Abstract
Catechin content, the ratio of tea polyphenols and free amino acids (TP/FAA), as well as the ratio of theaflavins and thearubigins (TFs/TRs) are important biochemical indicators to evaluate fermentation quality. To achieve rapid determination of such biochemical indicators, synergy interval partial least square and extreme learning machine combined with an adaptive boosting algorithm, Si-ELM-AdaBoost algorithm, were used to establish quantitative analysis models between near infrared spectroscopy (NIRS) and catechin content and between TFs/TRs and TP/FAA, respectively. The results showed that prediction performance of the Si-ELM-AdaBoost mixed algorithm is superior than that of other models. The prediction results with root-mean-square error of prediction ranged from 0.006 to 0.563, the ratio performance deviation values exceeded 2.5, and predictive correlation coefficient values exceeded 0.9 in the prediction model of each biochemical indicator. NIRS combined with Si-ELM-AdaBoost mixed algorithm could be utilized for online monitoring of black tea fermentation. Meanwhile, the AdaBoost algorithm effectively improved the accuracy of the ELM model and could better approach the nonlinear continuous function.
Keywords: Black tea, Fermentation, Near infrared spectroscopy, Nonlinear tools
Introduction
Black tea is the most widely consumed tea in the world, which has health care functions such as removing free radicals and anti-oxidation, and fermentation is the key process for forming the quality of black tea and its flavor characteristics [1, 2]. Fermentation is a chemical process during which polyphenols [3], mainly catechin, produce water-soluble colored oxidation products such as theaflavins (TFs), thearubigins (TRs), theabrownines (TBs), and fragrant substances after enzymatic oxidation reactions and eventually produce a black unique color, aroma, taste, and other chemical qualities in a time period of approximately 3–4 h.
Numerous studies [4–6] have showed that the quality of black tea ferment is mainly determined by the following biochemical indicators: catechin content (total amount of EC (epicatechin), GC (gallocatechin), EGC (epigallocatechin), ECG (epicatechin gallate), and EGCG (epigallocatechin gallate)), tea polyphenols (TP), the ratio of free amino acids (FAA), and the ratio of TFs to TRs. Polyphenols are “bitter,” and phenolic acid is “sweet.” A suitable ratio of phenol to ammonia forms the “fresh” characteristic taste of tea. Catechins are the basis of TFs and TRs formation; its content is positively correlated with the sensory quality of tea. TFs are the chemicals responsible for the brightness of black tea, which affects the concentration, intensity, and freshness of the tea. TRs are the chemicals that lead to the redness of black tea and mainly affect the concentration of flavor. TF and TR content changes with changing process time; that is, the optimum fermentation interval when the TFs/TRs approach 1:10–1:9 [7]. However, excessive fermentation causes continued decrease of TFs, imbalance of TFs and TRs, decrease of brightness, and eventually decline in the sensory score of black tea. Therefore, the composition ratio of TFs and TRs is an important evaluating indicator of fermentation quality.
Additionally, the change in surface color of the fermenting leaf (from green to red and then to brown) is a representation of catechins transforming into tea pigments (TFs and TRs). During the fermentation process of black tea, it is precisely this representation that enables the manufacturer to determine whether the fermentation quality is moderate, which is professional, subjective, and instable. To accurately grasp the actual extent of fermentation, we have to rely on instruments for chemical analysis to detect biochemical compositions and determine the extent and quality of fermentation according to main components and trends. Moreover, the operation of a spectrophotometer requires expert manpower and is more time-consuming. Therefore, it is essential to seek a method that can be used for rapid detection of biochemical composition and proportion when it comes to real-time identification of quality of tea during fermentation.
NIRS is applicable in detecting the composition of finished green tea (polyphenols, catechins, caffeine, TFs, amino acids, and water content) as well as its provenance [8, 9]. However, few studies have been conducted on the use of NIRS in tea processing [6, 10], especially in the non-destructive detection of the biochemical components during black tea fermentation [11, 12].
Extreme learning machine (ELM), a kind of single layer feed forward neural network (SLFN) learning algorithm [13, 14], has better robustness in performance and a shorter operation cycle than back-propagation artificial neural network (BP-ANN). As a machine learning algorithm, AdaBoost and other weak learning algorithms are often used together to improve model performance [15]. The ELM-AdaBoost algorithm was established, which takes ELM as the weak predictor for AdaBoost.
