Abstract
Inhibitory effects of soy-protein edible coatings incorporated with 1, 2, or 3% of thyme or oregano essential oils (EOs) were determined against Escherichia coli O157:H7 (EC), Listeria monocytogenes (LM), and Staphylococcus aureus (SA) in vitro and on fresh beef during refrigerated storage for 14 days. The soy-protein coatings with oregano and thyme EOs exhibited similar antimicrobial activity against the test bacteria. Greater antimicrobial activity of soy edible coatings was demonstrated against SA when 3% of EOs were added. Coatings with 3% thyme and oregano EOs exhibited 2.86 and 2.59, 1.97 and 1.90, and 1.87 and 1.83 log CFU/g reductions in SA, LM, and EC populations, respectively, as compared with the control by Day 14. This study demonstrated that application of edible coatings containing oregano and thyme EOs on fresh beef cuts could have a potential for controlling pathogenic bacteria and enhancing color stability with acceptable sensory characteristics.
Keywords: Edible coatings, Oregano essential oil, Thyme essential oil, Antibacterial activity, Beef
Introduction
Meat and meat products can be contaminated during the slaughter process with a variety of foodborne pathogenic bacteria that may cause serious foodborne illnesses and death. Listeria monocytogenes, Salmonella spp., Escherichia coli O157:H7, and Staphylococcus aureus are common meatborne pathogens, which are responsible for a significant number of foodborne outbreaks [1, 2]. An important priority of the meat processing industry is to prevent the growth of pathogenic bacteria associated with fresh meats and meat products. Bioactive packaging systems have been considered as a promising technology that has a significant effect on shelf-life extension and safety of product. Antimicrobial packaging has drawn much attention from the food industry in the recent years owing to the increase in consumer demand for minimally processed, natural, and more stable and safe foods. In this context, antimicrobial edible films and coatings have gained great interest in food preservation owing to their advantages such as biodegradability, environmentally friendly nature, and protection after the package is opened [3, 4]. Many studies have demonstrated that antimicrobial agents, when incorporated into packaging films and coatings, could be effective for reducing levels of foodborne microorganisms [5, 6].
Essential oils (EOs) and their components have great potential as natural antimicrobial agents to control the growth of pathogenic and spoilage bacteria in foods. The antimicrobial properties of many plant EOs have been reported by several researchers [7–9]. The applications of EOs in the food industry for controlling the growth of foodborne pathogens and food spoilage bacteria have been performed by directly adding an EO into foods as an ingredient or incorporating it into the edible coatings and films to surround the surface of the food.
Among EOs, thyme and oregano oils have been widely used flavoring agents in meat and meat products. Thyme and oregano EOs containing high concentrations of phenolic compounds such as thymol, carvacrol, p-cymene, and γ-terpinene have strong antibacterial properties against foodborne pathogens [10]. Phenolic active compounds are known to cause structural and functional damages to the bacterial cell membrane. Inhibition is believed to result, in part, from increased membrane permeability, leading to the loss of intracellular molecules such as protein, nucleic acids, inorganic ions, and ATP [11].
The antibacterial activity of thyme or oregano EOs against foodborne pathogens has been extensively examined in many in vitro studies; nevertheless, limited data exist on the application of antimicrobial edible coatings or films incorporated with EOs in meat systems. The objectives of this study were to evaluate the inhibitory effects of isolated soy-protein-based edible coatings incorporated with three different concentrations (1, 2, or 3%) of EOs from oregano (Oreganum heracleoticum L.) or thyme (Thymus vulgaris L.) against E. coli O157:H7, L. monocytogenes, and S. aureus on fresh beef cuts during refrigerated storage. Moreover, the pH value, instrumental color (lightness, redness and yellowness values), and sensory properties (appearance, color, odor, flavor, texture, and overall acceptability) were evaluated.
Materials and methods
Materials
Isolated soy protein with 90% protein content and two types of commercial EOs (EO) from O. heracleoticum L. and T. vulgaris L. were kindly supplied by GURHAK Food and Chemicals Corp., (Istanbul, Turkey), ERDOGMUS Fragrances Company (İstanbul, Turkey), and TALYA Herbal Products Company (Antalya, Turkey), respectively.
Bottom round beef was purchased from local butcher shops for the two replications on the day of slaughter, transferred to the meat technology laboratory of the Food Engineering Department at Ankara University in refrigerated containers, and stored at 4 °C for 48 h for aging purposes. Following aging, beef was cut into approximately 2.5 cm × 2.5 cm × 2.5 cm pieces using a stainless steel knife for microbiological, physicochemical, and sensory analysis, placed in sterile plastic stomacher bags, and irradiated at 10 kGy in a cobalt-60 gamma-irradiator at the Turkish Atomic Energy Authority (Ankara, Turkey) for sterilization. Irradiated beef pieces were then frozen at −18 °C for not more than 1 month for further analysis. Beef pieces were thawed at 4 °C for 24 h before the experiment. The sterility of the beef cuts was tested throughout the experiment, and there was no detectable contamination of beef cuts over the storage period.
Preparation of soy-protein-based coating formulation
Isolated soy-protein-based edible coating solutions were prepared using the methods described by Choi et al. [12] and Zivanovic et al. [5] with slight modifications. The coating solution was prepared by slowly dissolving 5% isolated soy protein (w/v) in distilled water while stirring. Glycerol (3.5% wt/vol) was added as a plasticizer and the pH was adjusted to 10.0 with 0.1 N NaOH. The coating solutions were heated to 90 °C in a water bath for 30 min followed by cooling to 40 °C and then filtering through four layers of cheese cloth by a vacuum pump. EO from O. heracleoticum L. (O) or T. vulgaris L. (T) were incorporated into the coating solution at various concentrations of 0% (SC as control), 1, 2, and 3% (v/v) of coating solution.
