Abstract
A face-centered factorial design was used to study the influence of temperature, cellulase, and pectinase concentration on the production of Saccharomyces boulardii cells during simultaneous saccharification and fermentation of organic and conventional apple substrate pulp. The effects of the variables fermentation temperature (25–35 °C), pectinase concentration (5–25 μL/100 g), and cellulase concentration (4–8 μL/100 g) were analyzed by multiple regression and polynomial models of second order, providing the ideal conditions for yeast cultivation. Cellular production of apple substrates was expressed in log CFU/mL. The optimum condition for temperature was 27.5 °C, and 20 and 5 μL/100 g for pectinase and 8 and 7 μL/100 g for cellulase concentrations for organic and conventional apple pulp, respectively. The observed viability values were in agreement with the predicted values of 8.352 log CFU/mL (organic) and 8.317 log CFU/mL (conventional) apple pulps, thus proving the effectiveness of the models.
Keywords: Probiotic production, SSF, Cellulase, Pectinase, Malus domestica
Introduction
The probiotic yeast, Saccharomyces boulardii, has great therapeutic potential. Its efficacy against gastrointestinal diseases has been associated with the fact that they block cell signaling molecules that promote intestinal inflammation. They also maintain cell physiology and interfere with attachment of pathogens, interacting with normal microbiota and acting as immune regulators [1–3].
As it is Saccharomyces spp., the fermentation process leads to the production of alcohol, which is considered the major metabolite. The formation of secondary compounds through other metabolic pathways for growth and cellular mass production is also reported [4]. The addition of oxygen, an important factor in the synthesis of lipid constituents of the cell membrane of the yeast cell mass, promotes greater synthesis [5].
Soil quality, climate, and crop nutrition are factors that can impact directly on the characteristics of the fruit [6, 7]. According to Knight and Newman [8], organic cultivation is gaining wide recognition for being more sustainable. Some studies reported that vegetables grown on an organic system contain higher levels of vitamins, minerals, and bioactive compounds than conventional cultivation [9–11].
Apple also has other carbohydrates such as pectin and cellulose, which provide viscosity to the fruit pulp. The synergistic effect of the combination of cellulase and pectinase is a decisive part in the enzymatic treatment for the liquefying process. The enzymatic hydrolysis of cell walls increases the reducing sugar content, total soluble solids, galacturonic acid, and titratable acidity of the pulp, generating a product with low viscosity [12–14].
When simultaneous saccharification and fermentation takes place, enzymes hydrolyze polysaccharides to sugars, which are immediately consumed by the yeasts [15]. This process requires an ideal temperature condition for the enzymes and yeasts as well as an enzyme concentration sufficient to release the sugars [14, 16].
The objective of this study was to check the influence of temperature and concentrations of cellulase and pectinase on the production of Saccharomyces boulardii cells during simultaneous saccharification and fermentation in conventional and organic apple pulp substrates using factorial design with face-centered star points (α = ±1).
Materials and methods
Raw material
Raw materials tested as a carbon source in the experiments were apples grown in organic (ORG) and conventional (CONV) systems. The cultivar “Fuji” industrial standard (season 2014/2015) purchased from Urupema region producers, Santa Catarina (Brazil) were used. The apples were washed, sanitized, and crushed in a processor (Walita Master RI7633, Brazil) with 5 g/L of ascorbic acid to prevent browning of the fruit. After that, the obtained apple pulp was packed in polythene bags and stored at −20 °C.
Enzymes
The enzymes Celluclast® 1.5 L (cellulase) and Novozym® 33095 (pectinase) were provided by NOVOZYMES®, Denmark. The Celluclast® 1.5 L, according to the manufacturer, has enzymatic activity of 700 EGU/g (endo-glucanase units/g), which is considered an endo-glucanase that hydrolyzes the (1,4)-β-d-glucosidic bonds in cellobiose and glucose. The optimum conditions of the enzyme activity according to the International Union of Pure and Analytical Chemistry (IUPAC) occurs at pH 4.8 and 50 °C [17].
Novozym® 33095 has an enzyme activity of 10,000 PECTU/mL (pectin transeliminase units/mL) and its main component is pectin lyase, which catalyzes the cleavage of bonds (1,4)-α-d-galacturonan methyl ester, acting from 15 to 60 °C and pH 3.0–5.0.
Characterization of organic and conventional apple substrates
The characterization of organic and conventional apples was conducted through the determination of protein, moisture, pH, titratable acidity, and total soluble solids, according to AOAC [18]. The minerals were determined in a plasma spectrophotometer induced by argon (ICP/OES, Optima 8300—Perkin Elmer®, USA) and the reducing and total sugars were determined by UV–Vis (Genesys 10S UV–Vis, Thermos ScientificTM) according to the methodologies described by Somogyi [19] and Dubois et al. [20], respectively.
