Abstract
Recently, considerable attention has been paid to drug exploration from natural sources for treating memory loss, a major manifestation of various neurodegenerative diseases. Increasing evidences implicate brain serotonin metabolism in learning and memory, supporting the notion that targeting 5-HT (5-hydroxytryptamine) and its receptors would be beneficial in the treatment of cognitive disorders. In the present study, behavioral and neurochemical effects were examined following administration of Sargassum swartzii extracts in albino Wistar rats. Increase in spatial working memory and recognition memory was exhibited by the seaweed-treated rats as compared to controls. Plasma tryptophan, brain 5-HT, and 5-hydroxyindoleacetic acid levels were measured using HPLC–ECD, and a significant increase in brain 5-HT metabolism was observed in the seaweed-treated rats. The increase in memory functions following repeated administration of S. swartzii extracts is suggested to be due to the increased serotonergic neurotransmission in the brain of seaweed-treated rats.
Keywords: 5-HT, Tryptophan, Seaweed, Water maze, Novel object recognition
Introduction
Neurodegenerative diseases are increasing exponentially around the globe and would surpass the second major cause of death among the aged population by 2050 [1]. Most common features of neurodegenerative disorders include impaired memory, mood disturbance, and reduced learning abilities. Abundant evidence supports the hypothesis that serotonin receptors play an important role in cerebral formation or development, such as cellular and subcellular regions in the hippocampus, which are implicated in learning and memory [2]. The involvement and subsequent modulation of memory function receptors have been attributed to 5-hydroxytryptamine (5-HT) and its metabolites [3]. It has been suggested that an increase in 5-HT concentration in the brain enhances the learning capability, whereas a hypofunction of the 5-HT system has emerged as the leading cause of memory impairment [4]. A linkage between long-term potentiation and the water maze (WM) test has been suggested; thus, the WM test is considered an important tool for investigating hippocampal circuitry [5]. The serotonin receptors are present in cellular and subcellular regions of the hippocampus, and thus, the spatial memory of test animals scored in the WM test is attributed to the serotonergic system [2]. Similarly, the novel object recognition (NOR) test is another well-characterized method to assess recognition memory in test animals [6, 7]. It has been shown that the recognition memory would be impaired when the brain serotonin level diminishes [8]. It is therefore suggested that spatial and recognition memory functions are impaired in patients with neurodegenerative diseases, and involvement of 5-HT receptors is implied in its pathophysiology [9].
A number of synthetic agents used to treat memory disorders have deleterious side effects [10]. Marine environment represents the largest closet for naturally occurring compounds of therapeutic interest, with marine algae representing one of the potential candidates as pharmacologically active treasure of novel natural products. Lower incidences of neurodegenerative diseases encountered in countries consuming marine algae in their diet as opposed to the non-consumers have developed interest in the use of seaweeds as bioprospecting agent [11]. Furthermore, it has been reported that seaweeds have inhibitory effects against nitric oxide production in the brain, which is involved in the pathogenesis of many neurodegenerative diseases [12]. It has also been demonstrated that an isolated fucoidan from Fucus vesiculosus inhibits cholinergic neuronal death induced by Aβ1−42 in a rat model [13]. In addition, Laminaria japonica is reported to protect against the neurotoxic effects of MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) to induce Parkinsonism [14]. Despite the importance of seaweeds in neurodegenerative disorders, they are not thoroughly assessed for their effects on memory functions.
In comparison to chlorophta (green) and rhodophyta (red) classes of seaweeds, phaeophyta (brown) seaweeds have been most extensively studied, as they possess fucoidan, a unique structural compound different in structure and function from compounds of terrestrial plants origin [15]. Among phaeophyta, Sargassum is exclusively studied for various pharmacological aspects, for example, antioxidant, anti-inflammatory, analgesic, nematicidal, and acetylcholinesterase inhibitory activities [16, 17]. Along the coast of Pakistan, Sargassum is one of the diverse genera exhibiting seven species of which S. swartzii is most commonly available in abundance. Keeping in view the properties and abundance of S. swartzii along the Pakistan coast, the present study was designed to evaluate its neurochemical and behavioral alterations related to memory functions in a rat model following long-term administration of methanolic extracts.
