PRDR
| Sample | IC50 (mg/mL) | ||
|---|---|---|---|
| DPPH | Nitrite | Superoxide anion | |
| DR | 2.61 ± 0.12 | 10.54 ± 0.35 | 15.33 ± 1.12 |
| PRDR | 0.68 ± 0.02* | 2.10 ± 0.05* | 3.48 ± 0.12* |
Values are the mean ± standard deviation
* Significantly different by Student’s t-test (p < 0.001) between DR and PRDR. DPPH, 2,2-diphenyl-1-picrylhydrazyl; DR, dried radish; PRDR, pressure-roasted dried radish
PRDR suppressed hepatic oxidative stress and lipid peroxidation induced by HFD
HFD-fed mice showed significantly higher body weight gains and food efficiency ratios than the chow diet-fed mice (p < 0.05, Table 2). However, there were no significant differences among the HFD-fed groups. These data indicated that HFD increased body weights for experimental period.
Table 2.
Body weight, body weight gain, and food efficiency ratio of C57BL/6 mice fed a high-fat diet for 12 weeks
| Groupa | Body weight gain (g) | Food efficiency ratiob |
|---|---|---|
| NOR | 6.98 ± 1.23b | 0.024 ± 0.003b |
| CON | 9.15 ± 1.32a | 0.036 ± 0.004a |
| DR | 8.30 ± 1.57ab | 0.032 ± 0.007a |
| PRDR | 7.94 ± 1.15ab | 0.031 ± 0.031a |
Data are the mean ± standard deviation (n = 8 each group)
aNOR, mice fed a chow diet and administrated distilled water (DW) for 12 weeks; CON, mice fed a high-fat diet (HFD) and administrated DW for 12 weeks; DR or PRDR, mice fed a HFD and administrated 237 mg/kg body weight of hot water extract of dried radish or pressure-roasted dried radish, respectively
bFood efficiency ratio = total weight gain/total food intake
a,bData with different letters in the column are significantly different with one-way ANOVA followed by Duncan’s multiple-range test at p < 0.05
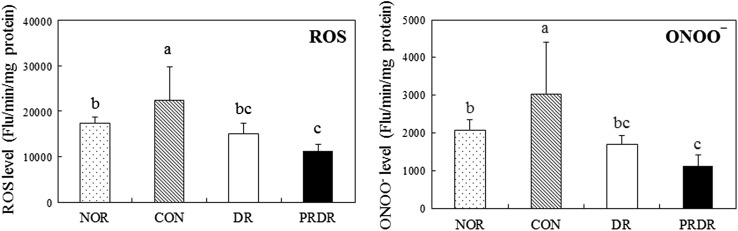
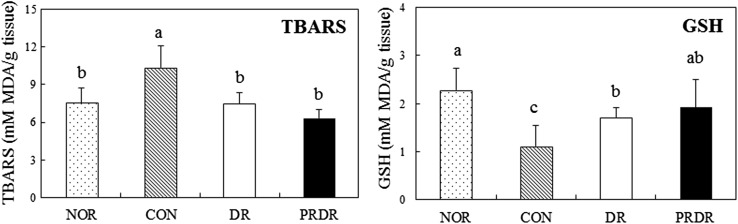
Compared with that in the NOR group, the hepatic ROS and ONOO− concentrations were significantly higher in the CON group (Fig. 1, p < 0.05). In contrast, such elevation was suppressed in the DR and PRDR groups (p < 0.05). Hepatic ROS and ONOO− levels in the PRDR group were significantly reduced by 24.98% and 34.62%, respectively, compared with the DR group (p < 0.05). The level of hepatic TBARS in the CON group was significantly higher than that in the NOR group (Fig. 2, p < 0.05). However, the level in the DR and PRDR groups was lower than that in the CON group (p < 0.05). Compared with the DR group, the TBARS level of PRDR group was lower by 15.47% but it was not significant. The GSH level in the CON group was decreased by 50% relative to that of the NOR group, due to the HFD (p < 0.05). The DR and PRDR groups had significantly higher GSH levels (by 53.15 and 72.92%, respectively) than the CON group (p < 0.05).
Fig. 1.
Effects of hot water extracts of DR and PRDR on hepatic ROS and ONOO− levels in C57BL/6 mice fed a high-fat diet for 12 weeks. Data are the mean ± SD (n = 8 each group). See the legend of Table 2 for experimental groups. a–cData with different letters are significantly different with one-way ANOVA followed by Duncan’s multiple-range test at p < 0.05. DR dried radish, PRDR pressure-roasted dried radish, ROS reactive oxygen species, ONOO −, peroxynitrite
Fig. 2.
