Abstract
Green microalga Chlorella (Chlorella vulgaris) powder was employed as a natural antioxidant in virgin olive oil and its antioxidant activity was compared with those of β-carotene and α-tocopherol during 16 days of accelerated storage. Furthermore, the synergistic effects of Chlorella and citric acid were investigated. The primary, secondary, and total oxidation products of Chlorella samples (with and without citric acid) were lower than those of the control samples. Induction period of Chlorella samples were significantly higher than those of the control, β-carotene, and α-tocopherol samples. Furthermore, carotenoid and chlorophyll contents of Chlorella samples were significantly higher than those of the control samples. These pigments can delay the oxidation process. Using Chlorella and citric acid in combination with each other showed no synergistic effect against the oxidation of virgin olive oil. In conclusion, Chlorella can be affirmed as a natural antioxidant, which extends the shelf life of virgin olive oil.
Keywords: Chlorella, Citric acid, Oxidation, Synergistic effect, Virgin olive oil
Introduction
Virgin olive oil (VOO) is obtained from the pressed olive fruit with distinct nutritional and sensory characteristics. VOO exhibits high stability due to its specific compositions, including high amounts of monounsaturated fatty acids (MUFAs) and a range of natural antioxidants [1]. However, VOO easily undergoes oxidation due to the presence of polyunsaturated fatty acids (PUFAs), namely, linoleic and linolenic acids [2]. Oxidation of VOO leads to the formation of oxidation compounds such as free radicals, hydro-peroxides, and harmful degraded oxidation products, which is associated with discoloration, rancid odor, and depletion in quality of VOO [1].
Green eukaryotic microalga Chlorella (Chlorella vulgaris), belonging to the family Chlorellaceae, has an annual production of 2000 tons [3]. Chlorella comprises high protein content (>55% dry weight) with essential and non-essential amino acids, which are comparable to the standard profile of human nutrition. It also contains lipids with essential fatty acids, carbohydrates, high levels of pigments such as chlorophylls and carotenoids, vitamins including both water- and fat-soluble, and minerals such as zinc and potassium [3]. According to investigations, Chlorella has reducing potential and free radical scavenging activity [4, 5].
Antioxidants can be categorized as primary and secondary types based on their mechanism of action. Primary antioxidants act as a chain-breaker such as tocopherols [6]. Secondary (preventive) antioxidants present different mechanisms for slowing down the oxidation rate and are titled as synergists because of their promoting activity of primary antioxidants [7]. Citric acid (CA) is frequently used as a synergistic antioxidant, which may function by chelating a metal [8].
The purpose of this study was to investigate the oxidative stability of VOO by supplementing VOO with Chlorella and CA. The two supplements were added either individually or in combination with each other. Moreover, the antioxidant activity of Chlorella was compared with that of α-tocopherol and β-carotene.
Materials and methods
Chemicals and reagents
2,2-Diphenyl-1-picrylhydrazyl (DPPH), gallic acid, quercetin, ascorbic acid, CA, β-carotene, α-tocopherol, Folin–Ciocalteu reagent, and p-anisidine reagent (4-methoxyaniline) were purchased from Sigma-Aldrich Company (St. Louis, MO). All other chemical substances and solvents used in this study were of analytical/chromatography grade and were made by Merck Company (Darmstadt, Germany).
Chlorella
Spray-dried Chlorella was purchased from Lifestream International Ltd. (Aukland, New Zealand) and was stored in vacuum-packed conditions at 4 °C until further use. The proximate chemical composition of Chlorella was investigated according to the Official Methods of Analysis of the Association of Official Analytical Chemists [9].
Fatty acid composition of Chlorella
Fatty acid methyl esters (FAMEs) were prepared according to the method described by Golmakani et al. [10] from relevant research. A flame ionization detector and a BPX70 fused silica capillary column (30 m long, 0.25 mm internal diameter, and 0.25 µm film thickness) were used for gas chromatography (GC) analysis. The carrier gas was nitrogen. The injection volume was 1 µL and the split ratio was 1:10. The injector and detector temperatures were set at 250 and 300 °C, respectively. The initial oven temperature was set at 140 °C for 5 min and increased to 180 °C at a rate of 20 °C/min and was maintained for 9 min. Finally, the temperature was further increased to 200 °C at 20 °C/min and was maintained for 3 min. FAMEs were identified through a comparison of their retention times against pure standards analyzed under the same chromatographic conditions. The percentage for each fatty acid was expressed as the relative area percent of the total and was also reported based on their unsaturation degree, as saturated fatty acid (SFA), MUFA, and PUFA.
Antioxidant properties of Chlorella
To determine bioactive substances and antioxidant properties, a methanolic extract of Chlorella was prepared. The extraction method was applied in several steps as follows: 5 g of Chlorella ground powder was mixed with 25 mL methanol and was shaken vigorously for 2 min. The mixture was centrifuged (SW14R, Froilabo, Lyon, France) for 5 min at 2588×g. The supernatant phase was filtered through a Whatman No. 1 filter paper and the residue was re-extracted twice following the above mentioned procedure. The supernatants were combined, centrifuged, and filtered. The volume of Chlorella extract was increased to 100 mL by adding methanol. The extract was then refrigerated until future use.