Materials and methods
Algal material and extraction
Brown seaweed, formally confirmed as S. swartzii (archived at Center of Excellence in Marine Biology (CEMB), University of Karachi), was collected from the Baluchistan coast during the time of its abundant growth between April and May 2012. A sample specimen was archived at CEMB for future reference. Healthy specimens of whole seaweeds were handpicked with care, rinsed thoroughly with tap water to remove any debris, air dried at room temperature in dark, and ground and stored for further analysis. Known weight of S. swartzii (200 g) was successively soaked in methanol (5 × 2 L) at room temperature for 24 h. Each extract was filtered (Whatman No. 1), pooled, and concentrated under reduced pressure using a rotary evaporator. The syrupy extracts were further dried under vacuum and finally freeze dried to get the desired methanolic extract (24.16 g of gummy residue).
Animals and dosing
Twelve locally bred adult, 8–12 weeks old, male albino Wistar rats were procured from International Centre for Chemical and Biological Sciences, University of Karachi. Animals were acclimatized in a ventilated room at 22 ± 2 °C under a 12 h light/dark cycle for 4 days before the experiment. Animals were randomly assigned as control (distilled water administered) and seaweed extract-treated (SET) groups. Each group consisted of six male rats. The seaweed extract mixed with distilled water were administered orally (intragastric; 60 mg/kg/ml; daily) for 4 weeks using a small stainless steel feeding tube attached to a 1 ml syringe. A single dose of 60 mg/kg was selected after evaluation of different doses (data not shown). Standard rodent diet (Table 1) and water were provided ad libitum to the control and SET rats during acclimatization and experimental periods. All procedures conducted were approved by the local animal care committee following the standard guidelines prescribed for the handling of animals by National Research Council (US) committee for the care and use of laboratory animals (2011) [18].
Table 1.
Composition of the standard diet used in the current study
| Ingredients | gm/kg |
|---|---|
| Wheat flour | 282.1 |
| Oat flour | 282.1 |
| Corn gluten | 141 |
| Gram flour | 188.1 |
| Milk powder | 11.7 |
| Fish meal | 47 |
| Soybean oil | 47 |
| Vitamins | 0.6 |
Values of ingredients are expressed in gm per kg of diet
Chemicals
The chemicals used in the present study include, sodium metabisulfite, ethylenediaminetetraacetic acid (EDTA), cysteine, octyl sodium sulfate, methanol, orthophosphoric acid, sodium perchlorate, hydrochloric acid, 5-hydroxytryptamine (5-HT), 5-hydroxyindoleaceticacid (5-HIAA), and tryptophan. The chemicals were procured from Sigma USA and Merck.
Food intake and body weight
The known quantity of food was placed in the hopper of the cage of each animal. Food intake was quantified weekly by weighing the leftover food. The rats were weighed every week for 4 weeks to evaluate the effect of seaweed extract on growth and mean weight gain was recorded.
Morris water maze (MWM) test
Nootropic effects of the extract were examined by elucidating the performance of rats in a MWM apparatus [19] modified from the original MWM design. The WM tank was filled with clean tap water up to a depth of 18 cm. The water temperature was maintained at 23 ± 1 °C. A wooden platform (16 cm2) was carefully placed 2 cm below the water level. The water was made opaque by adding powdered milk so that the platform could not be seen. After 4 weeks of extract administration, rats were first trained to find the platform. Upon finding the platform rats were left there for 20 s before removing them. The rats were again exposed to the WM apparatus after 24 h of training to assess the effect of the extract on spatial working memory, indicated by the time taken by the rat to find the platform in comparison to the control rats.
Novel object recognition (NOR) test
This test was established by Ennaceur and Delacour [6] to assess the cognitive ability of animals by distinguishing the novel environment and recognizing the differences in the surroundings. This test was used in the present study with slight modifications. The apparatus consisted of a gray colored wooden box and the objects to be discriminated were two analogous glass containers and a metallic container of the same size (novel object). The test was executed in three phases. In the first habituation phase on day 1, the rats were being acquainted with the NOR apparatus for 10 min. In the training phase on day 2, the rats were placed inside the box again with two similar glass containers for 10 min. The test phase was performed on day 3 where the rats were exposed to a known (glass container) and a novel (metallic container) object for 3 min each, and the time spent by the rats facing the novel object and sniffing or touching it was measured.
