Abstract
Pretreatments with different concentrations of sulfuric acid (0, 0.5, and 1% v/v) and temperatures (28 and 121 °C at 103 kPa in an autoclave) were performed on banana peels (BP) milled by mechanical grinding and grinding in a blender as well as without grinding. Cellulose, hemicellulose, lignin, ash, and total and reducing sugar contents were evaluated. The highest yields of cellulose enzymatic hydrolysis (99%) were achieved with liquefied autoclaved BP treated with 0.5 and 1% acid after 48 h of hydrolysis. Ethanol production by Kluyveromyces marxianus fermentation was assayed using hydrolyzed BP at 10, 15, and 20% (w/w). The highest ethanol level (21 g/L) was reached after 24 h of fermentation with 20% (w/w) BP. Kinetics of the consumption of reducing sugars under this fermentation condition demonstrates the presence of a lag period (about 8 h). Thus, BP are a good source for ethanol production.
Keywords: Banana peel, Physicochemical pretreatment, Enzymatic hydrolysis, Ethanol production
Introduction
Banana is an important crop in tropical and sub-tropical regions, mainly in Asia, South America, and Africa [1]. Currently, it is the second most produced fruit in the world. Its production is estimated at approximately 101 million tons [2]. Significant amounts of banana peels (BP), equivalent to 35–40% of the total weight of fresh banana, are generated as waste from the industrial production of frozen, fried, or dehydrated banana, banana puree, and banana flour [3]. This waste is frequently disposed into the environment without any treatment, which leads to potential serious environmental risks. Therefore, BP accumulation represents an issue that must be attended immediately.
BP utilization in biotechnological processes, such as biofuels and enzymes production, animal feed protein enrichment, and other important industrial applications, have been reported previously [4–10].
BP are characterized as a source of lignocellulosic compounds as well as carbohydrates, vitamins, and minerals (essential and non-essential). The lignocellulosic materials exist as three important components: cellulose, hemicellulose, and lignin. BP composition depends on the plant variety. Cellulose is a linear biopolymer that contains glucose molecules connected by β-1,4-glycosidic bonds and is considered a material suitable for the production of second-generation bioethanol [11, 12]. Hemicellulose is a heterogeneous polymer comprising five and six carbon monosaccharides such as fructose, mannose, galactose, xylose, and arabinose [11, 13]. Both biopolymers interact with lignin [14], which is formed by three types of aromatic alcohols: p-coumaryl, coniferyl, and sinapyl alcohol. During the production of second-generation bioethanol, lignin acts as a barrier for the enzymes that cause cellulose hydrolysis, and it is difficult to degrade. Physicochemical pretreatments are commonly applied prior to enzymatic hydrolysis to release hemicellulose, decrease cellulose interaction with lignin, and transform its crystalline form to an amorphous state [1, 15, 16].
Previous studies on BP as the lignocellulosic material were carried out using dry pulverized material and acids under autoclave conditions [1, 4, 15]. Previous research showed that mechanical pretreatment of lignocellulosic materials, such as the milling process, help to increase the yield of enzymatic cellulose hydrolysis. However, these pretreatments are characterized by high energy requirements and therefore high cost [17]. Other faster and cheaper operations must be tested. The liquefying process is one such method, which can be easily performed in industrial blenders. An alternative method is to not perform operations that are intended to reduce the particle size, which is otherwise achieved by pulverization and trituration in a blender.
According to our knowledge, BP fermentation by Kluyveromyces marxianus has not been studied previously. Ethanol fermentation of the thermotolerant yeast K. marxianus is characterized by the ability to utilize various sugars, including glucose, mannose, galactose, xylose, and fructose, except arabinose [18].
Aims of the present study were as follows: (1) to compare the composition of dry-pulverized, liquefied, or unmilled BP pretreated with different concentrations of sulfuric acid, (2) to evaluate the susceptibility of cellulose from pretreated materials to enzymatic hydrolysis, (3) to determine ethanol production kinetics on K. marxianus fermentation of hydrolyzed BP at 10, 15, and 20% dry biomass after their pretreatment with grinding in a blender for 20 s and autoclaving at 121 °C for 15 min without acid.
