Abstract
In this study, the inhibitory effects of bamboo leaf extracts on adipogenesis were investigated by evaluating their activity against adipogenic transcription factors and enzymes in 3T3-L1 adipocytes. Bamboo leaf extracts significantly decreased triglyceride levels, and increased glycerol release in adipocytes. Cells treated with the water extract showed significantly higher glycerol release as well as lower triglyceride contents than those treated with the ethanol extract. Both bamboo leaf extracts significantly inhibited the expression of adipogenic transcription factors and enzymes, such as CCAAT/enhancer-binding protein α, sterol regulatory element binding protein 1c, peroxisome proliferator-activated receptor γ, acetyl-coenzyme A carboxylase, and fatty acid synthase, and increased the expression of phospho-adenosine monophosphate-activated protein kinase. These results show that bamboo leaf extracts inhibited adipogenesis in 3T3-L1 adipocytes and that the water extract was more efficacious than the ethanol extract.
Keywords: Bamboo leaf, Adipogenesis, Obesity, 3T3-L1, Adipocyte
Introduction
The prevalence of obesity has increased markedly in recent years and has become a worldwide public health problem [1]. Obesity is the leading metabolic disease globally and is closely associated with coronary heart disease, hypertension, type 2 diabetes mellitus, cancer, respiratory complications, and osteoarthritis [2, 3]. Consumption of high-calorie diets promotes the mitotic clonal expansion of preadipocytes and stimulates adipogenesis, leading to the development of obesity [4]. Intra-abdominal visceral fat accumulation, which depends on adipocyte proliferation and differentiation of preadipocytes, directly contribute to the occurrence of obesity [5].
The differentiation of preadipocytes is controlled by several transcription factors, namely, CCAAT/enhancer-binding protein α (C/EBPα), sterol regulatory element binding protein 1c (SREBP-1c), and peroxisome proliferator-activated receptor γ (PPARγ) [6]. They are known as critical activators of adipogenesis and regulate the action of enzymes, such as acetyl-coenzyme A carboxylase (ACC) and fatty acid synthase (FAS). In addition, the activation of adenosine monophosphate-activated protein kinase (AMPK) in adipocytes attenuates lipid synthesis through modulation of lipogenic enzyme activities [7].
Bamboo (Phyllostachys bambusoides) leaf was usually as used as tea, it have been consumed for more than 1000 years in Asia for nutrition and as folk medicine. Bamboo leaf has gained attention for their various biological functions, such as anti-aging, antibacterial, antiviral, and anti-atherosclerosis activities, as well as for their role in enhancing immunity and preventing degenerative diseases [8–10]. However, no studies have yet been reported on the efficacy of bamboo leaf extract in inhibiting adipogenesis. In this study, the effects of ethanol and water extracts of bamboo leaf in 3T3-L1 adipocytes were investigated by measuring glycerol, and triglyceride contents. Furthermore, to investigate the inhibitory mechanism of bamboo leaf extracts on adipogenesis in 3T3-L1 adipocytes, we measured the expression levels of adipogenic transcription factors and their target genes.
Materials and methods
Extraction of bamboo leafs
Bamboo leaf (P. bambusoides Siebold & Zucc) was purchased from Human Herb (Kyungsan, Korea). The samples were rinsed carefully with fresh water and freeze-dried, before extraction with water and 30% ethanol. The water extract (BW) was subjected to extraction for 2 h at 100 °C, while the ethanol extract (BE) was subjected to extraction for 3 h at 80 °C (1:10, v/v of sample). The extracts were spray-dried using a spray dryer (Tokyo, Japan). Compressed air was used as the atomizing gas while conditioned room air served as the inlet drying gas. After spray drying, the dried extracts were powdered prior to use in experiments.
Cell culture and adipocyte differentiation
Mouse 3T3-L1 preadipocytes were grown to confluence in Dulbecco’s modified Eagle’s medium (DMEM) with 10% bovine calf serum at 37 °C in a humidified atmosphere of 5% CO2. One day post-confluence (designated “day 0”), cell differentiation was induced with a mixture of methylisobutylxanthine (0.5 mM), dexamethasone (0.25 μM), and insulin (5 μg/mL) in DMEM containing 10% fetal bovine serum (FBS). After 48 h (day 2), DMEM containing 10% FBS and insulin (5 μg/mL) was replaced every two days. The sample was added to the culture medium from days 3 to 8 to investigate its effect on lipid accumulation and triglyceride hydrolysis.
MTT assay
The 3T3-L1 adipocytes were seeded at a density of 1 × 104 cells/well in 96-well plates and treated with various concentrations of the sample for 72 h. After treatment, the adipocytes were incubated with MTT solution for 4 h at 37 °C. The supernatants were aspirated and dimethyl sulfoxide was added to each well. After 15 min of incubation, absorbance was measured at 540 nm by using an enzyme-linked immunosorbent assay (ELISA) plate reader.