Effects of hot water extracts of DR and PRDR on hepatic TBARS and GSH levels in C57BL/6 mice fed a high-fat diet for 12 weeks. Data are the mean ± SD (n = 8 each group). See the legend of Table 2 for experimental groups. a–cData with different letters are significantly different with one-way ANOVA followed by Duncan’s multiple-range test at p < 0.05. DR dried radish, PRDR pressure-roasted dried radish, TBARS thiobarbituric acid reactive substances, GSH glutathione
High consumption of fat elevates oxidative stress by producing lipid radicals that propagates free radical productions in the body [28]. Oxidative stress is common pathophysiological condition observed in various disease conditions. Subsequently the importance of the antioxidative systems in aerobic organisms is emphasized. Superoxide anion is generated during energy metabolism, which interacts with other radicals to generate ROS and reactive nitrogen oxygen species such as peroxynitrite, the most potent free radical in the body. Lipid peroxidation is a common pathophysiological condition observed in obesity associated liver diseases [29]. Hepatic tissue injury and TBARS level that reflects the lipid peroxidation are positively associated. GSH can act either to detoxify activated oxygen species such as H2O2 or to reduce lipid peroxides. The tissue GSH concentration reflects the potential for detoxification [30]. Diverse foods with antioxidant activity including tea have been studied with regard to their regulatory function against oxidative stress. The health promoting effects of tea, in particular, polyphenols compounds in green tea in regard to pharmacological activities of epicatechin, epicatechin-3-gallate, epigallocatechin, and epigallocatecin-3-gallate have been extensively studied and their effects are well documented [4, 5, 12]. Our previous study showed that scavenging of the superoxide anion, ONOO−, and hydroxyl radicals was increased in cells treated with PRDR, compared with those treated with DR [13]. LLC-PK1 cells induced by oxidative stress showed increased TBARS levels, which was reduced in the PRDR-treated cells compared with the DR-treated and control cells [13]. These results suggested that PRDR had antioxidative effects against the formation of free radicals. Total phenolic compounds of DR were increased by pressurized roasting [27].
PRDR increased hepatic antioxidant enzyme status via up-regulation of Nrf2
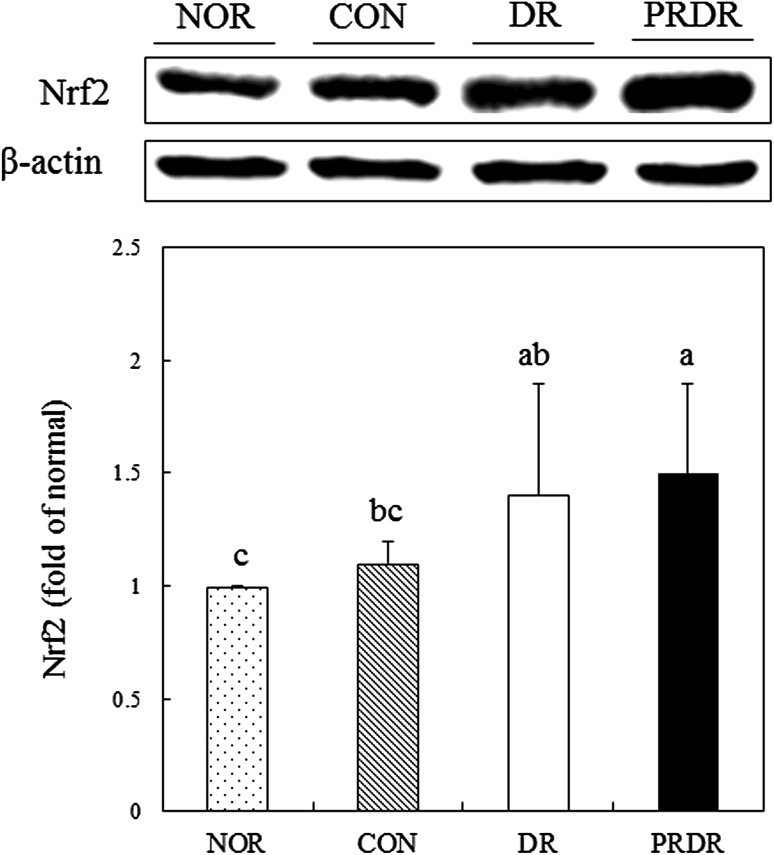
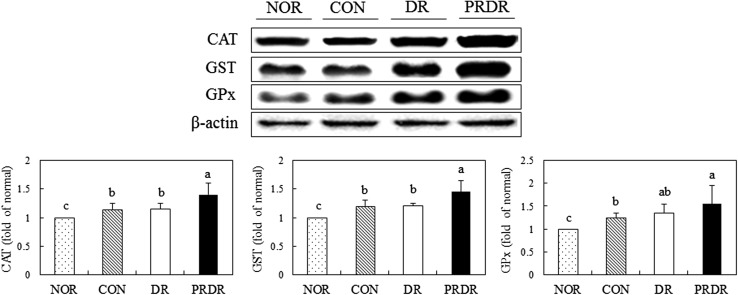
As shown in Fig. 3, the protein expression level of Nrf2 in DR and PRDR groups significantly increased compared with the CON group (p < 0.05). Particularly, that of the PRDR groups was significantly higher by 39.26% than the CON group. The protein expression levels of CAT, GST, and GPx in the PRDR group were significantly increased by 22.55, 20.78, and 27.19%, respectively, relative to the levels in the CON group (Fig. 4, p < 0.05). Particularly, the CAT and GST expression levels in the PRDR group were significantly higher than those in the DR groups (p < 0.05).
Fig. 3.
