Abstract
Mutants of Yarrowia lipolytica with high erythritol production were generated through an atmospheric and room temperature plasma (ARTP) mutation system. Among these mutants, Y. lipolytica M53 exhibited the highest erythritol yield. In a batch culture, M53 produced 64.8 g/L erythritol from 100 g/L glycerol. The yields of byproducts (e.g. mannitol, arabitol, and α-ketoglutaric acid) were low, and the mechanisms underlying these changes were examined by measuring enzyme activities in the pentose phosphate pathway. Up to 145.2 g/L erythritol was produced by M53 from 200 g/L of glycerol, and erythritol accumulation was promoted by 3.7 mg/L of Cu2+, 10.15 mg/L of Mn2+, and 30.37 g/L of NaCl. Fed-batch cultivation of M53 in a 5-L fermentor produced 169.3 g/L erythritol with low levels of byproducts within 168 h. This finding confirmed the potential of M53 as an erythritol producer on a commercial scale.
Keywords: Erythritol, Atmospheric and room temperature plasma mutation system, Yarrowia lipolytica, Fermentation optimization, Fed-batch fermentation
Introduction
Erythritol (1,2,3,4-butanetetrol) is a low-calorie (0.3 kcal/g) sweetener with 70–80% of the sweetness of sucrose [1]. Almost all (>90%) of the ingested erythritol cannot be utilized by the human body; thus the ingested erythritol is directly excreted by the excretory system without changing the glycemic index. Erythritol has been demonstrated to be safe in animal toxicological and clinical studies [2]. Given these properties, erythritol plays an important role as a functional sugar substitute in special diets for diabetic or obese patients. Erythritol can also be used as a substitute for xylitol in dental care because the former is unfermentable for bacteria causing dental caries [3]. Hence, erythritol is becoming increasingly important, and its demand in health care has been consistently growing since the recent years.
Chemical and fermentative processes have been regarded as the principal pathways for large-scale production of erythritol. However, chemical processes were considered to be poorly efficient [4]. In the past, erythritol was commercially produced from glucose by using Aureobasidium sp. and Pseudozyma tsukubaensis [5]. At present, the unconventional yeast Y. lipolytica has received considerable attention because of its biotechnological potential. Y. lipolytica exhibits obvious advantages over other strains in fermentation processes; for instance, a larger substrate variety, low sensitivity to heavy metals, and low concentrations of dissolved oxygen. These properties can ensure the possibility of using low-priced or less-refined substrates for erythritol production by Y. lipolytica [6–8]. Pure or crude glycerol can be converted to erythritol by Y. lipolytica in specific cultivation systems. Currently, many studies are focusing on improving the erythritol yield and productivity by optimizing the fermentation conditions or through microbial mutation [7, 9]. Under the optimal osmotic pressure conditions, the erythritol yield of Y. lipolytica can reach as high as 0.65 g/g of carbon source [9].
In biotechnological research and industries, breeding is an efficient way to obtain strains with new characteristics. Traditionally, cells with desirable phenotypes were obtained after exposure to chemical, physical, or biological mutagens. However, these processes are usually accompanied with environmental pollution. The atmospheric and room temperature plasma (ARTP) mutation system, a novel mutagenic breeding method, is a recently developed technique. ARTP provides many attractive advantages including low jet temperature, uniform generated plasma, rapid mutation, highly diverse mutants, low cost, and no-pollution. The simple and safe operation also enables diverse and rapid genome mutation in microbes in a non-GMO manner and sequentially alters the metabolic networks in target microbes [10]. Nowadays, ARTP has been successfully employed in mutation breeding (e.g., improvement in productivity, toxicity tolerance, or growth rate) of more than 40 kinds of microorganisms such as microalgae, fungi, and bacteria [10, 11]. Additionally, ARTP is more efficient than the traditional mutagenic methods. Zhang et al. [12] reported that the mutation rate of ARTP mutagenesis is higher than that of other mutation methods.
Yarrowia lipolytica SWJ-1b isolated from marine fish gut was used for citric acid production in our previous studies [13]. When Y. lipolytica SWJ-1b was cultivated in a citric acid fermentation medium, a large amount of citric acid was produced. In contrast, Y. lipolytica SWJ-1b produced considerable amounts of erythritol instead of citric acid in an erythritol fermentation medium. The erythritol productivity of Y. lipolytica SWJ-1b is comparable (48.3 g/L) with that of the majority of other microorganism previously reported. Therefore, the present work aims to establish a mutation library of Y. lipolytica SWJ-1b by using the ARTP mutagenesis. This study also aims to screen mutation with high erythritol production (Y. lipolytica M53). Then, the erythritol bioconversion rate of M53 was improved by optimizing the fermentation conditions and its influence factors in an erythritol production medium.
Materials and methods
Chemicals and microorganism
A wild strain of Y. lipolytica SWJ-1b was isolated from marine fish gut from the Bohai Sea. Cells of Y. lipolytica SWJ-1b were kept at 4 °C on a yeast peptone dextrose (YPD) agar slant containing 10 g/L yeast extract, 20 g/L peptone, 20 g/L glucose, and 20 g/L agar. Cell viability was maintained by transferring the culture to fresh agar slants each month.