Bacterial strains
E. coli O157:H7, L. monocytogenes (ATCC 7644), and S. aureus cultures obtained from the culture collections of the Food Engineering Department of Ankara University were used. All cultures were kept at −18 °C in 20% glycerol and grown in Tryptic Soy Broth (Merck, Darmstadt, Germany) for 24 h at 37 °C.
Antibacterial activity of the coating solutions
Antibacterial activity testing was conducted using agar well diffusion assay. The test microorganisms (~106 CFU/mL) were inoculated into 20 mL of Tryptic Soy Agar (TSA; Merck, Darmstadt, Germany) and poured onto a petri dish. Next, the agar was allowed to solidify at 4 °C for 1 h. Wells of 9-mm diameter were aseptically punched into the agar, and 100 µL from the edible coating solutions were transferred into each well. The isolated soy-protein-based edible coating solutions incorporated with three different concentrations (1, 2, or 3%) of EO from oregano or thyme. The isolated soy-protein coating solution without the addition of EO (SC) was used as control. All the preloaded plates were incubated at 37 °C for 24 h. After the incubation period, the inhibition zone diameters were measured and recorded in millimeter. All the tests were performed in duplicate and their means were recorded [13].
Application of coatings on fresh beef
The coatings were applied on fresh beef according to the method described by Ekinci [14], with slight modifications to suit the conditions of this experiment. E. coli O157:H7, L. monocytogenes (ATCC 7644), and S. aureus were used as the test microorganisms. Individual pieces of meat were inoculated by submersion in 100 mL of a solution containing approximately 106 CFU/mL test microorganism for 15 min at room temperature and then dried for 10 min on a sterile petri dish under a sterile laminar flow hood. For color analysis and sensory evaluation, part of the meat pieces was not inoculated with test bacteria. Each piece of inoculated and non-inoculated meat was immersed individually into 100 mL of the coating solutions for 15 min at room temperature and then transferred to sterile plastic sample cups with lids and stored at 4 °C. Sampling was conducted on Days 0, 1, 4, 7, 10, and 14 for microbiological and physicochemical analyses. Treatment groups comprised (1) control samples (C), (2) samples coated with soy-protein coating solution without the addition of EO (SC), (3) samples coated with 1, 2, or 3% oregano EO added coating solution (O1, O2, or O3, respectively), (4) samples coated with 1, 2, or 3% thyme-EO-added coating solution (T1, T2, or T3, respectively). Experiments were performed in duplicate.
Microbiological analysis of fresh beef
Randomly selected pieces of beef from each group were aseptically weighed in a stomacher bag. A calculated amount of 0.1% sterile peptone water was added and the pieces were homogenized in a stomacher (BagMixer®, Interscience, France) for 1 min to obtain the initial dilution. Appropriate serial dilutions were spread plated on TSA, followed by incubation at 37 °C for 48 h. Bacterial counts were expressed as log CFU/g sample.
Instrumental color
The changes of meat color (Commission Internationale de L’Eclairage L*-lightness, a*-redness, b*-yellowness) were monitored using Lovibond Tintometer (Lovibond RT-300, Reflactance Tintometer, UK) on the surface of the duplicate beef samples at five random sites during the storage period. The redness index (redness to yellowness ratio, a*/b*) was used as an index of change in redness [15].
Sensory evaluation
The sensory evaluation of cooked beef cuts was conducted on Day 1 after the coating was applied in the Food Engineering Department at Ankara University with a semi-trained panel comprising 10 assessors experienced in meat product evaluation, who were selected for their ability to recognize, differentiate, and rank basic tastes and their ability to detect and distinguish a set of odors [16]. The samples from each group were individually cooked on Teflon pans with 200 mL distilled water for 20 min, covered with an aluminum foil, and kept in a warm oven (approximately 40 °C) before the analysis. Each sample was placed in white plastic dishes coded with three-digit random numbers and served simultaneously in a random order to the panelists in individual booths without any disturbances from the surroundings. Water and unsalted crackers were provided to cleanse the palate between samples. A 9-point hedonic scale (9: like extremely, 8: like very much, 7: like moderately, 6: like slightly, 5: neither like nor dislike, 4: dislike slightly, 3: dislike moderately, 2: dislike very much, 1: dislike extremely) was used to assess the appearance, color, odor, flavor, texture, and overall acceptability attributes.
Statistical analysis
Two independent replications were performed for each experimental condition. Data were subjected to analysis of variance, and means were separated with Duncan’s multiple range tests at 5% level of probability using SPSS 20.0 Statistics Software (SPSS, Chicago, IL, USA). Sensory data were analyzed by the non-parametric Kruskal–Wallis test.