Materials and methods
Materials
K. marxianus was obtained from the Food Research Department, School of Chemistry, Autonomous University of Coahuila. This strain was isolated previously from a sample obtained from the Mexican semi-desert as a xerophytic microorganism. Bananas (Tabasco variety) were obtained in the local market (Saltillo, Coahuila, Mexico). Fruit maturation was achieved in a laboratory at room temperature (20–25 °C). BP were removed from the fruit at maturation stage No. 7 (yellow/a few brown spots), according to a previously defined scale [18, 19]. This maturation stage was selected due to its common application in different industrial and traditional culinary uses of banana, during which BP waste is generated. BP were dried at 72 °C for 48 h. Subsequently, BP were processed into three different forms: unmilled, liquefied, and pulverized. For the pulverized form, BP were milled in a mechanical grinder (Pulvex Mini 100, Mexico) using a 1.5 mm mesh. The powder was stored in polypropylene plastic bags at 25 °C before use [1, 18]. To obtain the liquefied form, BP were homogenized in a 2 L domestic blender in an aqueous medium [4]. Unmilled BP was used without any physical process. The cellulolytic complex Celluclast 1.5 L was purchased from Sigma-Aldrich (St. Louis, Mo, USA) to conduct assay enzymatic hydrolysis on pretreated BP. All other chemicals were obtained from Sigma-Aldrich (St. Louis, Mo, USA); they were of the analytical grade and used without further purification.
Physicochemical pretreatments
BP (pulverized, liquefied, and unmilled) were treated in 500 mL flasks with sulfuric acid at three different concentrations (0, 0.5, 1% v/v), autoclaved at 121 °C for 15 min or maintained at 28 °C for 45 min without thermal pretreatment [4]. All samples were assayed in triplicate, containing the same amount of water: 8 g of BP in 92 mL of aqueous phase.
Proximal composition analysis
Before the composition analysis, pretreated samples were dried in hot air oven at 72 °C without separation of the liquid phase, and then stored in polypropylene bags at 25 °C. The samples were then pulverized to a particle size smaller than 1 mm in a mechanical grinder. Neutral detergent fiber (NDF), acid detergent fiber (ADF) (obtained data not presented), and acid detergent lignin (ADL) were analyzed using an ANKOM 200 fiber analyzer (ANKOM Technology, USA) following the procedure specified by the manufacturer. The differences between ADF and ADL as well as NDF and ADF were reported as cellulose and hemicellulose, respectively [20]. The total sugar and reducing sugar contents were analyzed by the sulfuric phenol method and the 3–5 dinitrosalicylic acid (DNS) method [17, 20, 21], respectively, using a dilution (1/5) at each sample. The ash content was analyzed by the AOAC method 2000 [22]. All assays were duplicated.
Enzymatic assay with pretreated material
The enzymatic reaction was performed for 48 h at 50 °C and 150 rpm using materials prepared as described previously [23]. The particle size in all samples was less than 1 mm to avoid the effect of this parameter on enzymatic hydrolysis. Reaction was carried in a 100 mM acetate buffer at pH 5 using 15 FPU g−1 (Celluclast 1.5 L) and 0.05 g of each pretreated BP samples. Glucose release was analyzed spectrophotometrically using a commercial Kit (RANDOX) at 0, 24, and 48 h of hydrolysis. All assays were performed in triplicate.
The hydrolysis yield was calculated through the following equation [23]:
where (Glucose) is the glucose concentration (g/L) after hydrolysis, f is cellulose fraction in biomass (g/g), (Biomass) is the dry biomass concentration (g/L) at the beginning of hydrolysis, and 1.11 is the factor calculated as the ratio of glucose molecular weight (MW = 180.16 g/mol) to MW glucan (162.14 g/mol).
Ethanol production by Kluyveromyces marxianus fermentation
BP (10, 15, and 20% w/w) were groundn a blender along with 200 mL of distilled water for 20 s; this was then autoclaved at 121 °C for 15 min. Hydrolysis was performed in 100 mL Erlenmeyer flasks at 50 °C for 48 h using Celluclast 1.5 L at 15 FPU/dry BP g. Before the enzymatic hydrolysis, pH was adjusted with 50 mM citrate buffer at pH 5. Glucose and fructose contents were analyzed through high-performance liquid chromatography combined with a refractive index detector (HPLC–IR) according to a previously reported technique [24]. All measurements were carried out in duplicate.
Fermentation was carried out using K. marxianus at 6.5 × 107 cells/mL in a rotatory shaker at 42 °C and 150 rpm; for this, 10 mL of hydrolyzed BP was placed in 50 mL Erlenmeyer flasks along with 2 g/L yeast extract, 1 g/L NH4Cl, 1 g/L KH2PO4, 0.3 g/L MgSO4–7H2O. Ethanol was quantified by HPLC [24] at 0, 12, 24, and 30 h of fermentation.
Statistical analysis
For the ANOVA test, considering p < 0.05 as the significance level, the software Statistica 7.0 (Stat Soft, Tulsa, OK, USA) was employed. To compare results of materials characterization, ANOVA factorial Tukey’s range test was performed with a significance level at p < 0.05. Kinetic data were analyzed by ANOVA with a significance level at p < 0.05 and plotted in Excel with corresponding standard deviations.
