Abstract
Polyethylene glycol (PEG) and polyethylene glycol–polylactic acid (PEG–PLA) have an organic structure and no negative effect on human health. The present study compares the antimicrobial effectiveness of PEG and PEG–PLA on microbial growth. The following pathogens and fungi were examined: seven bacteria strains and 10 fungi (four yeasts and six molds). PEG, a non-modified polymer, exhibited no inhibition effect on all test microorganisms. However, the antimicrobial effect increased with the concentration of PEG–PLA. Bacteria showed more sensitivity to PEG–PLA compared with the other microorganisms used in this study. Enterobacter ATCC 19434 was found to be the most resistant bacteria. Molds and yeasts were more resistant than bacteria against PEG–PLA. MIC and MFC could not be determined on the tested fungi owing to the level of concentrations used, with the exception of the yeast Candida albicans and the molds Penicillium expansum and Aspergillus parasiticus.
Keywords: PEG–PLA, Biodegradable, Microbial growth, MIC, MBC
Introduction
Foodborne diseases encompass a wide spectrum of illnesses and are a growing public health problem worldwide. Foodborne diseases are caused by contamination by a variety of pathogens, including bacteria, viruses, and parasites [1]. Many foodborne pathogens can cause serious illness, hospitalization, and even death. Human foodborne infections often lead to a shutdown of the food supply network with serious negative financial consequences for producers. In addition, food-spoilage microorganisms produce off-flavors, affect taste, lead to discoloration of food products on the shelf, and, finally, rejection of the product by the consumer. Spoilage leads to loss of food, producer’s reputation, and economic value of food products [2].
A function of a food packaging material is to protect food products from various physical, chemical, biological, and environmental conditions such as oxygen, moisture, light, microorganisms, and physical stress. In recent years, there has been an increased demand for packaging that offers an improved shelf life for fresh, high-quality food products [3]. Polymers have replaced conventional materials in packaging applications in the food industry owing to their functionality, lightweight, ease of processing, and low cost. Unlike other chemicals used in the food industry that have toxic effects, polyethylene glycol (PEG) is a flexible, water-soluble polymer that is non-toxic, odorless, neutral, non-volatile, and non-irritating. It has an organic structure and no negative effect on human health [4]. The antioxidant and antimicrobial properties of such polymers have been of great interest because of their possible use as food-coating materials for the prevention of oxidation and for controlling pathogens and/or toxin-producing microorganisms in foods [5–7].
The rise of environmental pollution caused by synthetic polymers in developing countries has reached dangerous levels. Biodegradable polymers have a shelf life and involve the biodegradation process. Bio-based polymers are materials produced from renewable resources. Recent technological advancements have substantially improved the properties of some bio-based polymers, e.g., heat-resistant polylactic acid (PLA), thereby enabling a wider range of applications [8]. PLA can be synthesized either via condensation polymerization of lactic acid or ring-opening polymerization of lactide in the presence of a catalyst [9, 10]. It exhibits a balance of performance properties that are comparable to those of traditional thermoplastics. It can be fabricated through a variety of familiar processes and brings a new combination of attributes to packaging, including stiffness, clarity, dead fold and twist retention, low-temperature heat sealing ability, as well as an interesting combination of barrier properties such as flavor, aroma, and grease resistance. It possesses good mechanical properties, thermal plasticity, and biocompatibility; moreover, it is readily fabricated. Therefore, it is a promising polymer for various end-use applications. PLA is an inherently polar material owing to its basic repeated unit of lactic acid. Another benefit of this polymer is its resistance to aliphatic molecules such as oils and terpenes [11, 12]. PLA possesses high transparency and is an excellent material for packaging. In addition, a PLA film can be formed using conventional film-extrusion methodology. For these reasons, PLA is an excellent applicant for producing a commercial and biodegradable packaging material [3].
PEG is a flexible, water-soluble polymer and is non-toxic, odorless, neutral, nonvolatile, and non-irritating. It has no negative effect on human health. PEG has good biocompatibility and hydrophilicity as well as rapid degradability [13]. Lactic acid is used for the production of green biodegradable solvents, such as butyl lactate and other lactic acid esters, as well as for the production of non-toxic propylene glycol, which is widely used in pharmaceuticals and food processing. There is a growing demand for lactic acid in the production of biodegradable polymers [10].