Total phenolic content of Chlorella extract (10 mg/mL) was measured using the method described by Habibi et al. [11]. Different concentrations of gallic acid (0.02–0.10 mg/mL) were used to create a standard curve. Total flavonoid content of Chlorella extract (10 mg/mL) was determined using the spectrophotometric method as described by Habibi et al. [11]. Different concentrations of quercetin (0.02–0.10 mg/mL) were used to create a standard curve. The method by Dere et al. [12] was employed to estimate the carotenoid and chlorophyll contents of Chlorella.
The DPPH radical scavenging activity of different concentrations of Chlorella extract (0.01–1.00 mg/mL) was measured according to the method of Shalaby and Shanab [13]. The result was recorded as a sample concentration leading to 50% DPPH radical scavenging activity (IC50 value). Cupric reducing antioxidant capacity (CUPRAC) of Chlorella extract (1 mg/mL) was determined according to the method of Apak et al. [14]. For preparation of a standard curve, different concentrations of ascorbic acid (0.01–0.10 mg/mL) were used.
VOO
VOO used in this project was provided by the Edible Oil Industries Group of Etka Organization. VOO was stored in amber glass bottles with no head space and was kept at 4 °C until future analysis.
Quality and oxidation parameters of VOO
Free acidity (Ca 5a-40), peroxide value (PV; Cd 8-53), anisidine value (AV; Cd 18-90), and K232 and K268 (Ch 5-91) were measured using the Official Methods of American Oil Chemists’ Society [15]. Totox value (TV) was calculated as 2PV + AV. The maximum PV, K232 value, and K268 value for VOO was established as 20 meq O2/kg, 2.60, and 0.25, respectively [16].
Fatty acid composition of VOO
FAMEs of VOO were prepared according to Golmakani et al. [17]. All technical features and GC system conditions were the same as those described for fatty acid composition of Chlorella. The results were expressed as the percentage of relative peak area for each detected fatty acid.
Oxidative stability of VOO supplemented with Chlorella
Microalga Chlorella was milled into uniform dry powder using a mill grinder (MJW176P, Matsushita Electric Industrial Company, Ltd. Osaka, Japan). Milled Chlorella was added to VOO at a concentration of 1% (w/w) and was sonicated by immersing a clean ultrasonic probe (Bandelin Electronics, Berlin, Germany). The sonication condition provided 50 W for 10 min of total working time (20 s sonication time and 10 s interval time) at 25 °C. For comparison, β-carotene and α-tocopherol were also added to VOO at a concentration of 0.01% (w/w). Furthermore, the synergistic effects of different antioxidants with CA (at a concentration of 0.01%, w/w) were evaluated.
Samples were kept in closed amber glass bottles and were heated in an incubator (Memmert GmbH + Co. KG, Schwabach, Germany) at 60 ± 1 °C for 16 days in darkness. Chlorella samples (with and without CA) were initially filtered through a Whatman No. 1 filter paper and were then analyzed. In order to explore to what extent oxidation occurs in each sample, the PV, AV, TV, K232 value, and K268 value were measured every 4 days. Moreover, carotenoid and chlorophyll contents of samples were measured at the beginning and at the end of storage period and were respectively expressed in terms of mg lutein and pheophytin-a per kg of VOO [18]. Antioxidant indices, including protection factor (PF), antioxidant activity (AA), improved oxidative stability (IOS), and synergism degree, were measured according to the following equations:
| 1 |
| 2 |
| 3 |
| 4 |
Induction period (IP) was defined as the time (in days) taken to reach the maximum PV for VOO, namely, 20 meq O2/kg [16, 19]. The IP was calculated by extrapolation of PV curve. The AA index is a function of antioxidant concentration [20]. Accordingly, the AA values of samples containing synergistic modes of action were estimated on the basis of both antioxidant and CA concentrations.
Color attributes (L*a*b*) were evaluated for the samples at the beginning and at the end of storage period according to the method described by Habibi et al. [11]. In the L*a*b* coordinate system, the L* value indicates that brightness ranges from 0 (black) to 100 (white), whereas a* value ranges from −100 (greenness) to +100 (redness) and b* value ranges from −100 (blueness) to +100 (yellowness). Furthermore, the color difference () was calculated and set to be compared with the control samples (with and without CA).
Statistical analysis
All experiments and analyses were performed in triplicates. Results were reported as mean values ± standard deviation. Statistical analyses were conducted using Statistical Analysis Software (SAS) version 9.1 (SAS Institute Inc., Cary, NC). All data were treated with the general linear model procedure. Significant differences (p < 0.05) among the mean values were determined using Duncan’s multiple range test.
Results and discussion
Chlorella
The chemical composition of the Chlorella comprised 64.20% protein, 13.21% fat, 10.70% carbohydrates, 7.22% ash, and 4.67% moisture.
Fatty acid composition of Chlorella
Palmitic acid (28.02%), α-linolenic acid (24.95%), and linoleic acid (15.11%) were the major types of fatty acids present in Chlorella (Table 1). Only small amounts of PUFA (0.05%) can be liberated into VOO by adding 1% Chlorella. Therefore, adding Chlorella cannot significantly increase the PUFA content of VOO.
