Abstract
This study aimed to evaluate the effectiveness of aerosolized chlorine dioxide (ClO2) in reducing Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on washed carrots at various time durations and conditions. Populations of the bacteria on carrots were reduced by 1.5, 1.5, and 1.3 log CFU/g, respectively, in each inoculum after exposure to 300 ppm of aerosolized ClO2 for 30 min. Populations were further reduced by 2.4, 2.3, and 2.1 log CFU/g, respectively, at 400 ppm, showing a positive correlation between the concentrations of ClO2 and microbial control. The D-value was 13, 14, and 15 min for E. coli O157:H7, S. Typhimurium, and L. monocytogenes, respectively. ClO2 residues were 1 ppm or less in all treated carrots, showing no appearance or discoloration defects. As a result, effectiveness of aerosolized ClO2 in reducing bacterial pathogens and maintaining the quality of fresh carrots is signifying the prospects of aqueous ClO2 as a non-thermal disinfectant.
Keywords: Washed carrot, Chlorine dioxide, Antimicrobial effect, Aerosolization
Introduction
Demand for fresh produce is rising among today’s health-conscious, busy consumers as they look for fresh and ready-to-eat nutritious meals. Minimally processed fruits and vegetables are peeled, trimmed, sliced, and washed before packaging in various ready-to-eat formats. However, fresh produce represents a critical issue given that microbial contamination of fruits and vegetables may not be completely removed through the non-thermal process. That is, microbial contamination of fresh produce can pose a threat to the health of the people consuming fresh product. The most significant pathogens in minimally processed fresh produce include E. coli O157:H7, Salmonella spp., and L. monocytogenes [1–3]. Carrot is one of the most common raw vegetables and prone to microbial contamination. Carrots are consumed directly as juice or in salads, thus threatening food safety. However, pathogenic microbes on the surface of carrots after washing and bacterial biofilm formation on the surface may pose a food safety risk [4]. Non-thermal treatments including gamma ray exposure [5], UV-C exposure and immersion in ozone water [6], electrolyzed water [7], and aqueous chlorine [8] have been applied to freshly cut carrots. Among those treatments, chlorine is the most commonly used sanitizer for reducing pathogens on fresh produce. Use of chlorine solutions lead to bacterial counts of 1–2 log CFU/g or less on the surface of fruits and vegetables [9] and lose their effectiveness by reacting with organic compounds in foods, resulting in halogenated compounds [10]. Chlorine dioxide (ClO2) has emerged as an alternative to chlorine as it has better antimicrobial effectiveness with a higher solubility, shorter response time, and wider pH range [11]. It does not react with ammonia to form chloramines. Many studies are underway to explore the applications of ClO2 in gaseous and aqueous formations in the produce industry [12, 13]. However, regulatory permission of gaseous ClO2 for application to food is not established. Gaseous ClO2 cannot be stored or transported in its radical forms owing to its explosive nature, and sophisticated apparatus is required for ClO2 gas generation.
Sanitizers are applied to the food industry by the spraying or dipping methods. However, these methods decrease the antimicrobial activity in fresh produce by affecting the surface conformation and substances that obstruct the sanitizer to contact the pathogens. Aerosol technology is designed to generate aerosol mist by turning liquid into finedroplets. Aerosolized sanitizers overcome the drawbacks of aqueous solutions of sanitizers [14, 15]. Aerosol technologies have been applied to medical treatment for respiratory diseases [16] and disinfection purposes in farms and hospitals [17, 18]. However, its applications to the food industry have been insignificant. This study was therefore aimed to investigate the antimicrobial effectiveness of aerosolized ClO2 for fresh carrots.
Materials and methods
Bacterial strains and cell suspension
A streptomycin-resistant (StrR) mutants of E. coli O157:H7 (ATCC 43895), S. Typhimurium KCTC 2515, and L. monocytogenes 4244 were used as experimental bacteria. Each strain was sub-cultured more than three times in tryptic soy broth (TSB, Difco, USA) on a tryptic soy agar plate containing 0.1% streptomycin (TSA-STR, Sigma, St. Louis, MO, USA) and incubated at 37 °C for 24 h. The strains showed a time-related growth pattern similar to that of the parent strain in TSB. A single colony from each StrR strain was transferred to 15 mL TSB and incubated for 24 h at 37 °C and 100 rpm in a shaking incubator (VS-8480SF, Vision Scientific Co., Ltd., Osan, Korea). Following incubation, the culture suspension was centrifuged (VS-5000 N, Vision Scientific Co.) for 10 min at 4000 rpm. Isolated pellets were diluted in 0.1% peptone water. The culture suspension with a microbial population of approximately 108–109 log CFU/mL was used to inoculate the prepared carrots.
Sample preparation and inoculation
Pre-washed fresh carrots (Daucus carota, L) were purchased at a supermarket (Mokpo, Korea) and washed for 2 min with running water. Washed carrots were cut into pieces of the same size (3 × 3 × 1 cm3) with a sterile knife on a cutting board. A spot inoculation method was used to inoculate the pathogenic bacteria on carrots [19]. A 100-μL aliquot of each culture was spotted (10 droplets) with a micropipette on the surface of carrots in a biosafety hood (VS-1400, Vision Scientific Co.). Next, the carrots were placed on a sterile perforated tray and air-dried in a biosafety hood at room temperature for 30 min prior to aerosol ClO2 treatments.