During the last decade, there has been notable research in the field of polymeric materials with antimicrobial activity, which have considerable applications. The purpose of this study is to characterize the antimicrobial activity of PEG and PEG–PLA against food-spoilage microorganisms.
Materials and methods
Microorganism strains
Bacteria: Bacillus cereus ATCC 6464, Escherichia coli ATCC 25922, Salmonella Enteritidis ATCC 13076, Staphylococcus aureus ATCC 6538, Klebsiella pneumonia ATCC 700603, Enterobacter ATCC 19434, and Yersinia enterocolitica ATCC 29913; yeasts: Saccharomyces cerevisiae DSMZ 2548, Metschnikowia fructicola CBS 8853, Candida albicans ATCC 10231, and Candida oleophila ATCC 28137; and Molds: Aspergillus niger ATCC 16604, Aspergillus parasiticus ATCC 22789, Aspergillus oryzae ATCC 11499, Rhizopus oryzae ATCC 24536, Fusarium oxysporum ATCC 7601, and Penicillium expansum ATCC 16104 were all obtained from Department of Food Engineering, Faculty of Agriculture of Uludag University, Bursa, Turkey.
Preparation of polymer solutions
PEG has a molecular weight of 400 kDa, CAS Number 25322-68-3; density of 1.128 g/cm3; and melting point of 4–8 °C, (LD50 30 mL/kg); it was purchased from Sigma-Aldrich Chemie (GmbH, Munich, Germany). In addition, it is a water-soluble, clear, colorless, and viscous liquid.
Polymer synthesis was achieved via chain-opening polymerization using Sn(II)-ethyl hexanoate as the catalyst. In addition, 1 mol of PEG and 2 mol of lactic acid were placed in a 250 mL glass balloon with the addition of Sn(II)-ethyl hexanoate. This was followed by 24 h of stirring at 300 rpm at 180 °C in an oil bath attached with reverse cooling. Then, an ethyl alcohol–ether polymer solution was dissolved in dichloromethane and kept at 25 °C with petroleum ether. Purified PEG–PLA polymers were vacuum-dried at 70 °C and stored in a vacuum desiccator [14].
Concentrations of PEG and PEG–PLA with the final pH of 4.7 were prepared by dissolving PEG and PEG–PLA in distilled water at a concentration of 2 mL/100 mL (v/v). Experiments were performed with both PEG and PEG–PLA, each dissolved in three different solutions with concentrations of 1, 5, and 10%.
Antimicrobial assay
Antimicrobial activity of the polymer was tested using the plate count method [15]. In this study, Nutrient Broth (NB-Oxoid CM0501) and Nutrient Agar (NA-Oxoid CM0309) were used as bacterial growth media. Sabouraud Dextrose Broth (SDB-Difco 234000) and Sabouraud Dextrose Agar (SDA Difco 212000) were used as growth media for molds and yeasts. The microbial strains inserted were EMB (Eosin-Methylene Blue-Lactose-Saccharose-Merck 101347) agar and Blood agar (Merck 110886) from stock cultures, and they were incubated for 24 h at 37 and 30 °C [16]. Seventeen different microorganisms were used: seven bacteria strains and 10 fungi (four yeasts and six molds). Spore suspension was used for the 24-h mold culture.
Experiments were performed five times for each isolate. Fungi were cultured on Sabouraud Dextrose Agar (Difco, Detroit, MI) plates at 30 °C for 7 days. Moreover, 1 mL of spore suspension was inserted into 59 mL of the Sabouraud Dextrose broth medium. Then, 10 mL of sterile Tween 80 (1%) was added to the spore collection in order to allow the mold spores to pass through the solution. After 7 days, conidia were harvested via centrifugation (Hettich, Eba 3S, Germany) at 1000 rpm for 15 min and washed with 10 mL of sterile distilled water. This step was repeated three times, and the spore suspension was stored in sterile, distilled water (30 mL) at 4 °C until used. The concentration of spores in the suspension was determined through a viable spore count on the Sabouraud Dextrose Agar plates using the spread plate and surface count techniques [17, 18]. After incubation, the young cultures were used for microbial growth analysis.