Table 1.
Fatty acid composition of Chlorella and virgin olive oil
| Fatty acid | Relative peak area (%) | |
|---|---|---|
| Chlorella | Virgin olive oil | |
| Myristic acid (C14:0) | 4.16 ± 0.49a | ND |
| Palmitic acid (C16:0) | 28.02 ± 1.63 | 17.14 ± 0.05 |
| Margaric acid (C17:0) | 5.92 ± 1.00 | ND |
| Stearic acid (C18:0) | 14.85 ± 0.02 | 0.42 ± 0.16 |
| Oleic acid (C18:1 ω − 9) | 7.01 ± 0.22 | 75.44 ± 3.77 |
| Linoleic acid (C18:2 ω − 6) | 15.11 ± 1.34 | 5.71 ± 3.03 |
| α-Linolenic acid (C18:3 ω − 3) | 24.95 ± 0.45 | 1.29 ± 0.53 |
| ∑ Saturated fatty acid (SFA) | 52.94 ± 1.11 | 17.59 ± 0.16 |
| ∑ Monounsaturated acid (MUFA) | 7.01 ± 0.22 | 74.90 ± 2.75 |
| ∑ Polyunsaturated fatty acid (PUFA) | 40.06 ± 0.90 | 7.51 ± 2.60 |
ND not detected
aMean ± SD (n = 3)
Antioxidant properties of Chlorella
The total phenolic and flavonoid contents of Chlorella extract were 23.74 ± 1.50 mg gallic acid equivalent/g and 9.16 ± 0.10 mg quercetin equivalent/g, respectively. The carotenoid and chlorophyll contents of Chlorella were 2.7 and 32.6 mg/g dry weight, respectively. High content of chlorophyll is a distinctive characteristic of Chlorella [3].
Chlorella extract exhibited an IC50 value of 0.745 ± 0.023 mg/mL, indicating that Chlorella extract is able to transfer electrons to neutralize free radicals. This ability can be attributed to the bioactive components of Chlorella. In Cu (II)–Cu (I) transformation experiments, the reductive capacity of Chlorella extract was 29.42 ± 0.77 mg ascorbic acid equivalent/g.
Quality and oxidation parameters of VOO
The free acidity, PV, AV, TV, K232 value, and K268 value of olive oil were 1.76 g oleic acid/100 g, 4.18 meq O2/kg, 3.77 mg/kg, 12.13, 1.25, and 0.10, respectively. The quality parameters for the analyzed olive oil were free acidity, PV, K232 value, and K268 value, which could fall within the ranges established for the category of VOO, as stated by the International Olive Council [16].
Fatty acid composition of VOO
The major fatty acids identified in olive oil were oleic acid (75.44%), palmitic acid (17.14%), and linoleic acid (5.71%) (Table 1). Fatty acid composition of the olive oil in this case is likely to follow the ranges set for VOO [16]. Moreover, PUFA accounted for 7.51% of the total fatty acids in VOO.
Oxidative stability of VOO supplemented with Chlorella
PV and antioxidant indices
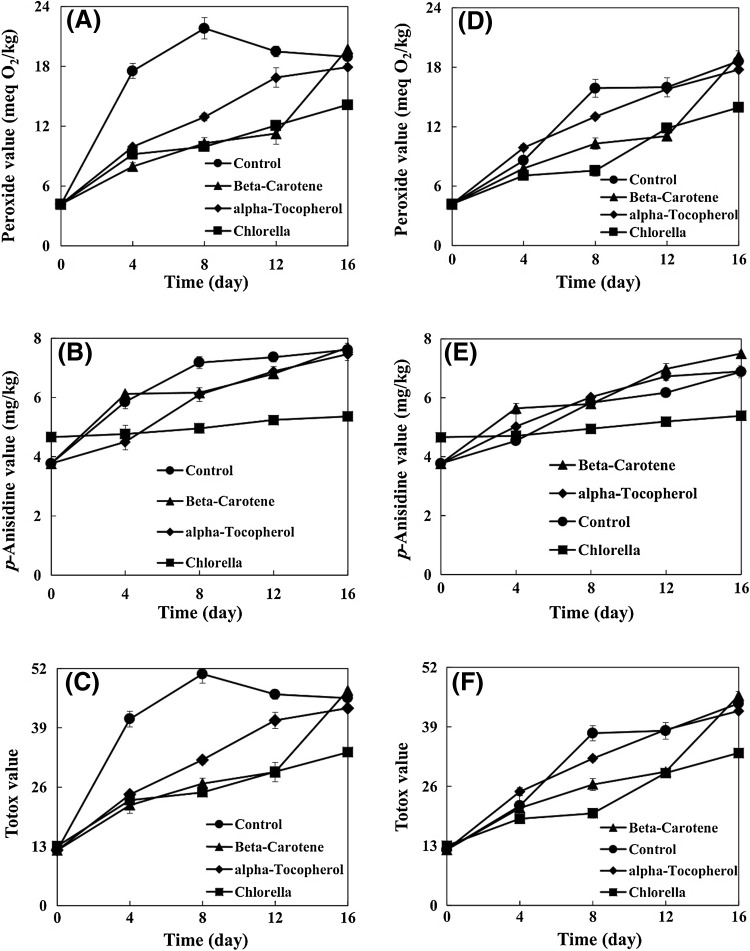
The oxidative stability of samples was assessed under accelerated storage conditions and was subjected to evaluate their primary, secondary, and total oxidation products. The PV of the control increased with increasing storage period, showing the maximum value of 21.81 meq O2/kg after 8 days (Fig. 1A). The PV of control was 40.82–54.38% higher than that of the samples containing natural and synthetic antioxidants. Thereafter, the PV of the control showed a decreasing trend. As the PV of control decreased, a progressive increase in the AV of control occurred. This behavior can be explained by the instability of hydro-peroxide products of the control and their conversion into secondary oxidation products.