Preparation of aerosol ClO2
A ClO2 stock solution was prepared from sodium chlorite and phosphoric acid using a ClO2 generator (K-CIDE, Envio Corp., Korea). This stock solution was used to prepare various working solutions (100, 200, 300, and 400 ppm) based on the content of the total available ClO2 (TACD expressed as mg/mL ClO2), as determined by iodometric and N,N-diethyl-p-phenylene-diamine (DPD) ferrous titration methods [20]. Both the stock and working ClO2 solutions were freshly prepared on each day of experimentation. The ultrasonic nebulizer (MH5518, Woori-Tec Co., Korea) used in this study had an aerosol output of 80 mL/h, and an ultrasonic vibrator used in the humidifier (MH-150B, M-Tec Co., Gimhae, Korea) had a frequency of 1.6 MHz.
Treatment of inoculated carrots with aerosol ClO2
All experiments were conducted using the plexiglass glove-box chamber (100 × 60 × 80 cm3). The flow of aerosol ClO2 was controlled by a regulation valve to maintain the desired ClO2 concentration in the treatment chamber. The aerosol ClO2 content in the treatment chamber was constantly mixed using a fan (UF12A series, Fulltech Electric Co. Ltd., Taiwan) to maintain constant relative humidity and ClO2 concentration during treatment. While samples were being treated in the test chamber, ClO2 aerosol density was measured at 5 min intervals using a continuous monitoring system (Interscan™, Intererscan Corp., Chatsworth, CA, USA).
Measurement of the concentration and pH of aqueous ClO2
After aerosol delivery by an ultrasonic nebulizer at 100, 200, 300, and 400 ppm of ClO2 working solutions, 10-mL aerosol liquid was collected into a 50-mL tube to measure the pH (420A + , Thermo Scientific Orion) and concentration of ClO2. The concentration of aqueous ClO2 was measured using the iodometric and DPD ferrous titration methods [20]. Antimicrobial effects were measured at 5, 10, and 30 min after an hour of aerosol stream flow of ClO2 into the plexiglass glove-box chamber using an ultrasonic nebulizer at a velocity of 80 ± 10 mL/h. Inoculated carrots were placed in sterile plastic containers inside the treatment chamber. Carrot samples were removed from the treatment chamber at 5, 10, and 30 min for microbial enumeration to determine the surviving cell populations.
Bacterial enumeration and D-value determination
Inoculated and treated carrots were transferred to sterile 400-mL stomacher bags (Fisher Scientific, Pittsburgh, NJ) and stomached for 2 min with a neutralizing buffer (1:5 w/v, Difco Laboratories, Sparks, MD) in a bag mixer (Interscience Lab, Inc., Boston, USA). Serial 10-fold dilutions were prepared in 0.1% peptone water (Difco Laboratories, Sparks, MD), and then the samples were spread on TSA-STR and incubated for 24 h at 37 °C. Colonies were counted, and results were expressed as log CFU/g of carrots. A first-order kinetic model (linear model) was used to analyze each of the data value for obtaining the log of the surviving microorganisms per treatment time. The efficacy of four different concentrations, 100, 200, 300, and 400 ppm, of ClO2 treated for 0, 5, 10, and 30 min was determined.
Aqueous ClO2 residues and changes in chromaticity
Each ClO2-treated carrot sample was blended with 16-mL distilled water in a 50-mL cap test tube for 2 min and filtered through a paper filter (No. 1, Advantec, Toyo Roshi Kaisha, Japan). The ClO2 residue in the solution was measured using a pocket colorimeter (Pocket Colorimeter II Analysis System, Hach Co., USA). The chromaticity of the ClO2-treated carrots was measured three times at different spots on the surface of the samples before and after each treatment using CR-300 (Konica Minolta Holdings Inc., Tokyo, Japan) and expressed as lightness (L), red (a), and yellow (b) values. The Hunter scale L, a, and b values based on standards of whiteness were 97.06, +0.04, and +1.84, respectively. The values of the changed colors after treatment were expressed as chromaticity difference (ΔE = ΔL2 + Δa2 + Δb2). The whiteness index (WI) was indicated as WI = 100 − [(100 − L)2 + a2 + b2]0.5 by adjusting the L, a, and b values according to the Bolin and Huxsoll method [21].
Statistical analysis
The results were statistically analyzed using SPSS 19 (IBM, New York, USA). Significance levels of the difference between the experimental bacteria was evaluated by Duncan‘s multiple range test following the analysis of variance (p ≤ 0.05).
Results and discussion
Preparation and purity of aqueous ClO2
The concentration of aqueous ClO2, comprising 8% sodium chlorite and 8% phosphoric acid, was 700 ± 20 ppm. The purity ranged between 96 and 98%, which met the FDA requirement of over 90% purity.
Concentration and pH of aerosolized ClO2
Aqueous ClO2 was introduced into the test chamber at 100, 200, 300, and 400 ppm using an ultrasonic nebulizer. Next, 10-mL aerosol liquid was collected into a 50-mL tube to measure the pH and concentration of ClO2 using the iodometric and DPD methods (Table 1). The concentration of aerosolized ClO2 was 23, 34, 45, and 61 ppm, respectively, at 100, 200, 300 and 400 ppm of ClO2 working solution concentration, respectively, showing significant differences from baseline measurements. The significant low concentration in aerosolized ClO2 likely resulted from the evaporation of ClO2 during the process of particles formation in the nebulizer. The pH level was negatively correlated with the concentration of aqueous ClO2. The pH level decreased after aerosolization, but the difference was not significant. The changes in the concentration of ClO2 with time were measured after ClO2 was introduced into the test chamber at a rate of 150 mL/min.