Results and discussion
Antibacterial activity of the coating solutions
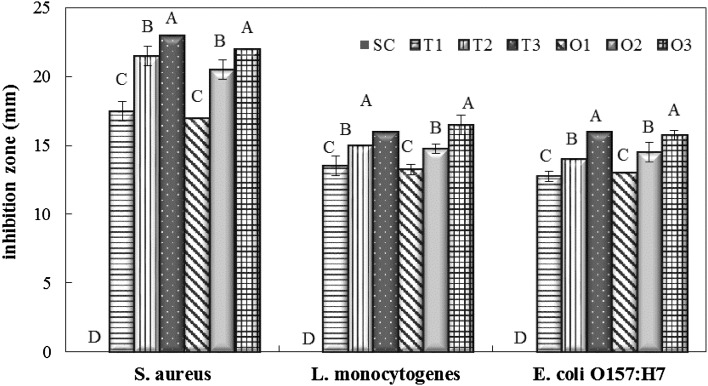
To confirm the antibacterial effects of thyme- and oregano-EO-incorporated coatings in vitro against E. coli O157:H7, L. monocytogenes, and S. aureus, the agar well diffusion test was conducted and the growth inhibition zones measured are presented in Fig. 1. Data from the agar well diffusion assay reveal that soy-protein coating solution without the addition of EO had no inhibitory effect against any of the pathogenic bacteria tested. Inhibition of four of the pathogenic bacteria in vitro by oregano- and thyme-EO-incorporated coatings (at 1, 2, and 3% levels) in the current study was concentration-dependent (p < 0.05). Oregano and thyme EOs showed inhibition against all bacteria, even at minimum concentration (1.0%). No significant difference (p > 0.05) was observed between oregano and thyme EOs added coatings in inhibiting the tested pathogens in the agar well diffusion assay. Greater antimicrobial activity of soy edible coatings were demonstrated against S. aureus, followed by L. monocytogenes and E. coli O157:H7 with inhibition zone diameters 23, 16, and 16 mm, respectively, when thyme EO was added at 3% level. Thyme- and oregano-EO-incorporated coatings presented the greater zone of inhibition on S. aureus at all concentrations (p < 0.05). Similarly, Sağdıç [17] determined the sensitivity of E. coli, E. coli O157:H7, Yersinia enterocolitica, and S. aureus to Turkish thyme and oregano hydrosols and noted that both hydrosols were inhibitive on the bacteria tested in broth, with the greatest effect observed on S. aureus. Gram positive bacteria have been reported to be more susceptible to plant EOs than their gram negative counterparts owing to the restriction of hydrophobic compound diffusion through the outer cell wall of gram negative bacteria [11, 13].
Fig. 1.
The inhibition zone of the coating solutions (mm) against S. aureus, L. monocytogenes, and E. coli O157:H7. SC: the isolated soy-protein-based edible coating solutions without essential oil addition. T1, T2, and T3: the isolated soy-protein-based edible coating solutions incorporated with 1, 2, and 3% thyme essential oils. O1, O2, and O3: the isolated soy-protein-based edible coating solutions incorporated with 1, 2, and 3% oregano essential oils. A–D: bars with different letters refer to statistically significant differences (p < 0.05)
As previously noted by several researchers, the antibacterial efficiency of oregano and thyme EOs result mostly from their major active phenolic components, thymol, carvacrol, p-cymene, and γ-terpinene [9]. With the introduction of edible coatings and films in food processing, current research on antimicrobial edible coatings and films has been directed toward antimicrobial packaging with the incorporation of natural agents, mostly EOs, into edible coatings or films, and it was shown that oregano and thyme EOs are effective against foodborne pathogens when applied in the coating or film formulation [1, 6, 18, 19]. In a previous study, the antibacterial activity of soy-protein edible films incorporated with oregano or thyme EOs was determined against E. coli, E. coli O157:H7, S. aureus, Pseudomonas aeruginosa, and Lactobacillus plantarum by the inhibition zone test, and it was reported that E. coli, E. coli O157:H7, and S. aureus were significantly inhibited by antimicrobial films, with weaker effect against L. plantarum and P. aeruginosa [20]. In another study, Lim et al. [21] examined the antimicrobial effects of Gelidium corneum edible films containing carvacrol, one of the major components of oregano and thyme EOs, at different concentrations against E. coli O157:H7 and L. monocytogenes with an inhibition zone assay and reported that the addition of 0.4–1.0% carvacrol inhibited growth of both pathogens.