Neurochemical estimation
At the end of behavioral activity tests, the rats were decapitated and their blood and brains were collected and stored at −70 °C for neurochemical assessment. Plasma tryptophan, brain 5-HT, and 5-HIAA were assessed using HPLC–ECD (electrochemical detection) method [20]. Prior sample evaluation by HPLC the system was calibrated using standard concentrations of l-tryptophan 1000 ng/ml, 5-HTP 100 ng/ml, and 5-HIAA 100 ng/ml. For the estimation of amines, the known weight of frozen brain samples were homogenized in 5–10 volume of extraction medium (0.4 M perchlorate containing 0.1% sodium metabisulfite, 0.01% EDTA, and 0.1% cysteine) and centrifuged at 20,160 g at 4 °C to obtain a protein-free supernatant. The extraction medium for plasma tryptophan comprised of 1 ml of 0.4 M perchlorate containing 0.01% EDTA, 0.1% sodium metabisulfite, and 0.1% cysteine for 0.05 ml plasma. The mixture was centrifuged at 20,160 g for 10–15 min at 4 °C and the supernatant was injected for determination of tryptophan. The HPLC (LC-20A pump, SIL 20-A autosampler, Prominence, Shimadzu, Japan)—ECD (L-ECD-6A) equipped with an analytical column (150 mm × 4.6 mm ID; 5 II Shim-pack ODS®) was used. The mobile phase (methanol (14%), octyl sodium sulfate (0.023%), and EDTA (0.0035%) had pH maintained at 2.9 with the help of 0.1 M phosphate buffer. The mobile phase was filtered using a 0.2 µm filter and degassed by vacuum before use. The electrochemical detection was achieved at an operating potential of +0.8 V for brain 5-HT and 5-HIAA and +1.0 V for plasma tryptophan.
Statistical analysis
Results are expressed as mean ± SD. In general student’s t test was performed to assess the significant differences in data recorded for SET and control rats in behavioral experiments as well as brain 5-HT and 5-HIAA determination. Differences were considered significant at p < 0.05.
Results and Discussion
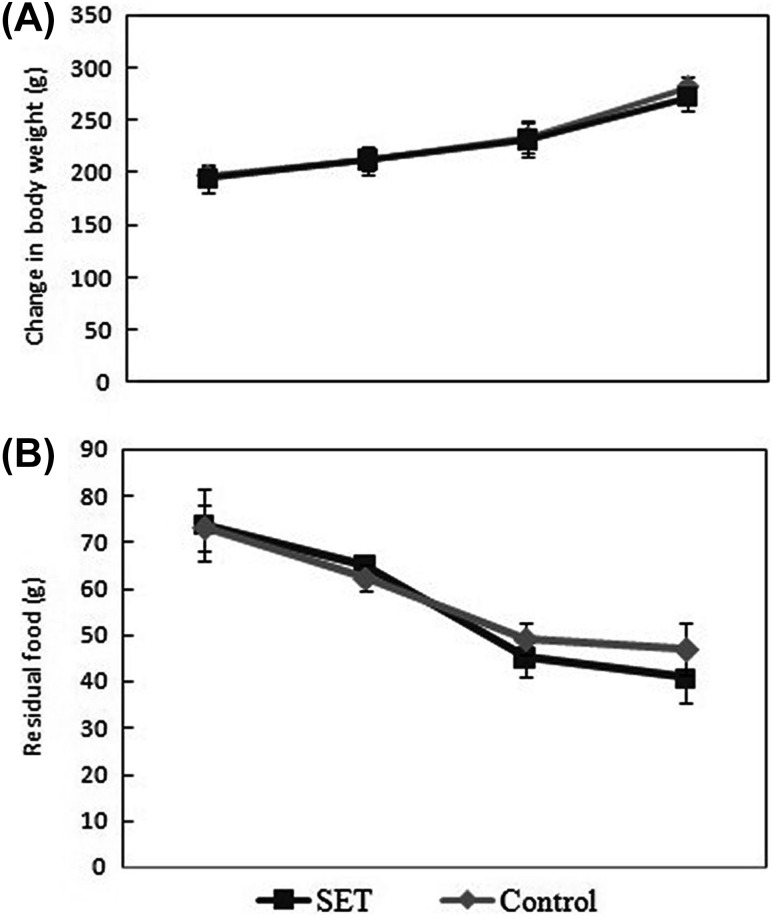
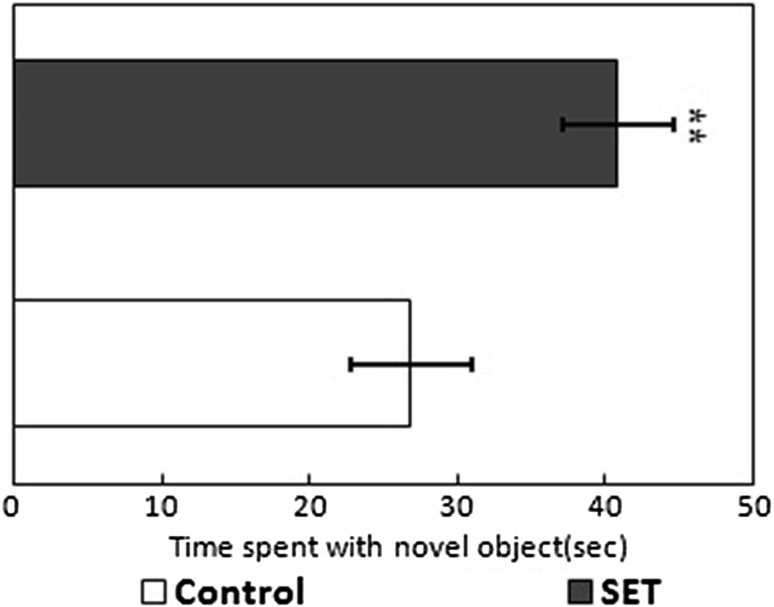
Primary focus of the present study was to elucidate the effect of S. swartzii extract administration on memory functions in rats. Seaweeds have long been used as food, fodder, and medicine. In recent years, there have been a significant number of studies on the bioprospecting of seaweeds [15–17] but the effect of seaweeds on memory functions has not gained considerable attention. Brown