Result values (triplicates) were subjected to Tukey’s test at 5% significance level using Statistica software version 10.0 for comparing possible differences in the composition of organic and conventional apple pulp substrates.
Microorganism and inoculum preparation
The culture of Saccharomyces boulardii was obtained from commercial products in a lyophilized form (Floratil® Merck S.A. Brazil). Each capsule of 100 mg contained at least 0.5 × 109 cells of S. boulardii-17.
Lyophilized yeast was reactivated in 50 mL YPD broth (yeast extract 10 g/L; bacteriological peptone, 20 g/L; dextrose, 20 g/L) previously sterilized by autoclaving (121 °C for 20 min) and maintained at 30 °C, 120 rpm for 24 h on a rotatory shaker incubator (CT-712 Cientec, Brazil). Results of the preliminary tests indicated that after 24 h, the microorganisms were in the stationary phase of proliferation.
Simultaneous saccharification and fermentation (SSF)
SSF was conducted by cell mass inoculation (S. boulardii) in the conventional or organic apple substrate in the presence of enzymes Celluclast® 1.5 L and Novozym® 33095.
The yeast biomass obtained after incubation in YPD broth (100 g/L) was centrifuged at 3000×g for 15 min (Eppendorf 5804 R, Germany) and was inoculated into vials containing 100 g of apple pulp that is previously pretreated at 80 °C for 20 min. The initial count at zero time for both the organic and conventional substrates was about 6 log CFU/mL.
The optimization of the cellular production of S. boulardii (Table 1) was evaluated by means of three independent variables. The growth temperature values (X1) were preset at 25–35 °C for growth of S. boulardii according to the literature [21, 22]. The concentration of pectinase (X2) ranged from 5 to 25 μL/100 g, according to the manufacturer’s specifications (NOVOZYMES®, Denmark), and the concentration of cellulase (X3) varied from 4 to 8 μL/100 g.
Table 1.
Coded and real values and the response obtained by the application of a central composite design during SSF in organic (YORG) and conventional (YCONV) apples
| Run order | Process parameters | Responses parameters | |||
|---|---|---|---|---|---|
| Levels, coded and real variables | YORG | YCONV | |||
| x1/X1 | x2/X2 | x3/X3 | |||
| 1 | −1 (25) | −1 (5) | −1 (4) | 8.237 ± 0.006 | 8.176 ± 0.001 |
| 2 | −1 (25) | −1 (5) | 1 (8) | 8.290 ± 0.001 | 8.279 ± 0.011 |
| 3 | −1 (25) | 1 (25) | −1 (4) | 8.217 ± 0.013 | 8.204 ± 0.013 |
| 4 | −1 (25) | 1 (25) | 1 (8) | 8.284 ± 0.017 | 8.190 ± 0.001 |
| 5 | 1 (35) | −1 (5) | −1 (4) | 8.224 ± 0.020 | 8.051 ± 0.010 |
| 6 | 1 (35) | −1 (5) | 1 (8) | 8.255 ± 0.001 | 8.161 ± 0.001 |
| 7 | 1 (35) | 1 (25) | −1 (4) | 7.989 ± 0.011 | 8.097 ± 0.001 |
| 8 | 1 (35) | 1 (25) | 1 (8) | 8.190 ± 0.001 | 8.011 ± 0.053 |
| 9 | −1 (25) | 0 (15) | 0 (6) | 8.306 ± 0.005 | 8.312 ± 0.011 |
| 10 | 1 (35) | 0 (15) | 0 (6) | 8.114 ± 0.017 | 8.138 ± 0.024 |
| 11 | 0 (30) | −1 (5) | 0 (6) | 8.267 ± 0.001 | 8.284 ± 0.017 |
| 12 | 0 (30) | 1 (25) | 0 (6) | 8.243 ± 0.001 | 8.267 ± 0.001 |
| 13 | 0 (30) | 0 (15) | −1 (4) | 8.224 ± 0.006 | 8.230 ± 0.001 |
| 14 | 0 (30) | 0 (15) | 1 (8) | 8.347 ± 0.005 | 8.249 ± 0.006 |
| 15 | 0 (30) | 0 (15) | 0 (6) | 8.322 ± 0.001 | 8.301 ± 0.021 |
| 16 | 0 (30) | 0 (15) | 0 (6) | 8.322 ± 0.021 | 8.301 ± 0.001 |
| 17 | 0 (30) | 0 (15) | 0 (6) | 8.301 ± 0.001 | 8.236 ± 0.006 |
| 18 | 0 (30) | 0 (15) | 0 (6) | 8.337 ± 0.015 | 8.267 ± 0.001 |
x1/X1: temperature, codified/real (°C); x2/X2: concentration of pectinase, codified/real (μL/100 g substrate); x3/X3: concentration of cellulase encoded/real (μL/100 g substrate); YORG and YCONV: cell viability in log CFU/mL in fermented organic and conventional apple pulp, respectively. The randomization of codified data is generated by the Statistica software
Fermentation occurred in a shaker incubator at 120 rpm and different temperatures, according to the experimental design (Table 1). Samples were taken after 24 h of fermentation and viability of cells were counted in plates.