Table 1.
Changes in the concentration and pH of chlorine dioxide before and after aerosolization
| Before aerosolization | After aerosolization | ||
|---|---|---|---|
| Chlorine dioxide concentration (ppm) | pH | Chlorine dioxide concentration (ppm) | pH |
| 100 | 4.39 ± 0.04Aa | 23.73 ± 3.90a | 4.36 ± 0.01Aa |
| 200 | 4.07 ± 0.05Ab | 33.73 ± 6.75b | 3.87 ± 0.03Ab |
| 300 | 3.95 ± 0.03Abc | 44.97 ± 4.12c | 3.71 ± 0.02Abc |
| 400 | 3.71 ± 0.03Ac | 60.71 ± 7.13d | 3.69 ± 0.02Ac |
Mean values with different lowercase letters in the same column for pH are significantly different (p < 0.05)
Mean values with different uppercase letters in the same row are significantly different (p < 0.05)
Effects of aerosolized ClO2 on pathogenic microbes
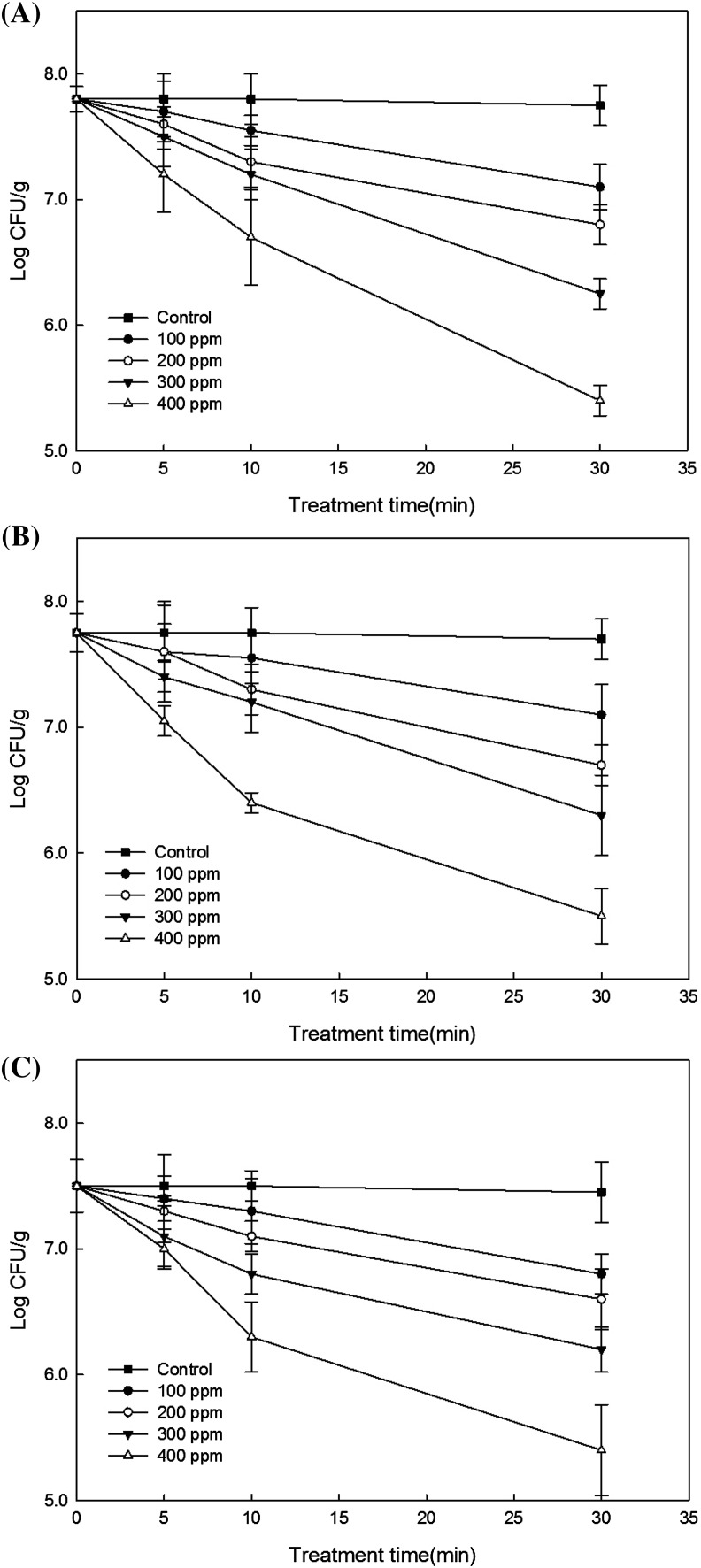
To facilitate the differentiation of natural flora on carrots from inoculated bacteria, the StrR mutant of pathogen was used to spike carrot samples. Fresh carrots artificially contaminated with E. coli O157:H7, S. Typhimurium, and L. monocytogenes were exposed to aerosolized ClO2 in a plexiglass glove-box chamber in which aerosolized ClO2 was introduced at 100, 200, 300, and 400 ppm, and the air was circulated by a fan. The changes in bacterial numbers in carrots inoculated with E. coli O157:H7 were measured at different times after exposure to aerosolized ClO2 (Fig. 1A). The baseline concentration was 7.8 log units before aerosolization. After exposure to aerosolized ClO2 at 100 ppm, the total bacterial count was 7.7, 7.6, and 7.1 log, respectively, after 5, 10, and 30 min, exhibiting a negative correlation with treatment time. The negative correlation became more significant as the concentrations of aerosolized ClO2 and time increased, showing 7.6, 7.3, and 6.8 log at the same intervals at 200 ppm, 7.5, 7.2, and 6.3 log at 300 ppm, and 7.2, 6.7, and 5.4 log at 400 ppm, respectively. The total bacterial count was reduced by 0.7, 1.0, 1.5, and 2.4 log units, respectively, at each concentration level of aerosolized ClO2 after 30 min of treatment. When samples were treated with 400 ppm of aerosolized ClO2 for 5 and 10 min, the total bacterial count was reduced by 0.6 and 1.1 log, respectively, showing antimicrobial effects similar to those obtained after exposure to 100 and 200 ppm aerosolized ClO2 for 30 min. The D-value was 43, 31, 20, and 13 min, respectively, at 100, 200, 300, and 400 ppm (Table 2). The results of fresh carrots inoculated with S. Typhimurium are presented in Fig. 1(B). The baseline concentration was 7.8 log, which is similar to that of E. coli O157:H7. The total bacterial count after treating it with aerosolized ClO2 for 5, 10, and 30 min each was 7.6, 7.6, 7.1 log, 7.6, 7.3, 6.7 log, 7.4, 7.2, 6.3 log, 7.1, 6.4, 5.5 log, respectively, at each concentration. The total bacterial count was reduced by 0.7 log at 400 ppm for 5 min, showing a microbial effect similar to that obtained at 100 ppm for 30 min. These results were identical with those of E. coli O157:H7. After treatment for 10 min, the bacterial population was reduced by 1.4 log. A similar reduction was also observed at 300 ppm after 30 min. However, the reduction rates were different from those of E. coli O157:H7 at each concentration and time. After treatment with 100, 200, 300, and ppm of aerosolized ClO2 for 30 min, the total bacterial count was reduced by 0.7, 1.1, 1.5, and 2.3 log, respectively. This result is similar to that of E. coli O157:H7. The D-value was 47, 28, 21, and 13 min, respectively. The results of fresh carrots inoculated with L. monocytogenes are presented in Fig. 1(C). The baseline concentration was 7.5 log, which