Effects of hot water extracts of DR and PRDR on the Nrf2 level in C57BL/6 mice fed a high-fat diet for 12 weeks. Data are the mean ± SD (n = 8 each group). See the legend of Table 2 for the experimental groups. a–cData with different letters are significantly different with one-way ANOVA followed by Duncan’s multiple-range test at p < 0.05. DR dried radish, PRDR pressure-roasted dried radish, Nrf2 nuclear factor (erythroid-derived 2)-like
Fig. 4.
Effects of hot water extracts of DR and PRDR on CAT, GST, and GPx levels in C57BL/6 mice fed a high-fat diet for 12 weeks. Data are the mean ± SD (n = 8 each group). See the legend of Table 2 for the experimental groups. a–cData with different letters are significantly different with one-way ANOVA followed by Duncan’s multiple-range test at p < 0.05. DR dried radish, PRDR pressure-roasted dried radish, CAT catalase, GST glutathione S-transferase, GPx glutathione peroxidase
Antioxidant enzymes such as CAT, GST, GPx, heme oxygenase-1, and NAD(P)H:quinone oxido-reductase are regulated by Nrf2 [3]. As oxidative stress is elevated then, Nrf2-mediated transcription for antioxidant enzymes are up-regulated [3, 31]. In this study, we observed that protein expression of CAT, GST, and GPx in the PRDR group was significantly increased through Nrf2 up-regulation. Although Nrf2 levels of the DR and PRDR groups were not significant, 7.1% increase in Nrf2 expression of PRDR than DR has been demonstrated. This slight increase in Nrf2 expression might partially attribute to significant differences in CAT and GST expressions between PRDR and DR group. In addition, radical scavenging activities of PRDR from in vitro test were significantly higher than DR. Our results were in good agreement with a previous study, wherein radish improved the antioxidant status and protected liver cells against oxidative stress in BALB/c mice [10, 11]. Sulforaphane, active compound in radish, up-regulated the expression of GST via the Nrf2 pathway in rat cardiomyocytes [32]. Unexpectedly, the expression of Nrf2 and its target genes in the HFD group were higher than the NOR group due to an adaptive response occurred during long-term feeding of a HFD [3]. This result was in line with a previous study that also showed elevated Nrf2 and its target genes in HFD-fed mice [33].
In this study, we observed that pressure roasting process used for radish tea production has beneficial effects against oxidative stress. In Asian countries, radish tea has been consumed to prevent coughing [34]. In addition, radish revealed its health benefits on antioxidative effects [10, 11], antimicrobial [7], lipid lowering [8], and anticancer [9]. Glucosinolates, isothiocyanates, flavonoids, and phenolics in radish are cited as functional compounds [35]. In particular, pharmaceutical effects of sulfur containing compounds such as glucosinolates and isothiocyanates in radish have been recognized. In conclusion, PRDR tea consumption might improve the hepatic antioxidant status thereby decrease hepatic oxidative stress via up-regulation of Nrf2 and antioxidant enzymes. Our study would contribute to the study for health benefits of radish tea.