This study was designed to establish a rapid quantitative detection method to detect the key biochemical indicators during black tea fermentation using the NIRS detection technique, as well as linear (PLS and Si-PLS) and nonlinear (Si-ELM and Si-ELM-AdaBoost) methods. Herein, we aim to optimize the performance parameters and improve the prediction capability and robustness of nonlinear models through the combination and comparison of different intelligence algorithms (ELM and AdaBoost). Then, we will be able to predict the contents and trends of biochemical substances in fermentation and lay the theoretical foundation for further development of special fermentation quality online monitoring equipment.
Materials and methods
Sample collection
For raw tea leaves, Fuding White varieties were used, the main tea variety used for producing black tea in China. The test was implemented in China’s largest organic tea processing base (Zhejiang Gengxiang tea factory) in April 2015. The tests were conducted 6 times at different fermentation temperatures (25, 27, 29, 31, 33, and 35 °C). Relative humidity is greater than 90%, the tenderness of a bud, withering, rolling and drying processes are consistent, the fermentation period of each was 360 min, and there was sampling every 30 min before the scanning of near infrared spectroscopy. Chemical analyses of the samples were conducted after the spectrum acquisition. The analysis of catechins, TFs, TRs, TP, and FAA was completed in the tea quality inspection laboratory of Ministry of Agriculture of China.
In total, 78 samples were collected. Among the 78 samples through the Kennard–Stone method (KS) [16], 52 samples were counted as the calibration set and the others were counted as the prediction set. The range of the calibration set is larger than the prediction set range. This can better assure the extensiveness of the prediction model.
Physicochemical analysis
Reference analysis of each biochemical component including the catechins, TFs/TRs, and TP/FAA, for each fermented sample of black tea was completed according to national standards of China (GB/T8313-2008, GB/T 30483-2013, and GB/T 23193-2008). Table 1 summarizes the data for the catechins, TFs/TRs, and TP/FAA.
Table 1.
Reference result for each biochemical component in the calibration and prediction set
| Parameters | Subsets | S.N.a | Range | Mean ± SDb |
|---|---|---|---|---|
| Catechin content (%) | Calibration set | 52 | 1.93–9.15 | 3.14 ± 1.772 |
| Prediction set | 26 | 1.93–8.90 | 3.52 ± 1.878 | |
| TFs/TRs | Calibration set | 52 | 0.095–0.148 | 2.01 ± 0.015 |
| Prediction set | 26 | 0.096–0.147 | 2.13 ± 0.014 | |
| TP/FAA | Calibration set | 52 | 2.846–6.478 | 0.69 ± 0.813 |
| Prediction set | 26 | 2.847–6.111 | 0.64 ± 0.802 |
a S.N. number of samples
b SD standard deviation
Spectral measurement
A portable near infrared analyzer (SupNIR-1520TM series; Focused Photonics Inc., Hangzhou, China) was used for diffuse reflectance spectroscopy scanning of the tea samples. The range of wavelength coverage was 1000–1799 nm, and 800 variables were obtained for measuring the once every 1 nm. The absorbance data were saved as log (1/R), where R represented reflectance. 50 g of each sample were put in the sample pool before near infrared spectral scanning, and after each sample was scanned thrice (32 scans each time), the mean value was recorded as the spectral value of the sample. To avoid the influence of the ambient conditions on the spectrophotometer, it is necessary to maintain the experimental temperature at 25 °C.
Chemometric methods
Multivariate analysis methods have extremely important functions for predicting biochemical components in black tea fermentation by means of the NIRS system. In this study, Si-PLS was applied to model biochemical indicators in fermentation. The entire spectrum (1000–1799 nm) was partitioned into multiple intervals (11, 12…20) combined with limited subintervals (3 or 4) [3–5, 15, 17–21]. Coefficient of calibration (Rc), coefficient of prediction (Rp), root-mean-square error of calibration (RMSEC), and root-mean-square error of prediction (RMSEP) as the performance parameters in the literature were used for reference for the evaluation indices of the model performances [22–24]. The Rc and Rp were used in all the processes. Additionally, the ratio performance deviation (RPD) of the prediction models was calculated as defined by SD/RMSEP to evaluate its practical utility. The best model was selected with respect to the highest Rc, RP, and RPD, the minimum RMSECV, RMSEP, and the number of principal components (PCs).
MATLAB® 2014b (Math Works, Natick, MA) was used to conduct all of the data processing and analyses. An Si-PLS algorithm using MATLAB functions and PLS Toolbox 6 (Eigenvector Research, Wenatchee, WA) were used as plug-ins for MATLAB.