Antibacterial activity of the coatings against pathogenic bacteria on beef cuts
Antibacterial activity against E. coli O157:H7
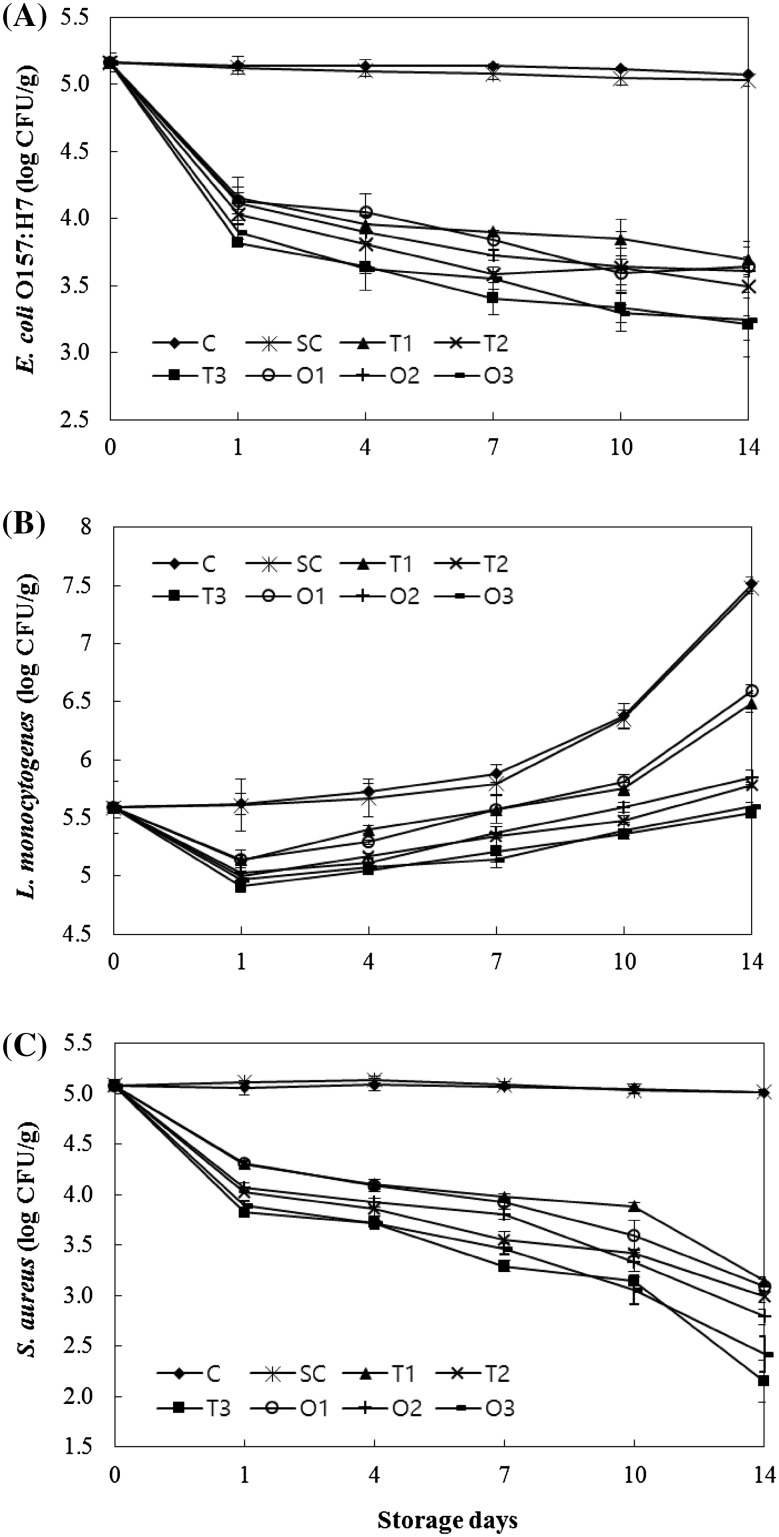
The initial counts of E. coli O157:H7 (5.16 log CFU/g) were significantly reduced in all the samples coated with oregano- or thyme-EO-incorporated edible coatings on Day 1 [Fig. 2(A)]. The reduction level by Day 1 with EO-incorporated coatings was around 1.01–1.34 log CFU/g depending on the concentration of the EO added. No significant change of E. coli O157:H7 counts from the initial value were determined in C and SC groups over the 14-day refrigerated storage. The antimicrobial activity of both oregano- and thyme-incorporated-edible coatings against E. coli O157:H7 on beef cuts was concentration-dependent and concentration was a significant factor in inhibiting E. coli O157:H7 for oregano- and thyme-incorporated edible coatings on Day 14, where 3% levels had greater (p < 0.05) antimicrobial activity than 1 and 2% level for each EO. Coatings with 3% oregano and thyme incorporation indicated 1.83 and 1.87 logarithmic unit reduction in E. coli O157:H7 populations as compared with C on Day 14.
Fig. 2.
Changes in E. coli O157:H7 (A), L. monocytogenes (B), and S. aureus (C) counts of beef samples during refrigerated storage. C: uncoated control, SC: the group coated with soy protein without essential oil addition. T1, T2, and T3: the isolated soy-protein-based edible coating solutions incorporated with 1, 2, and 3% thyme essential oils. O1, O2, and O3: the isolated soy-protein-based edible coating solutions incorporated with 1, 2, and 3% oregano essential oils. Error bars represent standard errors
Burt and Reinder [7] determined the antibacterial activity of selected plant EOs against E. coli O157:H7 and noted that oregano and thyme EOs exhibited stronger antimicrobial properties than clove and bay in the disc diffusion test. Results of the study showed that oregano and thyme EOs possessed significant in vitro colicidal and colistatic properties for a broad range of temperatures. The inhibitory effects of oregano and thyme EOs against E. coli O157:H7 have been reported to be attributed to their active components, carvacrol and thymol [2, 10, 11], which are effective for disintegrating the outer membrane of the microorganism and releasing the outer membrane-associated materials from the cells to the external medium [9]. Similar to the results of the present study, Oussalah et al. [1] showed that milk-protein-based bioactive films containing 1% oregano EO inhibited E. coli O157:H7 when applied on beef muscle slices with a 1.12 log CFU/cm2 reduction in E. coli O157:H7 population over 7-day refrigerated storage. Ravishankar et al. [22] reported that edible apple films containing 0.5 and 3% carvacrol ensured 2.8 and 6.8 log reductions, respectively, at 23 °C and 1 and 3 log reductions, respectively, at 4 °C against E. coli O157:H7 on chicken breast. Lim et al. [21] applied G. corneum edible films containing carvacrol on hams inoculated with E. coli O157:H7 and noted that the active edible film successfully inhibited the growth of this pathogen with a decrease in E. coli O157:H7 population by 0.75 log CFU/g, as compared to the control after nine days of storage.