Determination of the minimum inhibition concentration (MIC) using a tube-dilution method
Rapid identification and quantitative determination of antimicrobial susceptibility based on the minimum inhibitory concentration (MIC) was performed using a tube-dilution method [19–21]. The inhibition effect of the three polymer concentrations was measured. PEG and PEG–PLA concentrations were applied frequently instead of two times per dilution method, as reported previously. For this reason, the microbial inhibition effect was observed at every concentration level. In addition, 4 mL of the serial dilutions were inserted in NB (for bacterial growth) and SDB (for yeast and mold growth) media. The maximum dose was 100 mg/mL. Next, 1-mL portions of each concentration were added to test tubes containing 4 mL of the special medium. The microbial inoculation level for each dilution tube was 50 µL (bacterial cell account 106, yeast and mold account 104), which was prepared from 24-h broth cultures and added to the tubes that contained the PEG and PEG–PLA concentrations and the appropriate medium. These tubes were then incubated at 30 °C for 72 h. The lowest concentration at which there was no visible turbidity was defined as the MIC concentration. The results (shown in Table 1) were recorded in terms of MIC (mg/mL) percent activity values, which demonstrate the total antimicrobial potency of each polymer concentration, as described by Rangasamy et al. [22].
Table 1.
Antimicrobial characterization of PEG–PLA (10%) on the tested microorganisms
| Microorganisms | MIC | MBC–MFC |
|---|---|---|
| (mg/mL) | ||
| Bacillus cereus ATCC 6464 | 25 | 100 |
| Escherichia coli ATCC 25922 | 37.5 | 50 |
| Salmonella Enteritidis ATCC 13076 | 50 | 75 |
| Staphylococcus aureus ATCC 6538 | 50 | 100 |
| Klebsiella pneumonia ATCC 700603 | 50 | 75 |
| Enterobacter ATCC 19434 | 75 | 100 |
| Yersinia enterocolitica ATCC 29913 | 25 | 50 |
| Candida albicans ATCC 10231 | 100 | 100 |
| Aspergillus parasiticus ATCC 22789 | 100 | 100 |
| Penicillium expansum ATCC16104 | 50 | 100 |
MIC Minimum inhibitory concentration, MBC minimum bactericidal concentration, MFC minimum fungicidal concentration
Determination of the minimum bactericidal concentration (MBC) and minimum fungicidal concentration (MFC)
Using the results obtained from the MIC assay, the concentrations showing complete absence of visual growth of microorganisms were identified, and 100 μL of each culture broth was transferred and spread on NA for bacteria and on SDA for molds and fungi in preparation for colony counting. The plates were incubated at 37 °C for 48 h for bacteria, at 30 °C for 48 h for yeasts, and at 30 °C for 72–96 h for fungi. The complete inhibition of growth on the agar surface at the lowest concentration was defined as the minimum bactericidal concentration (MBC) and minimum fungicidal concentration (MFC) [23].
Statistical analysis
Data analyses were performed using the SPSS software (SPSS 11.5 SPSS Inc, Chicago, IL, USA). Standard deviation was calculated via analysis of variance using the Minitab 14.0 software (Minitab 14.0 software, State College, PA, USA). Duncan’s multiple-range test (P < 0.05; P < 0.01) determined the differences between variances using an MSTAT statistical package. The reported values of microbial growth are the mean ± standard deviation of triplicate determinations. Results of the variance analysis showed that each significance level in the LSD multiple-range test for three factors (i.e., PEG type, time, and interaction between microbial growth and polymer types) was determined to be P < 0.01.
Results and discussion
Polymer
PEG–PLA has a molecular weight of 30.000 (5.000–100.000) kDa, density of 1400 kg/m3 (1100–1700 kg/m3), melting point of 145 °C (130–180 °C), and a pH of 3.7–5.7. PEG–PLA has an organic structure and no negative effect on human health, unlike the toxic effects found with other chemicals. It has the capacity to negatively influence microorganisms.