Determining the number of viable cells
The cell counts were taken after serial dilutions in 1 g/L sterile peptone water up to 10−8. Aliquots (100 µL) of the appropriate dilutions were plated on YPD agar and incubated at 30 °C for 24 h. The results are expressed as log CFU/mL.
Experimental design
For the optimization processes, a 23 factorial design with face-centered star points (α = ±1) and two repetitions of central points (total of 18 experiments) were selected. This design was chosen because the axial points are at the center of each face of the factorial space, requiring only three levels for each factor [23]. The levels of factors used are shown in Table 1, where (−1), (0), and (+1) indicate the low level of each factor, mid-level, and the high level, respectively.
The experimental data were designed using Statistica software version 10.0. A complete quadratic polynomial regression model was used to correlate the experimental data using the following equation:
| 1 |
where Y is the cell viability response variable (log CFU/mL), xi are the factors of the process, including fermentation temperature and concentration of cellulase and pectinase, β0 is the compensation coefficient, βi are linear coefficients, βii are quadratic coefficients, and βij are interaction coefficients [24].
The polynomial equations established were used to map three-dimensional (3D) and two-dimensional (2D) surfaces in order to view the individual and interactive effects of the process when one of the parameters is set at its optimum value. The impact and meaning of each term (linear, quadratic, and interactions) in the regression equation was evaluated by analysis of variance (ANOVA).
Validation of optimal conditions and predictive model
Validation of optimized conditions and predictive models was tested using the ideal conditions provided. Results of experiments performed under optimal conditions of each substrate and the average experimental values were compared with the expected values to determine the validity of the models. The growth of S. boulardii in both apple substrates, organic and conventional, was compared by Tukey’s test at a 5% level of significance in order to check the interference of the substrate.
SSF characteristics during S. boulardii cell growth using ORG and CONV optimized media
The following characteristic variables of the SSF were calculated: Maximum concentration in a dry weight of the yeast, Xmax; substrate conversion factor in cells, YX/S; substrate consumption rate, dS/dt; rate of cell production, dX/dt and % saccharification = (final reducing sugar × 0.9/carbohydrate in the substrate) × 100.
Results and discussion
Characterization of raw material
The data obtained in the characterization of apple pulp must (Table 2) showed that the system of farming, organic and conventional, affects significantly (p < 0.05) in fruit characteristics. The organic apple had higher soluble solid concentration, acidity, total sugars, proteins, and minerals. The characteristics of the substrate are of great importance for the fermentation process; many of the components, including minerals, may positively or negatively influence the proliferation and cell growth [25].
Table 2.
Physical and chemical characteristics of dry apple pulp must edible parts (with peels) cultivated by organic (ORG) and conventional (CONV) methods
| Composition of Fuji pulp apple* | ||
|---|---|---|
| ORG | CONV | |
| pH | 3.515 ± 0.002a | 3.640 ± 0.004a |
| g/100 g | ||
| Total soluble solids | 17.376 ± 0.002ª | 14.772 ± 0.063b |
| Moisture | 86.240 ± 0.027b | 88.342 ± 0.079a |
| Acidity in malic acid | 0.532 ± 0.003a | 0.424 ± 0.015b |
| Total Sugars | 12.738 ± 0.148a | 9.404 ± 0.361b |
| Reducing Sugars | 9.537 ± 0.050a | 8.512 ± 0.285a |
| Total Carbohydrates | 13.282 ± 0.005a | 11.355 ± 0.283b |
| Proteins | 0.160 ± 0.005a | 0.070 ± 0.010b |
| Ash | 0.344 ± 0.007a | 0.238 ± 0.010b |
| Micronutrients | mg/kg | |
| Aluminum | 10.390 ± 0.250ª | 9.000 ± 0.008b |
| Calcium | 110.280 ± 0.200ª | 104.150 ± 0.150b |
| Magnesium | 42.020 ± 0.020ª | 38.301 ± 0.001b |
| Nitrogen | 256.000 ± 0.002a | 112.000 ± 0.007b |
| Phosphorus | 233.650 ± 0.090ª | 178.100 ± 0.100b |
| Potassium | 933.801 ± 0.033ª | 713.700 ± 0.095b |
| Sodium | 217.200 ± 0.020ª | 196.400 ± 0.050b |
| Sulfur | 26.450 ± 0.100ª | 21.930 ± 0.010b |
* Means in the same line followed by the same letter in a row are not significantly different (p < 0.05)
Saccharomyces boulardii requires a carbon source of chemical energy obtained by carbohydrates, especially sugars. Other micronutrients are also required, such as nitrogen, phosphorus, sulfur, potassium, magnesium, calcium, aluminum, sodium, and other trace elements that play important roles in cellular metabolism, essential for cell growth and multiplication [25].