Results and discussion
Spectral features and pretreatment
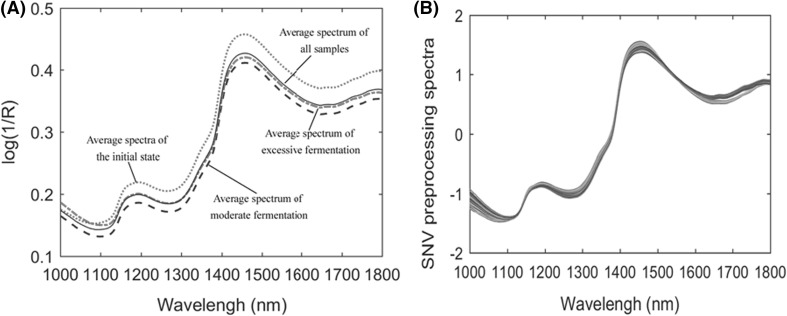
The raw NIRS profiles of black tea fermentation samples are shown in Fig. 1(A). In this research, the standard normal variate (SNV) transformation was selected as the preprocessing approach, which can effectively remove slope variation and correct light scattering in samples [23]. Figure 1(B) describes the spectra after SNV preprocessing.
Fig. 1.
Average NIRS raw absorbance spectra (A) and SNV spectra (B) of samples
Si-PLS model of catechin content
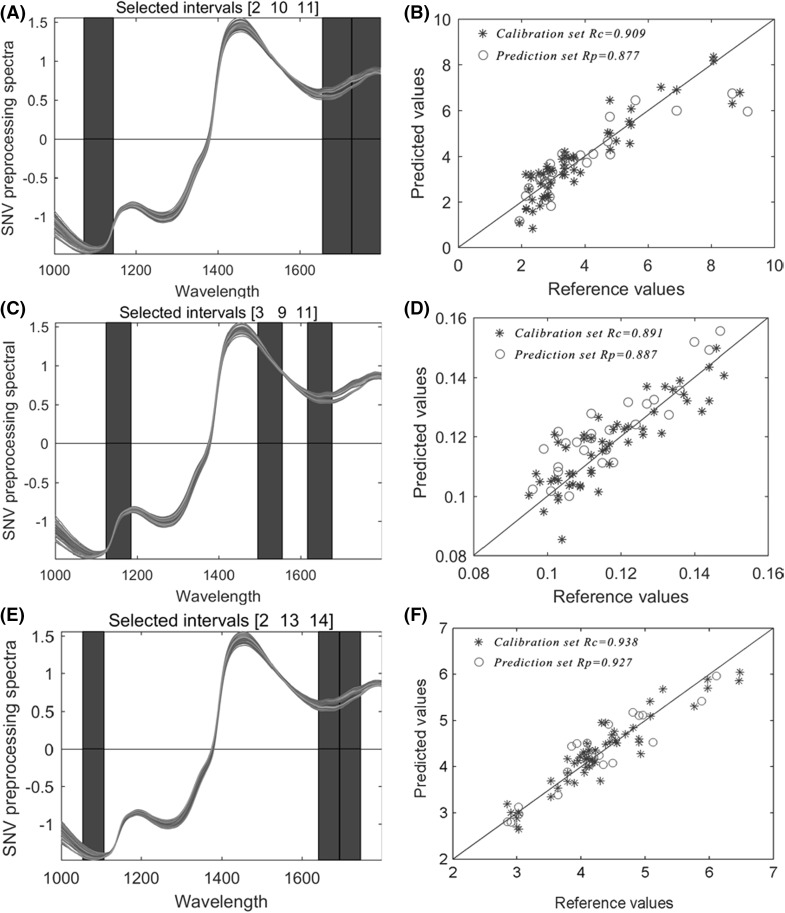
The results of the Si-PLS model for the catechin content are shown in Table 1. It is observed that the optimal model was gained with the combination of 3 of the 11 intervals; the most satisfactory model is highlighted with bold numbers in Table 2. Additionally, the optimal number of PCs was 8, and the most appropriate combinations of selected intervals were [2, 10, 11], which corresponded to 1073–1145, 1656–1727, and 1728–1799 nm, respectively [Fig. 2(A)]. There were 217 variables in the combination of subintervals that showed the optimal combination of intervals acquired by Si-PLS and was directly or indirectly related to catechin content. Figure 2(B) illustrated the relationship between the actual values and the NIRS predicted values.
Table 2.