Bacterial growth
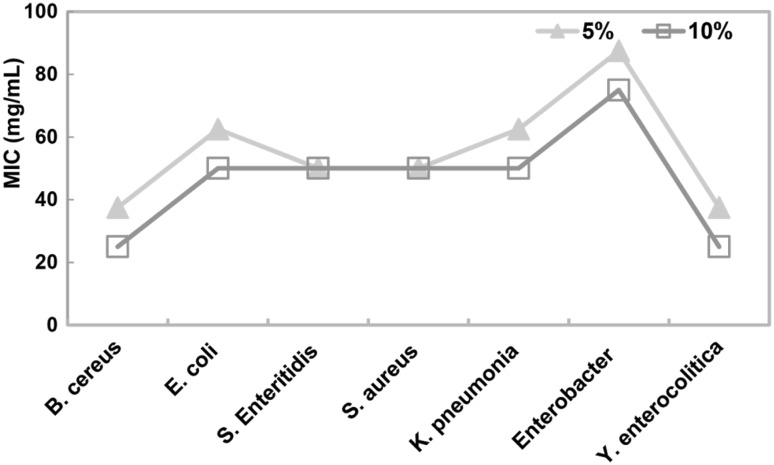
MIC was established as the lowest concentration of polymer that inhibited the visible growth of bacteria. PEG, in each of the three concentrations, did not show any inhibition effect on any of the microorganisms tested. In addition, PEG–PLA did not display antimicrobial effect at a 1% concentration. Steven and Hotchkiss [24] reported that PEG exhibits antimicrobial activity only at high concentrations (80 mg/mL in liquid media). Bacteria showed more sensitivity to PEG–PLA than other microorganisms used. Lou et al. [25] reported that the inhibition activity of QCRAg nanocomposites (Quaternized chitosan with Ag NP) against gram-positive bacteria was the best and the smallest MIC value, while their inhibitory activity against fungus was the worst. This effect is due to differences in the cell structures of microorganisms. As the concentration of PEG–PLA increased, the antimicrobial effect increased. The highest antimicrobial activity was determined at the 10% concentration levels (Fig. 1). Figure 1 shows that the smallest MICs of 10% PEG–PLA were shown by all the bacteria tested, with the exception of S. Enteritidis and S. aureus.
Fig. 1.
Evaluation of the MIC (mg/mL) values for bacterial growth at different concentrations of PEG–PLA
MBC was determined as the lowest concentration of the polymer at which no growth of bacteria was observed on the polymer-free medium after incubation. Enterobacter ATCC 19 434 was found to be the most resistant bacteria, followed by S. aureus. Shen et al. [26] showed that the bagasse–AgNP aerogel had a limited inhibition effect on S. aureus. Meanwhile, the thick peptidoglycan layer of the S. aureus cell wall prevented the penetration of polymers into the cytoplasm, thereby limiting their antimicrobial effect. The most sensitive bacteria used was Y. enterocolitica, followed by B. cereus (Table 1).