was lower than that of E.coli O157:H7 and S. Typhimurium. The total bacterial count after treating it with aerosolized ClO2 for 5, 10, and 30 min each was 7.4, 7.3, 6.8 log, 7.3, 7.1, 6.6 log, 7.1, 6.8, 6.2 log, 7.0, 6.3, 5.4 log, respectively, at each concentration. After treatment at 100 ppm for 30 min, the bacterial population was reduced by 0.7 log, which is similar to that of E. coli O157:H7 and S. Typhimurium. After treatment at 400 ppm for 5 min, the bacterial population was reduced by 0.5 log, showing a lower reduction compared to that of E. coli O157:H7 and S. Typhimurium. After treatment for 10 min, the bacterial population was reduced by 1.2 log, showing an antimicrobial effect similar to that of S. Typhimurium but different from that of E. coli O157:H7. After treatment at 100, 200, 300, and 400 pm for 30 min, the total bacterial count was reduced by 0.7, 0.9, 1.3, and 2.3 log, respectively, exhibiting somewhat lower reductions at 200, 300, and 400 ppm than E. coli O157:H7 and S. Typhimurium. The D-value was 42, 34, 25, and 15 min, respectively. After treatment at 400 ppm for 30 min, E. coli O157:H7, S. Typhimurium, and L. monocytogenes were reduced by 2.4, 2.3, and 2.1 log, respectively. This result is similar to the reduction levels of 2.47, 2.55, and 2.17 log found in lettuce inoculated with the same bacterial population and treated with 2% malic acid for 30 min [14]. However, Oh et al. [15] reported the bacterial reductions of 2.2, 3.3, and 2.7 log after treatment with peroxyacetic acid for 30 min. Thus, the microbial control for S. Typhimurium varies among studies. Kim et al. [22] claimed higher antimicrobial activity of smaller particle sizes after treating iceberg lettuce inoculated with the same bacteria used in this study. They aerosolized 200 ppm of sodium hypochlorite solution using vibrators with frequencies of 1.6 and 2.4 MHz. They have also reported reduction of E. coli O157:H7, S. Typhimurium, and L. monocytogenes of 0.2, 0.3, and 0.3 log at vibration frequency of 1.6 MHz and 1.6, 0.6, and 0.5 log at 2.4 MHz for 30 min treatment. However, Lee et al. [23] asserted a higher antimicrobial activity at 1.6 MHz against E. coli O157:H7 after aerosolizing five different disinfectants at vibration frequencies of 1.6 and 2.4 MHz. They suggested a higher microbial activity at 2.4 MHz against L. monocytogenes, concluding that no correlation was established between the sizes of aerosol particles and antimicrobial effects. Their study also revealed that hydrogen-peroxide-based disinfectants proved better performance in controlling E. coli O157:H7, S. Typhimurium, and L. monocytogenes among disinfectants for commercial use. Chlorine-based disinfectants demonstrated a reduction of L. monocytogenes by 1.56 log and a reduction of E. coli O157:H7 and S. Typhimurium by 0.33 log or lower. ClO2 is considered as an alternative to chlorine and an effective sanitizer for fresh produce in gaseous and aqueous formations [14]. This study also confirmed high microbial activity of aerosolized ClO2. Izumi [24] reported that the total microbial count of carrot slices treated with electrolyzed water (pH 6.8) containing 50 ppm chlorine for 3 min was reduced by 1.1 log10CFU/g on the surface. Choi et al. [25] reported a reduction of over 3 log in a stainless steel coupon inoculated with E. coli O157:H7, S. Typhimurium, and L. monocytogenes and treated with disinfectants containing 0.25 and 0.5% hydrogen peroxide-based disinfectants. Park et al. [26] reported bacterial reduction in range of 0.5–3.6 and 2.8–5.8 log, respectively, in their study in which stainless steel and PVC coupon