Results of Si-PLS calibration model selected optimal spectral subintervals for prediction of each biochemical component in black tea fermentation
| Parameters | Number of intervals | Selected subintervals | PCs | RC | RMSECV | RP | RMSEP |
|---|---|---|---|---|---|---|---|
| Catechin content (%) | 11 | 2 10 11 | 8 | 0.909 | 0.742 | 0.878 | 0.916 |
| 12 | 2 6 12 | 4 | 0.894 | 0.790 | 0.857 | 0.953 | |
| 13 | 2 3 4 12 | 6 | 0.903 | 0.763 | 0.860 | 0.957 | |
| 14 | 2 3 4 7 | 6 | 0.905 | 0.761 | 0.871 | 0.911 | |
| 15 | 2 3 11 14 | 7 | 0.918 | 0.701 | 0.872 | 0.911 | |
| 16 | 3 13 16 | 6 | 0.925 | 0.673 | 0.850 | 0.973 | |
| 17 | 3 14 17 | 7 | 0.925 | 0.671 | 0.860 | 0.845 | |
| 18 | 1 2 3 | 6 | 0.955 | 0.521 | 0.877 | 1.050 | |
| 19 | 3 10 19 | 6 | 0.925 | 0.673 | 0.870 | 0.913 | |
| 20 | 4 10 18 | 7 | 0.920 | 0.692 | 0.870 | 1.070 | |
| TFs/TRs | 11 | 2 9 11 | 10 | 0.864 | 0.007 | 0.817 | 0.010 |
| 12 | 2 3 7 12 | 7 | 0.860 | 0.007 | 0.787 | 0.009 | |
| 13 | 3 9 11 | 7 | 0.891 | 0.007 | 0.887 | 0.009 | |
| 14 | 3 8 12 14 | 8 | 0.907 | 0.006 | 0.801 | 0.011 | |
| 15 | 3 9 13 15 | 10 | 0.915 | 0.006 | 0.812 | 0.010 | |
| 16 | 3 4 16 | 7 | 0.855 | 0.007 | 0.815 | 0.009 | |
| 17 | 3 14 17 | 7 | 0.871 | 0.007 | 0.840 | 0.010 | |
| 18 | 3 15 18 | 6 | 0.864 | 0.007 | 0.822 | 0.010 | |
| 19 | 3 4 7 19 | 7 | 0.915 | 0.006 | 0.813 | 0.010 | |
| 20 | 4 14 17 | 10 | 0.885 | 0.007 | 0.891 | 0.009 | |
| TP/FAA | 11 | 2 4 10 | 9 | 0.925 | 0.318 | 0.926 | 0.318 |
| 12 | 1 8 9 | 5 | 0.910 | 0.347 | 0.884 | 0.368 | |
| 13 | 1 2 6 12 | 8 | 0.931 | 0.306 | 0.908 | 0.337 | |
| 14 | 1 2 3 13 | 8 | 0.931 | 0.306 | 0.875 | 0.398 | |
| 15 | 2 13 14 | 8 | 0.938 | 0.287 | 0.927 | 0.300 | |
| 16 | 1 9 15 | 9 | 0.935 | 0.298 | 0.881 | 0.386 | |
| 17 | 2 9 15 16 | 9 | 0.929 | 0.311 | 0.924 | 0.306 | |
| 18 | 1 2 3 | 7 | 0.951 | 0.260 | 0.893 | 0.379 | |
| 19 | 1 2 3 | 6 | 0.952 | 0.258 | 0.898 | 0.360 | |
| 20 | 1 2 3 | 6 | 0.952 | 0.258 | 0.904 | 0.354 |
Fig. 2.
The optimal spectral intervals selected by the Si-PLS of catechin content (A), TFs/TRs (C) and TP/FAA (E); reference values versus NIR predicted values of catechin content (B), TFs/TRs (D) and TP/FAA (F) in the calibration set and prediction set of Si-PLS models
Si-PLS model of TFs/TRs
The optimal combination of 3 from 13 intervals for the Si-PLS model of TFs/TRs is marked with a blue bar, and the optimal number of PCs was 7. Meanwhile, [3, 9, 11] were selected as the best combination of intervals, which correspond to 1124–1185, 1495–1555, and 1617–1677 nm, respectively [Fig. 2(C)]. In this combination of subintervals, there are 184 variables altogether. A scatter diagram showed the estimated performance of TFs/TRs [Fig. 2(D)].
Si-PLS model of TP/FAA
As shown in Table 2 (bold text), a combination of 3 of the 15 intervals was achieved with the optimal Si-PLS model for TP/FAA. The optimal number of PCs was 8, and this combination of subintervals had 160 variables in all. The most appropriate combination of intervals was [2, 13, 14], corresponding to 1054–1107, 1641–1693, and 1694–1746 nm in the spectral ranges, as shown in Fig. 2(E). Figure 2(F) represents the relationship of the reference and the NIRS predicted values of TP/FAA. It turned out that these spectral regions highly correlated with TP/FAA during black tea fermentation.