Helium-based ARTP mutation of Y. lipolytica
The protocols for helium-based ARTP mutation were described in detail by Lu et al. [14]. In the current study, for mutation of Y. lipolytica SWJ-1b, 50 μL of the culture (OD600nm = 30) was dropped onto a stainless steel plate (8 mm in diameter) and then was treated by a helium plasma jet. The working helium gas flow rate was QHe = 10 standard liters per minute (SLM), and 120 W of radio frequency (RF) power was used to obtain a low-temperature glow discharge plasma jet, which is favorable for treating the yeast cell samples. The treatment time of the cells of Y. lipolytica SWJ-1b by ARTP was 2 min. Then, the metal plate was oscillated in 1.5 mL of sterilized normal saline for 1 min. Subsequently, 200 μL of the diluent elute was spread on the YPD agar plate and cultivated at 28 °C until colonies were obtained. The mutants were then cultured in an erythritol fermentation broth for screening strains with high erythritol production.
Culture methods
Yarrowia lipolytica SWJ-1b and its mutants were incubated for 24 h at 28 °C and agitated at 180 rpm. Approximately 1 mL of the culture (OD600nm = 30) was transferred into 50 mL of the erythritol fermentation medium. The basal medium for erythritol production comprised the following: 100 g/L glycerol, 2.3 g/L (NH4)2SO4, 1 g/L MgSO4·7H2O, 0.23 g/L KH2PO4, 1 g/L yeast extract, and 26.4 g/L NaCl. In microelement optimization experiments, different concentrations of Cu2+ (2–8 mg/L) and Mn2+ (5–20 mg/L) were added to the basal medium for erythritol fermentation. Various initial concentrations of glycerol (100–300 g/L) and NaCl (0–40 g/L) were added to the basal media, respectively, to investigate the effects of glycerol concentration and osmotic pressure on erythritol production. Osmotic pressure was analyzed using a freezing-point osmometer (Osmomat 030, Gonotec). Samples were withdrawn for analysis at regular intervals, and assays were performed in triplicate (data varying less than 5%). All the results were expressed by their averages.
Cu2+, Mn2+, and NaCl were selected as the fundamental factors influencing erythritol production and were subjected to response surface methodology to obtain the maximum concentrations. Cultures were prepared as designed and were incubated at 30 °C, 200 rpm for 7 days. Samples were analyzed as follows: Design-Expert V8.0.6 was used to design the experiments as well as to model and analyze the results.
Preparation of crude extracts and activity assays of key enzymes
Up to 100 mL of cultured broth was centrifuged at 4 °C (10,000×g, 1 min), and 5 g of the wet cells obtained was washed and suspended in 5 mL of phosphate buffer (20 mM, pH 6.5). After ultrasonic decomposition of the suspension for 20 min at 4 °C, cell extracts were centrifuged at 10,000×g and 4 °C for 10 min to remove the cell debris. According to the method previously described, the activities of erythrose reductase (ER), transketolase (TK), transaldolase (TA), hexokinase (HK), glucose-6-phosphate dehydrogenase (G6PDH), 6-phosphogluconate dehydrogenase (6PGDH), pyruvate dehydrogenase (PDH), and citrate synthase (CS) were determined as previously described [15–22]. Protein concentrations in cell extracts were determined by the Lowry method [23]. A unit of enzyme activity represents the amount of enzyme required to convert the substrate into 1 μmol product per minute.
Analytical methods of products
Samples from shake-flask experiments and bioreactor cultivations were centrifuged (3063×g; 10 min). The supernatant was used to determine the yields of the products, and the cell pellet was collected and washed for biomass determination. The supernatant was filtered and diluted in deionized water (1:9). Levels of glycerol, erythritol, mannitol, arabitol, citric acid, and α-ketoglutaric acid in the supernatant were determined by HPLC (Agilent 1200 infinity series, Santa Clara, CA, USA) with an Aminex HPX-87H ion exclusion column (300 × 7.8 mm2; Bio-Rad Laboratories Inc., Hercules, CA, USA). Up to 5 mmol/L H2SO4 was used as the mobile phase, which flowed at the rate of 0.6 mL/min to elute the column at 35 °C. The sample injection volume was 10 μL. The concentrations of the products were detected using a refractive index detector (RID). All values were expressed as averages of three independent extraction processes. The cell dry weight used for biomass determination was measured as reported by Liu et al. [13].
Erythritol production by batch cultivation
Erythritol fermentation was scaled up in a 5-L fermentor. The fermentor was equipped with an oxygen sensor, an alkali pump, a heating element, a stirrer, and a temperature sensor. 60 mL of the seed (OD600nm = 30) culture was transferred into 3 L of the erythritol fermentation medium. Fermentation was performed under an agitation speed of 250 rpm, with an aeration rate of at least 3 L/min/L medium and a temperature of 28 °C. During the fermentation, the dissolved oxygen value (DO) was maintained at 20–30% of air saturation. In the fed-batch cultures, the initial glycerol concentration was measured at 200 g/L. The glycerol concentration was maintained at 150 g/L by adding glycerol into the culture broth at an interval of 12 h until the productivity of erythritol began to decline. During the fermentation, 20 mL of the culture was collected at 24-h intervals for determination.