Results and discussion
Pretreatment effect on banana peel composition
BP used in the present study contained 86% moisture before drying. Table 1 summarizes the results of the compositional characterization conducted for BP after different pretreatments. These results indicate that BP is a lignocellulosic material rich in polysaccharides (i.e., cellulose, hemicellulose), lignin, and minerals. The amount of cellulose in the pulverized material treated with 0.5% sulfuric acid at 121 °C was higher than the amount in the liquefied and unmilled materials. Acid use at both temperatures in the liquefied BP led to a decrease in the cellulose content. The same effects were observed for the unmilled BP only at room temperature, while at autoclave conditions with and without acid, similar values were obtained.
Table 1.
Pulverized, liquefied, and unmilled banana peel composition after pretreatments with and without sulfuric acid at room and autoclave conditions
| BP sample | Acid (%) | Temperature (°C) | Cellulose (%) | Hemicellulose (%) | Acid-detergent lignin (ADL) (%) | Ash (%) | Total sugars (g g−1BP) | Reducing sugars (g g−1BP) |
|---|---|---|---|---|---|---|---|---|
| Pulverized | 0.0 | 28 | 25.84b | 19.55ab | 18.14abc | 15.92bc | 0.24de | 0.11de |
| 121 | 22.79bcd | 16.22a | 18.93bcd | 14.59d | 0.36cde | 0.17de | ||
| 0.5 | 28 | 21.35cde | 8.09bcde | 18.34bcde | 16.62ab | 0.27de | 0.12de | |
| 121 | 30.15a | 0.00f | 27.59a | 10.71g | 0.27de | 0.15de | ||
| 1.0 | 28 | 21.19de | 0.00f | 17.88bcde | 17.31a | 0.21de | 0.09e | |
| 121 | 20.08de | 0.00f | 16.87cdef | 17.72a | 0.20e | 0.14de | ||
| Liquefied | 0.0 | 28 | 24.83b | 1.80def | 25.23a | 13.37ef | 0.21de | 0.15de |
| 121 | 18.75ef | 2.48ef | 23.41ab | 11.34g | 0.69ab | 0.26bc | ||
| 0.5 | 28 | 15.53g | 9.85abc | 12.17f | 13.09ef | 0.72a | 0.29b | |
| 121 | 15.25g | 6.77bcd | 12.25f | 12.94ef | 0.84a | 0.27bc | ||
| 1.0 | 28 | 15.64g | 7.35bcd | 12.00f | 13.52cd | 0.77a | 0.16de | |
| 121 | 14.24g | 1.78def | 13.41def | 14.86de | 0.61abc | 0.26bc | ||
| Unmilled | 0.0 | 28 | 23.37bc | 12.33abc | 12.33def | 12.75f | 0.43bcd | 0.22bcd |
| 121 | 14.42g | 5.19cde | 17.28cdef | 11.3g | 0.31cde | 0.30b | ||
| 0.5 | 28 | 16.4fg | 18.60a | 13.8def | 12.72f | 0.40cde | 0.20cd | |
| 121 | 15.93g | 5.10cde | 14.65def | 11.66g | 0.39cde | 0.37a | ||
| 1.0 | 28 | 16.26fg | 6.45abc | 16.26ef | 13.84de | 0.38cde | 0.17de | |
| 121 | 14.57g | 4.27cde | 14.57f | 13.96de | 0.40cde | 0.21bcd |
Data in a column followed by the same letter are not significantly different according to the Tukey’s test (p = 0.05)
In all tested samples, the trend of decreasing hemicellulose amount with an increase in temperature and with acid treatment was observed. Similar trends were observed in case of hemicellulose amounts in liquefied and unmilled materials. However, the highest concentration of hemicellulose was detected in unmilled material treated with 0.5% sulfuric acid at 28 °C. Similar hemicellulose levels were detected in pulverized BP treated without acid. Pulverized materials treated with 0.5 or 1% sulfuric acid at 121 °C and with 1% acid at 28 °C showed no presence of hemicellulose (Table 1). This may be related to the size of raw materials, which affects porosity, maximizing the contact between the material and acid to increase hemicellulose hydrolysis; since the smaller size of raw materials is associated with a larger surface area, the hemicellulose hydrolysis is easier [23]. High temperature also increases hemicellulose hydrolysis.
Comparing the total sugar contents obtained from the liquid fraction showed that the liquefied BP had the highest total sugar levels in comparison with pulverized and unmilled BP. These levels were achieved with 0.5% sulfuric acid at 121 °C and with 1% acid at 28 °C (Table 1).