Fungal growth
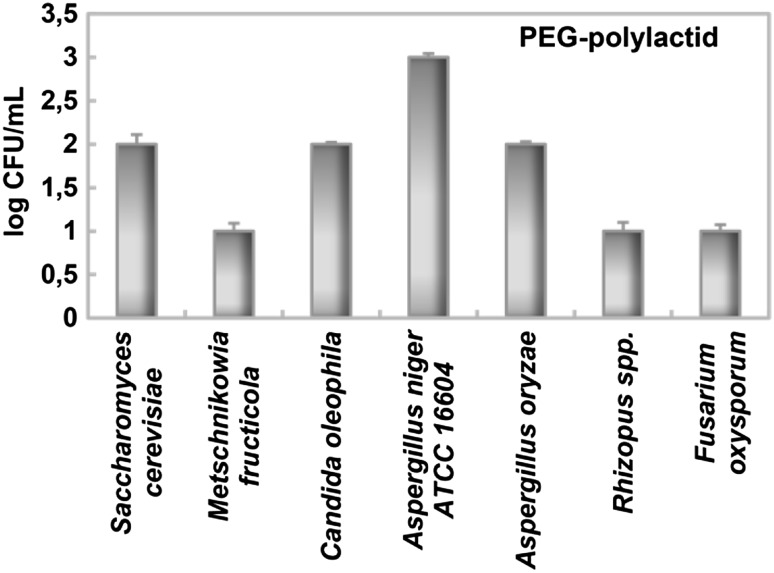
Compared with bacteria, molds and yeasts were found to be more resistant to PEG–PLA (Table 1). While MIC and MFC could not be determined on most of the fungi tested because of the concentrations used, the yeast C. albicans and molds P. expansum and A. parasiticus were impacted sufficiently to perform these measurements. Meng et al. [27] was previously reported a high inhibition effect determined on C. albicans by the PDMS/OMMT polymer. Figure 2 shows the impact of PEG–PLA on the fungi in the form of a log decimal reduction. As stated earlier, there was no evidence of inhibition by PEG in any of the fungi. However, PEG–PLA did have a measurable impact. In all the microorganisms tested, A. niger ATCC 16604 was determined to be the most resistant microorganism to PEG–PLA (Fig. 2). Huang et al. [28] observed that different microbial cell structures may be an important reason for the different antimicrobial effects. Unlike the other microorganisms, the fungus A. niger has a denser cell wall; moreover, and it is very difficult to inhibit its fungal spores. In comparison, with an MIC of 50 mg/mL, the fungi P. expansum was the most sensitive microorganism, followed by A. parasiticus and C. albicans (MIC = 100 mg/mL).
Fig. 2.
Effect of PEG–PLA on the microbial growth of fungi
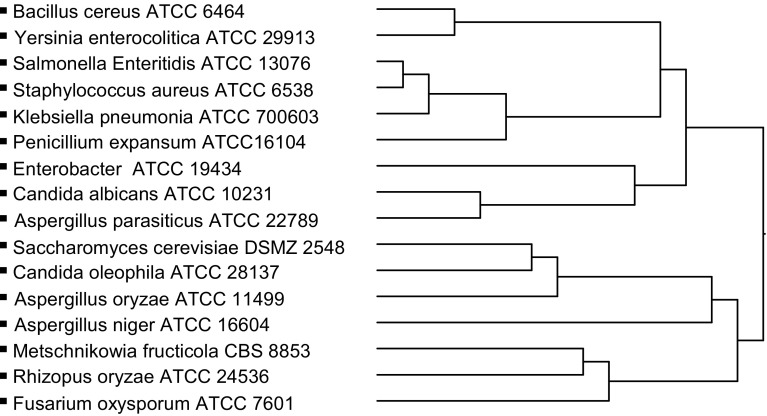
A hierarchical cluster analysis (shown in Fig. 3) permitted us to establish two main groups for PEG–PLA (10%). Cluster analysis was used for dividing homogenous groups according to some similarity or differences calculated between the variables [29]. In Fig. 3, the upper main group, which contained all the tested bacteria except C. albicans and A. parasiticus, was more affected by PEG–PLA (10%), while the main group below was less influenced.
Fig. 3.
Dendrogram based on a cluster analysis grouping the microorganisms tested for PEG–PLA (10%)
The results show that PEG–PLA exhibits significant antimicrobial properties. It can be used for preserving some raw and processed foods and/or for extending their shelf life. The findings of this study provide the basis for further research on new bioactive polymers that are not hazardous to human health and are more trustworthy, especially for the food industry. However, it should be mentioned that the effectiveness against bacteria and fungi depends on the chemistry of the polymer, its structure, and its concentration.
Thus far, there has been an insufficient amount of research conducted to successfully identify the specific mechanisms used by PEG–PLA to inhibit certain microorganisms, e.g., the ones used in this study. PEG–PLA should be studied more extensively for the preservation of food quality and safety and/or extension of the shelf life of fresh as well as processed foods.
Acknowledgements
The authors would like to thank Research Fund of the University of Uludag for their financial support to this research Project (Project No: KUAP(Z)- 2013/16).