Fig. 1.
Effect of Chlorella on peroxide, anisidine, and Totox values of virgin olive oil with (D–F) and without (A–C) citric acid
At the end of storage period, the Chlorella sample showed the lowest PV. Therefore, adding Chlorella can be a suitable approach to retard the VOO oxidation. Eliciting bioactive components from Chlorella can be a cause for lowering the PV level in comparison with the control. In Chlorella, compounds with known antioxidant capacity include carotenoids (approximately 4 mg per gram), chlorophylls (approximately 21 mg per gram), phenolic compounds (approximately 15 mg gallic acid equivalent per gram of dry extract), and also trace elements of several other compounds [4, 21]. Santoso et al. [22] investigated the antioxidant capacity of seven species of Indonesian seaweeds in an emulsion system of fish oil. They observed that seaweed extracts had lower PVs than that of control. In this study, the Chlorella sample had a significantly lower PV than the PVs of β-carotene and α-tocopherol samples at the end of storage period.
The PVs of samples containing CA increased with increasing storage period (Fig. 1D). Throughout the storage period, the PV of Chlorella sample was significantly lower than that of the control. The Chlorella sample had significantly lower PV (by 25.05%) than that of the control at the end of storage period. The PV of Chlorella sample was significantly lower than those of the α-tocopherol and β-carotene samples at the end of storage period. At the same time, there were no significant differences between the PV of β-carotene sample and that of the control. At the end of storage period, there were no significant differences between PVs of all samples without CA and those with CA.
Estimation of antioxidant indices can allow researchers to study the effects of antioxidants on resistance of VOO. All samples showed significantly higher IP values than that of the control (Table 2). The Chlorella sample had the highest IP value, which can be attributed to the release of Chlorella bioactive components. Moreover, the IP values of α-tocopherol and β-carotene samples were observed to be similar. The IP value of Chlorella sample containing CA was significantly higher than its corresponding control. There were no significant differences among the IP values of control, α-tocopherol, and β-carotene samples containing CA. The IP values of all samples containing CA, other than the control, were the same as their corresponding samples without CA.
Table 2.
Effect of Chlorella on antioxidant indices of virgin olive oil
| Sample | Induction period (IP; day) | Protection factor (PF) | Antioxidant activity (AA) | Improved oxidative stability (IOS; %) |
|---|---|---|---|---|
| Without citric acid | ||||
| Control | 6.50 ± 0.22c1 | 1.00 ± 0.00c | – | – |
| Chlorella | 25.72 ± 1.10a | 3.96 ± 0.17a | 2.95 ± 0.17b | 295.69 ± 16.87a |
| β-Carotene | 18.13 ± 1.71b | 2.82 ± 0.30b | 178.87 ± 26.33a | 178.87 ± 26.33b |
| α-Tocopherol | 16.87 ± 0.55b | 2.59 ± 1.11b | 159.59 ± 8.53a | 159.59 ± 8.53b |
| With citric acid | ||||
| Control | 16.15 ± 0.97b | 1.00 ± 0.00c | – | – |
| Chlorella | 26.24 ± 0.55a | 1.63 ± 0.03a | 0.63 ± 0.03a | 62.50 ± 3.40a |
| β-Carotene | 18.20 ± 1.90b | 1.13 ± 0.11b | 6.33 ± 5.91a | 12.49 ± 11.55b |
| α-Tocopherol | 17.51 ± 0.07b | 1.08 ± 0.01bc | 4.20 ± 0.22a | 8.40 ± 0.45b |
In each column and for each part (i.e., with or without citric acid), means with different letters are significantly different (p < 0.05)
1Mean ± SD (n = 3)
According to Table 2, the Chlorella samples (both with and without CA) showed the highest PF values. No significant differences were observed between the PF values of α-tocopherol and β-carotene samples.
AA is an index depending on antioxidant concentration [20]. Chlorella sample had the lowest AA value. AA values of α-tocopherol and β-carotene samples were the same, whereas they were higher than that of the Chlorella sample.
Chlorella significantly increased the IOS value of VOO (295.69%), as shown in Table 2. Moreover, the IOS values of VOO significantly increased to 178.87 and 159.59% by the incorporation of β-carotene and α-tocopherol, respectively. Chlorella sample containing CA had the highest IOS value (62.50%). The IOS values of β-carotene and α-tocopherol samples containing CA were the same.