Reducing sugar levels were higher in the liquefied and unmilled BP than in pulverized BP. The highest level of reducing sugars was detected in the unmilled BP treated with 0.5% acid at 121 °C.
The obtained results demonstrate that a pretreatment process with diluted acid and high temperature can dissolve almost all hemicellulose, but not the lignin component (Table 1). In pulverized BP, a higher lignin level was observed in the material treated with 0.5% acid under the autoclave condition; acid treatment and high temperature did not lead to lignin removal. However, in the case of liquefied BP, minor lignin levels were detected with acid treatments, while in unmilled BP, increase in acid concentration led to minor lignin removal (Table 1).
The mineral content was not significantly different between the evaluated treated materials. However, the highest values were detected in pulverized BP treated with 1.0% sulfuric acid at 121 °C (Table 1).
Thus, pulverized BP were more susceptible to hemicellulose but not to lignin removal. Liquefied BP preserved sugar content, while pulverized material maintained a higher level of mineral content under similar conditions of treatment.
The results of BP composition (Table 1) confirm that this organic waste is a source of cellulose, hemicellulose, lignin, and inorganic compounds. Cellulose and hemicellulose values detected in the present study (Table 1) correlate with the previously reported data [3, 15, 18]. However, the ADL levels are higher than those reported by the same authors. This behavior can be attributed to the different variety of banana used for the present study [17].
Higher cellulose values quantified in the pulverized BP after pretreatment can be related to less effective hydrolysis (even with acid treatment and high temperature) than in the case of treatments performed on liquefied and unmilled materials (Table 1). The increase in total sugar levels revealed that polysaccharides were transformed to low-weight saccharides, which also might proceed from hemicellulose. Applied treatments led to partial hemicellulose hydrolysis in all samples, which was more effective in the presence of acid and at high temperature of the autoclave. However, hydrolysis occurred even in the absence of acid and at room temperature. These results agree with those reported by Mosier [26], who considered that at high temperature, the water steam acts as a weak acid and can partially hydrolyze compounds such as hemicellulose. Souza et al. [4] reported high total sugar content in the BP used for bioethanol production. However, they did not recommend using acids for polysaccharide hydrolysis in BP pretreatment because acid application did not lead to an increase in the bioethanol levels.
The presence of detectable ADL levels indicates that lignin is not degraded, not even with acid at room temperature or autoclave conditions. The same observations were reported by Happi Emaga [18], who assayed pulverized banana peels (unknown variety).
Ash percentage is associated with the presence of mineral [3]. According to the results shown in Table 1, the ash percentage was higher in the case of pulverized BP treated with 1% acid. A lower level of ash was observed in the case of pulverized and liquefied BP treated with 0.5% acid at the autoclave condition. Because the ash percentage is a relative value, the removal of components such as hemicellulose and lignin after treatment corresponds to an increase of ash percentage, while a higher content of lignocellulosic components is associated with a lower percentage of minerals. Ash content was reported to be in a range 6.4–12.8% for bananas of different varieties [3, 18, 27]. Among the main minerals in BP, potassium, phosphorus, magnesium, and calcium [3] were identified. In the present study, the level of ash quantified for pretreated Tabasco variety BP was within or above the aforementioned range.
Susceptibility of pretreated banana peels to enzymatic cellulose hydrolysis
Figures 1 and 2 describe the cellulose hydrolysis yield. This was considered as the criterion for cellulose susceptibility to enzymatic hydrolysis in pretreated BP. A tendency of an increase in the susceptibility with increase in the pretreatment temperature was observed. The yield of cellulose hydrolysis was higher at 48 h than at 24 h of reaction for all assayed samples.
Fig. 1.
Yield of cellulose enzymatic hydrolysis of banana peels (BP) pretreated at 28 °C for 45 min in the absence 0% and the presence of 0.5 and 1.0% (v/v) sulfuric acid: (A) pulverized BP; (B) liquefied BP; (C) unmilled BP
Fig. 2.
Yield of cellulose enzymatic hydrolysis of banana peels (BP) pretreated by autoclaving at 121 °C for 15 min in the absence 0% and presence of 0.5 and 1.0% (v/v) sulfuric acid: (A) pulverized BP; (B) liquefied BP; (C) unmilled BP
For BP treated at 28 °C, the greatest cellulose hydrolysis yield was observed in the liquefied BP treated with 0.5% sulfuric acid, while the lower levels were quantified for the pulverized BP treated with 1% sulfuric acid and the unmilled samples (Fig. 1).
For BP treated at autoclave conditions, high cellulose hydrolysis yields were detected after 48 h for the liquefied BP pretreated with 0.5 and 1% sulfuric acid, as well as without acid, and for the unmilled BP treated with 1% sulfuric acid. A lower yield was detected in the case of pulverized BP without acid treatments (Fig. 2). The lowest cellulose conversion was observed in case of the unmilled BP treated with 0.5% sulfuric acid.