Complianc with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Huang Q, Wang X, Lu L, Deng X, Chen Z, Liang J, Li J, Huang X, Zhang Y, Yang X. The effectiveness of food-borne diseases training among clinicians in Guangdong, China. Food Control. 2013;33:268–273. doi: 10.1016/j.foodcont.2013.03.003. [DOI] [Google Scholar]
- 2.De Koster CG, Brul S. MALDI-TOF MS identification and tracking of food spoilers and food-borne pathogens. Curr. Opin. Food Sci. 2016;10:76–84. doi: 10.1016/j.cofs.2016.11.004. [DOI] [Google Scholar]
- 3.Byun Y, Kim YT, Whiteside S. Characterization of an antioxidant polylactic acid (PLA) film prepared with α-tocopherol, BHT and polyethylene glycol using film cast extruder. J. Food Eng. 2010;100:239–244. doi: 10.1016/j.jfoodeng.2010.04.005. [DOI] [Google Scholar]
- 4.Shankaraiah GK, Shaikh BM, Chavan SA, Dawane BS. Polyethylene glycol (PEG-400): An efficient and recyclable reaction medium for the synthesis of novel 1; 5-benzodiazepines and their antimicrobial activity. Chinese Chem. Let. 2011;22:65–68. doi: 10.1016/j.cclet.2010.09.012. [DOI] [Google Scholar]
- 5.Quintavalla S, Vicini L. Antimicrobial food packaging in meat industry. Meat Sci. 2002;62:373–380. doi: 10.1016/S0309-1740(02)00121-3. [DOI] [PubMed] [Google Scholar]
- 6.Zivanovic S, Chi S, Draughon AF. Antimicrobial activity of chitosan films enriched with essential oils. J Food Sci. 2005;70(1):45–51. doi: 10.1111/j.1365-2621.2005.tb09045.x. [DOI] [Google Scholar]
- 7.Goncagul G, Gurbuz O, Sahan Y, Kara A. Polyethylene glycol coating of fresh eggs, Turk. Pat. Appl. 8 pp. CODEN: TRXXB5 TR 2009002991 B 20100721 CAN 154:309357 AN 2011:341719 CAPLUS (2010)
- 8.Ashter SA. Introduction to Bioplastics Engineering, 1st edition, Elsevier, 81–151 (2016)
- 9.Sung SY, Sina LT, Tee TT, Bee ST, Rahmat AR, Rahman WAWA, Tan AC, Vikhraman M. Antimicrobial agents for food packaging applications. Trends Food Sci. Tech. 2013;33:110–123. doi: 10.1016/j.tifs.2013.08.001. [DOI] [Google Scholar]
- 10.Kozlovskiy R, Shvets V, Kuznetsov A. Technological aspects of the production of biodegradable polymers and other chemicals from renewable sources using lactic acid. J. Clean Prod. 10.1016/j.jclepro.2016.08.092 (2016) [DOI]
- 11.Gupta AP, Vimal Kumar V. New emerging trends in synthetic biodegradable polymers poly- lactide: a critique. Eur. Polym. J. 2007;43:4053. doi: 10.1016/j.eurpolymj.2007.06.045. [DOI] [Google Scholar]
- 12.Rudnik E. Compostable Polymer Properties and Packaging Applications. In: Plastic Films in Food Packaging, Materials, Technology and Applications, 1st Edition, (Ed. By. S Ebnesajjad), 217–248 (2013)
- 13.Chen Y, Park Y, Noda I, Jung YM. Influence of polyethylene glycol (PEG) chain length on the thermal behavior of spin-coated thin films of biodegradable poly(3- hydroxybutyrate-co-3-hydroxyhexanoate)/PEG blends. J. Mol. Struct. 2016;1124:159–163. doi: 10.1016/j.molstruc.2016.02.059. [DOI] [Google Scholar]
- 14.Riley T, Stolnik S, Heald CR, Xiong CD, Garnett MC, Illum L, Davis SS. Physicochemical evaluation of nanoparticles assembled from poly lactic acid-poly ethylene glycol) (PLA-PEG) block copolymers as drug delivery vehicles. Langmuir. 2001;17:3168–3174. doi: 10.1021/la001226i. [DOI] [Google Scholar]
- 15.Anonymous. Aerobic colony count by pour plate method. HPA Standard Method. Standards Unit, Evaluations and Standards Laboratory in Conjunction with the Regional Food, Water and Environmental Coordinators Forum, Issue 4.2, p14 (2008)