seaweed, S. swartzii, is known to have antimicrobial, antioxidant, analgesic, and anti-inflammatory activities [17]. The present study reports improved spatial working memory and recognition memory following administration of S. swartzii extract in rats. Phytochemical analysis of the extract using GC–MS revealed the presence of bioactive compounds such as oleic acid, palmitic acid, stearic acid, and beta-sitosterol (data not shown). The memory functions were assessed using MWM and NOR tests. Performance in the MWM test is reported to be associated with the effect of the drug on neurotransmitters [21]. The technique is widely used for scoring spatial or place learning. Similarly, NOR is another well-characterized test for the assessment of memory functions and is considered to be involved in episodic rather than working memory [7]. The increase in memory functions was apparent in SET rats assessed through MWM and NOR tests. The effect of long-term administration of S. swartzii extracts on growth and food intake in the rat model is shown in Fig. 1. The SET rats showed no significant treatment effect on both food intake [p = 0.30: 1 (A)] as well as growth rates [p = 0.31: 1 (B)]. However, the oral (intragastric) administration of S. swartzii extracts for 4 weeks has significantly increased spatial working memory in rats, which is evident from the WM test data (Fig. 2). The SET rats were able to find the hidden platform in significantly (t = 20.3, p < 0.001) shorter time as compared to control rats. Similarly, the NOR test depicts that seaweed administration ameliorates recognition memory. The SET rats spent significantly higher time (t = 6.43, p < 0.001) in exploring the novel object compared to control rats, indicating increased recognition memory (Fig. 3).
Fig. 1.
Panels (A) and (B) Variations in growth rate and food intake of albino Wistar rats following oral (intragastric) administration of S. swartzii extract for 4 weeks. Values are mean ± SD (n = 6). Data analyzed by student’s t test revealed no significant difference among treatment groups (p > 0.05)
Fig. 2.
Changes in spatial working memory of rats assessed through the water maze test following oral (intragastric) administration of S. swartzii extract for 4 weeks. Results are expressed as mean ± SD (n = 6). Asterisk (*) indicates significant difference against control rats (p < 0.001, Student’s t test)
Fig. 3.