Results and discussion
Mutation and selection of the mutants with improved erythritol productivity
High-lethality radiation can obtain more mutants with changed physiology [14]; thus 50 μL of the culture (diluted to 1 × 106 cells/mL) was treated with the helium-based ARTP for 2 min, and the lethality rate of Y. lipolytica SWJ-1b reached 97%. In spite of the high lethality rate, approximately 1.5 × 103 cells survived, and all the survived colonies were conserved for further screening. The mutants were selected on the basis of the high yields of erythritol in the erythritol production medium, and 104 strains of the mutants showed improved erythritol productivities. Among the positive mutants, strain M53 showed the highest erythritol production (64.8 g/L) and was selected for further research (Data not shown).
In the past few years, random mutagenesis by ultraviolet (UV) or chemical mutagens has been performed to enhance the erythritol production. Lin et al. [24] obtained a high-erythritol-producing (59.5% yield) mutant of Moniliella sp. from nitrosoguanidine (NTG) treatment. Rywinska et al. [8] reported the mutant Y. lipolytica Wratislavia 1.31, which was isolated after exposure to UV irradiation of the wild strain Y. lipolytica A-101, for the first time to improve the erythritol production of Y. lipolytica through mutagenesis. However, the erythritol productivities of the mutants obtained using methods mentioned above were relatively low. Moreover, all the chemical mutagenesis approaches are hazardous to the operator and the environment. Recently, the ARTP mutation system was developed and successfully employed for mutation breeding, and researches have confirmed that ARTP was powerful and environmentally friendly for generating a microbial mutant library [10]. In the present study, mutant M53 obtained from ARTP can give high yields of erythritol. After 20 subcultures, the erythritol production of M53 maintained stability. This finding suggests that M53, the mutant of Y. lipolytica SWJ-1b generated by ARTP, can be used for further study.
Metabolic flux analysis by determining the activities of the key enzymes
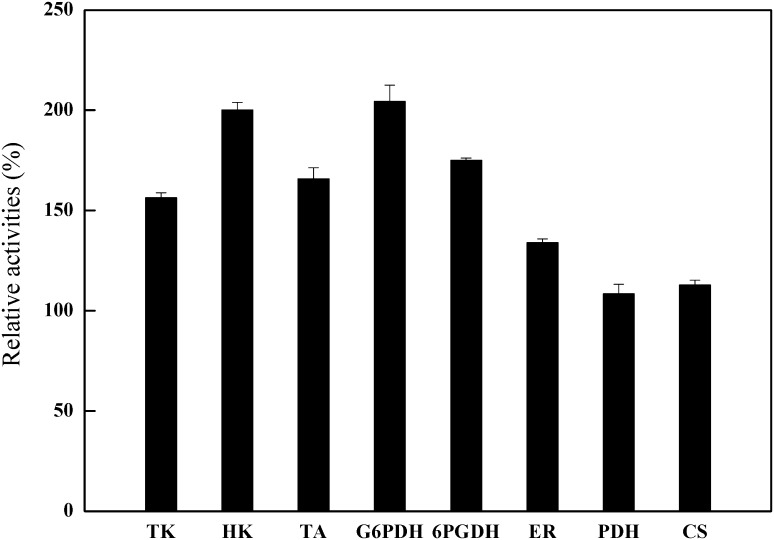
For erythritol accumulation, an imbalance should exist between the metabolic fluxes in the pentose phosphate pathway [7]. However, the pathways involved in regulating erythritol production by Y. lipolytica have not been studied in detail. Thus, enzymes related to erythritol synthesis in M53 were determined when they were grown on various media. In particular, Y. lipolytica M53 was cultured in a 500-mL Erlenmeyer flask for 72 h, and the activities of the main enzymes in the pentose phosphate pathway (such as TA, HK, TK, G6PDH, 6PGDH, and ER) and the tricarboxylic acid cycle cycle (PDH and CS) were determined. As shown in Fig. 1, all the measured enzyme activities in M53 tended to be higher than those of Y. lipolytica SWJ-1b, and the relative activities of enzymes involved in the pentose phosphate pathway were obviously higher than those involved in the TCA cycle.
Fig. 1.
The changes in relative activities of the enzymes involved in products synthesis in M53. Data are given as mean ± SD, n = 3
Researchers suggested that less inhibition of ER resulted in a high yield of erythritol in Torula corallina [25]. The improved erythritol biosynthesis in Y. lipolytica was also correlated with an enhanced activity of ER [26]. In Trichosporonoides megachiliensis, higher erythritol productivity might be ascribed to the higher activity of the enzymes involved in the pentose phosphate pathway, such as TK and TA [27]. ER activity in Y. lipolytica mutants generated by UV was enhanced, which thereby promoted the erythritol production [28]. Consequently, the high erythritol productivity of Y. lipolytica M53 may be attributed to the increased activity of these enzymes in the pentose phosphate pathway.