with biofilm cells of E. coli O157:H7, S. Typhimurium, and L. monocytogenes were treated with aerosolized sodium hypochlorite and peracetic acid at 100 ppm for 50 min. Ha et al. [27] reported that bacterial population was reduced in the range of 0.3–3.8 log when stainless steel coupons inoculated with E. coli O157:H7, S. Typhimurium, and L. monocytogenes were treated with aerosolized grapefruit extract and acetic acid, citric acid, and lactic acid for 5 min. They confirmed the effectiveness of the combined use of natural and chemical sanitizers in aerosol formation in the food industry. ClO2 gas treatment needs humidity for its antimicrobial activity and therefore requires a humidifier. However, the advantage of aerosol ClO2 is that it does not need humidifier. Relative humidity in the chamber can be generated and controlled using an ultrasonic humidifier.
Fig. 1.
Viable cell numbers of E. coli O157:H7 (A), S. Typhimurium (B), and L. monocytogenes (C) on inoculated carrot surface after aerosolized chlorine dioxide treatment by an ultrasonic nebulizer for various times and concentrations
Table 2.
D-values of E. coli O157:H7, S. Typhimurium, and L. monocytogenes on freshly cut carrot by aerosolized chlorine dioxide treatment
| Chlorine dioxide concentration (ppm) | D-value (min) | ||
|---|---|---|---|
| E. coli O157:H7 | S. Typhimurium | L. monocytogenes | |
| 100 | 42.6 | 47.2 | 42.4 |
| 200 | 30.8 | 28.5 | 34.2 |
| 300 | 19.6 | 21.3 | 24.7 |
| 400 | 13.0 | 14.4 | 14.9 |
Residual concentration and changes in chromaticity after exposure to aerosolized ClO2
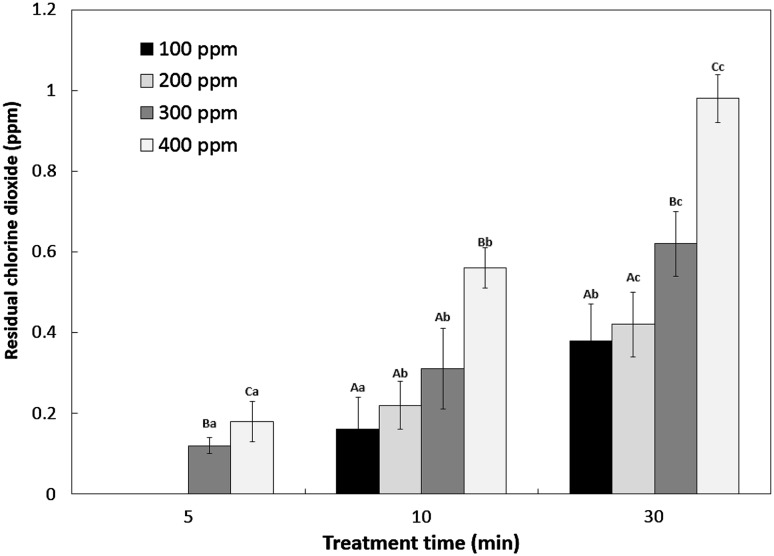
ClO2 residues in fresh carrots after exposure are presented in Fig. 2. Aerosolized ClO2 residues were not detected when samples were treated with 100 and 200 ppm aerosolized ClO2 for 5 min. However, residues of 0.16 and 0.38 ppm, respectively, were detected at 100 ppm of aerosolized ClO2 for 10 and 30 min and 0.22 and 0.42 ppm at 200 ppm aerosolized ClO2 for the same treatment times. Residues of 0.12, 0.31, and 0.62 ppm were detected at 300 ppm and 0.18, 0.56, and 0.98 ppm were detected at 400 ppm. That is, ClO2 residues were correlated with concentration and time. However, residue concentrations were below 1 ppm in all experimental bacteria. Gómez-López et al. [28] described that no residue was detected after 6 min when carrots were treated with gaseous ClO2 of 1.33 mg/L for 30 s. Lee [29] suggested that ClO2 residues are affected by temperature and pH and decrease with time. Thus, ClO2 is known to be decomposed by heat and light. Changes in color are presented in Table 3. Color changes in fresh produce are an important factor affecting the quality of produce. Bleaching is defined as the color change induced when lignin and pigment such as β-carotene are decomposed [28, 30]. Bleaching is caused by the contamination of microbes during storage and distribution or by textural damage or dryness during the process. Moreover, a study claimed that bleaching is accelerated when the concentration of ClO2 increases [31]. Minimally processed carrots lose their bright orange color quickly during storage, developing a white blush on its surface [21]. Cisneros–Zevallos et al. [32] reported a biopolymer-based edible coating that can control microbial growth and white blush on the surface of minimally processed carrots. However, it was found that WI decreased when carrots were treated with aerosolized ClO2. Low bleaching is explained by the aerosol technology in which particles stick to the surface without affecting the surface. Moreover, lightness is improved by aerosol particles just after treatment.