Oil red O staining
On day 8, 3T3-L1 cells were washed twice with PBS and fixed with 10% (v/v) fresh formalin for 2 h. Cells were stained with Oil red O working solution for 1 h. After the staining solution was removed, stained lipid droplets in 3T3-L1 cells were washed four times with distilled water. The cells stained with Oil red O were visualized using an microscopy with image analysis (Leica Microsystems, Bensheim, Germany).
Measurement of triglyceride content
The triglyceride content of the adipocyte lysates was determined by using the triglyceride quantification colorimetric/fluorometric kit (Biovision Inc., Mountain View, CA, USA) according to the manufacturer’s instructions.
Measurement of glycerol content
Glycerol levels in the medium were measured using a free glycerol determination kit (Sigma, St. Louis, MO, USA) with glycerol standards for calibration. Briefly, 200 μL of free glycerol reagent reconstituted in distilled water was mixed with 50 μL of distilled water (blank), glycerol standard, or test samples in the presence of adipocytes. Thereafter, the mixtures were incubated at 37 °C for 15 min, and the absorbance of the solution was measured at 540 nm by using a microplate reader.
Immunoblotting
Adipocytes were homogenized with ice-cold lysis buffer containing 250 mM NaCl, 25 mM Tris-HCl (pH 7.5), 1% (v/v) NP-40, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and protein inhibitor cocktail (10 μg/mL aprotinin and 1 μg/mL leupeptin). The adipocytes were centrifuged at 20,000 g for 15 min at 4 °C. The supernatants were used as total protein extracts. The total protein content was determined using a Bio-Rad protein kit, with bovine serum albumin as the standard. The lysate containing 20 μg of protein was subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were transferred electrophoretically onto a pure nitrocellulose membrane, blocked with 5% skimmed milk solution for 1 h, and incubated with primary antibodies (1:1000; Abcam, Cambridge, UK) overnight at 4 °C. After washing, the blots were incubated with goat anti-rabbit or goat anti-mouse IgG horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature (37 °C). Each antigen–antibody complex was visualized using ECL Western blotting detection reagents and detected using chemiluminescence with a LAS-1000 plus imaging system (FUJIFLIM, Tokyo, Japan). Band densities were determined using an image analyzer (FUJIFILM) and normalized to β-actin.
Statistical analysis
Each experiment was performed in triplicate. The results were expressed as the mean ± standard deviation (SD). Statistical analysis, including analysis of variance, was performed using SAS 9.1 software. Significant differences (P < 0.05) between the means were determined using Duncan’s multiple range test.
Results and discussion
Effect of bamboo leaf extracts on cell viability
Inhibition of adipogenesis is emerging as a therapeutic alternative for the prevention and treatment of obesity and obesity-mediated metabolic syndromes [11]. However, currently available drugs for the treatment of obesity cause undesirable side effects. Consequently, the use of natural products to combat obesity is rapidly increasing. In Korean and Chinese traditional medicine, bamboo leaf extract is used to treat various diseases [12], owing to the presence of numerous naturally occurring organic compounds. The ethanol and water extracts of bamboo leaf mainly contain flavonoids, phenolic acids, phytosterols, amino acids, and other microelements [13, 14]. In this study, we compared the adipogenesis inhibiting effects of bamboo leaf ethanol extract (BE) and water extract (BW) via adipogenic transcription factor regulation in 3T3-L1 adipocytes.
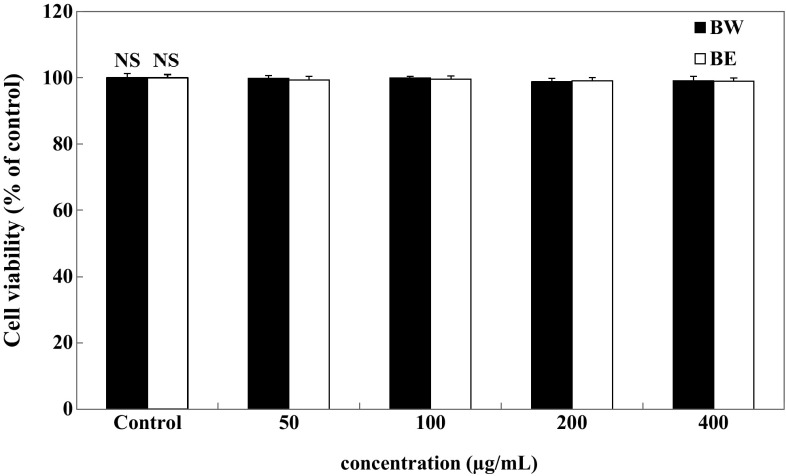
We first examined the effect of the bamboo leaf extracts on the viability of 3T3-L1 adipocytes. The adipocytes were exposed to various concentrations (50–400 μg/mL) of BE and BW. Intracellular toxicity was measured using MTT assay. The results showed that BE and BW did not decrease cell viability, with no significant cytotoxic effect observed up to an extract concentration of 400 μg/mL (Fig. 1).