It was evident that CA showed antioxidant capacity in VOO but no synergistic relationship appeared to exist between Chlorella and CA (−46.97%). Therefore, it means that CA is unable to further increase the oxidative stability of the Chlorella sample. Similarly, CA had no synergistic effect on improving the oxidative stability of α-tocopherol (−81.92%) and β-carotene samples (−83.68%).
AV and TV
A continuous increase in the AVs of samples was observed during the entire storage period (Fig. 1B). As far as the accelerated storage is concerned, the AV of Chlorella sample was significantly lower than that of the control. This behavior of the Chlorella sample can be attributed to the antioxidant properties of bioactive components, which it releases into the VOO. Hermund et al. [23] evaluated the effects of brown alga (Fucus vesiculosus) extracts on the oxidation of fish-oil-enriched mayonnaises. They suggested that the efficiency of alga can be attributed to its compounds (carotenoid and phenolic) and also its radical scavenging activity and metal chelating ability.
The AV of Chlorella sample was significantly lower than the AVs of α-tocopherol and β-carotene samples. After 12 days of storage, the AV of control was significantly higher than the AVs of β-carotene and α-tocopherol samples. However, there were no significant differences among them at the end of storage period. The control sample reached its maximum AV after 8 days, and then it exhibited a relatively constant value until the end of storage period. Even though β-carotene and α-tocopherol samples had lower AVs compared to the control after 8 and 12 days, there were no significant differences among their AVs at the end of storage period due to the continuous increase in the AVs of β-carotene and α-tocopherol samples.
The AVs of samples containing CA were observed to increase with increasing storage period (Fig. 1E). In general, the Chlorella sample showed the lowest AV during the storage period. At the end of storage period, the AV of Chlorella sample was significantly lower (by 21.66%) than that of the control. However, the β-carotene sample exhibited the highest AV throughout the storage period. Therefore, this sample was not successful in holding back the secondary oxidation phase. At the end of storage period, the α-tocopherol sample presented an AV similar to that of the control. At the end of storage period, the AVs of control and α-tocopherol sample containing CA were significantly lower than those of their corresponding samples without CA. However, there were no significant differences between the AVs of the Chlorella samples with and without CA.
The TV trends of samples were similar to the pattern of PV trends. The TV of control was increased by 8 days during storage and reached a maximum value of 50.80 (Fig. 1C). At this point, TVs of Chlorella, β-carotene, and α-tocopherol samples were 51.08, 47.30, and 37.09%, respectively, lower than the TV of control. Between the eighth and sixteenth day of storage, a decreasing trend was observed in the TV of control, which decreased to 45.55 ultimately. A possible explanation for this decrease in PV, during the aforementioned period, is that primary oxidation products convert to secondary ones. It is further notable to state that the Chlorella sample showed the lowest TV.
The TVs of samples containing CA increased with increasing storage period (Fig. 1F). In general, the Chlorella sample was identified as having the lowest TV during the storage period. At the end of storage period, the TV of α-tocopherol sample was significantly lower than that of the control but the TV of β-carotene sample was significantly higher. The TVs of all samples containing CA were significantly lower than those of their corresponding samples without CA at the end of storage period.
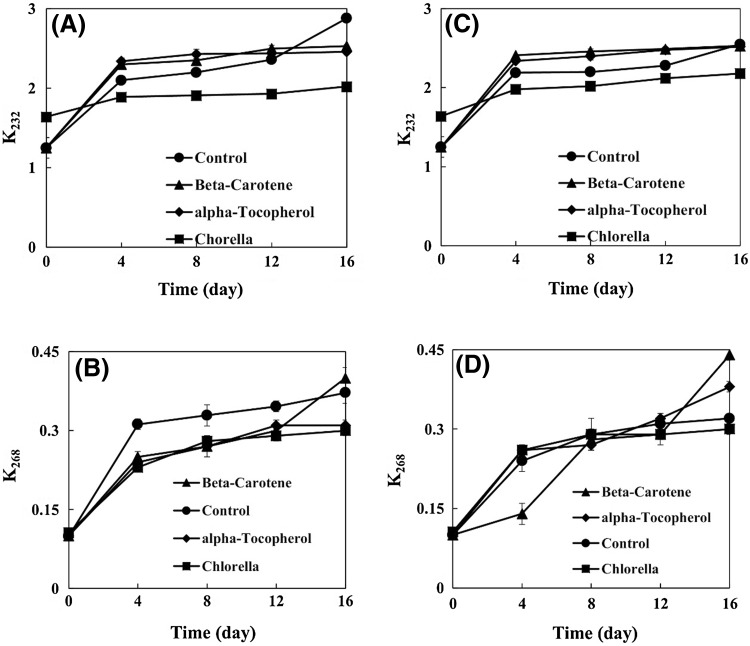
K232 and K268 values
K232 and K268 values are parameters for the measurement of diene and triene compounds, respectively [15]. The K232 values of samples increased with increasing storage period (Fig. 2A). The K232 value of Chlorella sample was significantly lower than that of the control during the storage period. Athukorala et al. [24] measured the conjugated diene contents (K234) in fish oil containing red alga Grateloupia filicina extract. They found that alga extract samples had lower conjugated diene contents in comparison with the control.