Thus, BP grinding in a blender followed by autoclaving is a good method to increase cellulose susceptibility to enzymatic hydrolysis. Moreover, after autoclaving, the unmilled BP were susceptible to enzymatic hydrolysis. This indicates that pulverization of BP may be avoided during biomass pretreatment.
The analysis of cellulose susceptibility to enzymatic hydrolysis allows to conclude that liquefying may be applied as a part of BP treatment instead of pulverization of dry BP. By means of sulfuric acid addition and autoclaving, the highest susceptibility to cellulose enzymatic hydrolysis was achieved (Fig. 2). However, acid use leads to environmental problems, generates fermentation inhibitors, and requires an additional step of acid neutralization [16]. Thus, liquefying treatment without acid was selected for the next assay.
The highest value of glucose concentration (Fig. 3) achieved in the present study with the liquefied material at 20% of dry biomass (w/w) was higher than those reported by other authors (17–27.8 g glucose/L) [16, 28, 29]. This may be related to the use of different banana varieties, different fruit maturation stage, higher BP concentrations, and other types of enzymatic preparations applied for celullase hydrolysis.
Fig. 3.
Glucose (top) and fructose (bottom) levels during enzymatic hydrolysis (15 FPU/dry biomass g) of banana peel (BP) applied at different concentrations: 10, 15, and 20% dry biomass (pretreatment conditions: grinding in a blender for 20 s, autoclaving at 121 °C for 15 min)
The use of enzymes with cellulolytic and pectinolytic activities, applied at different proportions for polysacharide hydrolysis was reported to increase the amount of BP sugars available for ethanol production [1, 15, 16, 20]. A high glucose level is essential for the ethanolic fermentation process. Liquefied BP pretreated under the autoclave condition is a good source of glucose obtained by enzymatic hydrolysis as well as fructose obtained during pretreatment (Fig. 3). An increase in the biomass content led to an increase in the glucose level to 32 g/L, although the percentage of cellulose hydrolysis slightly decreased. It may be related to enzyme inhibition with the reaction product [16].
Ethanol production by Kluyveromyces marxianus fermentation
The assay was performed using liquefied BP pretreated in an autoclave. This treatment was selected because the glucose level after enzymatic hydrolysis was high and because the pretreated material may be applied directly in hydrolysis and in the subsequent fermentation process without acid neutralization. Moreover, sulfuric acid application requires work safety control, as it can cause corrosion and has an environmental impact.
Figure 3 shows glucose and fructose concentration detected in liquefied BP used at different concentrations (10, 15, and 20% w/w) at different times of enzymatic hydrolysis. The levels of fermentable sugars were increased with the biomass content increase. However, fructose concentration was not changed after the reaction time, while glucose concentration increased after 24 h of enzymatic hydrolysis. Glucose concentrations did not change after 48 h of hydrolysis and achieved the values at 18, 26, and 32 g/L, respectively, for the three BP concentrations. These levels correspond to 96, 94, and 88% hydrolysis yield, respectively, for each biomass concentration. While glucose derives from cellulose, the presence of fructose is associated with the hemicellulose hydrolysis during pretreatment [3].
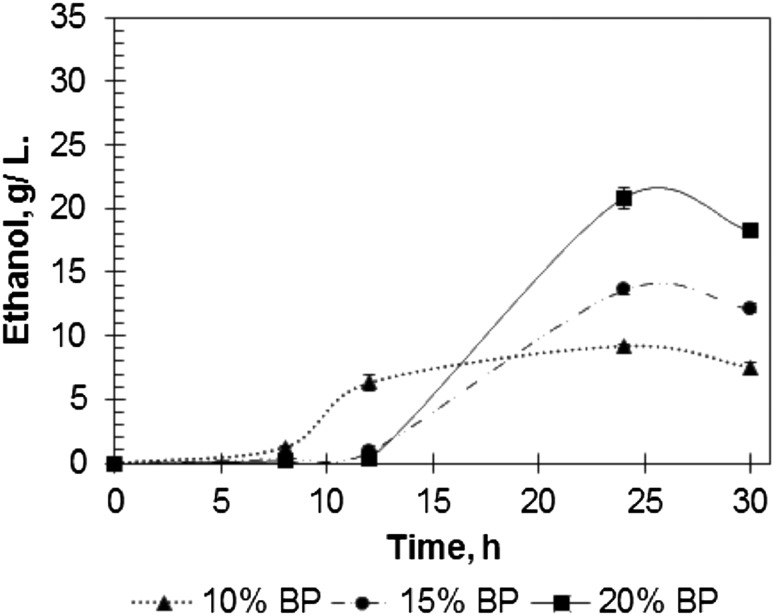
Figure 4 shows the kinetics of ethanol production from hydrolyzed BP during K. marxianus fermentation. The highest levels of ethanol (9, 14, and 21 g/L, respectively) were detected at 24 h of fermentation. It is known that theoretically from 1 g of glucose, only 0.51 g of ethanol can be obtained. The comparison of glucose levels with ethanol concentrations (Figs. 3, top, 4) indicates that during BP fermentation by K. marxianus, 98, 117, and 129% of theoretical ethanol production from glucose were achieved. These high ethanol levels appear as fructose participates in the yeast metabolism and ethanol production. The ethanol production yields calculated from the theoretical value for both hexoses (glucose and fructose) were estimated as 56, 65 and 70%, respectively. Similar effects were reported previously [25]. Thus, the selected pretreatment technique applied to BP and enzymatic hydrolysis allowed the obtainment of the fermentable system with high ethanol production.