Results of the ELM-AdaBoost model based on optimal spectral intervals (Si-ELM-AdaBoost)
In this work, ELM-AdaBoost modeling with optimal intervals chosen by Si-PLS, named as Si-ELM-AdaBoost model, was used for predicting the main biochemical component in black tea fermentation. The optimal variable PCs were used as the inputs of the ELM mode, the number of weak classifiers in the ELM-AdaBoost algorithm was set as T = 10. The prediction error threshold (Φ) and PCs largely affected the prediction precision of the Si-ELM-AdaBoost algorithm. Thus, we selected 20 values of Φ (0.01 ~ 1, with a step length of 0.05) and 10 values of PCs (1–10, with step length 1); PCs and Φ were optimized by the lowest RMSECV.
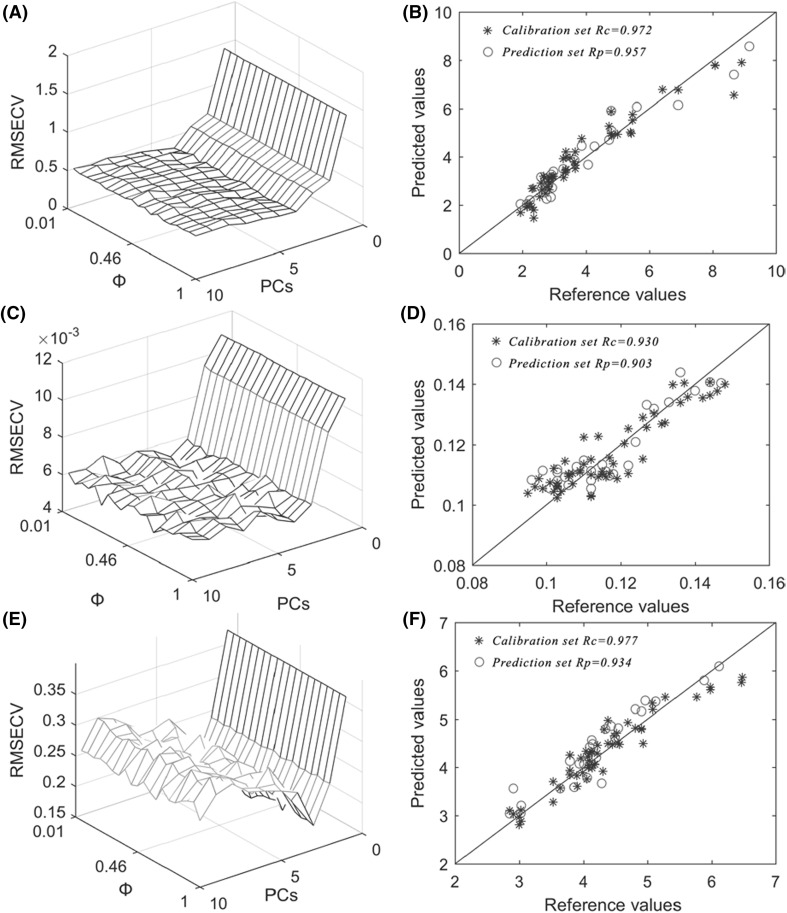
Figure 3(A) shows that the optimum Si-ELM-AdaBoost model of catechin content was achieved with PCs = 4 and Φ = 0.01. Figure 3(C) shows that the optimum Si-ELM-AdaBoost model of TFs/TRs was achieved with PCs = 4 and Φ = 0.06. Figure 3(E) shows that the optimum Si-ELM-AdaBoost model of TFs/TRs was achieved with PCs = 3 and Φ = 0.51. Figure 3(B, D, and F) describe reference values and NIRS predicted values of each biochemical component (catechin content, TFs/TRs, and TP/FAA) in the Si-ELM-AdaBoost models.
Fig. 3.
RMSECV values of the Si-ELM-AdaBoost model obtained from different PCs and Φ of catechin content (A), TFs/TRs (C), and TP/FAA (E). Reference values versus NIR predicted values of catechin content (B), TFs/TRs (D), and TP/FAA (F) in the calibration set and prediction set of the Si-ELM-AdaBoost models
For the sake of finding an accurate model in the detection of vital biochemical indicators in black tea fermentation based on NIRS, four regression approaches including PLS (linear model), Si-PLS (linear model), Si-ELM (nonlinear model), and Si-ELM-AdaBoost (nonlinear model) were studied systematically and comparatively. Table 3 summarizes the consequences from different algorithmic models for predicting each biochemical indicator (catechin content, TFs/TRs, and TP/FAA).