Fig. 2.
Change of residual chlorine dioxide contents in washed carrot treated with aerosolized chlorine dioxide
Table 3.
Changes in the color of carrot treated with aerosolized chlorine dioxide for various times and concentrations
| Concentration and time (min) | Before treatment | After treatment | ΔE | Whiteness index | ||||
|---|---|---|---|---|---|---|---|---|
| L | a | b | L | a | b | |||
| Control | ||||||||
| 5 | 53.1 ± 0.18Ad | 15.3 ± 0.23Aa | 27.8 ± 0.19Aa | 54.0 ± 0.17Bf | 15.2 ± 0.24Aa | 28.2 ± 0.15Aab | 1.07 ± 0.35bcde | 43.35 ± 0.02e |
| 10 | 52.9 ± .011Ad | 15.3 ± 0.13Aa | 27.7 ± 0.14Aa | 53.9 ± 0.12dBef | 15.1 ± 0.21Aa | 27.8 ± 0.22Aa | 1.05 ± 0.28bcde | 43.16 ± 0.19e |
| 30 | 52.8 ± 0.14Acd | 15.3 ± 0.23Aa | 27.7 ± 0.13Aa | 53.6 ± 0.16Ad | 15.6 ± 0.16Bb | 28.3 ± 0.23Bb | 1.07 ± 0.22cde | 43.09 ± 0.12e |
| 100 | ||||||||
| 5 | 53.7 ± 0.24Ae | 16.7 ± 0.10Ab | 29.7 ± 0.16Ad | 53.7 ± 0.11Adef | 16.2 ± 0.14Bc | 29.3 ± 0.13Ad | 0.64 ± 0.06abc | 42.52 ± 0.20d |
| 10 | 53.9 ± 0.13Ae | 16.8 ± 0.21Ab | 29.8 ± 0.17Ad | 53.7 ± 0.17Adef | 16.3 ± 0.19Ac | 29.4 ± 0.20Ade | 0.62 ± 0.35abc | 42.62 ± 0.12d |
| 30 | 53.8 ± 0.15Ae | 16.8 ± 0.15Ab | 29.9 ± 0.12Ad | 53.7 ± 0.16Adef | 16.3 ± 0.26Ac | 29.3 ± 0.14Bd | 0.78 ± 0.30abcd | 42.49 ± 0.19d |
| 200 | ||||||||
| 5 | 53.8 ± 0.13Ae | 16.8 ± 0.19Ab | 29.7 ± 0.16Ad | 53.9 ± 0.19Adef | 16.4 ± 0.20Acd | 29.4 ± 0.22Adef | 0.51 ± 0.29abc | 42.57 ± 0.13d |
| 10 | 53.9 ± 0.19Ae | 16.8 ± 0.21Ab | 29.9 ± 0.10Ad | 54.0 ± 0.21Aef | 16.9 ± 0.11Ade | 29.5 ± 0.26Adef | 0.51 ± 0.18abc | 42.48 ± 0.21d |
| 30 | 53.9 ± 0.24Ae | 16.9 ± 0.11Ab | 29.9 ± 0.23Ad | 54.0 ± 0.18Aef | 16.8 ± 0.13Ade | 29.8 ± 0.14Af | 0.21 ± 0.23a | 42.45 ± 0.17d |
| 300 | ||||||||
| 5 | 52.8 ± 0.17Ad | 17.3 ± 0.21Ac | 29.4 ± 0.10Ac | 53.1 ± 0.29Ac | 16.9 ± 0.12Ae | 29.8 ± 0.17Bef | 0.66 ± 0.08abcd | 41.81 ± 0.12c |
| 10 | 52.4 ± 0.21Abc | 16.9 ± 0.19Ab | 29.1 ± 0.16Abc | 51.6 ± 0.11Bde | 16.4 ± 0.18Bc | 29.3 ± 0.23Ad | 1.31 ± 0.19e | 41.73 ± 0.17bc |
| 30 | 52.1 ± 0.19Aab | 16.8 ± 0.15Ab | 29.9 ± 0.13Ad | 53.1 ± 0.15Bc | 16.5 ± 0.19Acd | 29.3 ± 0.16Bd | 1.26 ± 0.21de | 41.06 ± 0.05a |
| 400 | ||||||||
| 5 | 51.8 ± 0.21Aa | 16.7 ± 0.20Ab | 28.9 ± 0.11Ab | 51.9 ± 0.13Ab | 16.7 ± 0.25Acde | 28.2 ± 0.17Bb | 0.64 ± 0.27abcd | 41.39 ± 0.19ab |
| 10 | 51.8 ± 0.11Aa | 16.7 ± 0.14Ab | 28.8 ± 0.23Ab | 49.9 ± 0.18Ba | 16.6 ± 0.15Acde | 28.4 ± 0.10Ab | 1.97 ± 0.28f | 41.44 ± 0.20b |
| 30 | 51.9 ± 0.13Aa | 16.7 ± 0.21Ab | 28.8 ± 0.19Ab | 52.1 ± 0.15Ab | 16.7 ± 0.25Acde | 28.9 ± 0.15Ac | 0.23 ± 0.13ab | 41.53 ± 0.20bc |
Mean values with different lowercase letters in the same column are significantly different (p < 0.05)
Mean values with different uppercase letters in the same row are significantly different (p < 0.05)
This study provides information for aerosolized ClO2 applications with traditional sanitizing procedures. Application of aerosolized ClO2 can be incorporated into the existing process during post-harvest treatment. In conclusion, the aerosolized ClO2 treatment on carrots can be useful in controlling foodborne pathogens. In addition, different components in various types of fresh produce may influence the interaction with aerosolized ClO2, and other environmental factors affecting antimicrobial effectiveness need to be studied.