Fig. 2.
Effect of Chlorella on the K232 and K268 values of virgin olive oil with (C, D) and without (A, B) citric acid
The K232 values of β-carotene and α-tocopherol samples were significantly higher than that of the Chlorella sample but were significantly lower than that of the control at the end of storage period.
The K232 values of samples containing CA increased with increasing storage period (Fig. 2C). As far as the accelerated storage is concerned, the K232 value of Chlorella sample was significantly lower than that of the control and even lower than the values of β-carotene and α-tocopherol samples. There were no significant differences among the K232 values of control, β-carotene, and α-tocopherol samples containing CA.
The K268 values of samples increased with increasing storage period (Fig. 2B). At the end of storage period, the K268 values of Chlorella and α-tocopherol samples were significantly lower than that of the control. The K268 value of β-carotene sample reached 0.40, being significantly higher than that of the control.
The K268 values of samples containing CA increased with increasing storage period (Fig. 2D). At the end of storage period, the Chlorella sample exhibited significantly lower K268 value than that of the control. At the same time, the K268 value of β-carotene sample was significantly higher than the values of the control and α-tocopherol samples.
Carotenoid and chlorophyll contents
Carotenoids can prevent oxidation by scavenging free radicals or trapping the singlet oxygen. Carotenoids reduction of the control indicates that carotenoids can act as a natural antioxidant (Table 3). The highest carotenoid degradation was observed in β-carotene sample. The carotenoid content of β-carotene sample decreased more rapidly than other samples, which can be attributed to its consumption as an antioxidant and also its decomposition under thermal conditions. The initial carotenoid content of Chlorella sample was 4.75 times higher than that of the control. This high content can be attributed to the release of Chlorella carotenoids into VOO. At the end of storage period, an increase of 2.18% was observed in the carotenoid content in the Chlorella sample. The slight increase in carotenoid content of the Chlorella sample can be explained by a higher rate of carotenoid release than its consumption.
Table 3.
Effect of Chlorella on carotenoid and chlorophyll contents of virgin olive oil
| Sample | Carotenoid | Chlorophyll | ||||
|---|---|---|---|---|---|---|
| Initial content (mg/kg) | Final content (mg/kg) | Relative change (%) | Initial content (mg/kg) | Final content (mg/kg) | Relative change (%) | |
| Without citric acid | ||||||
| Control | 6.16 ± 0.04c1 | 5.16 ± 0.06c | −16.17 ± 1.52c | 15.49 ± 0.22b | 10.04 ± 0.19c | −32.78 ± 0.27c |
| Chlorella | 29.28 ± 0.57b | 29.91 ± 0.41b | +2.18 ± 2.16a | 134.19 ± 1.64a | 134.56 ± 0.96a | +00.28 ± 0.55a |
| β-Carotene | 103.61 ± 1.57a | 55.36 ± 2.09a | −46.57 ± 1.65d | 15.49 ± 0.22b | 9.95 ± 0.11c | −35.73 ± 1.64d |
| α-Tocopherol | 6.16 ± 0.04c | 5.46 ± 0.03c | −11.36 ± 1.22b | 15.49 ± 0.22b | 12.00 ± 0.14b | −22.53 ± 0.66b |
| With citric acid | ||||||
| Control | 6.16 ± 0.04c | 5.30 ± 0.03c | −13.90 ± 1.09b | 15.49 ± 0.22b | 10.34 ± 0.19b | −33.25 ± 1.72c |
| Chlorella | 29.39 ± 0.52b | 30.16 ± 0.11b | +2.62 ± 1.98a | 134.48 ± 0.52a | 135.94 ± 1.34a | +1.08 ± 0.97a |
| β-Carotene | 102.04 ± 0.13a | 36.28 ± 0.17a | −64.00 ± 0.78c | 15.49 ± 0.22b | 10.04 ± 0.06b | −35.15 ± 1.28c |
| α-Tocopherol | 6.16 ± 0.04c | 5.34 ± 0.07c | −13.30 ± 1.77b | 15.49 ± 0.22b | 11.08 ± 0.11b | −28.47 ± 0.51b |
In each column and for each part (i.e., with or without citric acid), means with different letters are significantly different (p < 0.05)
1Mean ± SD (n = 3)
The initial carotenoid content of Chlorella sample containing CA was 4.77 times higher compared to its corresponding control (Table 3). Although the loss of carotenoids in the control was 13.90% of its initial content, the carotenoid content of Chlorella sample slightly increased at the end of storage period.
Chlorophyll can act as an antioxidant agent in a dark environment. Chlorophyll contents of Chlorella samples (with and without CA) were higher than those of their corresponding control samples (with and without CA), both at the beginning and at the end of storage period (Table 3). The chlorophyll content of Chlorella sample remained almost constant at the end of storage period but the chlorophyll content of the control reduced by 32.78% during the same course of time.
Color attributes
The initial L* values of Chlorella samples (with and without CA) were significantly lower compared with those of their corresponding control samples (Table 4). The low initial L* values indicate that the release of Chlorella pigments leads to the decrease in the brightness of VOO. The L* values of Chlorella samples decreased through the course of storage period.