Optimization of supplemental minerals in erythritol production medium
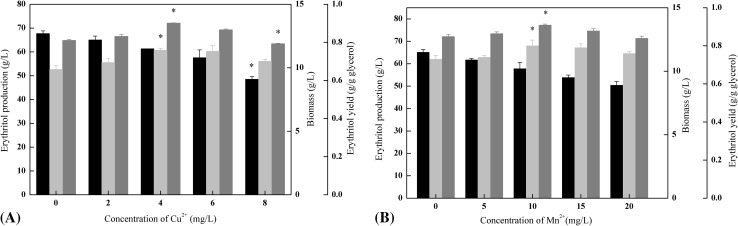
Minerals such as Cu2+, Fe3+, Mg2+, and Zn2+ affected the ER activities in Y. lipolytica during erythritol fermentation [26]. Among these minerals, Cu2+ and Mn2+ were reported to exert the most obvious effect on erythritol biosynthesis [29]. To investigate the effects of metal ions on erythritol biosynthesis in M53, Cu2+ and Mn2+ were added to the erythritol fermentation medium with the concentrations varying from 0 to 8 mg/L and 0 to 20 mg/L, respectively. As shown in Fig. 2(A), both cellular growth and erythritol yield were influenced by the medium with additional Cu2+, the biomass decreased with the increasing concentration of Cu2+, and the maximal erythritol production (72.1 g/L, 0.76 g/g glycerol) was obtained in the cultures containing 4 mg/L of Cu2+. Cell growth was also negatively affected when Mn2+ was supplemented to the erythritol fermentation medium. However, the cells’ capability for erythritol bioconversion was observed to be more sensitive to Mn2+ than to biomass, and the highest erythritol yield (77.2 g/L, 0.8 g/g glycerol) was obtained when 10 mg/L Mn2+ was added to the medium (Fig. 2(B)).
Fig. 2.
Effects of various concentrations of Cu2+ and Mn2+ on erythritol production and biomass by M53. Erythritol production (gray), yield (light gray), biomass (black). Data are given as mean ± SD, n = 3. * represents 0.05 < p < 0.1
In the report by Lee et al. [29], the erythritol yield by Torula sp. was improved when Mn2+ and Cu2+ were supplemented. Moreover, the ER activities in cells can be significantly increased by adding Cu2+ in the medium. As such, supplemental Cu2+ can reduce the production of fumarate, a strong inhibitor of ER [25]. Unlike Cu2+, Mn2+ exerts entirely different effects on erythritol production. In particular, Mn2+ can alter cell permeability [30], thereby increasing the export of the intracellular erythritol. Consequently, intracellular erythritol would be immediately transferred out of the cells. However, an excess of Mn2+ might break the intracellular balance, thus reducing the erythritol production. Therefore, the increased cell permeability caused by the addition of 10 mg/L Mn2+ and the elevated activity of ER improved by 4 mg/L Cu2+ resulted in high erythritol productivities [26, 29]. These finding agreed with the reports of Lee et al. [25, 29].
Effects of initial glycerol concentrations on erythritol production by M53
For osmophilic microorganisms, glycerol acts as both an osmotic regulator and a carbon source. Therefore, high initial concentration of glycerol is beneficial to erythritol synthesis [7]. As Y. lipolytica can tolerate high osmotic pressure, the effect of glycerol concentration on the fermentation parameters of M53 should be evaluated. As such, erythritol fermentation experiments were conducted in media with glycerol concentrations varying from 100 to 300 g/L. As shown in Table 1, 100 to 200 g/L glycerol yielded relatively high erythritol concentrations (0.77–0.69 g/g). The maximum volumetric production rate of erythritol was detected in the medium containing 200 g/L of glycerol. Most glycerol in the fermentation medium was consumed by M53. However, the erythritol yield and productivity decreased significantly when the initial glycerol concentration was higher than 200 g/L. When the initial glycerol concentration was as high as 300 g/L, the biomass was 12.6 g/L, the yield of erythritol decreased to 0.52 g/g, and the erythritol productivity decreased to 0.73 g/L/h because of the prolonged fermentation time. At the end of cultivation, up to 47.9 g/L of residual glycerol remained in the medium. On the basis of the above results, the optimal initial concentration of glycerol was determined to be 200 g/L.
Table 1.