Acknowledgements
This work was supported by a 2-year Research Grant from Pusan National University.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interests.
References
- 1.Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, Dhama K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. Biomed. Res. Int. 2014;2014:761264. doi: 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valko M, Jomova K, Rhodes CJ, Kuča K, Musílek K. Redox-and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016;90:1–37. doi: 10.1007/s00204-015-1579-5. [DOI] [PubMed] [Google Scholar]
- 3.Regoli F, Giuliani ME. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014;93:106–117. doi: 10.1016/j.marenvres.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Yen GC, Chen HY. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995;43:27–32. doi: 10.1021/jf00049a007. [DOI] [Google Scholar]
- 5.Azam S, Hadi N, Khan NU, Hadi SM. Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: implications for anticancer properties. Toxicol. in vitro. 2004;18:555–561. doi: 10.1016/j.tiv.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Kapoor LD. Handbook of Ayurvedic medicinal plants: Herbal reference library. Boca Raton, USA: CRC Press; 2000. [Google Scholar]
- 7.Beevi SS, Mangamoori LN, Dhand V, Ramakrishna DS. Isothiocyanate profile and selective antibacterial activity of root, stem, and leaf extracts derived from Raphanus sativus L. Foodborne Pathog. Dis. 2009;6:129–136. doi: 10.1089/fpd.2008.0166. [DOI] [PubMed] [Google Scholar]
- 8.An SJ, Kim MK. Effect of dry powders, ethanol extracts and juices of radish and onion on lipid metabolism and antioxidative capacity in rats. Korean J. Nutr. 2001;34:513–524. [Google Scholar]
- 9.Murillo G, Mehta RG. Cruciferous vegetables and cancer prevention. Nutr. Cancer. 2001;41:17–28. doi: 10.1080/01635581.2001.9680607. [DOI] [PubMed] [Google Scholar]
- 10.Salah-Abbès JB, Abbès S, Abdel-Wahhab MA, Oueslati R. Raphanus sativus extract protects against Zearalenone induced reproductive toxicity, oxidative stress and mutagenic alterations in male Balb/c mice. Toxicon. 2009;53:525–533. doi: 10.1016/j.toxicon.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Salah-Abbès JB, Abbès S, Ouanes Z, Houas Z, Abdel-Wahhab MA, Bacha H, Oueslati R. Tunisian radish extract (Raphanus sativus) enhances the antioxidant status and protects against oxidative stress induced by zearalenone in Balb/c mice. J. Appl. Toxicol. 2008;28:6–14. doi: 10.1002/jat.1240. [DOI] [PubMed] [Google Scholar]
- 12.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 13.Song YB, Choi JS, Lee JE, Noh JS, Kim MJ, Cho EJ, Song YO. The Antioxidant effect of hot water extract from the dried radish (Raphanus sativus L.) with pressurized roasting. J. Korean Soc. Food Sci. Nutr. 39: 1179–1186 (2010)
- 14.Kitts DD, Chen XM, Jing H. Demonstration of antioxidant and anti-inflammatory bioactivities from sugar–amino acid Maillard reaction products. J. Agric. Food Chem. 2012;60:6718–6727. doi: 10.1021/jf2044636. [DOI] [PubMed] [Google Scholar]
- 15.Wu S, Hu J, Wei L, Du Y, Shi X, Zhang L. Antioxidant and antimicrobial activity of Maillard reaction products from xylan with chitosan/chitooligomer/glucosamine hydrochloride/taurine model systems. Food Chem. 2014;148:196–203. doi: 10.1016/j.foodchem.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 16.Reichardt N, Gniechwitz D, Steinhart H, Bunzel M, Blaut M. Characterization of high molecular weight coffee fractions and their fermentation by human intestinal microbiota. Mol. Nutr. Food Res. 2009;53:287–299. doi: 10.1002/mnfr.200700509. [DOI] [PubMed] [Google Scholar]
- 17.Hatani T, Edamatsu R, Hiramatsu M, Mori A, Fujta Y, Yasuhara T, Yoshida T, Okuda R. Effect of the interaction of tannins with Co-existing substances. VI. Effects of tannins and related polyphenols on superoxide anion radical, and on 1,1-diphenyl-2-picrylhydrazyl radical. Chem. Pharm. Bull. 1989;37:2016–2021. doi: 10.1248/cpb.37.2016. [DOI] [Google Scholar]