Fig. 1.
Effect of bamboo leaf extracts on cell viability of 3T3-L1 adipocytes. Differentiated 3T3-L1 adipocytes were treated with various concentrations (0, 50, 100, 200, and 400 μg/mL) of bamboo leaf extracts and incubated for 72 h at 37 °C in a humidified incubator containing 5% CO2. Each value is expressed as mean ± SD (n = 3). NS not significant, BE bamboo leaf ethanol extract, BW bamboo leaf water extract
Effect of bamboo leaf extracts on lipid accumulation
Fat accumulation in the adipose tissue occurs at the late stage of adipogenesis and is associated with increased triglyceride concentrations [15]. Therefore, triglyceride accumulation in 3T3-L1 adipocytes was measured as an index of adipogenesis. The images of Oil red O staining shown in Fig. 2(A) suggested that bamboo leaf extract suppressed lipid accumulation. Untreated control cells were observed with plenty of lipid droplets. On the other hand, the bamboo leaf exract-treated cells were observed with less lipid droplets compared to the control cells. Triglyceride content was quantified by measuring triglyceride quantification colorimetric/fluorometric kit. Triglyceride contents in adipocytes treated with the bamboo leaf extracts were significantly lower than those in untreated adipocytes. Triglyceride content decreased from 126.2 μg/mg protein to 116.4 and 112.8 μg/mg protein (BE), and 116.1 and 93.3 μg/mg protein (BW), upon administration of 50 and 100 μg/mL extracts, respectively [Fig. 2(B)]. Hence, BW was more potent than BE in reducing triglyceride content in adipocytes. Treatment with hydroxycitric acid (HCA), the positive control, reduced triglyceride content to 98.01 μg/mg protein at a concentration of 100 μg/mL, which was not significantly different compared with the 100 μg/mL BW treatment.
Fig. 2.
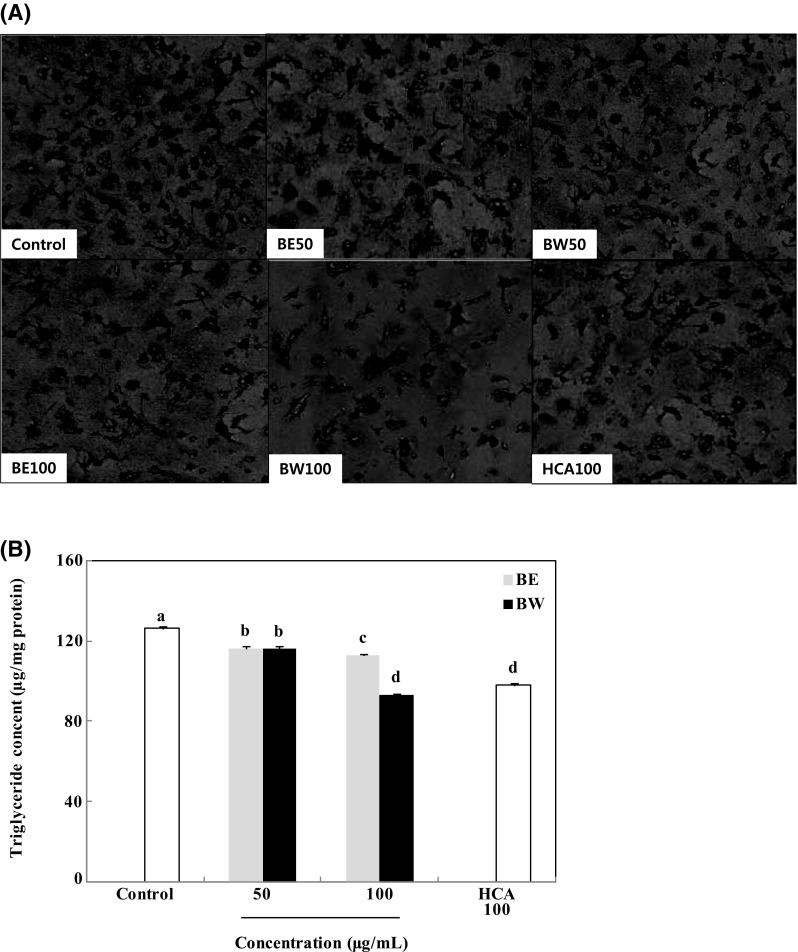
Effect of bamboo leaf extracts on Oil red O staining images (A) and triglyceride content (B) in 3T3-L1 adipocytes. Differentiated 3T3-L1 adipocytes were treated with different concentrations (0, 50, and 100 μg/mL) of bamboo leaf extracts and incubated for 8 days at 37 °C in a humidified incubator containing 5% CO2. Each value is expressed as mean ± SD (n = 3). a–dValues with different letters are significantly different (P < 0.05) as analyzed using Duncan’s multiple range test. BE bamboo leaf ethanol extract, BW bamboo leaf water extract, HCA hydroxycitric acid
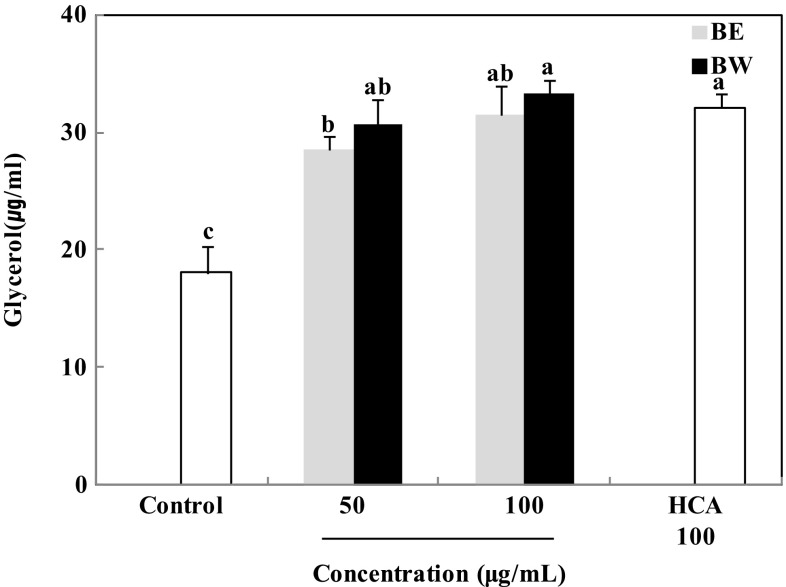
Triglyceride hydrolysis in adipocytes releases glycerol and free fatty acids [16]. To determine whether the reduction in triglyceride content was associated with lipolysis, the amount of glycerol released into the medium was measured. As shown in Fig. 3, treatment with bamboo leaf extracts significantly increased glycerol release. The amount of glycerol released increased from 18.1 μg/mL to 28.5 and 31.5 μg/mL (BE), and 30.6 and 33.4 μg/mL (BW), after treatment with 50 and 100 μg/mL extracts, respectively. This clearly shows that the bamboo leaf extracts significantly increased glycerol release and that BW induced a stronger effect than BE. Glycerol release after 100 μg/mL HCA treatment was 32.09 μg/mL, similar