Effects of the initial glycerol concentrations on erythritol biosynthesis in M53
| Glycerol concentration (g/L) | Erythritol (g/L) | Productivity (g/L/h) | YP/S of erythritol (g/g) | Residual glycerol (g/L) | Biomass (g/L) |
|---|---|---|---|---|---|
| 100 | 77.2 ± 1.9 | 0.64 ± 0.02 | 0.77 ± 0.2 | 3.9 ± 0.7 | 9.5 ± 0.9 |
| 150 | 114.3 ± 2.1 | 0.79 ± 0.01 | 0.76 ± 0.1 | 5.4 ± 1.1 | 10.4 ± 0.6 |
| 200 | 138.7 ± 1.0 | 0.82 ± 0.01 | 0.69 ± 0.1 | 10.7 ± 2.5 | 11.9 ± 0.3 |
| 250 | 149.5 ± 1.1 | 0.77 ± 0.01 | 0.60 ± 0.1 | 28.1 ± 2.7 | 12.4 ± 0.2 |
| 300 | 158.3 ± 1.5 | 0.73 ± 0.01 | 0.52 ± 0.0 | 47.9 ± 6.7 | 12.6 ± 0.1 |
Data are given as mean ± SD, n = 3
Oh et al. [31] proposed that an increased initial concentration of glucose usually caused an osmotic effect on cells or substrate repression on glucose-consuming enzymes, and the optimum glucose concentration for erythritol production by Torula sp. was determined to be 300 g/L. Yang et al. [7] also reported that exposing the yeast to an environment with high osmotic pressure was the prerequisite for erythritol production, and the osmotic pressure was usually provided by high concentrations of glycerol or NaCl. It was observed that Y. lipolytica CICC 1675 could produce high levels of erythritol in an environment with high osmotic pressure caused by high concentration of glycerol [7]. When an acetate-negative mutant of Y. lipolytica was grown with 300 g/L raw glycerol, 170 g/L erythritol was produced, corresponding to a yield of 56% [32]. By contrast, the identified concentration of glycerol for erythritol production was not high (200 g/L) in the present study. To achieve relatively high osmotic pressure in the medium for erythritol production, additional NaCl was added and optimized in the following experiments.
Optimization of initial NaCl concentration in erythritol production medium
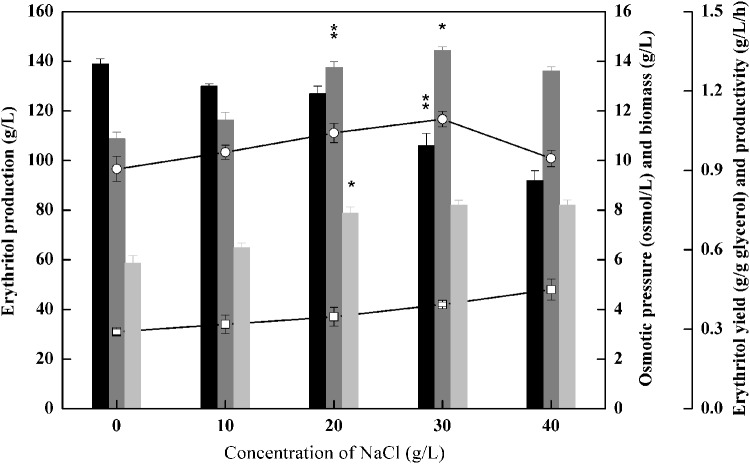
Osmotic pressure significantly influences the growth and intracellular metabolism of cells [33]. Thus, an appropriate increase of osmotic pressure in the fermentation medium can effectively enhance erythritol production by Y. lipolytica. The concentrations of the carbon source and NaCl were two key factors influencing osmotic pressure. Nevertheless, exceeding high concentrations of glycerol caused a sharp decrease in erythritol yield (Table 1). Yang et al. [7] supposed that the initial concentration of NaCl rather than the substrate exerted more important effects on osmotic pressure. Thus, in the current study, NaCl was used as the osmotic agent to adjust the initial osmotic pressure of the culture system. NaCl was added to the medium (200 g/L glycerol, 4 mg/L Cu2+, 10 mg/L Mn2+) with the concentrations ranging from 0 to 40 g/L, and the results are presented in Fig. 3. The maximum erythritol concentration was achieved (144.4 g/L) at the initial osmotic pressure of 4.2 osmol/kg when 30 g/L of NaCl was added to the medium. This finding indicated that higher osmotic pressure was beneficial for erythritol biosynthesis in M53. However, when NaCl concentration was extremely high (i.e., 40 g/L), cell growth and the erythritol production were obviously inhibited.
Fig. 3.
Effects of initial NaCl concentrations on erythritol biosynthesis in M53. Erythritol production (gray), yield (light gray), biomass (black), productivity (white circle), osmotic pressure (white square). Data are given as mean ± SD, n = 3. ** represents 0.001 < p < 0.05, * represents 0.05 < p < 0.1
Optimization of production medium using response surface analysis
Considering that the yield of erythritol depends on the supplemental minerals (Cu2+ and Mn2+) and the osmotic pressure (NaCl) in the production medium, response surface methodology was then used for statistical optimization of media to increase the erythritol production by M53. In the preliminary one-factor experiments, 4 mg/L Cu2+, 10 mg/L Mn2+, and 30 g/L NaCl were the optimal conditions for erythritol production. After analysis using Design-Expert V8.0.6 software, the response data were obtained, and 3D contour plots were generated (Data not shown). A response surface analysis model of three component-three level-one response was then applied to examine the combined effects of these three factors. The theoretical optimal concentrations of the three compositions were determined to be 10.15, 3.7, and 30.37 g/L, respectively. Under these conditions, the yield of erythritol increased to 145.2 g/L, which was close to the yield analyzed by the model (146.8 g/L).