According to Amarante et al. [26], the organic production system improves the physical, chemical, and biological soil properties when compared to conventional production system, which needs constant fertilization. The organic production system enables more homogeneous and integrated soil, directly influencing the quality of the fruits.
Face-centered central composite design to optimize parameters in the production of S. boulardii by SSF
The responses obtained from the experimental data for cell viability during SSF of organic (YORG) and conventional (YCONV) apples are shown in Table 1.
The experimental data (Table 1) were used to develop a quadratic polynomial regression with linear and quadratic terms. The mathematical statistical models represent the response function equivalent to Eqs. 2 and 3, organic and conventional, respectively. The significant terms at 5% are shown with an asterisk (*)
| 2 |
| 3 |
Equations (2) and (3) demonstrated that linear and quadratic temperature values (x1) were the only significant values (p < 0.05) in both models. For the ORG model, linear x1 presented a p = 0.0013 and quadratic of p = 0.0042, and for CONV model, linear x1 presented a p = 0.0055 and quadratic p = 0.0372.
The interactions x1 and pectinase concentration (x2) were significant (p = 0.0074) in the ORG model and the interaction x2 and cellulase concentration (x3) was significant (p = 0.0373) in the CONV model; this means that the main effects (x1, x2, and x3) cannot be evaluated separately.
All significant terms had negative coefficients, except for linear x3 in the model for the organic apple pulp. The negative coefficient showed that with increasing pectinase concentration and temperature, the production of S. boulardii cells decreased, which is different for cellulase, whose coefficient was positive. The signal and the value of the quantitative effect represent the trend and magnitude of the influence on the response, respectively [27, 28].
To verify the adequacy and fit of the developed regression equations, ANOVA was performed. For both SSF in the organic and conventional apple substrates, the developed models were significant at a 5% probability level.
The non-significant terms were maintained in the model, because when they were removed, there was a reduction in the value of R2. The regression models were able to explain the values observed in 89 and 96% (R2) for the production of S. boulardii cells in organic and conventional apples, respectively. It was also reported that both models were significant (p < 0.05) since the values of F calculated in ORG (F cal = 6.466) and CONV (F cal = 16.522) were higher than F tabulated (F tab = 3.39).
Lack of fit was significant (p = 0.0363) for organic apples only, suggesting that the model does not accurately fit to the data. However, Waszczynskyj et al. [29] suggested that the test of lack of fit may be considered irrelevant when the mean square experimental error is low (MS E = 0.0002), which occurred in this study, confirming the validity of the predictive analytics model.
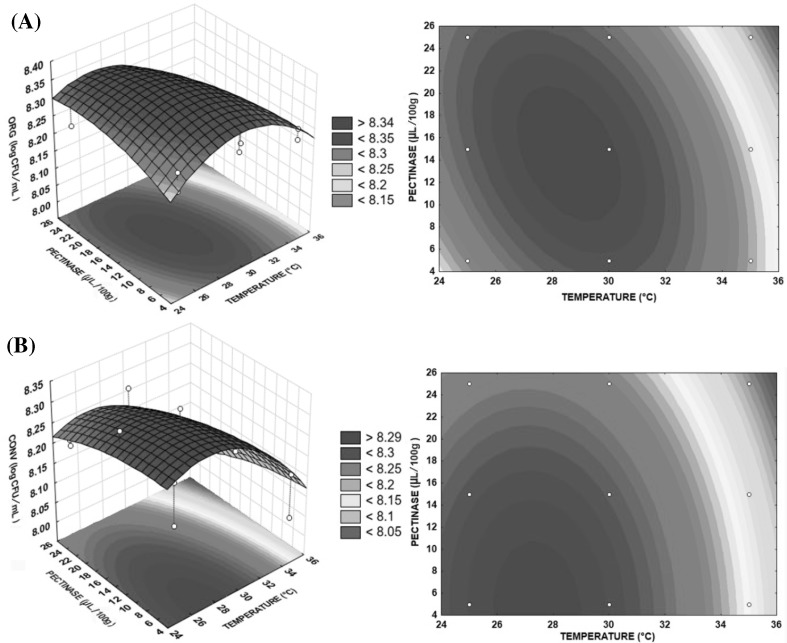
The response surfaces were generated in order to optimize the process. For each 3D surface response curve, a 2D contour corresponding curve was also constructed in order to express an infinite number of points between two independent variables, wherein the color level reflects the different responses to a concentration of S. boulardii.