Changes in recognition memory of rats assessed through the novel object recognition test following oral (intragastric) administration of S. swartzii extract for 4 weeks. Results are expressed as mean ± SD (n = 6). Asterisk (*) indicates a significant difference against control rats (p < 0.001, Student’s t test)
Spatial working and recognition memories are considered to play a significant role in everyday living, and these functions are severely impaired in subjects with neurodegenerative diseases possibly due to decreased brain 5-HT levels [22]. Although some reports do not readily recognize the link between memory functions and 5-HT [23, 24], there exist many instances where significant role of the serotonergic system in cognition and memory is evident. For example fluoxetine, a widely used antidepressant drug that is supposed to increase extracellular brain 5-HT levels by inhibiting its reuptake into the presynaptic cell, when administered to rats, showed marked improvement in memory functions [25]. Further findings appeared to be consistent with the argument that increase levels of brain 5-HT improves memory and learning [26], while depleted levels of 5-HT and its metabolites are shown to be associated with impaired memory functions [27]. Studies that involve advance methodologies such as positron emission tomography (PET) and a knockout mouse model have also validated the relation between serotonin and memory consolidation [28–30]. The linkage between memory functions and 5-HT is evident from our data, where low levels of total plasma tryptophan and increased levels of 5-HT and 5-HIAA in the brain were recorded in SET rats. Previous studies have shown that tryptophan administration in rodents ameliorates brain 5-HT metabolism [31, 32], whereas its depletion results in decreased levels of 5-HT in the brain [33]. Although total plasma tryptophan was not increased in the present study, a significant changes in tryptophan and brain 5-HT were observed. It appears that free tryptophan as a precursor of 5-HT enters the brain in excess and metabolizes to produce high levels of 5-HT [34]. In plasma, the amino acid (precursor of serotonin) tryptophan is bound to albumin protein. Excess tryptophan is released from albumin when free fatty acids are available for replacement after augmented lipid metabolism [35]. Seaweeds are reported to have a lipolytic effect [36], and thus might be able to mediate the release of excess free fatty acids and free tryptophan in plasma. Convincing evidence supports the idea that there is an inverse relationship between total plasma tryptophan and plasma-free tryptophan [37, 38]. It is shown that increase in free tryptophan in plasma is associated with a decrease in total plasma tryptophan when noradrenaline or L-DOPA injection was administered, while a converse relationship is observed when the subjects were injected with insulin [39]. Decrease in plasma total tryptophan in the present study exhibited the similar effect of S. swartzii administration as in the case of noradrenaline or L-DOPA injection. Total plasma tryptophan is decreased significantly as compared with control, indicating an increase in plasma-free tryptophan, which is concordant with the increase in brain 5-HT levels. Effects of long-term administration of methanolic extracts of S. swartzii on tryptophan in plasma and on 5-HT and its metabolite 5-HIAA in the brain were evaluated using HPLC–ECD. Figure 6 shows HPLC chromatograms of standards, controls, and test samples. The plasma level of tryptophan, a precursor of 5-HT, in SET rats showed significant decrease (t = 13.04, p < 0.001) as compared to control rats (Fig. 4). On the other hand, significant increases in brain 5-HT (t = 10.12, p < 0.001) and 5-HIAA levels (t = 13.46, p < 0.001) exhibiting increase in 5-HT metabolism were recorded (Fig. 5). The SET rats therefore appeared to exhibit enhanced memory function through plasma-free tryptophan and serotonin modulation.
Fig. 6.
Typical chromatogram of standards and samples: Panel (A) exhibits a standard sample containing 5HT and 5HIAA, each at 100 nM. Panel (B) shows the standard of tryptophan. Separation conditions were as follows. Column: 150 mm × 4.6 mm ID; 5 II Shim-pack ODS®. Mobile phase: pH 2.9 0.1 M phosphate buffer methanol 14%, octyl sodium sulfate 0.023%, and EDTA 0.0035%. Injection volume: 20 µL. Panels (C) and (D) display chromatograms of plasma tryptophan of control and SET rats. Panels (E) and (F) show chromatograms of brain 5-HT and 5-HIAA of control and SET rats, respectively
Fig. 4.
Changes in plasma tryptophan levels in rats through HPLC–ECD analysis following oral (intragastric) administration of S. swartzii extract for 4 weeks. Results are expressed as mean ± SD values (n = 6). Asterisk (*) indicates a significant difference against control rats (p < 0.001, Student’s t test)
Fig. 5.
Panels (A) and (B): Changes in 5-HT and 5-HIAA levels in brain tissues of rats following oral (intragastric) administration of S. swartzii extract for 4 weeks. Results are expressed as mean ± SD values (n = 6). Asterisks (*) indicates a significant difference against control rats (p < 0.001, Student’s t test)
Previous study carried out by our group with regards to the bioprospecting potential of selected brown seaweeds, including S. swartzii, have demonstrated that administration of the seaweed extracts was able to increase brain norepinephrine (NE) levels in rats. In addition, the study revealed that seaweed extracts exhibited significant anxiolytic and psychostimulant activity [40]. It is important to note that besides their direct effect, neurotransmitters are known to also influence other neurotransmitters. In case of 5-HT, its interaction with NE and acetylcholine has been given much importance recently. Studies are in agreement that the monoaminergic system act in an interacting fashion and there are many behavioral overlaps that exhibit interaction among the neurotransmitters. For instance, vigilance is managed by NE, such as 5-HT; it also controls irritability and anxiety [41]. Moreover, increasing evidence suggests that increase in 5-HT levels in the brain also modulates cholinergic activity [42]. The enzyme that is responsible for the catalytic break down of acetylcholine and its subsequent decrease in the synaptic cleft is acetylcholinesterase (AChE). A recent study showed that inhibition of AChE increases activation of 5-HT receptors synergistically [43]. The synergy between 5-HT and acetylcholine has also been proven through neuroanatomical assessments of the rat brain [44]. It is interesting to note that methanolic extracts of Sargassum is known to inhibit the cholinesterase activity [45]. This AChE inhibitory effect of Sargassum has been attributed to two plastaquinones present, thus suggesting possible inclusion of Sargassum in the treatment regime of Alzheimer’s disease [46]. The increase in memory functions by increased 5-HT metabolism may additionally be attributed to already established acetylcholinesterase inhibitory activity of Sargassum species and to the synergistic relation between acetylcholine and 5-HT. However, the exact mechanism of the interaction of Sargassum warrants further investigation.