Fed-batch of erythritol production by M53 in 5-L bioreactor
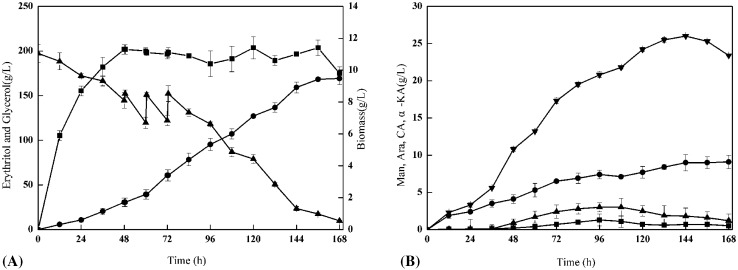
As the carbon source also acts as an osmotic regulator in erythritol fermentation mediums [7], the consumption of glycerol during the fermentation did not only reduce the carbon supply but also decreased the osmotic pressure in the medium, thereby influencing the production of erythritol [33]. Consequently, the addition of glycerol into the culture broth at certain intervals to maintain the concentration of the carbon source is a potentially effective way to improve the erythritol production by the microorganism. Fed-batch fermentation of erythritol was performed in a 5-L fermenter containing 3 L of the optimal medium. During the experiment, the glycerol concentration was maintained at a constant level, and the erythritol productivity remained at a relatively high level during the glycerol-feeding stage. After the feeding stage, the fermentation process was continued until 168 h. The time course of the fed-batch fermentation is shown in Fig. 4. At the end of the fermentation, the maximum concentration of erythritol reached 169.3 g/L (0.65 g/g of erythritol yield), whereas the biomass showed no obvious increase after the glycerol feeding (Fig. 4(A)). The preparation of byproducts is shown in Fig. 4(B). Among the byproducts, mannitol showed the highest production, which was 26.2 g/L at 144 h of cultivation. The yields of citric acid and α-ketoglutaric acid were obviously inhibited (9.1 and 3.0 g/L, respectively) during erythritol fermentation. Except for citric acid, all the byproducts slightly decreased at the end of cultivation. Similar results were reported by Mironczuk et al. [32].
Fig. 4.
The changes in main products and byproducts, biomass and residual glycerol during 5 L fermentation. (A) Erythritol (black circle), biomass (black square), glycerol (black up-pointing triangle); (B) mannitol (black down-pointing triangle), arabitol (black square), citric acid (black circle), α-ketoglutarate (black up-pointing triangle). Data are given as mean ± SD, n = 3
Erythritol production of various yeasts is summarized in Table 2. Moniliella sp. N61188-12 produced 237.8 g/L erythritol when grown in a medium containing glucose [8], which provided the highest yield (0.6 g/g) among the reported results. Erythritol production was also reported by Rywinska et al. [9]; in their report, 132 g/L (0.44 g/g) of erythritol was produced by Y. lipolytica Wratislavia K1 in the fed-batch mode and optimized medium. In the current study, a culture of Y. lipolytica M53 exhibited the highest erythritol yield (0.65 g/g) from glycerol in the fed-batch fermentor compared with most of the other strains of Y. lipolytica that were ever reported (Table 2). Table 2 shows that the erythritol productivity of M53 was higher than most of the other erythritol producers in the list.
Table 2.
Comparison of the reported erythritol fermentation using various microorganisms
| Strains | Carbon source | Erythritol (g/L) | YP/S (g/g) | Erythritol productivity (g/L/h) | References |
|---|---|---|---|---|---|
| Torula sp. | Glucose | 192 | 0.48 | 2.26 | [31] |
| Moniliella sp. N61188-12 | Glucose | 237.8 | 0.60 | 1.98 | [24] |
| Candida sorbosivorans SSE-24 | Glucose | 60.2 | 0.38 | 0.50 | [34] |
| Y. lipolytica A16 | Crude glycerol | 110.7 | 0.48 | 1.01 | [35] |
| Y. lipolytica Wratislavia K1 | Raw glycerol | 170 | 0.56 | 1.0 | [32] |
| Y. lipolytica Wratislavia K1 | Glycerol | 220 | 0.43 | 0.54 | [36] |
| Y. lipolytica M53 | Glycerol | 164.1 | 0.65 | 1.05 | This study |
Data are given as mean ± SD, n = 3
Acknowledgements
The Y. lipolytica SWJ-1b was graciously provided by Professior Chi, Ocean University of China. This work was supported by the National Natural Science Foundation of China (21406083 and 31600047), Natural Science Foundation of Jiangsu Province (BK20150412 and BK20160430), Natural Science Fund for Colleges and Universities in Jiangsu Province (15KJD180006), Opening Fund of Jiangsu Provincial Engineering Laboratory for Biomass Conversion and Process Integration (Huaiyin Institute of Technology) (JPELBCPI2015001), and Project Funded by China Postdoctoral Science Foundation (2016M591757).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Moon HJ, Jeya M, Kim IW, Lee JK. Biotechnological production of erythritol and its applications. Appl. Microbiol. Biot. 2010;86:1017–1025. doi: 10.1007/s00253-010-2496-4. [DOI] [PubMed] [Google Scholar]