Figure 1 shows the interactive effect of temperature and pectinase concentration on the cellular concentration of S. boulardii during SSF in organic (A) and conventional (B) apples. The regions with high concentrations of cells were near 28 °C, 15 μL/100 g for (A), and 5 μL/100 g for (B) revealing that increasing the temperature and the pectinase concentration at an optimum level of cellulase does not favor the growth of the yeasts studied.
Fig. 1.
Response surface 3D and 2D contour plots. S. boulardii cells (log CFU/mL) during SSF in organic (A) and conventional (B) apples, according to temperature (X1) and the pectinase concentration (X2), when fixed cellulase concentration (X3) at its optimum point
As the pH values of both apples were about 3.5 (Table 2), which were within the range of activity of the enzyme pectinase, and the temperature was in an optimum range, probably the best enzyme/substrate ratio was observed in these conditions, increasing the fluidity of the must. The enzyme Novozym® 33095 degraded pectin polymers directly by β-elimination mechanism that resulted in the formation of 4,5-unsaturated oligogalacturonides. The consequence was an increase in juice extraction, lower viscosity of the apple pulp, and a reduced quantity of waste pomace [30, 31].
However, the temperature effect was more significant in the model than the effect of pectinase concentration. As both linear and quadratic temperature terms were significant (p < 0.05) in each substrates and the quadratic term has a negative sign, a maximum is observed within the range studied (Fig. 1). According to Pardo et al. [32], the best biomass production of S. boulardii in shake flasks occurred at 28 °C, with a production of 8.462 log cel/mL; these values were very close to those obtained in this study, because between 25 and 30 °C, the cell production was more than 8.340 ± 0.020 log CFU/mL (YORG) and 8.290 ± 0.020 log CFU/mL (YCONV) (Fig. 1). This suggests that in this temperature range, the yeasts found better conditions for cellular multiplication.
Figure 2 shows the effect of the interaction between temperature and concentration of cellulase on the cell concentration of S. boulardii during SSF in organic (A) and conventional (B) apples at the optimal pectinase concentration. In both (A) and (B) cases, the optimum growth of the yeast corresponded to 6 and 8 μL/100 g for cellulase enzyme and T ≈ 28 °C.
Fig. 2.
Response surface 3D and 2D contour plots. S. boulardii cells (log CFU/mL) during SSF in organic (A) and conventional (B) apples, according to temperature (X1) and the celullase concentration (X3), when fixed pectinase concentration (X2) at its optimum point
The enzyme Celluclast® 1.5 L, even under unfavorable conditions of pH and temperature, proved to be significantly efficient in the SSF process (Eq. 1). The endoglucanases promoted hydrolysis of internal cellulose chains producing oligosaccharide, cellobiose and glucose [33]. The increase in the concentration of cellulase to the maximum level, probably caused release of more glucose units, which became available to the yeast during the fermentation process.
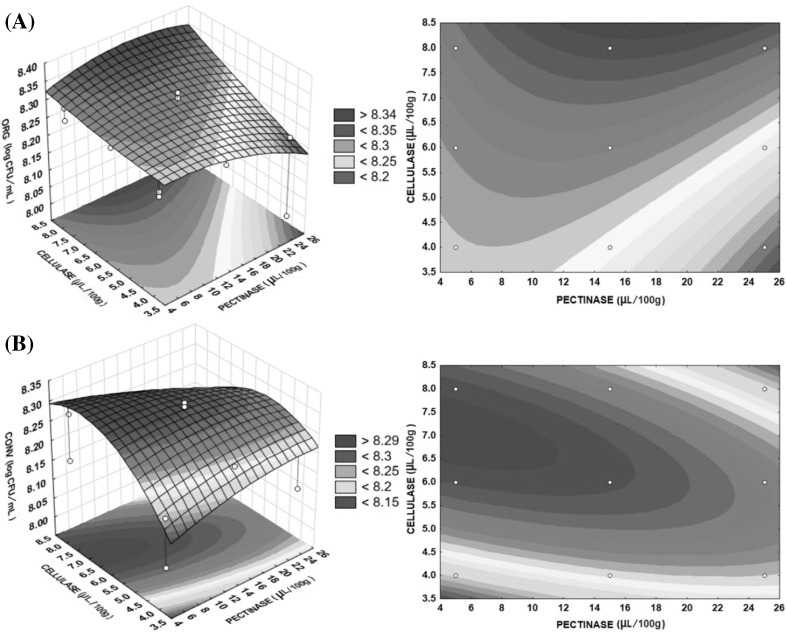
The effects of the interaction of cellulase and pectinase addition, when setting the temperature at the optimum point are indicated in Fig. 3. In conventional apple pulp, the 2D contour plot as well as the 3D response surface reiterated the tendency of increased production of yeast biomass when cellulase concentration was higher or close to 8 μL/100 g and pectinase concentration was between 5 and 15 μL/100 g. For organic apples, the better cellulase and pectinase concentrations were between 6 and 8 μL/100 g and less than 10 μL/100 g, respectively. For Wilkins et al. [15], enzymes hydrolyze polysaccharides into simple sugars, which are immediately consumed by the yeasts as they are formed, avoiding their accumulation in the medium and consequent enzymatic inhibition.