Table 3.
Results of different models for predicting each biochemical component
| Properties | Methods | PCs | Calibration set | Prediction set | |||
|---|---|---|---|---|---|---|---|
| Rc | RMSECV | Rp | RMSEP | RPD | |||
| Catechin content | PLS | 9 | 0.898 | 0.776 | 0.861 | 0.920 | 1.603 |
| Si-PLS | 8 | 0.909 | 0.742 | 0.878 | 0.916 | 1.612 | |
| Si-ELM | 5 | 0.921 | 0.587 | 0.904 | 0.736 | 2.005 | |
| Si-ELM-AdaBoost | 4 | 0.972 | 0.416 | 0.957 | 0.563 | 2.621 | |
| TFs/TRs | PLS | 8 | 0.831 | 0.008 | 0.851 | 0.009 | 1.574 |
| Si-PLS | 7 | 0.891 | 0.007 | 0.887 | 0.009 | 1.827 | |
| Si-ELM | 4 | 0.912 | 0.006 | 0.894 | 0.008 | 2.003 | |
| Si-ELM-AdaBoost | 4 | 0.930 | 0.005 | 0.903 | 0.006 | 2.544 | |
| TP/FAA | PLS | 8 | 0.916 | 0.333 | 0.887 | 0.367 | 1.631 |
| Si-PLS | 8 | 0.938 | 0.287 | 0.927 | 0.300 | 2.630 | |
| Si-ELM | 4 | 0.952 | 0.206 | 0.929 | 0.302 | 2.675 | |
| Si-ELM-AdaBoost | 3 | 0.977 | 0.176 | 0.934 | 0.227 | 3.472 | |
By comparing the results in Table 3, Si-ELM-AdaBoost models obtained the best performance among all quality indicators; it is feasible to estimate the main biochemical component during black tea fermentation using the NIRS technique. The prediction results of the catechin content model were as follows: RP was 0.957, RMSEP was 0.563, and RPD was 2.621. The prediction results of the TFs/TRs model were as follows: RP was 0.903, RMSEP was 0.006, and RPD was 2.544. The prediction results of the TFs/TRs model were as follows: RP was 0.934, RMSEP was 0.227, and RPD was 3.472. The evaluated RPD value (>2.5) indicated that this can serve as a correct model to predict the indicators.
Additionally, the models established on the base of the optimal intervals performed better than the full spectrum model, and compared with linear algorithm (PLS and Si-PLS), the nonlinear (Si-ELM) and nonlinear-hybrid algorithms (Si-ELM-AdaBoost) could further enhance the performance of model. The main reasons could be generalized as follows: For the full wavelength variables (1000–1799 nm) PLS model, there was a multiple collinear relation among variables or uncorrelated variables to the biochemical component of black tea. More significantly, the broad water absorption bands were in excess at approximately 1450 nm. In contrast, Si-PLS proved more suitable for modeling [18]. As far as the Si-PLS model is concerned, it singled out the most relevant NIRS wavelength variables for the biochemical components of black tea, getting rid of much of the irrelevant information, particularly water molecule (O–H bond) absorption spectrum bands, and the number of spectral variables reduced to 160–216.
Polyphenols (C22H18O11), catechins (C15H14O6), theanines (C7H14N2O3), TFs (C29H24O12), and TRs (C33H47 NO13) exist in organic forms with a complex molecular structure, and their content changes along with the fermentation process and results in complex near infrared spectral absorption characteristics. Polyphenols, catechins, theanine, TFs, and TRs have this characteristic mainly because of the stretching vibration of C–H and O–H groups and more C–C and C–O absorption in the benzene ring [17, 20]. Free O–H radicals in polyphenols have similar frequency multiplication, and the first frequency multiplication is between 1408 and 1519 nm. The first frequency multiplication of C–H is between 1690 and 1755 nm, and the third frequency multiplication of C–C is between 1625 and 1875 nm. The first frequency multiplication of the theanine carboxyl OH group was between 1436 and 1571 nm. The effective spectra variables regions selected by Si-PLS for the biochemical components of black tea corresponded to 1054–1185 and 1495––1799 nm. This range of wavelength avoided the main absorption peak of the water contained in the fermented leaves, and the filtered wave band results were consistent with other studies [25, 26].