Antibacterial activity against L. monocytogenes
The initial L. monocytogenes count of beef cuts was 5.59 log CFU/g, which showed significant increase (p < 0.05) on Day 14 as compared to other periods of the refrigerated storage for C and SC that reached 7.51 and 7.48 log CFU/g, respectively [Fig. 2(B)]. Nevertheless, in the thyme- or oregano-EO-incorporated edible coated group, L. monocytogenes counts decreased significantly ranging from 0.68 to 0.44 log unit on the first day of storage and afterward increased more gradually as compared with C and SC during 14 days of storage (p < 0.05). When compared to uncoated beef cuts, the significant bacterial reductions by coating with 1, 2, and 3% thyme and oregano EOs were 1.02, 1.73, and 1.97 log CFU/g and 0.91, 1.66, and 1.90 log CFU/g, respectively, at the end of storage (p < 0.05). Although the inhibitory effects of thyme- and oregano-EO-incorporated soy edible coatings were determined against L. monocytogenes in vitro in the current study, the same inhibitory effect was not observed on the beef cuts over 14-day refrigerated storage. Thus, the limited antibacterial effects of thyme- and oregano-EO-incorporated edible coatings could be attributed to the unexpected behavior of L. monocytogenes on beef or the interference of the bacterium with the extrinsic factors of beef. L. monocytogenes is known to adapt to lower temperatures by producing phospholipids with shorter and more branched fatty acids. The increased resistance of L. monocytogenes toward antibacterial coatings at low storage temperature might be linked to the psychrotrophic nature of the bacterium [22, 23]. Veldhuizen et al. [23] examined the antimicrobial effect of carvacrol, a major component of thyme and oregano EOs against L. monocytogenes and noted that carvacrol had strong antilisterial activity in the growth medium, but no effect was observed when carvacrol was tested in steak tar-tare. Two reasons for this reduced activity were reported to be the antilisterial activity of carvacrol that was strongly reduced at lower temperatures (10 vs. 30 °C) and the presence of food components interfering with the activity of carvacrol. Carvacrol was found to bind to albumin, suggesting that the reduced antilisterial activity of carvacrol in foods such as dairy products and uncooked meats is the result of fewer free unbound carvacrol molecules available to interact with the bacteria. In another study, Tammineni et al. [24] showed that growth of L. monocytogenes was reduced by greater than 2 log units on cold smoked salmon stored at 4 °C using potato-peel-waste-based films containing 1.9% oregano oil.
Antibacterial activity against S. aureus
Similar inhibitory effects of oregano- and thyme-EO-incorporated soy edible coatings against S. aureus determined in well diffusion test was also observed on coated beef. No significant change in the initial number of S. aureus (5.08 log CFU/g) was determined in C and SC groups during refrigerated storage [Fig. 2(C)]. Soy-protein coatings, regardless of the concentrations of oregano and thyme, significantly reduced S. aureus growth by 0.75–1.17 log CFU/g and 0.75–1.23 log CFU/g, respectively, as compared with C on Day 1 (p < 0.05). The results indicate that oregano- and thyme-EO-incorporated soy edible coatings exhibited similar antibacterial activity against S. aureus. Increasing the concentration of both EOs in edible coatings resulted in significantly higher antibacterial activity for S. aureus. Storage time also significantly influenced the antibacterial activity. The coatings incorporated with oregano and thyme EOs at 3% level exhibited reductions of 1.78 and 1.6 log unit on Day 7 and 2.86 and 2.59 log unit on Day 14, respectively, in S. aureus counts as compared with C. In agreement with the results of the current study, Jouki et al. [25, 26] reported the strong inhibitory effect of quince seed mucilage films prepared with oregano and thyme EOs on the growth of S. aureus. Similar antibacterial activities of EOs from thyme- and oregano-incorporated films or coatings against S. aureus were also determined by Benavides et al. [18], Emiroğlu et al. [20], and Fernandez–Pan et al. [27].
Instrumental color
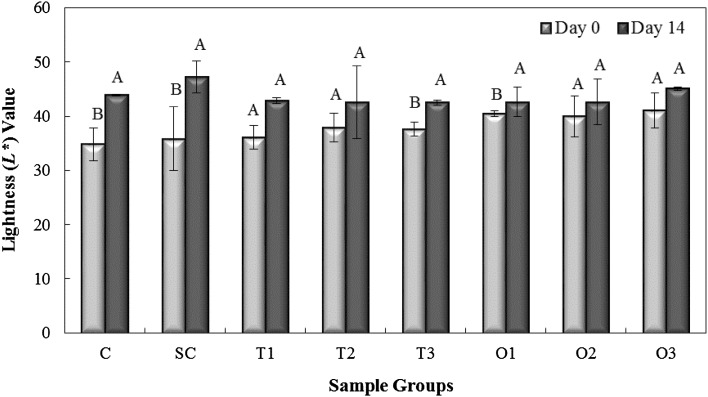
There was no significant difference in lightness (L*) values between the groups. Figure 3 shows L* values of beef samples on Day 0 and 14 to demonstrate the differences in samples between the initial and final values. L* values of C and SC groups exhibited significant increases (p < 0.05) during storage when compared to the initial L* value of 37.5 (Day 0). There were slight increases in the L* values of beef samples treated with edible coatings incorporated with different concentrations of oregano and thyme EOs during refrigerated storage; however, these changes were not significant (p > 0.05), indicating that oregano- and thyme-EO incorporation into the edible coatings did not result in significant changes in the L* value of the beef cuts. These results are in agreement with those of Ünalan et al. [15], who found that the active packaging by zein edible films incorporated with partially purified lysozyme and Na2EDTA did not have a significant effect on the lightness values of ground beef patties.
Fig. 3.