to that after 100 μg/mL BW treatment. These findings indicate that BW exerted a stronger inhibitory effect on lipid accumulation in 3T3-L1 adipocytes than BE. Water-soluble flavone C-glucosides, i.e. orientin, isoorientin, vitexin, and isovitexin, have been previously isolated from bamboo leaf and shown to possess biological functions [17]. Isoorientin and vitexin in particular, have been reported to inhibit triglyceride accumulation and increase glycerol release in 3T3-L1 adipocytes [18, 19]. Although water would favorably dissolve more polar plant polyphenols than other solvents, ethanol could also dissolve polar plant polyphenols [20]. Thus, both BE and BW were effective in reducing lipid accumulation because the compounds such as isoorientin and vitexin were soluble in not only water but also ethanol. However BW treatment might decrease more triglyceride content and increase more glycerol release in 3T3-L1 adipocytes than BE treatment since the compounds were more favorably soluble in water than ethanol.
Fig. 3.
Effect of bamboo leaf extracts on glycerol release in 3T3-L1 adipocytes. Differentiated 3T3-L1 adipocytes were treated with different concentrations (0, 50, and 100 μg/mL) of bamboo leaf extracts and incubated for 8 days at 37 °C in a humidified incubator containing 5% CO2. Each value is expressed as mean ± SD (n = 3). a–cValues with different letters are significantly different (P < 0.05) as analyzed using Duncan’s multiple range test. BE bamboo leaf ethanol extract, BW bamboo leaf water extract, HCA hydroxycitric acid
Effect of bamboo leaf extracts on the protein expression of adipogenic factors
Adipogenesis involves a highly regulated and coordinated cascade of transcription factors, such as members of the PPARγ, C/EBPs, and SREBP family of proteins, which together contribute to adipocyte development [21]. Fat accumulation is associated with PPARγ activation. Thus, modulation of PPARγ activity may be an effective way to control obesity [22]. In this study, 50 and 100 μg/mL BE and BW treatments considerably reduced the expression level of PPARγ in 3T3-L1 adipocytes to 79 and 68% (BE), and 81 and 70% (BW), respectively, relative to that in untreated adipocytes (Fig. 4). In addition, PPARγ expression levels after BE and BW treatments (100 μg/mL) were significantly lower than that after HCA treatment (100 μg/mL). Adipocyte differentiation promotes PPARγ activity, which induces the binding of C/EBPα to its promoter region. The transcription factor C/EBPα is critical to adipogenesis [23] and is induced relatively late during adipocyte differentiation—after the induction of PPARγ, but before the synthesis of enzymes and proteins characteristic of fully differentiated cells [24]. Both BE and BW treatments also significantly decreased the expression of C/EBPα in the adipocytes (Fig. 4). The C/EBPα expression level in the adipocytes treated with 50 and 100 μg/mL BE and BW decreased significantly to 83 and 63% (BE), and 79 and 59% (BW), respectively, relative to that in control adipocytes. Moreover, 100 μg/mL BW appeared a significantly stronger inhibitory effect than 100 μg/mL BE on C/EBPα expression.