Acknowledgements
This research was supported in part by the Research Funds of Mokpo National University in 2014.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Sivapalasingam S, Friedman CR, Cohen L, Tauxe RV. Fresh produce: A growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J. Food Prot. 2004;67:2342–2353. doi: 10.4315/0362-028X-67.10.2342. [DOI] [PubMed] [Google Scholar]
- 2.Gleeson E, O’Beirne D. Effects of process severity on survival and growth of Escherichia coli and Listeria innocua on minimally processed vegetables. Food Control. 2005;16:677–685. doi: 10.1016/j.foodcont.2004.06.004. [DOI] [Google Scholar]
- 3.Hwang JH, Yoon JH, Bae YM, Choi MR, Lee SY, Park KH. Effect of the precutting process on sanitizing treatments for reducing pathogens in vegetables. Food Sci. Biotechnol. 2017;26:531–536. doi: 10.1007/s10068-017-0073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patras A, Tiwari BK, Brunton NP, Butler F. Modelling the effect of different sterilization treatment on antioxidant activity and color of carrot slices during storage. Food Chem. 2009;114:484–491. doi: 10.1016/j.foodchem.2008.09.104. [DOI] [Google Scholar]
- 5.Byun MW, Lee NY, Jo C, Lee EY, Kim HJ, Shin DH. Effect of irradiation on the microbial content of ready-to-use cooked carrot. Food Sci. Biotechnol. 2007;16:138–141. [Google Scholar]
- 6.Selma MV, Allende A, López-Gálvez F, Conesa MA, Gil MI. Disinfection potential of ozone, ultraviolet-C and their combination in wash water for the fresh-cut vegetable industry. Food Microbiol. 2008;25:809–814. doi: 10.1016/j.fm.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Koide S, Shitanda D, Note M, Cao W. Effects of mildly heated, slightly acidic electrolyzed water on the disinfection and physicochemical properties of sliced carrot. Food Control. 2011;22:452–456. doi: 10.1016/j.foodcont.2010.09.025. [DOI] [Google Scholar]
- 8.Ruiz-Cruz S, Acedo-Felix E, Diaz-Cinco M, Islas-Osuna MA, Gonzalez-Aguilar GA. Efficacy of sanitizers in reducing Escherichia coli O157:H7, Salmonella spp. and Listeria monocytogenes populations on fresh-cut carrots. Food Control. 2007;18:1383–1390. doi: 10.1016/j.foodcont.2006.09.008. [DOI] [Google Scholar]
- 9.Beuchat LR. Survival of Escherichia coli O157:H7 in bovine faces applied to lettuce and ineffectiveness of chlorinated water as a disinfectant. J. Food Prot. 1999;62:845–849. doi: 10.4315/0362-028X-62.8.845. [DOI] [PubMed] [Google Scholar]
- 10.Wei CI, Cook DL, Kirk JR. Use of chlorine compounds in the food industry. Food Technol. 1985;39:107–115. [Google Scholar]
- 11.Kim JM, Huang TS, Marshall MR, Wei CI. Chlorine dioxide treatment of seafoods to reduce bacterial loads. J. Food Sci. 1999;64:1089–1093. doi: 10.1111/j.1365-2621.1999.tb12288.x. [DOI] [Google Scholar]
- 12.Singh N, Singh RK, Bhunia AK. Sequential disinfection of Escherichia coli O157:H7 inoculated alfalfa seeds before and during sprouting using aqueous chlorine dioxide, ozonated water, and thyme essential oil. LWT-Food Sci. Tech. 2003;36:235–243. doi: 10.1016/S0023-6438(02)00224-4. [DOI] [Google Scholar]
- 13.Mahmoud BSM, Vaidya NA, Corvalan CM, Linton RH. Inactivation kinetics of inoculated Escherichia coli O157:H7, Listeria monocytogenes and Salmonella Poona on whole cantaloupe by chlorine dioxide gas. Food Microbiol. 2008;25:857–865. doi: 10.1016/j.fm.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Choi MR, Lee SY, Park KH, Chung MS, Ryu SR, Kang DH. Effect of aerosolized malic acid against Listeria monocytogenes, Salmonella Typhimurium, and Escherichia coli O157:H7 on spinach and lettuce. Food Control. 2012;24:171–176. doi: 10.1016/j.foodcont.2011.09.022. [DOI] [Google Scholar]
- 15.Oh SW, Dancer GI, Kang DH. Efficacy of aerosolized peroxyacetic acid as a sanitizer of lettuce leaves. J. Food Prot. 2005;68:1745–1747. doi: 10.4315/0362-028X-68.8.1743. [DOI] [PubMed] [Google Scholar]