Fig. 4.
Ethanol production kinetics during Kluyveromyces marxianus fermentation of hydrolyzed banana peels (BP) used at 10, 15, and 20% of dry biomass
Ethanol production by K. marxianus fermentation of hydrolyzed BP was carried out with a high yield of glucose conversion. Moreover, fructose was fermented by this yeast, leading to ethanol production at levels higher than theoretical consideration of glucose transformation to ethanol (Fig. 5). Wilkins et al. [25] reported a similar effect during orange peel fermentation using K. marxianus. The consumption of glucose and fructose, and a slight decrease in galactose, was detected. The ethanol level reported by Wilkins et al. [25] was approximately 37 g/L, and concentrations of glucose and fructose were approximately 50 and 30 g/L, respectively. In the present study, the highest ethanol level was estimated at 21 g/L in the presence of 32 and 25 g/L of glucose and fructose (Figs. 3, 4).
Fig. 5.
Glucose and fructose consumption kinetics during K. marxianus fermentation on hydrolyzed banana peels (BP) at 20% of dry biomass
However, the kinetics of sugar consumption and ethanol production (Figs. 4, 5) indicate the presence of a lag period (approximately 8 h). In our previous study [30], the same behavior was observed for kinetics assayed with 50 g/L glucose in the synthetic medium. The lag period, in this case, was more than 18 h. This behavior is probably related to yeast adaptation to high glucose concentration. The presence of a lag period during glucose fermentation of a synthetic medium allows to conclude that the delay in ethanol production from BP is related to a specific yeast response to high sugar concentration and not to the presence of inhibitors. Saccharomyces cerevisiae BY4742 inhibition using a substrate and ethanol was reported previously by Zhang et al. [31].
Moreover, another yeast strain that should also be considered in the second-generation ethanol production from food industry wastes was applied. BP fermentation was carried out previously using S. cerevisiae strains or Pachysolen tannophilus MTCC 1077 strain [4, 6, 16, 20]. The advantage of K. marxianus in comparison with other yeast strains is its tolerability to a high temperature that may be useful for simultaneous saccharification and fermentation processes. In the present study, K. marxianus was isolated from a semi-desert environment, and therefore it is probably not the best suitable yeast for ethanol production due to the presence of a lag period. However, its ability to ferment fructose, which leads to an increase in ethanol concentration is favored.
Thus, BP are a rich glucose and fructose source. Their liquefaction in a blender is one possible form treatment; further acid and thermal treatments of this BP allows the obtainment of material more susceptible to cellulase hydrolysis than pulverized material treated under the same conditions. Unmilled banana peels treated with sulfuric acid at the autoclave condition also may be considered as an alternative form for material pretreatment due to high cellulose susceptibility to enzymatic hydrolysis. Therefore, material drying and milling before pretreatment may be avoided as an industrial process step. Through the use of liquefied BP treated at the autoclave condition, a high yield of enzymatic hydrolysis may be archived. The glucose and fructose may be transformed into ethanol by means of fermentation of K. marxianus, obtaining a high concentration of ethanol.
Obtained results demonstrated the conversion of BP sugars to ethanol (Fig. 4). Despite high conversion efficiency of lignocellulosic waste, relatively low ethanol volumetric productivity could restrain the commercialization of such process. Ethanol concentration increase is critically important in terms of industrial applications, as achieving high ethanol concentration in less time improves productivity, having a direct effect on the economic processes and commercial feasibility. The highest productivity could be achieved via process optimization by increasing BP loading, using other yeast strains, different fermentation process schemes under optimized parameters, such as control over pH during the entire process, agitation, and other operational conditions.