Changes in the lightness (L*) values of beef samples measured on Day 0 and Day 14 of refrigerated storage. C: uncoated control, SC: the group coated with soy protein without essential oil addition. T1, T2, and T3: the isolated soy-protein-based edible coating solutions incorporated with 1, 2, and 3% thyme essential oils. O1, O2, and O3: the isolated soy-protein-based edible coating solutions incorporated with 1, 2, and 3% oregano essential oils. A–B: bars with different letters refer to statistically significant differences (p < 0.05). Error bars represent standard errors
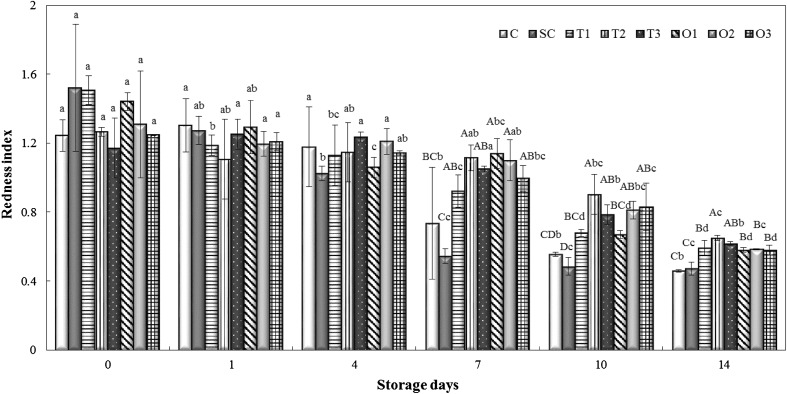
Redness index (a*/b* ratio) is used as an index to assess the discoloration in meats owing to apparent changes in redness, which is mainly related to the darkening of meats resulting from metmyoglobin formation [28]. Although the redness index of the beef samples was not significantly different (p > 0.05) among the groups on the first four days of storage (Fig. 4), the application of soy-protein coatings with thyme and oregano EOs, particularly at concentrations of 2 and 3% EO-incorporated groups, resulted in a higher redness index (p < 0.05) than C and SC at the later stages of storage (after Day 7). These higher redness indices in thyme- and oregano-incorporated samples could be explained by the antioxidant activity of oregano and thyme EOs. Zinoviadou et al. [29] noted that whey protein isolate films containing oregano EO showed better color retention on fresh beef. The redness index significantly decreased for all beef samples over time (p < 0.05). The loss of redness of beef samples is due to myoglobin oxidation, which leads to meat discoloration under aerobic storage conditions. Similar findings were reported by Shon et al. [30], who investigated the effect of soy-protein-isolate coating on the quality attributes of aerobically packaged cut raw beef and noted that redness values of coated beef decreased significantly on the fifth day of storage.
Fig. 4.
Changes in the redness index (a*/b* ratio) of beef samples during refrigerated storage. C: uncoated control, SC: the group coated with soy protein without essential oil addition. T1, T2, and T3: the isolated soy-protein-based edible coating solutions incorporated with 1, 2, and 3% thyme essential oils. O1, O2, and O3: the isolated soy-protein-based edible coating solutions incorporated with 1, 2, and 3% oregano essential oils. A–D: bars with different letters (within a storage day between groups) refer to statistically significant differences (p < 0.05). a–d: bars with different letters (within a group between storage days) refer to statistically significant differences (p < 0.05). Error bars represent standard errors
Sensory analysis
In sensory evaluation, there were no significant differences (p > 0.05) among the beef samples for the appearance, color, odor, and texture attributes (Table 1). In contrast, Skandamis and Nychas [31] reported that 1% of oregano EO positively affected the odor and color of minced meat. The addition of thyme or oregano EOs into the edible coating affected the flavor and overall acceptability scores of the beef samples (p < 0.05). The lowest flavor score was obtained with the samples treated with 3% oregano-EO-incorporated edible coating, which only differed from the samples C, SC, and T1 (p < 0.05). Edible coatings incorporated with thyme EO resulted in higher flavor scores than the ones incorporated with oregano EO. These differences might be attributed to the differences in the specific flavor compounds in the composition of EO used, which might also influence the sensory properties of beef. C had the highest overall acceptability score (7.82) followed by SC (7.23) and T1 (7.13). The overall acceptability scores for samples treated with oregano- or thyme-added edible coating were significantly lower than C (p < 0.05). Nevertheless, no significant differences were observed in the overall acceptability scores between the SC and other groups (p > 0.05). The overall acceptability and flavor scores decreased as the concentration of thyme and oregano EOs increased in the edible coating. Nonetheless, soy-protein coating containing oregano or thyme EOs were considered to be acceptable at all concentration levels used in this study with the lowest overall acceptability score (6.57) received in the O3 group (overall acceptability scores higher than five are considered to be acceptable). The practical application of EOs in foods is limited owing to their strong flavor characteristics [32]. In agreement to our results, Jouki et al. [19] reported that quince seed mucilage edible films containing oregano or thyme EOs at concentrations 1, 1.5, and 2% had no significant negative effect on the organoleptic acceptability of rainbow trout fillets.
Table 1.