- 16.Chung PY, Chung LY, Ngeow YF, Goh SH, Imiyabir Z. Antimicrobial activities of Malaysian plant Species. Pharm. Bio. 2004;42(4–5):292–300. doi: 10.1080/13880200490511837. [DOI] [Google Scholar]
- 17.Yin MC, Tsao SM. Inhibitory effect of seven Allium plants upon three Aspergillus species. Int. J. Food Microbiol. 1999;49:49–56. doi: 10.1016/S0168-1605(99)00061-6. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Malo A, Alzamora SM, Palou E. Aspergillus flavus Growth in the presence of chemical preservatives and naturally occurring antimicrobial compounds. Int. J Food Microbiol. 2005;99:119–128. doi: 10.1016/j.ijfoodmicro.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Chandrasekaran M, Venkatesalu V. Antibacterial and antifungal activity of Syzgium jambolanum seeds. J Ethnopharm. 2004;91:105–108. doi: 10.1016/j.jep.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Mathabe MC, Nikolova RV, Lall N, Nyazemac NZ. Antibacterial activities of medicinal plants used for the treatment of diarrhea in Limpopo Province, South Africa. J Ethnopharm. 2006;105:286–293. doi: 10.1016/j.jep.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 21.Fazeli MR, Amin G, Attari MMA, Ashtiani H, Jamalifar H, Samadi N. Antimicrobial activities of Iranian sumac and avishan-e shirazi (Zataria multiflora) against some food-borne bacteria. Food Control. 2007;18:646–649. doi: 10.1016/j.foodcont.2006.03.002. [DOI] [Google Scholar]
- 22.Rangasamy O, Raoelison G, Rakotoniriana FEJ. Screening for anti-effective properties of several medicinal plant of the Mauritius flora. Ethnopharmacol. 2007;109:331. doi: 10.1016/j.jep.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Korukluoglu M, Gurbuz O, Sahan Y, Yigit A, Kacar O, Rouseff RL. Chemical characterization and antifungal activity of Origanum onites L. essential oils and extracts. Journal of Food Safety. 2009;29:144–161. doi: 10.1111/j.1745-4565.2008.00124.x. [DOI] [Google Scholar]
- 24.Steven MD, Hotchkiss JH. Non-migratory bioactive polymers (NMBP) in food packaging. In; Novel food packaging techniques (Ed. by R. Ahvenainen), Woodhead Publishing Limited, 71–102 (2003)
- 25.Luo J, Xie M, Wang X. Green fabrication of quaternized chitosan/rectorite/Ag NP nanocomposites with antimicrobial activity. Biomedical Materials. 2014;9:011001. doi: 10.1088/1748-6041/9/1/011001. [DOI] [PubMed] [Google Scholar]
- 26.Shen Z, Han G, Wang X, Luo J, Sun R. An ultra-light antibacterial bagasse-AgNP aerogel. Journal of Materials Chemistry B. 2017;5:1155–1158. doi: 10.1039/C6TB02171A. [DOI] [PubMed] [Google Scholar]
- 27.Meng N, Zhou NL, Zhang SQ, Shen J. Synthesis and antifungal activities of polymer/montmorillonite–terbinafine hydrochloride nanocomposite films. Applied Clay Science. 2009;46:136–140. doi: 10.1016/j.clay.2009.07.003. [DOI] [Google Scholar]
- 28.Huang X, Hu N, Wang X, Zhang YS, Sun R. Copper Sulfide Nanoparticle/Cellulose Composite Paper: Room-Temperature Green Fabrication for NIR laser inducible Ablation of Pathogenic Microorganisms. ACS Sustainable Chemistry & Engineering. 2017;5:2648–2655. doi: 10.1021/acssuschemeng.6b03003. [DOI] [Google Scholar]
- 29.Ozdamar K. Statistical analysis by package programme. 2. Eskisehir, Turkey: Kaan Pub. Co.; 2003. [Google Scholar]