In conclusion, administration of S. swartzii extracts improve memory functions in the rat model, which may be related to the elevated levels of 5-HT and 5-HIAA in brain tissue concomitantly with a decrease in total plasma tryptophan levels. The specific mechanism underlying this relation remains to be resolved and further studies are recommended on S. swartzii and other seaweeds to evaluate their phychotherapeutic potentials in the treatment of memory loss and neurodegenerative diseases.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ansari A, Siraj A, Inamdar N. Pharmacotherapeutic approaches of Parkinson’s disease. Int. J. Pharma. 2010;6:584–590. doi: 10.3923/ijp.2010.584.590. [DOI] [Google Scholar]
- 2.Hernandez-Perez JJ, Gutierrez-Guzman BE, Lopez-Vazquez MA, Olvera-Cortes ME. Supramammillary serotonin reduction alters place learning and concomitant hippocampal, septal, and supramammillar theta activity in a Morris water maze. Front. Pharmacol. 2015;6:250. doi: 10.3389/fphar.2015.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buhot MC, Martin S, Segu L. Role of serotonin in memory impairment. Ann. Med. 2000;32:210–221. doi: 10.3109/07853890008998828. [DOI] [PubMed] [Google Scholar]
- 4.Lieben CK, Van Oorsouw K, Deutz NE, Blokland A. Acute tryptophan depletion induced by a gelatin based mixture impairs object memory but not affective behavior and spatial learning in the rats. Beh. Brain Res. 2004;151:53–64. doi: 10.1016/j.bbr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Morris RGM, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate antagonist, AP5. Nature. 1986;329:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 6.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav. Brain Res. 31: 47–59 (1998). [DOI] [PubMed]
- 7.Ennaceur A. One-trial object recognition in rats and mice: methodological and theoretical issues. Behav. Brain Res. 2010;215:244–254. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 8.Belcher AM, O’Dell SJ, Marshall JF. Impaired object recognition memory following methamphetamine, but not p-chloroamphetamine- or d-amphetamine-induced neurotoxicity. Neuropsychopharmacology. 2005;30:2026–2034. doi: 10.1038/sj.npp.1300771. [DOI] [PubMed] [Google Scholar]
- 9.Fowler KS, Saling MM, Conway EL, Semple JM, Louis WJ. Paired associate performance in the early detection of DAT. J. Int. Neuropsych. Soc. 2002;8:58–71. doi: 10.1017/S1355617702811067. [DOI] [PubMed] [Google Scholar]
- 10.Caroff SN, Mann SC, Campbell EC, Sullivan KA. Movement disorders associated with atypical antipsychotic drugs. J. Clin. Psychiat. 2002;63:12–19. [PubMed] [Google Scholar]
- 11.Jorm AF, Jolley D. The incidence of dementia: A meta-analysis. Neurology. 1998;51:728–733. doi: 10.1212/WNL.51.3.728. [DOI] [PubMed] [Google Scholar]
- 12.Heales S, Bolanos J, Stewart V, Brookes P, Land JM, Clark J. Nitric oxide, mitochondria and neurological disease. Biochem. Biophys. Acta. 1999;1410:215–228. doi: 10.1016/s0005-2728(98)00168-6. [DOI] [PubMed] [Google Scholar]
- 13.Jhamandas JH, Wie MB, Harris K, MacTavish D, Kar S. Fucoidan inhibits cellular and neurotoxic effects of β-amyloid (Aβ) in rat cholinergic basal forebrain neurons. Eur. J. Neurosci. 2005;21:2649–2659. doi: 10.1111/j.1460-9568.2005.04111.x. [DOI] [PubMed] [Google Scholar]
- 14.Luo D, Zhang Q, Wang H, Cui Y, Sun Z, Yang J, Zheng Y, Jia J, Yu F, Wang X. Fucoidan protects against dopaminergic neuron death in vivo and in vitro. Eur. J. Pharmacol. 1617: 33–40 (2009). [DOI] [PubMed]
- 15.Wijesinghe WAJP, Jeon YJ. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohyd. Polym. 2012;88:13–20. doi: 10.1016/j.carbpol.2011.12.029. [DOI] [Google Scholar]
- 16.Sadati N, Khanavi M, Mahrokh A, Nabavi SMB, Sohrabipour J, Hadjiakhoondi A. Comparison of antioxidant activity and total phenolic contents of some Persian Gulf marine algae. J. Medicin. Plants. 2011;10:37. [Google Scholar]