Sensory evaluation scores of beef samples coated with essential-oils-incorporated edible coatings
| Groups | Appearance | Color | Odor | Flavor | Texture | Overall acceptability |
|---|---|---|---|---|---|---|
| C | 7.84 ± 0.06 | 7.67 ± 0.23 | 7.53 ± 0.13 | 7.74 ± 0.04 A | 7.26 ± 0.14 | 7.82 ± 0.12 A |
| SC | 7.85 ± 0.15 | 7.48 ± 0.18 | 7.54 ± 0.34 | 7.28 ± 0.28 AB | 6.49 ± 0.29 | 7.23 ± 0.33 AB |
| T1 | 7.94 ± 0.06 | 7.32 ± 0.02 | 7.42 ± 0.08 | 6.90 ± 0.05 BC | 6.86 ± 0.24 | 7.13 ± 0.02 B |
| T2 | 7.68 ± 0.12 | 7.36 ± 0.24 | 7.13 ± 0.07 | 6.51 ± 0.39 BCD | 6.97 ± 0.53 | 6.85 ± 0.30 B |
| T3 | 7.78 ± 0.12 | 7.58 ± 0.02 | 7.20 ± 0.20 | 6.16 ± 0.44 CD | 6.83 ± 0.27 | 6.73 ± 0.23 B |
| O1 | 7.62 ± 0.28 | 7.25 ± 0.25 | 7.18 ± 0.18 | 6.49 ± 0.26 BCD | 6.68 ± 0.12 | 6.86 ± 0.19 B |
| O2 | 7.79 ± 0.09 | 7.58 ± 0.08 | 6.99 ± 0.11 | 6.31 ± 0.54 CD | 6.62 ± 0.18 | 6.73 ± 0.12 B |
| O3 | 7.84 ± 0.06 | 7.69 ± 0.19 | 7.15 ± 0.15 | 5.82 ± 0.38 D | 6.83 ± 0.17 | 6.57 ± 0.13 B |
Mean ± standard error
A–D: within an attribute, different letters indicate significant differences between samples (p < 0.05). C: uncoated control, SC: the group coated with soy protein without essential oil addition. T1, T2, and T3: the isolated soy-protein-based edible coating solutions incorporated with 1, 2, and 3 thyme essential oils. O1, O2, and O3: the isolated soy-protein-based edible coating solutions incorporated with 1, 2, and 3% oregano essential oils
The antibacterial effects of oregano- and thyme-EO-incorporated edible coatings were evident against three of the pathogenic bacteria evaluated in vitro and when applied on the surface of beef cuts, with no significant difference between the two EO-incorporated coatings. It was observed that the antibacterial effect against S. aureus in growth media and on the fresh beef was greater than against L. monocytogenes and E. coli O157:H7. While similar antibacterial activities of EOs from thyme- and oregano-incorporated coatings were observed against L. monocytogenes and E. coli O157:H7, in agar well diffusion test, it was interesting to note that when the antimicrobial coatings were applied on beef surfaces, oregano or thyme EOs showed limited antibacterial effects against L. monocytogenes on the beef refrigerated for over 14 days. The results indicate that the antibacterial activity against the pathogens was in the order of S. aureus > E. coli O157:H7 > L. monocytogenes. Thyme and oregano EO incorporation into soy edible coatings improved the color of the beef with acceptable sensory properties.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Oussalah M, Caillet S, Salmiéri S, Saucier L, Lacroix M. Antimicrobial and antioxidant effects of milk protein-based film containing essential oils for the preservation of whole beef muscle. J. Agric. Food Chem. 2004;52:5598–5605. doi: 10.1021/jf049389q. [DOI] [PubMed] [Google Scholar]
- 2.Solomakos N, Govaris A, Koidis P, Botsoglou N. The antimicrobial effect of thyme essential oil, nisin and their combination against Escherichia coli O157:H7 in minced beef during refrigerated storage. Meat Sci. 2008;80:159–166. doi: 10.1016/j.meatsci.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Cutter NC. Opportunities for bio-based packaging technologies to improve the quality and safety of fresh and further processed muscle foods. Meat Sci. 2006;74:131–142. doi: 10.1016/j.meatsci.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Sánchez-González L, Vargas M, González-Martínez C, Chiralt A, Cháfer M. Use of essential oils in bioactive edible coatings: A review. Food Eng. Rev. 2011;3:1–16. doi: 10.1007/s12393-010-9031-3. [DOI] [Google Scholar]
- 5.Zivanovic S, Chi S, Draughon AF. Antimicrobial activity of chitosan films enriched with essential oils. J. Food Sci. 2005;70:45–51. doi: 10.1111/j.1365-2621.2005.tb09045.x. [DOI] [Google Scholar]
- 6.Seydim AC, Sarikus G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res. Int. 2006;39:639–644. doi: 10.1016/j.foodres.2006.01.013. [DOI] [Google Scholar]
- 7.Burt S, Reinders RD. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett. Appl. Microbiol. 2003;36:162–167. doi: 10.1046/j.1472-765X.2003.01285.x. [DOI] [PubMed] [Google Scholar]
- 8.Dadalioglu I, Evrendilek GA. Chemical compositions and antibacterial effects of essential oils of Turkish oregano (Origanum minutiflorum), bay laurel (Laurus nobilis), Spanish lavender (Lavandula stoechas L.), and fennel (Foeniculum vulgare) on common foodborne pathogens. J. Agric. Food Chem. 2004;52:8255–8260. doi: 10.1021/jf049033e. [DOI] [PubMed] [Google Scholar]
- 9.Rojas-Graü MA, Avena-Bustillos RJ, Olsen C, Friedman M, Henika PR, Martin-Belloso O. Effects of plant essential oils and oil compounds on mechanical, barrier and antimicrobial properties of alginate-apple puree edible films. J. Food Eng. 2007;81:634–641. doi: 10.1016/j.jfoodeng.2007.01.007. [DOI] [Google Scholar]
- 10.Oussalah M, Caillet S, Salmiéri S, Saucier L, Lacroix M. Antimicrobial effects of alginate-based film containing essential oils for the preservation of whole beef muscle. J. Food Prot. 2006;69:2364–2369. doi: 10.4315/0362-028X-69.10.2364. [DOI] [PubMed] [Google Scholar]
- 11.Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Choi SG, Kim KM, Hanna MA, Weller CL, Kerr WL. Molecular dynamics of soy-protein isolate films plasticized by water and glycerol. J. Food Sci. 2003;68:2516–2522. doi: 10.1111/j.1365-2621.2003.tb07054.x. [DOI] [Google Scholar]