Black tea fermentation is accompanied by a series of oxidation, polymerization, condensation, and other complex chemical changes [1, 2], and the composition ratio and content of the main biochemical compositions shows characteristics of time sequence, variability, and nonlinearity. The nonlinear situation in this work could not be solved by linear regression tools. Furthermore, in the aspect of self-learning and self-adjustment, the nonlinear method had more advantages. Both Si-ELM and Si-ELM-AdaBoost are nonlinear regression tools, which can provide complete solutions of the complex issues and improve the performance of the model.
AdaBoost is a convenient and rapid iterative learning algorithm, which can combine many predictors with weak prediction ability to build a predictor with better performance. Integrating AdaBoost with ELM can further improve the model accuracy. Hence, by consolidating the advantages of Si-PLS and ELM-AdaBoost, this study can certainly enhance the model prediction performance for biochemical components of black tea using NIRS.
As a non-destructive and fast technique, NIRS could confirm that the changes are caused by important biochemical components in black tea at different fermentation stages, and can be potentially extended to quality parameters of other tea varieties such as Anhua dark tea and Pu-erh tea.
Acknowledgements
This work has been financially supported by the National Natural Science Foundation of China (31471646), the Natural Science Foundation of Zhejiang Province (Y16C160009), the Innovation Project of China Academy of Agricultural Sciences (CAAS-ASTIP-TRICAAS), and Science and Technology Project of Zhenjiang City (NY2016013).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Owuor PO, Obanda M, Nyirenda HE, Mandala WL. Influence of region of production on clonal black tea chemical characteristics. Food Chem. 2008;108:263–271. doi: 10.1016/j.foodchem.2007.09.017. [DOI] [Google Scholar]
- 2.Roberts E. The chemistry of tea manufacture. J. Sci. Food Agr. 1958;9:381–390. doi: 10.1002/jsfa.2740090701. [DOI] [Google Scholar]
- 3.Muthumani T, Kumar RS. Influence of fermentation time on the development of compounds responsible for quality in black tea. Food Chem. 2007;101:98–102. doi: 10.1016/j.foodchem.2006.01.008. [DOI] [Google Scholar]
- 4.Ghosh A, Tamuly P, Bhattacharyya N, Tudu B, Gogoi N, Bandyopadhyay R. Estimation of theaflavin content in black tea using electronic tongue. J. Food Eng. 2012;110:71–79. doi: 10.1016/j.jfoodeng.2011.12.007. [DOI] [Google Scholar]
- 5.Sharma P, Ghosh A, Tudu B, Sabhapondit S, Baruah BD, Tamuly P, Bhattacharyya N, Bandyopadhyay R. Monitoring the fermentation process of black tea using QCM sensor based electronic nose. Sensor. Actuat. B-Chem. 2015;219:146–157. doi: 10.1016/j.snb.2015.05.013. [DOI] [Google Scholar]
- 6.Gill G, Kumar A, Agarwal R. Monitoring and grading of tea by computer vision–A review. J. Food Eng. 2011;106:13–19. doi: 10.1016/j.jfoodeng.2011.04.013. [DOI] [Google Scholar]
- 7.Muthumani T, Kumar R. Influence of fermentation time on the development of compounds responsible for quality in black tea. Food Chem. 2007;101:98–102. doi: 10.1016/j.foodchem.2006.01.008. [DOI] [Google Scholar]
- 8.Cai J, Chen Q, Wan X, Zhao J. Determination of total volatile basic nitrogen (TVB-N) content and Warner-Bratzler shear force (WBSF) in pork using Fourier transform near infrared (FT-NIR) spectroscopy. Food Chem. 2011;126:1354–1360. doi: 10.1016/j.foodchem.2010.11.098. [DOI] [Google Scholar]
- 9.Chen Q, Ding J, Cai J, Sun Z, Zhao J. Simultaneous measurement of total acid content and soluble salt-free solids content in chinese vinegar using near-infrared spectroscopy. J. Food Sci. 2012;77:C222–C227. doi: 10.1111/j.1750-3841.2011.02549.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Zhang D, Pan W, Ouyang Q, Li H, Urmila K, Zhao J. Recent developments of green analytical techniques in analysis of tea’s quality and nutrition. Trends Food Sci. Tech. 2015;43:457–458. doi: 10.1016/j.tifs.2015.01.009. [DOI] [Google Scholar]