Fig. 4.
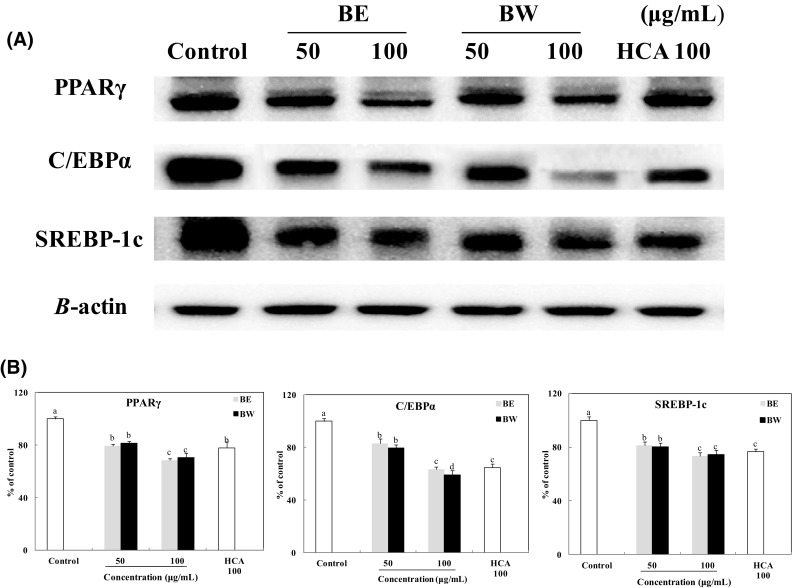
Effect of bamboo leaf extracts on the expression (A) and levels (B) of PPARγ, C/EBPα, and SREBP-1c in 3T3-L1 adipocytes. Western blot signal intensities were determined through densitometric analysis by using Multi Gauge V3.1 software. Representative blots are shown with expression levels quantified relative to those observed in untreated adipocytes. a–dValues with different letters are significantly different (P < 0.05) as analyzed using Duncan’s multiple range test
Another important transcription factor in adipogenesis, SREBP-1c, cross-activates a ligand binding domain of PPARγ and regulates the expression of enzymes involved in adipogenesis [6, 25]. The expression levels of SREBP-1c in the adipocytes treated with 50 and 100 μg/mL BE and BW decreased significantly to 81 and 73% (BE), and 80 and 74% (BW), respectively, compared with that in untreated adipocytes. Interestingly, there was no significant difference between the effects of BE and BW treatments. Comparing HCA treatment (100 μg/mL), there was no significant difference between HCA and BE or BW (100 μg/mL) in reducing SREBP-1c expression.
Further, SREBP-1c regulates the expression of the key enzymes of adipogenesis, FAS and ACC [6]. Both bamboo leaf extracts significantly inhibited the expression of these enzymes (Fig. 5). The role of FAS in lipogenesis is to regulate fatty acid synthesis [26]. The FAS expression level in adipocytes treated with 50 and 100 μg/mL BE and BW decreased to 91 and 80% (BE), and 85 and 70% (BW), respectively, compared with that in control adipocytes. Moreover, its expression levels in 100 μg/mL BW-treated cells were significantly lower than that in 100 μg/mL HCA-treated cells (positive control). The function of ACC is to convert acetyl-CoA, an essential substrate of fatty acids, to malonyl-CoA [27, 28]. The ACC expression level in the adipocytes treated with 50 and 100 μg/mL BE and BW decreased to 88 and 83% (BE), and 85 and 74% (BW), respectively, relative to that in untreated adipocytes. On the other hand, bamboo leaf extract treatment significantly increased the levels of p-ACC. The p-ACC expression level in adipocytes treated with 50 and 100 μg/mL BE and BW increased to 166 and 180% (BE), and 169 and 195% (BW), respectively, compared with that in control adipocytes. Since the phosphorylation of ACC inhibits the enzyme’s activity, increased levels of p-ACC by bamboo leaf extract treatment would lead to a decrease in fatty acid biosynthesis [29]. These results show that the inhibition of lipogenesis in 3T3-L1 adipocytes by bamboo leaf extracts may be mediated in part through downregulation of FAS and ACC and upregulation of p-ACC. In addition, BW treatment significantly decreased more FAS and ACC expression levels than BE treatment in the adipocytes. Lipogenesis is the enzymatic process by which glycerol is esterified with free fatty acids to form triglyceride [30]. Therefore, BW treatment inhibited more secretion of lipogenesis enzymes and decreased more triglyceride contents than BE.