- 2.Park JB, Seo BC, Kim JR, Park YK. Production of erythritol in fed-batch cultures of Trichosporon sp. J. Ferment. Bioeng. 1998;86:577–580. doi: 10.1016/S0922-338X(99)80010-5. [DOI] [Google Scholar]
- 3.Goldberg I. Functional foods: designer foods, pharma food, nutraceuticals. Chapman & Hall New York N Y (1994)
- 4.Pfeifer V, Sohns V, Conway H, Lancaster E, Dabic S, Griffin E. Two stage process for dialdehyde starch using electrolytic regeneration of periodic acid. Ind. Eng. Chem. Res. 1960;52:201–206. doi: 10.1021/ie50603a020. [DOI] [Google Scholar]
- 5.Ishizuka H, Wako H, Kasumi T. Sasaki T. Breeding of a mutant of Aureobasidium sp. with high erythritol production. J. Ferment. Bioeng. 1989;68:310–314. doi: 10.1016/0922-338X(89)90003-2. [DOI] [Google Scholar]
- 6.Tomaszewska L, Rymowicz W, Rywińska A. Mineral supplementation increases erythrose reductase activity in erythritol biosynthesis from glycerol by Yarrowia lipolytica. Appl. Biochem. Biotech. 2014;172:3069–3078. doi: 10.1007/s12010-014-0745-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang LB, Zhan XB, Zheng ZY, Wu JR, Gao MJ, Lin CC. A novel osmotic pressure control fed-batch fermentation strategy for improvement of erythritol production by Yarrowia lipolytica from glycerol. Bioresource. Technol. 2014;151:120–127. doi: 10.1016/j.biortech.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 8.Rywinska A, Bak M, Rakicka M, Tomaszewska L, Boruczkowski T, Lazar Z. Selection of the UV mutants of Yarrowia lipolytica yeast for erythritol biosynthesis from glycerol. Acta. Sci. Pol. Biotechnol. 2012;11:23–28. [Google Scholar]
- 9.Rywinska A, Marcinkiewicz M, Cibis E, Rymowicz W. Optimization of medium composition for erythritol production from glycerol by Yarrowia lipolytica using response surface methodology. Prep. Biochem. Biotechnol. 2015;45:515–529. doi: 10.1080/10826068.2014.940966. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Zhang XF, Li HP, Wang LY, Zhang C, Xing XH, Bao CY. Atmospheric and room temperature plasma (ARTP) as a new powerful mutagenesis tool. Appl. Microbiol. Biotechnol. 2014;98:5387–5396. doi: 10.1007/s00253-014-5755-y. [DOI] [PubMed] [Google Scholar]
- 11.Jiang M, Wan Q, Liu RM, Liang LY, Chen X, Wu MK, Zhang HW, Chen KQ, Ma JF, Wei P, Ouyang PK. Succinic acid production from corn stalk hydrolysate in an E. coli mutant generated by atmospheric and room temperature plasmas and metabolic evolution strategies. J. Ind. Microbiol. Biotechnol. 2014;41:115–123. doi: 10.1007/s10295-013-1346-7. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Zhang C, Zhou QQ, Zhang XF, Wang LY, Chang HB, Li HP, Oda Y, Xing XH. Quantitative evaluation of DNA damage and mutation rate by atmospheric and room-temperature plasma (ARTP) and conventional mutagenesis. Appl. Microbiol. Biotechnol. 2015;99:5639–5646. doi: 10.1007/s00253-015-6678-y. [DOI] [PubMed] [Google Scholar]
- 13.Liu XY, Lv JS, Zhang T, Deng YF. Direct conversion of pretreated straw cellulose into citric acid by co-cultures of Yarrowia lipolytica SWJ-1b and immobilized Trichoderma reesei mycelium. Appl. Biochem. Biotech. 2014;173:501–509. doi: 10.1007/s12010-014-0856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Y, Wang L, Ma K, Li G, Zhang C, Zhao H, Lai Q, Li HP, Xing XH. Characteristics of hydrogen production of an Enterobacter aerogenes mutant generated by a new atmospheric and room temperature plasma (ARTP) Biochem. Eng. J. 2011;55:17–22. doi: 10.1016/j.bej.2011.02.020. [DOI] [Google Scholar]
- 15.Park EH, Lee HY, Ryu YW, Seo JH, Kim MD. Role of Osmotic and Salt Stress in the Expression of Erythrose Reductase in Candida magnoliae. J. Microbiol. Biotechn. 2011;10:1064–1068. doi: 10.4014/jmb.1105.05029. [DOI] [PubMed] [Google Scholar]
- 16.Kochetov GA. Transketolase, in: N. O. Kaplan, S. P. Colovick, W. A. Methods in Enzymology, 9, Academic Press, New York, 209–217 (1982)
- 17.Ochoa T, Horecker BL. Methods in Enzymology, 9. New York: Academic Press; 1966. pp. 499–50525. [Google Scholar]