- 18.Kato H, Lee IE, Cheyen NV, Kim SB, Hayse F. Inhibition of nitrosamine formation by nondialyzable melanoidins. J. Agric. Food Chem. 1987;51:1333–1339. [Google Scholar]
- 19.Candan F, Sokme S. Effects of Rhus coriaria L. (Anacardiaceae) on lipid peroxidation and free radical scavenging activity. Phytother. Res. 2004;18:84–86. doi: 10.1002/ptr.1228. [DOI] [PubMed] [Google Scholar]
- 20.Sasazuki S, Inoue M, Hanaoka T, Yamamoto S, Sobue T, Tsugane S. Green tea consumption and subsequent risk of gastric cancer by subsite: the JPHC Study. Cancer Causes Control. 2004;15:483–491. doi: 10.1023/B:CACO.0000036449.68454.42. [DOI] [PubMed] [Google Scholar]
- 21.Ali SF, Lebel CP, Bondy SC. Reactive oxygen species formation as a biomaker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology. 1991;13:637–648. [PubMed] [Google Scholar]
- 22.Kooy NW, Royall JA, Ischiropoulos H, Beckman JS. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radical Biol. Med. 1994;16:149–156. doi: 10.1016/0891-5849(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 23.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 24.Ellman M. A spectrophotometric method for determination of reduced glutathione in tissues. Anal. Biochem. 1959;74:214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- 25.Jung K, Hong SH, Kim M, Han JS, Jang MS, Song YO. Antiatherogenic effects of Korean cabbage kimchi with added short arm octopus. Food Sci. Biotechnol. 2015;24:249–255. doi: 10.1007/s10068-015-0033-z. [DOI] [Google Scholar]
- 26.Kim S, Kim M, Song YB, Cho MK, Song YO. Development of low calorie roasted radish tea beverage with anti-oxidant activity. Food Sci. Biotechnol. 2016;25:113–118. doi: 10.1007/s10068-016-0107-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cioroi M. The antioxidant character of melanoidins. Czech J. Food Sci. 2000;18:103–105. [Google Scholar]
- 28.Le Lay S, Simard G, Martinez MC, Andriantsitohaina R. Oxidative stress and metabolic pathologies: from an adipocentric point of view. Oxid. Med. Cell. Longev. 2014;2014:908539. doi: 10.1155/2014/908539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Šebeková K, Kupčová V, Schinzel R, Heidland A. Markedly elevated levels of plasma advanced glycation end products in patients with liver cirrhosis–amelioration by liver transplantation. J. Hepatol. 2002;36:66–71. doi: 10.1016/S0168-8278(01)00232-X. [DOI] [PubMed] [Google Scholar]
- 30.Vijayakumar RS, Nalini SN. Antioxidant efficacy of black pepper (Piper nigrum L.) and piperine in rats with high fat diet induced oxidative stress. Redox Rep. 2004;9:105–110. doi: 10.1179/135100004225004742. [DOI] [PubMed] [Google Scholar]
- 31.Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radical Biol. Med. 2004;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 32.Leoncini E, Malaguti M, Angeloni C, Motori E, Fabbri D, Hrelia S. Cruciferous vegetable phytochemical sulforaphane affects phase II enzyme expression and activity in rat cardiomyocytes through modulation of Akt signaling pathway. J. Food Sci. 2011;76:175–181. doi: 10.1111/j.1750-3841.2011.02311.x. [DOI] [PubMed] [Google Scholar]
- 33.Kim S, Sohn I, Ahn JI, Lee KH, Lee YS, Lee YS. Hepatic gene expression profiles in a long-term high-fat diet-induced obesity mouse model. Gene. 2004;340:99–109. doi: 10.1016/j.gene.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Bae R, Lee YK, Lee SK. Changes in nutrient levels of aqueous extracts from radish (Raphanus sativus L.) root during liquefaction by heat and non-heat processing. Korean J. Hortic. Sci. Technol. 2012;30:409–416. doi: 10.7235/hort.2012.11141. [DOI] [Google Scholar]
- 35.Takaya Y, Kondo Y, Furukawa T, Niwa M. Antioxidant constituents of radish sprout (Kaiware-Daikon), Raphanus sativus. J. Agric. Food Chem. 2003;51:8061–8066. doi: 10.1021/jf0346206. [DOI] [PubMed] [Google Scholar]