Fig. 5.
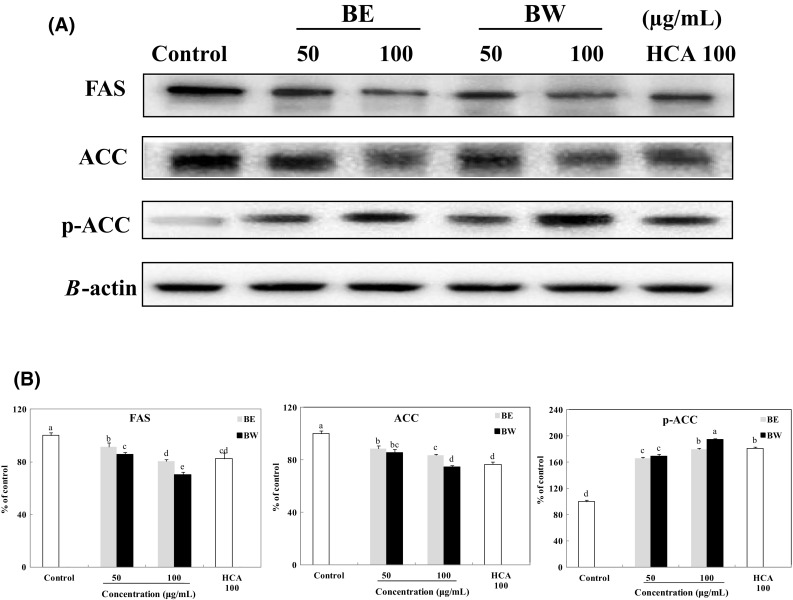
Effect of bamboo leaf extracts on the expression (A) and levels (B) of FAS, ACC, and p-ACC in 3T3-L1 adipocytes. Western blot signal intensities were determined through densitometric analysis by using Multi Gauge V3.1 software. Representative blots are shown with expression levels quantified relative to those observed in untreated adipocytes. a–eValues with different letters are significantly different (P < 0.05) as analyzed using Duncan’s multiple range test
Effect of bamboo leaf extracts on the expression of pAMPK
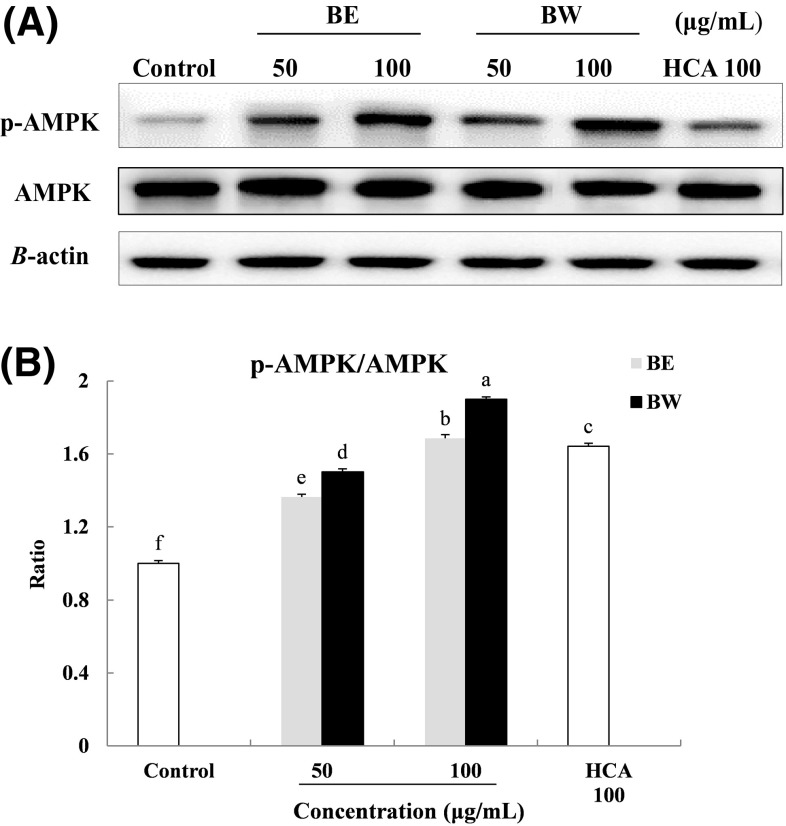
The regulatory factor, AMPK is involved in the regulation of body weight, systemic glucose homeostasis, lipid metabolism, mitochondrial biogenesis, and insulin signaling [31]. Activation of AMPK by phosphorylation may potentially inhibit adipocyte differentiation and control obesity [32]. To examine whether bamboo leaf extracts had an effect on the activity of AMPK, the levels of phosphorylated-AMPK and AMPK were determined by Western blot analyses. The p-AMPK/AMPK ratio in the adipocytes treated with 50 and 100 μg/mL BE and BW were increased to 1.4 and 1.7% (BE), and 1.5 and 1.9% (BW), respectively, relative to that in untreated adipocytes. BW treatment resulted in higher p-AMPK/AMPK ratio than BE treatment. Furthermore, p-AMPK/AMPK ratio after 100 μg/mL BW treatments was significantly higher than that after treatment with HCA, the positive control (Fig. 6).