- 17.Yende SR, Harle UN, Chaugule B. Therapeutic potential and health benefits of Sargassum species. Phycog. Rev. 2014;8:1–7. doi: 10.4103/0973-7847.125514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th edition, Washington (DC), USA: National Academies Press (2011). [PubMed]
- 19.Haider S, Saleem S, Perveen T, Tabassum S, Batool Z, Sadir S, Liaquat L, Madiha S. Age-related learning and memory deficits in rats: role of altered brain neurotransmitters, acetylcholinesterase activity and changes in antioxidant defense system. Age (Dordr). 2014;36:9653. doi: 10.1007/s11357-014-9653-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khaliq S, Haider S, Mukhtar A, Haleem DJ. Gender difference in memory functions following long term tryptophan supplementation in rats. Pak. J. Pharm. 2006;23:39–45. [Google Scholar]
- 21.McNamara RK, Skelton RW. The neuropharmacological and neuro chemical basis of place learning in the morris water maze. Brain Res. Rev. 1993;18:33–49. doi: 10.1016/0165-0173(93)90006-L. [DOI] [PubMed] [Google Scholar]
- 22.Morici JF, Ciccia L, Malleret G, Gingrich JA, Bekinschtein P, Weisstaub NV. Serotonin 2a receptor and serotonin 1a receptor interact within the medial prefrontal cortex during recognition memory in mice. Front. Pharmacol. 2015;6:298. doi: 10.3389/fphar.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sacaefer TL, Vorhees CV, Williams MT. Mouse Pet-1 knock-out induced 5-HT disruption results in a lack of cognitive deficits and an anxiety phenotype complicated by hypoactivity and defensiveness. Neuroscience. 2009;164:1431–1443. doi: 10.1016/j.neuroscience.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altman HJ, Normile HJ. What is the nature of the role of the serotonergic nervous system in learning and memory: prospects for development of an effective treatment strategy for senile dementia. Neurobiol. Aging. 1988;9:627–638. doi: 10.1016/S0197-4580(88)80124-6. [DOI] [PubMed] [Google Scholar]
- 25.Nowakowska E, Kus K, Chodera A, Rybakowski J. Behavioural effects of fluoxetine and tianeptine, two antidepressants with opposite action mechanisms, in rats. Arznei – Forsh. 2000;50:5–10. doi: 10.1055/s-0031-1300156. [DOI] [PubMed] [Google Scholar]
- 26.Ballaz SJ, Akil H, Watson SJ. The 5-HT7 receptor: role in novel object discrimination and relation to novelty-seeking behavior. Neuroscience. 2007;149:192–202. doi: 10.1016/j.neuroscience.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 27.Morley KC, Gallate JE, Hunt GE, Mallet PE, McGregor IS. Increased anxiety and impaired memory in rats 3 months after administration of 3,4-methylenedioxymethamphet-amine (ecstasy) Eur. J. Pharmacol. 2001;433:91–99. doi: 10.1016/S0014-2999(01)01512-6. [DOI] [PubMed] [Google Scholar]
- 28.Glikmann-Johnston Y, Saling MM, Reutens DC, Stout JC. Hippocampal 5-HT1A receptor and spatial learning and memory. Front. Pharmacol. 2015;6:289. doi: 10.3389/fphar.2015.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bert B, Fink H, Rothe J, Walstab J. Bönisch H Learning and memory in 5-HT(1A)-receptor mutant mice. Behav. Brain Res. 2008;16:78–85. doi: 10.1016/j.bbr.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 30.Glikmann-Johnston Y, Saling MM, Chen J, O’Keefe G, Gong S, Tochon-Danguy H, Mulligan R, Reutens DC. Hippocampal 5-HT1A receptor binding is related to object-location memory in humans. Brain Struct. Funct. 2015;220:559–570. doi: 10.1007/s00429-013-0675-7. [DOI] [PubMed] [Google Scholar]
- 31.Grahame-Smith DG. Studies in vivo on the relationship between brain tryptophan, brain 5-ht synthesis and hyperactivity in rats treated with a monoamine oxidase inhibitor and l-tryptophan. J. Neurochem. 1971;18:1053–1066. doi: 10.1111/j.1471-4159.1971.tb12034.x. [DOI] [PubMed] [Google Scholar]