- 16.Palmer LB, Smaldone GC, Simon SR, O’Riordan TG, Cuccia A. Aerosolized antibiotics in mechanically ventilated patients: delivery and response. Crit. Care Med. 1998;26:31–39. doi: 10.1097/00003246-199801000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Fišer A. Disinfection of air and dust in fattening houses for chickens by lactic acid aerosol. Acta Vet. Brno. 1978;47:173–183. doi: 10.2754/avb197847030173. [DOI] [Google Scholar]
- 18.Hiom SJ, Lowe C, Oldcorne M. Assessment of surface bioburden during hospital aseptic processing. Int. J. Pharm. Pract. 2003;11:R62. [Google Scholar]
- 19.Han Y, Selby TL, Schultze KK, Nelson PE, Linton RH. Decontamination of strawberries using batch and continuous chlorine dioxide gas treatments. J. Food Prot. 2004;67:2450–2455. doi: 10.4315/0362-028X-67.11.2450. [DOI] [PubMed] [Google Scholar]
- 20.American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 17th ed. American Public Health Association, Washington, DC. (1989)
- 21.Bolin HR, Huxsoll CC. Control of minimally processed carrot (Daucuscarota) surface discoloration caused by abrasion peeling. J. Food Sci. 1991;56:416–418. doi: 10.1111/j.1365-2621.1991.tb07975.x. [DOI] [Google Scholar]
- 22.Kim YH, Jo YJ, Kim YJ, Koo MS, Lee JK, Oh SW. Effects of aerosolized sanitizers of different droplet sizes on foodborne pathogen reduction. Food Sci. Biotech. 2008;17:664–668. [Google Scholar]
- 23.Lee SY, Jung JH, Jin HH, Kim YH, Oh SW. Inhibitory effect of aerosolized commercial sanitizers against foodborne pathogens. J. Food Hyg. Safety. 2007;22:235–242. [Google Scholar]
- 24.Izumi H. Electrolyzed water as a disinfectant for fresh-cut vegetables. J. Food Sci. 1999;64:536–539. doi: 10.1111/j.1365-2621.1999.tb15079.x. [DOI] [Google Scholar]
- 25.Choi NY, Baek SY, Yoon JH, Choi MR, Kang DH. Efficacy of aerosolized hydrogen peroxide-based sanitizer on the reduction of pathogenic bacteria on a stainless steel surface. Food Control. 2012;27:57–63. doi: 10.1016/j.foodcont.2012.02.027. [DOI] [Google Scholar]
- 26.Park SH, Cheon HL, Park KH, Chung MS, Choi SH, Ryu SR, Kang DH. Inactivation of biofilm cells of foodborne pathogen by aerosolized sanitizers. Int. J. Food Microbiol. 2012;154:130–134. doi: 10.1016/j.ijfoodmicro.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 27.Ha SJ, Yang SK, Park HJ, Kim CH, Oh SW. Efficacy of aerosolized natural antimicrobial and organic acids as a sanitizer foodborne pathogens on stainless steel. J. Food Hyg. Safety. 2011;26:336–341. [Google Scholar]
- 28.Gómez-López VM, Devlieghere F, Ragaert P, Debevere J. Shelf-life extension of minimally processed carrots by gaseous chlorine dioxide. Int. J. Food Microbiol. 2007;116:221–227. doi: 10.1016/j.ijfoodmicro.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Lee YJ. Impact of water quality parameters on the disinfection of total coliform with chlorine dioxide. Korean J. Environ. Health. 2006;32:211–215. [Google Scholar]
- 30.Kim JG, Luo Y, Lim CI. Effect of ozonated water and chlorine water wash on the quality and microbial de-contamination of fresh-cut carrots shreds. Korean J. Food Preserv. 2007;14:54–60. [Google Scholar]
- 31.Sy KV, Murray MB, Harrison MD, Beuchat LR. Evaluation of gaseous chlorine dioxide as a sanitizer for killing Salmonella, Escherichia coli O157:H7, Listeria monocytogenes, and yeasts and molds on fresh and fresh-cut produce. J. Food Prot. 2005;68:1176–1187. doi: 10.4315/0362-028X-68.6.1176. [DOI] [PubMed] [Google Scholar]
- 32.Cisneros-Zevallos L, Saltveit ME, Krochta JM. Hygroscopic coatings control surface white discoloration of peeled (minimally processed) carrots during storage. J. Food Sci. 1997;62:363–366. doi: 10.1111/j.1365-2621.1997.tb04002.x. [DOI] [Google Scholar]