One of the economic advantages of BP using as a raw material for the ethanol production is its large amount generated in the food industry as well as its low cost. In addition, obtained results demonstrate that pretreatment of this waste is much simpler in comparison to other lignocellulosic sources, for example, wood residues, since similar levels of fermentable sugars are achieved without the use of acid under thermal treatment. The fact that acid use can be avoided results in green and cleaner technology with less ecological impact. Moreover, apparently, the BP particle size control is not of great importance for the saccharification of this lignocellulosic material (Fig. 2). However, temperature plays an important role in its pretreatment to have a greater enzymatic hydrolysis yield. This also constitutes a great economic gain for the commercialization of ethanol production thanks to a simple process for lignocellulosic material pretreatment.
Acknowledgements
Authors would thank to the Mexican National Council of Science and Technology (CONACYT) for the financial support of the project PDCPN 2013-01-213844, Ph.D. scholarship, and for the financial support under the program “Cátedras-CONACyT” (Researcher No. 2498).
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
References
- 1.Oberoi HS, Vadlani PV, Saida L, Bansal S, Hughes JD. Ethanol production from banana peels using statistically optimized simultaneous saccharification and fermentation process. Waste Manage. 2011;31:1576–1584. doi: 10.1016/j.wasman.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Palacios-Ponce S, Ilyina A, Ramos-González R, Ruiz HA, Martínez-Hernández JL, Segura-Ceniceros EP, Aguilar MA, Sánchez O, Aguilar CN. Bioproducts obtained from the bioprocessing of the banana peel waste: an Overview. In: Applied Chemistry and Chemical Engineering. Vol. 5. Research Methodologies in Modern Chemistry and Applied Science. Part II - 10. Eds: Haghi AK, Faria Ribeiro AC, Pogliani L, Balköse D, Francisco Torrens F, Omari V, Mukbaniani OV. CRC Press. Taylor and Francis Group. India. (2017) (in Press)
- 3.Nagarajaiah SB, Prakash J. Chemical composition and antioxidant potential of peels from three varieties of banana. As. J. Food Ag-Ind. 2011;4:31–46. [Google Scholar]
- 4.Souza O, Schulz MA, Fischer GAA, Wagner TM, Sellin N. Energia alternativa de biomassa: bioetanol a partir de casca e polpa de banana. Rev. Bras. Eng. Agr. Ambient. 2012;16:915–921. doi: 10.1590/S1415-43662012000800015. [DOI] [Google Scholar]
- 5.Analysis Energy. Velásquez-Arredondo HI, Ruiz-Colorado AA, De Oliveira junior S. Ethanol production process from banana fruit and its lignocellulosic residues. Energy. 2010;35:3081–3087. doi: 10.1016/j.energy.2010.03.052. [DOI] [Google Scholar]
- 6.Dhabekar A, Chandak A. Utilization of banana peels and beet waste for alcohol production. Asiatic. J. Biotechnol. Res. 2010;1:8–13. [Google Scholar]
- 7.Clarke WP, Radnidge P, Lai TE, Jensen PD, Hardin MT. Digestion of waste bananas to generate energy in Australia. Waste Manage. 2008;28:527–533. doi: 10.1016/j.wasman.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Ilori MO, Adebusoye SA, Iawal AK, Awotiwon OA. Production of biogas from banana and plantain peels. Adv. Environ. Biol. 2007;1:33–38. [Google Scholar]
- 9.Bardiya N, Somayaji D, Khanna S. Biomethanation of banana peel and pineapple waste. Bioresour. Technol. 1996;58:73–76. doi: 10.1016/S0960-8524(96)00107-1. [DOI] [Google Scholar]
- 10.Gunaseelan VN. Biochemical methane potential of fruits and vegetable solid waste feedstocks. Biomass Bioenerg. 2004;26:389–399. doi: 10.1016/j.biombioe.2003.08.006. [DOI] [Google Scholar]
- 11.Pérez J, Muñoz-Dorado J, De la Rubia T, Martínez J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: an overview. Int. Microbiol. 2002;5:53–63. doi: 10.1007/s10123-002-0062-3. [DOI] [PubMed] [Google Scholar]
- 12.Ovando-Chacó SL, Waliszewski KN. Commercial cellulases preparations and their applications in extractives processes. Universidad y Ciencia. 2005;21:111–120. [Google Scholar]
- 13.Carmen S. Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009;27:185–194. doi: 10.1016/j.biotechadv.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Badui DS. Hidratos de carbono. 107-113. In: Química de los alimentos. Ed: Duarte EQ. Cuarta Edición. Pearson Educación. México (2006)