Fig. 3.
Response surface 3D and 2D contour plots. S. boulardii cells (log CFU/mL) during SSF in organic (A) and conventional (B) apples, according to pectinase concentration (X2) and celullase concentration (X3) and fixed optimum temperature (X1)
Each temperature and enzyme concentration can limit or stimulate growth and metabolic maintenance of S. boulardii. The maximum production points were temperature 27.5 °C, pectinase concentration 20 and 5 μL/100 g, and cellulase concentration 8 and 7 μL/100 g for the organic and conventional substrate apple pulp, respectively.
Comparing the concentrations of pectinase (X2) and cellulase (X3), the maximal response of the organic apple substrates was superior to that of conventional apple substrates. This can be attributed to the fact that the organic apple pulp have shown more soluble solids content and lower moisture (Table 2), thus requiring higher enzyme concentrations to achieve greater hydrolysis. This provides a less viscous substrate, allowing greater aeration of the medium and therefore more cell mass production.
Determination and validation of optimal conditions
The optimum condition for the growth of S. boulardii followed the polynomial models (Eqs. 2, 3). Validation experiments were conducted in order to compare the experimental results with predicted values, within the expected confidence interval of 95%, for the different apple substrates (Table 3).
Table 3.
Predicted and experimental values of S. boulardii cell concentration of the responses under optimal conditions for fermentation of organic (ORG) and conventional (CONV) apple pulp
| Organic | Conventional | |
|---|---|---|
| Variable | ||
| Independent | ||
| X1 (°C) | 27.5 | 27.5 |
| X2 (μL/100 g) | 20.0 | 5.0 |
| X3 (μL/100 g) | 8.0 | 7.0 |
| YORG (log CFU/mL) | YCONV log (CFU/mL) | |
|---|---|---|
| Variable dependent | ||
| Predict | 8.352a,A | 8.317a,A |
| Experimental | 8.349 ± 0.015a,A | 8.319 ± 0.016a,A |
Means followed by the same uppercase letter in the same column do not differ significantly (p < 0.05)
Means followed by the same lowercase letter in the same line do not differ significantly (p < 0.05)
Table 3 shows that although the organic apple presented higher levels of sugars (Table 2), yeast cell multiplication was not statistically higher when exposed to this substrate according to Tukey’s test (p < 0.05). Both culture media were considered suitable fermentative substrates, since products with a number higher than 8 log CFU/mL may show probiotic action.
SSF characteristics during S. boulardii cell growth using ORG and CONV optimized media
To better understand the effect of the optimized substrates on S. boulardii biomass production, SSF was conducted under optimized conditions for 24 h. At the end of this period the following mean values for sugars in ORG and CONV, respectively, were found: final total sugar concentration ATf = 112.8 and 81 g/L; final concentration of reducing sugar ARf = 86.5 and 79.22 g/L, respectively.
Therefore, the kinetic and saccharification parameters calculated for ORG and CONV were, respectively, maximum concentration of the yeast in dry weight Xmax = 4.917 and 4.812 g/L; substrate conversion factor in cells YX/S = 0.239 and 0.253; substrate consumption rate ds/dt = 0.607 and 0.617 g/L/h; rate of cell production dx/dt = 0.146 and 0.138 g/L/h; and percentage of saccharification = 58.613 and 62.790%.
The percentage of saccharification and the final concentration of total and reducing sugars were different (p < 0.05) between ORG and CONV. However, it was verified that this did not affect the culture of S. boulardii since the kinetic parameters were equal (p < 0.05) for both substrates.
Chin et al. [34] used the following optimized substrate conditions for S. boulardii culture glucose, 20; steep liquor, 15; NaNO3, 1.0; KH2PO4, 6.0; MgSO4.7H2O, 3.0; CuSO4.5H2O, 0.002; FeSO4. 7.10, 0.001; ZnSO4∙7H2O, 0.01 in g/L and found Xmax = 4 g/L at pH 4.4 and = 8.2 g/L at pH 5.5 after 16 h of culture.
The parameters found by Muller et al. [35] were close to our study: Xmax = 3.907 g/L and YX/S = 0.417 for S. boulardii cultured in a glucose-containing medium, 10; peptone, 2; yeast extract, 2; KH2PO4, 0.6; urea, 0.36; (NH4)2SO4, 0.12; and MgSO4, 0.24 g/L for 12 h.