- 13.Gutierrez J, Barry-Ryan C, Bourke P. Antimicrobial activity of plant essential oils using food model media: Efficacy, synergistic potential and interactions with food components. Food Microbiol. 2009;26:142–150. doi: 10.1016/j.fm.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Ekinci FY, Mode of action and injury-enhanced spectrum of jenseniin G, a Propionibacterium bacteriocin produced in fed-batch cultures. Clemson University, (2001)
- 15.Ünalan IU, Korel F, Yemenicioğlu A. Active packaging of ground beef patties by edible zein films incorporated with partially purified lysozyme and Na2EDTA. Int. J. Food Sci. Technol. 2011;46:1289–1295. doi: 10.1111/j.1365-2621.2011.02625.x. [DOI] [Google Scholar]
- 16.Meilgaard MC, Carr BT, Civille GV, Sensory Evaluation Techniques. 4th Ed. CRC Press, (2006)
- 17.Sağdıç O. Sensitivity of four pathogenic bacteria to Turkish thyme and oregano hydrosols. LWT - Food Sci. Technol. 2003;36:467–473. doi: 10.1016/S0023-6438(03)00037-9. [DOI] [Google Scholar]
- 18.Benavides S, Villalobos-Carvajal R, Reyes JE. Physical, mechanical and antibacterial properties of alginate film: Effect of the crosslinking degree and oregano essential oil concentration. J. Food Eng. 2012;110:232–239. doi: 10.1016/j.jfoodeng.2011.05.023. [DOI] [Google Scholar]
- 19.Jouki M, Yazdia FT, Mortazavia SA, Koocheki A, Khazaei N. Effect of quince seed mucilage edible films incorporated with oregano or thyme essential oil on shelf life extension of refrigerated rainbow trout fillets. Int. J. Food Microbiol. 2014;174:88–97. doi: 10.1016/j.ijfoodmicro.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Emiroğlu ZK, Yemiş GP. Coşkun; BK, Candoğan K, Antimicrobial activity of soy edible films incorporated with thyme and oregano essential oils on fresh ground beef patties. Meat Sci. 2010;86:283–288. doi: 10.1016/j.meatsci.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Lim GO, Hong YH, Song KB. Application of gelidium corneum edible films containing carvacrol for ham packages. J. Food Sci. 2010;75:90–93. doi: 10.1111/j.1750-3841.2009.01431.x. [DOI] [PubMed] [Google Scholar]
- 22.Ravishankar S, Zhu L, Olsen CW, McHugh TH, Friedman M. Edible apple film wraps containing plant antimicrobials inactivate foodborne pathogens on meat and poultry products. J. Food Sci. 2009;74:440–445. doi: 10.1111/j.1750-3841.2009.01320.x. [DOI] [PubMed] [Google Scholar]
- 23.Veldhuizen EJA, Creutzberg TO, Burt S, Haagsman HP. Low temperature and binding to food components inhibit the antibacterial activity of carvacrol against Listeria monocytogenes in steak tartare. J. Food Prot. 2007;70:2127–2132. doi: 10.4315/0362-028X-70.9.2127. [DOI] [PubMed] [Google Scholar]
- 24.Tammineni N, Ünlü G, Min SC. Development of antimicrobial potato peel waste-based edible films with oregano essential oil to inhibit Listeria monocytogenes on cold-smoked salmon. Int. J. Food Sci. Technol. 2013;48:211–214. doi: 10.1111/j.1365-2621.2012.03156.x. [DOI] [Google Scholar]
- 25.Jouki M, Yazdi FT, Mortazavi SA, Koocheki A. Quince seed mucilage films incorporated with oregano essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Food Hydrocoll. 2014;36:9–19. doi: 10.1016/j.foodhyd.2013.08.030. [DOI] [Google Scholar]
- 26.Jouki M, Mortazavi SA, Yazdi FT, Koocheki A. Characterization of antioxidant-antibacterial quince seed mucilage films containing thyme essential oil. Carbohydr. Polym. 2014;99:537–546. doi: 10.1016/j.carbpol.2013.08.077. [DOI] [PubMed] [Google Scholar]
- 27.Fernández-Pan I, Royo M, Ignacio Maté J. Antimicrobial activity of whey protein isolate edible films with essential oils against food spoilers and foodborne Pathogens. J. Food Sci. 2012;77:383–390. doi: 10.1111/j.1750-3841.2012.02752.x. [DOI] [PubMed] [Google Scholar]
- 28.Chaijan M, Benjakul S, Visessanguan W, Faustman C. Changes of pigments and color in sardine (Sardinella gibbosa) and mackerel (Rastrelliger kanagurta) muscle during iced storage. Food Chem. 2005;93:607–617. doi: 10.1016/j.foodchem.2004.10.035. [DOI] [Google Scholar]
- 29.Zinoviadou KG, Koutsoumanis KP, Biliaderis CG. Physico-chemical properties of whey protein isolate films containing oregano oil and their antimicrobial action against spoilage flora of fresh beef. Meat Sci. 2009;82:338–345. doi: 10.1016/j.meatsci.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Shon J, Eo JH, Eun JB. Effect of soy protein isolate coating on quality attributes of cut raw han-woo (Korean Cow) beef, aerobically packaged and held refrigerated. J. Food Qual. 2010;33:42–60. doi: 10.1111/j.1745-4557.2010.00332.x. [DOI] [Google Scholar]
- 31.Skandamis PN, Nychas GJE. Effect of oregano essential oil on microbiological and physico-chemical attributes of minced meat stored in air and modified atmospheres. J. Appl. Microbiol. 2001;91:1011–10220. doi: 10.1046/j.1365-2672.2001.01467.x. [DOI] [PubMed] [Google Scholar]
- 32.Tsigarida E, Skandamis P, Nychas GJE. Behaviour of Listeria monocytogenes and autochthonous flora on meat stored under aerobic, vacuum and modified atmosphere packaging conditions with or without the presence of oregano essential oil at 5 °C. J. Appl. Microbiol. 2000;89:901–909. doi: 10.1046/j.1365-2672.2000.01170.x. [DOI] [PubMed] [Google Scholar]