Fig. 6.
Effect of bamboo leaf extracts on the expression (A) and ratio (B) of AMPK and p-AMPK in 3T3-L1 adipocytes. Western blot signal intensities were determined through densitometric analysis by using Multi Gauge V3.1 software. Representative blots are shown with expression levels quantified relative to those observed in untreated adipocytes. a–fValues with different letters are significantly different (P < 0.05) as analyzed using Duncan’s multiple range test
In conclusion, bamboo leaf extracts inhibited adipogenesis in 3T3-L1 adipocytes by reducing lipid accumulation. Comparing the two extracts, BW showed stronger adipogenesis-suppressing effects than BE did. Treatment with BW led to lower FAS and ACC expression, as well as higher p-ACC and p-AMPK expression, than treatment with BE did. The results of the present study suggests that BW is a better candidate than BE as an alternative therapy to control obesity.
Acknowledgements
This work was supported by a grant from the Korea Bio Medical Science Institute.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Wu T, Guo Y, Liu R, Wang K, Zhang M. Black tea polyphenols and polysaccharides improve body composition, increase fecal fatty acid, and regulate fat metabolism in high-fat diet-induced obese rats. Food Funct. 2016;7:2469–2478. doi: 10.1039/C6FO00401F. [DOI] [PubMed] [Google Scholar]
- 2.Hsu CL, Yen GC. Effects of flavonoids and phenolic acids on the inhibition of adipogenesis in 3T3-L1 adipocytes. J. Agric. Food Chem. 2007;55:8404–8410. doi: 10.1021/jf071695r. [DOI] [PubMed] [Google Scholar]
- 3.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 4.Bae CR, Park YK, Cha YS. Quercetin-rich onion peel extract suppresses adipogenesis by down-regulating adipogenic transcription factors and gene expression in 3T3-L1 adipocytes. J. Sci. Food. Agric. 2014;94:2655–2660. doi: 10.1002/jsfa.6604. [DOI] [PubMed] [Google Scholar]
- 5.Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism. 1987;36:54–59. doi: 10.1016/0026-0495(87)90063-1. [DOI] [PubMed] [Google Scholar]
- 6.Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- 7.Choi KM, Lee YS, Kim W, Kim SJ, Shin KO, Yu JY, Yoo HS. Sulforaphane attenuates obesity by inhibiting adipogenesis and activating the AMPK pathway in obese mice. J. Nutr. Biochem. 2014;25:201–207. doi: 10.1016/j.jnutbio.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 8.He YJ, Yue YD. A review of the effective component and applications of extracts from bamboo leaves. Biomass Chemical Engineering. 2000;3:008. [Google Scholar]
- 9.Park EJ, Jhon DY. The antioxidant, angiotensin converting enzyme inhibition activity, and phenolic compounds of bamboo shoot extracts. Food Science and Technology. 2010;43:655–659. [Google Scholar]
- 10.Koide CLK. The effect of bamboo extract on hepatic biotransforming enzymes–Findings from an obese–diabetic mouse model. J. ethnopharmacology. 2011;133:37–45. doi: 10.1016/j.jep.2010.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lara-Castro C, Fu Y, Chung BH, Garvey WT. Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease. Curr. Opin. Lipidol. 2007;218(3):263–270. doi: 10.1097/MOL.0b013e32814a645f. [DOI] [PubMed] [Google Scholar]
- 12.Lee MJ, Park WH, Song YS, Lee YW, Song YO, Moon GS. Effect of bamboo culm extract on oxidative stress and genetic expression: bamboo culm extract ameliorates cell adhesion molecule expression and NFκB activity through the suppression of the oxidative stress. Clin. Nutr. 2008;2:755–763. doi: 10.1016/j.clnu.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Deng S, Fu H. Inhibition of the corrosion of steel in HCl, H 2 SO 4 solutions by bamboo leaf extract. Corrosion Science. 2012;62:163–175. doi: 10.1016/j.corsci.2012.05.008. [DOI] [Google Scholar]