The face-centered central composite design has been successfully used to study and optimize the individual and interactive effects of the process variables temperature, cellulase concentration, and pectinase concentration on the growth of S. boulardii cells. The model developed was able to predict the cell concentration taking into account the experimental values observed.
There were no differences between the apple substrates for the production of S. boulardii, indicating that the differences between conventional and organic apples (mainly sugar, protein, and mineral contents) did not significantly affect the cell growth.
Acknowledgements
This research was developed with the support of the Londrina State University, which provided the infrastructure and facilities. This study was supported by a scholarship from the National Council of Technological and Scientific Development and Coordination for the Improvement of Higher Education Personnel.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
References
- 1.McFarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J. Gastroentero. 2010;16(18):2202–2222. doi: 10.3748/wjg.v16.i18.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pothoulakis C. Recent advances in Saccharomyces boulardii research. Gastrin. Clin. Biol. 2010;34(1):S62–S70. doi: 10.1016/S0399-8320(10)70023-3. [DOI] [PubMed] [Google Scholar]
- 3.Czerucka D, Piche T, Rampal P. Review article: yeast as probiotics–Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2007;26:767–778. doi: 10.1111/j.1365-2036.2007.03442.x. [DOI] [PubMed] [Google Scholar]
- 4.Nelson DL, Cox MM. Lehninger Principles of Biochemistry. 4. New York: W.H. Freeman and Company; 2005. [Google Scholar]
- 5.Rosenfeld E, Beauvoit B, Blondin B, Salmon J. Oxygen consumption by anaerobic Sacharomyces cerevisiae in enological conditions: effect on fermentation kinetics. Appl. Environ. Microb. 2003;69(1):113–121. doi: 10.1128/AEM.69.1.113-121.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nogueira A, Santos LD, Paganini C, Wosiacki G. Evaluation of the alcoholic fermentation of aqueous extract of the apple pomace. Semina. 2005;26(2):187–194. [Google Scholar]
- 7.Peck GM, Andrews PK, Reganold JP, Reganold JK. Apple orchard productivity and fruit quality under organic, conventional, and integrated management. Hort. Sci. 2006;41(1):99–107. [Google Scholar]
- 8.Knight KW, Newman S. Organic agriculture as environmental reform: a cross-national investigation. Soc. Natur. Resour. 2013;26(4):369–385. doi: 10.1080/08941920.2012.687070. [DOI] [Google Scholar]
- 9.Bourn D, Prescott JA. Comparison of the nutritional value, sensory qualities, and food safety of organically and conventionally produced foods. Crit. Rev. Food Sci. 2002;42:1–34. doi: 10.1080/10408690290825439. [DOI] [PubMed] [Google Scholar]
- 10.Dangour AD, Dodhia SK, Hayter A, Allen E, Lock K, Uauy R. Nutritional quality of organic foods: a systematic review. Am. J. Clin. Nutr. 2009;90:680–685. doi: 10.3945/ajcn.2009.28041. [DOI] [PubMed] [Google Scholar]
- 11.Mazzoncini M, Antichi D, Silvestri N, Ciantelli G, Sgherri C. Organically vs conventionally grown winter wheat: effects on grain yield, technological quality, and on phenolic composition and antioxidant properties of bran and refined flour. Food Chem. 2015;175:445–451. doi: 10.1016/j.foodchem.2014.11.138. [DOI] [PubMed] [Google Scholar]
- 12.Drilleau JF. Biochemical characteristics of apple juices and fermented products from musts obtained enzymatically. Fruit Process. 1994;4:108–113. [Google Scholar]
- 13.Wilkins MR, Widmer WW, Grohmann K, Cameron RG. Hydrolysis of grapefruit peel waste with cellulase and pectinase enzymes. Bioresource Technol. 2007;98:1596–1601. doi: 10.1016/j.biortech.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 14.Parmar I, Rupasinghe HPV. Bio-conversion of apple pomace into ethanol and acetic acid: enzymatic hydrolysis and fermentation. Bioresource Technol. 2013;130:613–620. doi: 10.1016/j.biortech.2012.12.084. [DOI] [PubMed] [Google Scholar]
- 15.Wilkins M, Widmer WW, Grohmann K. Simultaneous saccharification and fermentation of citrus peel waste by Saccharomyces cerevisiae to produce ethanol. Process. Biochem. 2007;42:1614–1619. doi: 10.1016/j.procbio.2007.09.006. [DOI] [Google Scholar]