Table 4.
Effects of Chlorella on color attributes of virgin olive oil
| Sample | L* | a* | b* | ΔE1 | ||||
|---|---|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | Initial | Final | |
| Without citric acid | ||||||||
| Control | 57.67 ± 0.82a2 | 55.00 ± 0.71b | −5.50 ± 0.55b | −4.40 ± 0.55c | 56.83 ± 0.98a | 51.40 ± 0.55c | – | – |
| Chlorella | 34.00 ± 0.41c | 27.00 ± 1.00c | −11.00 ± 0.71c | 2.40 ± 0.55a | 40.17 ± 0.75d | 34.75 ± 0.50d | 29.44 ± 0.70a | 33.38 ± 0.48a |
| β-Carotene | 56.80 ± 0.84ab | 58.00 ± 0.82a | −4.25 ± 0.50a | −3.00 ± 0.00b | 54.50 ± 0.58c | 57.50 ± 0.58a | 2.81 ± 0.43b | 6.78 ± 0.00b |
| α-Tocopherol | 56.50 ± 0.58b | 56.00 ± 0.00b | −5.25 ± 0.50b | −5.00 ± 0.00d | 55.75 ± 0.50b | 55.50 ± 0.58b | 1.74 ± 0.78c | 4.18 ± 0.07c |
| With citric acid | ||||||||
| Control | 58.00 ± 0.00a | 57.50 ± 0.58a | ± 5.50 ± 0.58a | −4.75 ± 0.50c | 56.50 ± 0.58a | 54.50 ± 0.58b | – | – |
| Chlorella | 33.25 ± 0.50c | 25.25 ± 0.50b | −10.00 ± 0.71b | 3.20 ± 0.45a | 40.17 ± 0.75c | 34.75 ± 0.50c | 29.96 ± 0.59a | 38.41 ± 0.81a |
| β-Carotene | 56.40 ± 0.55b | 57.80 ± 0.45a | −4.75 ± 0.50a | −4.00 ± 0.00b | 54.25 ± 0.50b | 57.25 ± 0.50a | 2.90 ± 0.36b | 2.93 ± 0.48b |
| α-Tocopherol | 56.50 ± 0.58b | 57.00 ± 0.82a | −5.25 ± 0.50a | −5.00 ± 0.00c | 56.25 ± 0.50a | 57.50 ± 0.58a | 1.68 ± 0.47c | 3.12 ± 0.15b |
In each column and for each part (i.e., with or without citric acid), means with different letters are significantly different (p < 0.05)
1In comparison with its corresponding control as reference
2Mean ± SD (n = 3)
The initial a* values of Chlorella samples (with and without CA) were significantly lower than those of their corresponding control samples, confirming their greenish color (Table 4). The a* values of Chlorella samples increased during the period of accelerated storage. This increase possibly results from the pheophytinization of chlorophylls present in Chlorella, leading to the production of pheophytin [4]. Parallel to the decrease in chlorophyll contents of the control samples, their a* values increased at the end of storage period.
The initial b* values of Chlorella samples (with and without CA) were significantly lower than those of their corresponding control samples (Table 4). The b* values of control samples (with and without CA) decreased during the storage period, which can be attributed to the presence of oxidized VOO pigments. The b* values of β-carotene samples were observed to increase, which can be due to carotenoid oxidation.
Chlorella samples (with and without CA) had significantly higher ΔE values in comparison with β-carotene and α-tocopherol samples (Table 4). The ΔE value of β-carotene sample was significantly higher than that of α-tocopherol sample, both at the beginning and at the end of storage period.