- 32.Strasser B, Gostner JM, Fuchs D. Mood, food, and cognition: role of tryptophan and serotonin. Curr. Opin. Clini. Nutr. Metabol. Care. 2016;19:55–61. doi: 10.1097/MCO.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 33.Rutten K, Lieben C, Smiths L, Blokland A. The PDE4 inhibitor rolipram reverses object memory impairment induced by acute tryptophan depletion in the rat. Psychopharmacology. 2007;192:275–282. doi: 10.1007/s00213-006-0697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eccleston D, Ashcroft GW, Crawford TBB, Stanton JB, Wood D, McTurk PH. Effect of tryptophan administration on 5HIAA in cerebrospinal fluid in man. J. Neurol. Neurosurg. Psychiat. 1970;33:269–272. doi: 10.1136/jnnp.33.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salter M, Knowles RG, Pogson CI. How does displacement of albumin-bound tryptophan cause sustained increases in the free tryptophan concentration in plasma and 5-hydroxytryptamine synthesis in brain? Biochem. J. 1989;262:365–368. doi: 10.1042/bj2620365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maeda H, Hosokawa M, Sashima T, Funayama K, Miyashita K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005;332:392–397. doi: 10.1016/j.bbrc.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Knott PJ, Curzon G. Free tryptophan in plasma and brain tryptophan metabolism. Nature. 1972;239:452–453. doi: 10.1038/239452a0. [DOI] [PubMed] [Google Scholar]
- 38.Curzon G, Kantamaneni BD, Winch J, Rojas-Bueno A, Murray-Lyon IM, Williams R. Plasma and brain tryptophan changes in experimental acute hepatic failure. J. Neurochem. 1973;21:137–146. doi: 10.1111/j.1471-4159.1973.tb04233.x. [DOI] [PubMed] [Google Scholar]
- 39.Curzon G, Knott PJ. Effects on plasma and brain tryptophan in the rat of drugs and hormones that influence the concentration of unesterified fatty acid in the plasma. Br. J. Pharmac. 1974;50:197–204. doi: 10.1111/j.1476-5381.1974.tb08562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan A, Uddin N, Khaliq S, Nawaz S, Rasheed M, Dar A, Hanif M, Siddiqui PJA. Brown seaweeds generate psychotherapeytic response associated with brain norepinephrine modulation in rats. J. Pharmacog. Phytother. 2016;9:11–18. [Google Scholar]
- 41.Hamon M, Blier P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog. Neuropsychopharmacol. Biol. Psychiat. 2013;1:54–63. doi: 10.1016/j.pnpbp.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Consolo S, Arnaboldi S, Giorgi S, Russi G, Ladinsky H. 5HT4 receptor stimulation facilitates acetylcholine release in rat frontal cortex. Neuroreport. 1994;5:1230–1232. doi: 10.1097/00001756-199406020-00018. [DOI] [PubMed] [Google Scholar]
- 43.Freret T, Bouet V, Quiedeville A, Nee G, Dallemagne P, Rochais C, Boulouard M. Synergistic effect of acetylcholinesterase inhibition (donepezil) and 5-HT (4) receptor activation (RS67333) on object recognition in mice. Behav. Brain Res. 2012;230:304–308. doi: 10.1016/j.bbr.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 44.Gasbarri A, Sulli A, Pacitti C, McGaugh JL. Serotoninergic input to cholinergic neurons in the substantiainnominata and nucleus basalismagnocellularis in the rat. Neuroscience. 1999;91:1129–1142. doi: 10.1016/S0306-4522(98)00672-1. [DOI] [PubMed] [Google Scholar]
- 45.Natarajan S, Shanmugiahthevar KP, Kasi PD. Cholinesterase inhibitors from Sargassum and Gracilaria gracilis: seaweeds inhabiting South Indian coastal areas (Hare Island, Gulf of Mannar) Nat. Prod. Res. 2009;23:355–369. doi: 10.1080/14786410802156036. [DOI] [PubMed] [Google Scholar]
- 46.Choi BW, Ryu G, Park SH, Kim ES, Shin J, Roh SS, Shin HC, Lee BH. Anticholinesterase activity of plastoquinones from Sargassum sagamianum: lead compounds for Alzheimer’s disease therapy. Phytother. Res. 2007;21:423–426. doi: 10.1002/ptr.2090. [DOI] [PubMed] [Google Scholar]