- 16.Martin C, Marcet M, Thomsen AB. Comparison between wet oxidation and steam explosion as pretreatment methods for enzymatic hydrolysis of sugarcane bagasse. Bioresource Technol. 2008;3:670–683. [Google Scholar]
- 17.Ghose TK. Measurement of cellulase activities. Pure. Appl. Chem. 1987;59(2):257–268. doi: 10.1351/pac198759020257. [DOI] [Google Scholar]
- 18.AOAC. Official Method of Analysis of AOAC Intl. 16th ed. Association of Official Analytical Chemists, Arlington, VA, USA (1995)
- 19.Somogyi M. Notes on sugar determination. J. Biol. Chem. 1952;195(1):19–23. [PubMed] [Google Scholar]
- 20.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 21.Ali MAE, Abdel-Fatah OM, Janson J, Elshafei AM. Antimicrobial potential of Saccharomyces boulardii extracts and fractions. J. App. Sci. Res. 2012;8(8):4537–4543. [Google Scholar]
- 22.Nagashima AY, Pansiera PE, Baracat MM, Gómez RJHC. Development of effervescent products, in powder and tablet form, supplemented with probiotics Lactobacillus acidophilus and Saccharomyces boulardii. Food Sci. Technol. 2013;33(4):605–611. [Google Scholar]
- 23.Rezazadeh M, Yamini Y, Seidi S, Esrafili A. One-way and two-way pulsed electromembrane extraction for trace analysis of amino acids in foods and biological samples. Anal. Chim. Acta. 2013;773:52–59. doi: 10.1016/j.aca.2013.02.030. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Luo Z, Shahbazi A. Optimization of simultaneous saccharification and fermentation for the production of ethanol from sweet sorghum (Sorghum bicolor) bagasse using response surface methodology. Ind. Crop. Prod. 2013;42:280–291. doi: 10.1016/j.indcrop.2012.06.005. [DOI] [Google Scholar]
- 25.Stehlik-Tomas V, Zetic VG, Stanzer D, Grba S, Vahcic N. Zinc, Copper and Manganese Enrichment in S. cerevisiae. Food Technol. Biotech. 2004;42(2):115–120. [Google Scholar]
- 26.Amarante CVT, Da Rosa EFF, Albuquerque JA, Klauberg Filho O, Steffens CA. Soil attributes and fruit quality in organic and conventional apple production systems in southern Brazil. Rev. Cien. Agron. 2015;46(1):99–109. doi: 10.1590/S1806-66902015000100012. [DOI] [Google Scholar]
- 27.Verma S, Lan Y, Gokhale R, Burgess DJ. Quality by design approach to understand the process of nanosuspension preparation. Int. J. Pharm. 2009;377:185–198. doi: 10.1016/j.ijpharm.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W, Lin Y, Zhang Q, Wang X, Wu D, Kong H. Optimisation of simultaneous saccharification and fermentation of wheat straw for ethanol production. Fuel. 2013;112:331–337. doi: 10.1016/j.fuel.2013.05.064. [DOI] [Google Scholar]
- 29.Waszczynskyj N, Rao CS, Silva RSF. Extraction of proteins from wheat bran: application of carbohydrases. Cereal Chem. 1981;58(4):264–266. [Google Scholar]
- 30.Immobilized pectinase for mash treatment Demir N, Acar J, Sarioğlu K, Mutlu M. The use of commercial pectinase in fruit juice industry. Part 3. J. Food Eng. 2001;46:275–280. [Google Scholar]
- 31.Yadav S, Yadav PK, Yadav D, Yadav KDS. Pectin lyase: A review. Process. Biochem. 2009;44:1–10. doi: 10.1016/j.procbio.2008.09.012. [DOI] [Google Scholar]
- 32.Pardo S, Galvagno MA, Cerrutti P. Studies of viability and vitality after freezing of the probiotic yeast Saccharomyces boulardii: physiological preconditioning effect. Rer. Iberoam. Micol. 2009;26(2):155–160. doi: 10.1016/S1130-1406(09)70028-2. [DOI] [PubMed] [Google Scholar]
- 33.Karlsson J, Momcilovic D, Wittgren B, Schulein M, Tjerneld F, Brinkmalm G. Enzymatic degradation of carboxymethyl cellulose hydrolyzed by the endoglucanases Cel5A, Cel7B, and Cel45A from Humicola insolens and Cel7B, Cel12A and Cel45Acore from Trichoderma reesei. Biopolymers. 2002;63(1):32–40. doi: 10.1002/bip.1060. [DOI] [PubMed] [Google Scholar]
- 34.Chin TS, Othman NZO, Malek RA, Elmarzugi N, Leng OM, Ramli S, Musa NF, Aziz R, Enshasy HEL. Bioprocess optimization for biomass production of probiotics yeast Saccharomyces boulardii in semi-industrial scale. J. Chem. Pharm. Res. 2015;7(3):122–132. [Google Scholar]
- 35.Muller JL, Potti KL, Machado MS, Lacerda LV, Bresolin TMB, Podlech PS. Comparison of Saccharomyces boulardii growth in an air-lift fermentor and in a shaker. Food Sci. Technol. (Campinas) 2007;27(4):88–693. doi: 10.1590/S0101-20612007000400003. [DOI] [Google Scholar]