- 11.Li X, Nie P, Qiu Z, et al. Using wavelet transform and multi-class least square support vector machine in multi-spectral imaging classification of Chinese famous tea. Expert. Syst. Appl. 2015;38:11149–11159. doi: 10.1016/j.eswa.2011.02.160. [DOI] [Google Scholar]
- 12.Borah S, Bhuyan M. A computer based system for matching colours during the monitoring of tea fermentation. Int. J. Food Sci. Tech. 2005;40:675–682. doi: 10.1111/j.1365-2621.2005.00981.x. [DOI] [Google Scholar]
- 13.Huang GB, Chen L. Enhanced random search based incremental extreme learning machine. Neurocomputing. 2008;71:3460–3468. doi: 10.1016/j.neucom.2007.10.008. [DOI] [Google Scholar]
- 14.Huang GB, Zhu QY, Siew CK. Extreme learning machine: theory and applications. Neurocomputing. 2006;70:489–501. doi: 10.1016/j.neucom.2005.12.126. [DOI] [Google Scholar]
- 15.Freund Y, Schapire RE. A decision-theoretic generalization of on-line learning and an application to boosting. J. Comput. Syst. Sci. 1997;55:119–139. doi: 10.1006/jcss.1997.1504. [DOI] [Google Scholar]
- 16.Mir-Marqués A, Elvira-Sáez C, Cervera ML, Garrigues S, Guardia MDL. Authentication of protected designation of origin artichokes by spectroscopy methods. Food Control. 2016;59:74–81. doi: 10.1016/j.foodcont.2015.05.004. [DOI] [Google Scholar]
- 17.Lee M, Hwang Y, Lee J, Choung M. The characterization of caffeine and nine individual catechins in the leaves of green tea (Camellia sinensis L.) by near-infrared reflectance spectroscopy. Food Chem. 2014;158:351–357. doi: 10.1016/j.foodchem.2014.02.127. [DOI] [PubMed] [Google Scholar]
- 18.Ouyang Q, Zhao J, Chen Q. Measurement of non-sugar solids content in Chinese rice wine using near infrared spectroscopy combined with an efficient characteristic variables selection algorithm. Spectrochim. Acta A. 2015;151:280–285. doi: 10.1016/j.saa.2015.06.071. [DOI] [PubMed] [Google Scholar]
- 19.Ouyang Q, Zhao J, Chen Q, Lin H, Sun Z. Rapid measurement of antioxidant activity in dark soy sauce by NIR spectroscopy combined with spectral intervals selection and nonlinear regression tools. Anal. Methods. 2012;4:940–946. doi: 10.1039/c2ay05766b. [DOI] [Google Scholar]
- 20.Qi S, Ouyang Q, Chen Q, Zhao J. Real-time monitoring of total polyphenols content in tea using a developed optical sensors system. J. Pharmaceut. Biomed. 2014;97:116–122. doi: 10.1016/j.jpba.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 21.Tan C, Li M. Mutual information-induced interval selection combined with kernel partial least squares for near-infrared spectral calibration. Spectrochim. Acta A. 2008;71:1266–1273. doi: 10.1016/j.saa.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 22.Chen Q, Hu W, Su J, Li H, Ouyang Q, Zhao J. Nondestructively sensing of total viable count (TVC) in chicken using an artificial olfaction system based colorimetric sensor array. J. Food Eng. 2016;168:259–266. doi: 10.1016/j.jfoodeng.2015.08.003. [DOI] [Google Scholar]
- 23.Ouyang Q, Zhao J, Pan W, Chen Q. Real-time monitoring of process parameters in rice wine fermentation by a portable spectral analytical system combined with multivariate analysis. Food Chem. 2016;190:135–141. doi: 10.1016/j.foodchem.2015.05.074. [DOI] [PubMed] [Google Scholar]
- 24. Ouyang Q, Chen Q, Zhao J. Intelligent sensing sensory quality of Chinese rice wine using near infrared spectroscopy and nonlinear tools. Spectrochim. Acta A. 154: 42–46 (2016) [DOI] [PubMed]
- 25.Ren G, Wang S, Ning J, Xu R, Wang Y, Xing Z, Wan X, Zhang Z. Quantitative analysis and geographical traceability of black tea using Fourier transform near-infrared spectroscopy (FT-NIRS) Food Res. Int. 2013;53:822–826. doi: 10.1016/j.foodres.2012.10.032. [DOI] [Google Scholar]
- 26.Li X, Sun C, Luo L, He Y. Determination of tea polyphenols content by infrared spectroscopy coupled with iPLS and random frog techniques. Comput. Electron. Agr. 2015;112:28–35. doi: 10.1016/j.compag.2015.01.005. [DOI] [Google Scholar]