Acknowledgements
This research project was financially supported by the Shiraz University. We would like to thank the Edible Oil Industries Group of Etka Organization for providing VOO. We also thank the Persian editor, Mohsen Hamedpour-Darabi, for natively editing the English of the paper.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Frankel EN. Chemistry of extra virgin olive oil: Adulteration, oxidative stability, and antioxidants. J. Agric. Food Chem. 2010;58:5991–6006. doi: 10.1021/jf1007677. [DOI] [PubMed] [Google Scholar]
- 2.Morales MT, Przybylski R. Olive oil oxidation. In: Aparicio R, Harwood J, editors. Handbook of Olive Oil. New York: Springer; 2013. p. 482. [Google Scholar]
- 3.Safi C, Zebib B, Merah O, Pontalier PY, Vaca-Garcia C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sust. Energ. Rev. 2014;35:265–278. doi: 10.1016/j.rser.2014.04.007. [DOI] [Google Scholar]
- 4.Cha KH, Kang SW, Kim CY, Um BH, Na YR, Pan CH. Effect of pressurized liquids on extraction of antioxidants from Chlorella vulgaris. J. Agric. Food Chem. 2010;58:4756–4761. doi: 10.1021/jf100062m. [DOI] [PubMed] [Google Scholar]
- 5.Goiris K, Muylaert K, Fraeye I, Foubert I, De Brabanter J, De Cooman L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012;24:1477–1486. doi: 10.1007/s10811-012-9804-6. [DOI] [Google Scholar]
- 6.Wanasundara PKJPD, Shahidi F. Antioxidants: Science, technology, and applications. In: Shahidi F, editor. Bailey’s Industrial Oil and Fat Products. New York: John Wiley & Sons; 2005. p. 436. [Google Scholar]
- 7.Reische DW, Lillard DA, Eitenmiller RR. Antioxidants. In: Akoh CC, Min DB, editors. Food lipids: Chemistry, nutrition, and biotechnology. Boca Raton, FL, USA: CRC Press, Inc; 2008. pp. 410–412. [Google Scholar]
- 8.Pokorny J. Antioxidants in food preservation. In: Rahman MS, editor. Handbook of Food Preservation. Boca Raton, FL, USA: CRC Press; 2007. pp. 274–275. [Google Scholar]
- 9.AOAC. Official methods of analysis of AOAC intl. Association of Analytical Chemists, USA (1997)
- 10.Golmakani MT, Rezaei K, Mazidi S, Razavi SH. γ-Linolenic acid production by Arthrospira platensis using different carbon sources. Eur. J. Lipid Sci. Technol. 2012;114:306–314. doi: 10.1002/ejlt.201100264. [DOI] [Google Scholar]
- 11.Habibi M, Golmakani MT, Mesbahi G, Majzoobi M, Farahnaky A. Ultrasound-accelerated debittering of olive fruits. Innov. Food Sci. Emerg. Technol. 2015;31:105–115. doi: 10.1016/j.ifset.2015.06.014. [DOI] [Google Scholar]
- 12.Dere S. GÜNEŞ T, Sivaci R. Spectrophotometric determination of chlorophyll-A, B and total carotenoid contents of some algae species using different solvents. Turk. J. Bot. 1998;22:13–18. [Google Scholar]
- 13.Shalaby EA, Shanab SM. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Indian J. Geo-Mar. Sci. 2013;42:556–564. [Google Scholar]
- 14.Apak R, Güçlü K, Özyürek M, Karademir SE. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC Method. J. Agric. Food Chem. 2004;52:7970–7981. doi: 10.1021/jf048741x. [DOI] [PubMed] [Google Scholar]
- 15.AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society. Illinois (US), AOCS Press (1998)
- 16.IOC. International Olive Council. Trade Standard Applying to Olive oils and Olive -pomace oils. Decision COI/T.15/NC No 3/Rev. 8. Madrid (2015)
- 17.Golmakani MT, Rezaei K, Mazidi S, Razavi SH. Effect of alternative C2 carbon sources on the growth, lipid, and γ-linolenic acid production of spirulina (Arthrospira platensis) Food Sci. Biotechnol. 2012;21:355–363. doi: 10.1007/s10068-012-0047-8. [DOI] [Google Scholar]
- 18.Minguez-Mosquera MI, Rejano-Navarro L, Gandul-Rojas B, SanchezGomez AH, Garrido-Fernandez J. Color-pigment correlation in virgin olive oil. J. Am. Oil Chem. Soc. 1991;68:332–336. doi: 10.1007/BF02657688. [DOI] [Google Scholar]
- 19.Keramat M, Golmakani MT, Aminlari M, Shekarforoush SS. Comparative effect of Bunium persicum and Rosmarinus officinalis essential oils and their synergy with citric acid on the oxidation of virgin olive oil. Int. J. Food Prop. 2016;19:2666–2681. doi: 10.1080/10942912.2015.1126722. [DOI] [Google Scholar]
- 20.Antolovich M, Prenzler PD, Patsalides E, McDonald S, Robards K. Methods for testing antioxidant activity. Analyst. 2002;127:183–198. doi: 10.1039/b009171p. [DOI] [PubMed] [Google Scholar]
- 21.Cha KH, Lee HJ, Koo SY, Song DG, Lee DU, Pan CH. Optimization of pressurized liquid extraction of carotenoids and chlorophylls from Chlorella vulgaris. J. Agric. Food Chem. 2010;58:793–797. doi: 10.1021/jf902628j. [DOI] [PubMed] [Google Scholar]
- 22.Santoso J, Yoshie-Stark Y, Suzuki T. Anti-oxidant activity of methanol extracts from Indonesian seaweeds in an oil emulsion model. Fisheries science. 2004;70:183–188. doi: 10.1111/j.1444-2906.2003.00787.x. [DOI] [Google Scholar]
- 23.Hermund DB, Yeşiltaş B, Honold P, Jónsdóttir R, Kristinsson HG, Jacobsen C. Characterisation and antioxidant evaluation of Icelandic F. vesiculosus extracts in vitro and in fish-oil-enriched milk and mayonnaise. J. Funct. Foods. 2015;19:828–841. doi: 10.1016/j.jff.2015.02.020. [DOI] [Google Scholar]
- 24.Athukorala Y, Lee KW, Park EJ, Heo MS, Yeo IK, Lee YD, Jeon YJ. Reduction of lipid peroxidation and H2O2-mediated DNA damage by a red alga (Grateloupia filicina) methanolic extract. J. Sci. Food Agric. 2005;85:2341–2348. doi: 10.1002/jsfa.2246. [DOI] [Google Scholar]