- 18.Joshi MD, Jagannathan V. Hexokinase, in: W. A. Methods in Enzymology, 9, Academic Press, New York, 371–375 (1966)
- 19.Kornberg A, Horecker BL. Methods in Enzymology, 9. New York: Academic Press; 1966. pp. 323–324. [Google Scholar]
- 20.Pontremoli S, Grazi E. Methods in Enzymology, 9. New York: Academic Press; 1966. pp. 137–138. [Google Scholar]
- 21.Kresze GB, Ronft H. Pyruvate dehydrogenase complex from baker’s yeast. 1. Purification and some kinetic and regulatory properties. Eur. J. Biochem. 1981;119:573–579. doi: 10.1111/j.1432-1033.1981.tb05646.x. [DOI] [PubMed] [Google Scholar]
- 22.Ge Y, Cao Z, Song P, Zhu G. Identification and characterization of a novel citrate synthase from Streptomyces diastaticus No. 7 strain M1033. Biotechnol. Appl. Bioc. 62: 300–308 (2015) [DOI] [PubMed]
- 23.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 24.Lin SJ, Wen CY, Wang PM, Huang JC, Wei CL, Chang JW, Chu WS. High-level production of erythritol by mutants of osmophilic. Moniliella sp. Process. Biochem. 2010;45:973–979. doi: 10.1016/j.procbio.2010.03.003. [DOI] [Google Scholar]
- 25.Lee JK, Koo BS, Kim SY. Fumarate-Mediated inhibition of erythrose reductase, a key enzyme for erythritol production by Torula coralline. Appl. Environ. Microb. 2002;68:4534–4538. doi: 10.1128/AEM.68.9.4534-4538.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomaszewska L, Rywinska A, Rymowicz W. High selectivity of erythritol production from glycerol by Yarrowia lipolytica. Biomass. Bioener. 2014;64:309–320. doi: 10.1016/j.biombioe.2014.03.005. [DOI] [Google Scholar]
- 27.Sawada K, Taki A, Yamakawa T, Seki M. Key role for transketolase activity in erythritol production by Trichosporonoides megachiliensis SN-G42. J. Biosci. Bioeng. 2009;108:385–390. doi: 10.1016/j.jbiosc.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Ghezelbash GR, Nahvi I, Emamzadeh R. Improvement of erythrose reductase activity, deletion of by-products and statistical media optimization for enhanced erythritol production from Yarrowia lipolytica mutant 49. Curr. Microbiol. 2014;69:149–157. doi: 10.1007/s00284-014-0562-3. [DOI] [PubMed] [Google Scholar]
- 29.Lee JK, Ha SJ, Kim SY, Oh DK. Increased erythritol production in Torula sp. by Mn2+ and Cu2+ Biotechnol. Lett. 2000;22:983–986. doi: 10.1023/A:1005672801826. [DOI] [Google Scholar]
- 30.Mironczuk AM, Dobrowolski A, Rakicka M, Rywinska A, Rymowicz W. Newly isolated mutant of Yarrowia lipolytica MK1 as a proper host for efficient erythritol biosynthesis from glycerol. Process. Biochem. 2015;50:61–68. doi: 10.1016/j.procbio.2014.10.020. [DOI] [Google Scholar]
- 31.Oh DK, Cho CH, Lee JK, Kim SY. Increased erythritol production in fed-batch cultures of Torula sp. by controlling glucose concentration. J. Ind. Microbiol. Biotechnol. 2001;26:248–252. doi: 10.1038/sj.jim.7000122. [DOI] [PubMed] [Google Scholar]
- 32.Rymowicz W, Rywinska A, Marcinkiewicz M. High-yield production of erythritol from raw glycerol in fed-batch cultures of Yarrowia lipolytica. Biotechnol. Lett. 2009;31:377–380. doi: 10.1007/s10529-008-9884-1. [DOI] [PubMed] [Google Scholar]
- 33.Tomaszewska L, Rakicka M, Rymowicz W, Rywinska A. A comparative study on glycerol metabolism to erythritol and citric acid in Yarrowia lipolytica yeast cells. FEMS. Yeast. Res. 2014;14:966–976. doi: 10.1111/1567-1364.12184. [DOI] [PubMed] [Google Scholar]
- 34.Saran S, Mukherjee S, Dalal J, Saxena RK. High production of erythritol from Candida sorbosivorans SSE-24 and its inhibitory effect on biofilm formation of Streptococcus mutans. Bioresource. Technol. 2015;198:31–38. doi: 10.1016/j.biortech.2015.08.146. [DOI] [PubMed] [Google Scholar]
- 35.Yang LB, Zhan XB, Zhu L, Gao MJ, Lin CC. Optimization of a low-cost hyperosmotic medium and establishing the fermentation kinetics of erythritol production by Yarrowia lipolytica from crude glycerol. Prep. Biochem. Biotechnol. 2016;46:376–383. doi: 10.1080/10826068.2015.1045604. [DOI] [PubMed] [Google Scholar]
- 36.Mironczuk AM, Furgała J, Rakicka M, Rymowicz W. Enhanced production of erythritol by Yarrowia lipolytica on glycerol in repeated batch cultures. J. Ind. Microbiol. Biotechnol. 2014;41:57–64. doi: 10.1007/s10295-013-1380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]