- 15.Sharma N, Kalra KL, Oberoi H, Bansal S. Optimization of fermentation parameters for production of ethanol from kinnow waste and banana peels by simultaneous saccharification and fermentation. Indian J. Microbiol. 2007;47(4):310–316. doi: 10.1007/s12088-007-0057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itelima J, Onwuliri E, I. O, Oforji S. Bio-Ethanol Production from Banana, Plantain and Pineapple Peels by Simultaneous Saccharification and Fermentation Process. Int. J. Environ. Sci. Dev. 4 (2): 213–216 (2013)
- 17.Hendriks ATWM, Zeeman G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009;100(1):10–18. doi: 10.1016/j.biortech.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 18.Happi Emaga T, Andrianaivo RH, Wathelet B, Tchango JT, Paquot M. Effects of the stage of maturation and varieties on the chemical composition of banana and plantain peels. Food Chem. 2007;103(2):590–600. doi: 10.1016/j.foodchem.2006.09.006. [DOI] [Google Scholar]
- 19.Happi Emaga T, Robert C, Ronkart SN, Wathelet B, Paquot M. Dietary fibre components and pectin chemical features of peels during ripening in banana and plantain varieties. Bioresour. Technol. 2008;99(10):4346–4354. doi: 10.1016/j.biortech.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 20.Oberoi HS, Sandhu SK, Vadlani PV. Statistical optimization of hydrolysis process for banana peels using cellulolytic and pectinolytic enzymes. Food Bioprod. Process. 2012;90(2):257–265. doi: 10.1016/j.fbp.2011.05.002. [DOI] [Google Scholar]
- 21.King BC, Donnelly MK, Bergstrom GC, Walker LP, Gibson DM. An optimized microplate assay system for quantitative evaluation of plant cell wall-degrading enzyme activity of fungal culture extracts. Biotechnol. Bioeng. 2009;102(4):1033–1044. doi: 10.1002/bit.22151. [DOI] [PubMed] [Google Scholar]
- 22.Happi Emaga T, Andrianaivo RH, Wathelet B, Tchango JT, Paquot M. Effects of the stage of maturation and varieties on the chemical composition of banana and plantain peels. Food Chem. 2007;103:590–600. doi: 10.1016/j.foodchem.2006.09.006. [DOI] [Google Scholar]
- 23.Li A, Antizar-Ladislao B, Khraisheh M. Bioconversion of municipal solid waste to glucose for bio-ethanol production. Bioprocess Biosyst. Eng. 2007;30(3):189–196. doi: 10.1007/s00449-007-0114-3. [DOI] [PubMed] [Google Scholar]
- 24.Negro MJ, Ballesteros I, Manzanares P, Oliva JM, Sáez F, Ballesteros M. Inulin-containing biomass for ethanol production. Appl. Biochem. Biotechnol. 2006;132(1):922–932. doi: 10.1385/ABAB:132:1:922. [DOI] [PubMed] [Google Scholar]
- 25.Wilkins MR, Suryawati L, Maness NO, Chrz D. Ethanol production by Saccharomyces cerevisiae and Kluyveromyces marxianus in the presence of orange-peel oil. World J. Microbiol. Biotechnol. 2007;23(8):1161–1168. doi: 10.1007/s11274-007-9346-2. [DOI] [Google Scholar]
- 26.Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005;96:673–686. doi: 10.1016/j.biortech.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 27.Hammond JB, Egg R, Diggins D, Coble CG. Alcohol from bananas. Bioresour. Technol. 1996;56(1):125–130. doi: 10.1016/0960-8524(95)00177-8. [DOI] [Google Scholar]
- 28.Oberoi HS, Sandhu SK, Vadlani PV. Statistical optimization of hydrolysis process for banana peels using cellulolytic and pectinolytic enzymes. Food Bioprod. Process. 2012;90:257–265. doi: 10.1016/j.fbp.2011.05.002. [DOI] [Google Scholar]
- 29.Oberoi HS, Vadlani PV, Saida L, Bansal S, Hughes JD. Ethanol production from banana peels using statistically optimized simultaneous saccharification and fermentation process. Waste Manag. 2011;31:1576–1584. doi: 10.1016/j.wasman.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Cortés-Arganda JF, Ramos-González R, Segura-Ceniceros EP, Martínez-Hernández JL, Rutiaga Quiñones OM, Iliná A. Screening of Kluyveromyces marxianus strains for ethanol production. II435. In: IAFP’s 5th Latin American Symposium in Food Safety, 7th Food Science, Biotechnology and Safety Meeting. November 9–11, Convention Center, Cancún. Mexico (2016)
- 31.Zhang Q, Wu D, Lin Y, Wang X, Kong H, Tanaka S. Substrate and product inhibition on yeast performance in ethanol fermentation. Energy Fuels. 2015;29:1019–1027. [Google Scholar]