- 14.Choi S, Park MS, Lee YR, Lee YC, Kim TW, Do SG, Jeon BH. A standardized bamboo leaf extract inhibits monocyte adhesion to endothelial cells by modulating vascular cell adhesion protein-1. Nutr. Res. Pract. 2013;7:9–14. doi: 10.4162/nrp.2013.7.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao D, Rangwala SM, Bailey ST, Krakow SL, Reginato MJ, Lazar MA. Interdomain communication regulating ligand binding by PPAR-γ. Nature. 1998;396:377–380. doi: 10.1038/24634. [DOI] [PubMed] [Google Scholar]
- 16.Basu A, Sanchez K, Leyva MJ, Wu M, Betts NM, Aston CE, Lyons TJ. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J. Am. Coll. Nutr. 2010;29:31–40. doi: 10.1080/07315724.2010.10719814. [DOI] [PubMed] [Google Scholar]
- 17.Kweon MH, Hwang HJ, Sung HC. Identification and antioxidant activity of novel chlorogenic acid derivatives from bamboo (Phyllostachys edulis) J Agric Food Chem. 2001;49:4646–4655. doi: 10.1021/jf010514x. [DOI] [PubMed] [Google Scholar]
- 18.Park HS, Lim JH, Kim HJ, Choi HJ, Lee IS. Antioxidant flavone glycosides from the leaves of Sasa borealis. Arch Pharm Res. 2007;30:161–166. doi: 10.1007/BF02977689. [DOI] [PubMed] [Google Scholar]
- 19.Lam KY, Ling APK, Koh RY, Wong YP, Say YH. A review on medicinal properties of orientin. Adv Pharmacol Sci. 2016: 4104595 (2016) [DOI] [PMC free article] [PubMed]
- 20.Kim SH, Zhao X, Shen J, Chang KJ. Effects of phytosterols and fatty acids from lotus (Nelumbo Nucifera) seed on differentiation of human preadipocytes into adipocytes. The FASEB Journal. 2013;27:1079-30. [Google Scholar]
- 21.Ok E, Do GM, Lim Y, Park JE, Park YJ, Kwon O. Pomegranate vinegar attenuates adiposity in obese rats through coordinated control of AMPK signaling in the liver and adipose tissue. Lipids Health Dis. 2013;12:1. doi: 10.1186/1476-511X-12-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 23.Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM. C/EBPα induces adipogenesis through PPARγ: a unified pathway. Genes dev. 2002;16:22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Z, Xie Y, Morrison RF, Bucher NL, Farmer SR. PPARg induces the insulin-dependent glucose transporter GLUT4 in the absence of C/EBPa during the conversion of 3T3 fibroblasts into adipocytes. J. Clin. Invest. 1998;101:22–32. doi: 10.1172/JCI1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/S0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 26.Oh DR, Kim Y, Choi EJ, Jung MA, Bae D, Jo A, Kim S. Antiobesity effects of unripe rubus coreanus miquel and its constituents: An in vitro and in vivo characterization of the underlying mechanism. Evid. Based. Complement. Alternat. Med. 2016: 4357656 (2016) [DOI] [PMC free article] [PubMed]
- 27.Kwon TH, Wu YX, Kim JS, Woo JH, Park KT, Kwon OJ, Park NH. 6, 6′-Bieckol inhibits adipocyte differentiation through downregulation of adipogenesis and lipogenesis in 3T3-L1 cells. J. Sci. Food. Agric. 2015;95:1830–1837. doi: 10.1002/jsfa.6881. [DOI] [PubMed] [Google Scholar]
- 28.Kohjima M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, Takayanagi R. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int J Mol Med. 2008;21:507–512. [PubMed] [Google Scholar]
- 29.Min B, Lee H, Song JH, Han MJ, Chung J. Arctiin inhibits adipogenesis in 3T3-L1 cells and decreases adiposity and body weight in mice fed a high-fat diet. Nutrition research and practice. 2014;8:655–661. doi: 10.4162/nrp.2014.8.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kersten S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO. Rep. 2001;2:282–286. doi: 10.1093/embo-reports/kve071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Koo J, Eun J. Antioxidant activities of methanol extracts and phenolic compounds in Asian pear at different stages of maturity. Food. Sci. Biotechnol. 2006;15:44. [Google Scholar]
- 32.Wu CH, Yang MY, Chan KC, Chung PJ, Ou TT, Wang CJ. Improvement in high-fat diet-induced obesity and body fat accumulation by a Nelumbo nucifera leaf flavonoid-rich extract in mice. J. Agric. Food. Chem. 2010;58:7075–7081. doi: 10.1021/jf101415v. [DOI] [PubMed] [Google Scholar]