Abstract
To develop an antibacterial treatment for acne vulgaris using natural substance with few side effects, we investigated the antibacterial activities of oligochitosan against acne-related bacteria, particularly Propionibacterium acnes. Oligochitosan showed potent antibacterial effect on P. acnes. Especially, 10 kDa oligochitosan presented the highest antimicrobial effect with minimum inhibitory concentration values of 32–64 μg/mL on P. acnes. In addition, oligochitosan clearly reversed the antibacterial effect of tetracycline and erythromycin on P. acnes in the combination mode. The combination of tetracycline- or erythromycine-10 kDa oligochitosan resulted in a median ΣFIC range of 0.02–0.56, suggesting that the antibiotics–oligochitosan combination resulted in an antibacterial synergy against P. acnes. Thus, the results obtained in this research strongly supported that erythromycin and tetracycline will restore the antibacterial activity against P. acnes in the combination mode with 10 kDa oligochitosan.
Keywords: Acne-related bacteria, Antibacterial activity, Erythromycin, Oligochitosan, Synergistic effect, Tetracycline
Introduction
Acne vulgaris is one of the most typical skin diseases, affecting approximately 80% of adults aged from 11 to 30 years [1]. There are several significant factors causing acne in people such as skin pathogenic bacteria, keratinization of the follicle, and increase in sebum secretion [2]. Although it is not life threatening, it can cause a permanent scar and also negatively affects psychological developments, resulting in a severe emotional scar, which may lead to clinical depression and social phobias [3]. It has been known that Staphylococcus epidermidis, Propionibacterium acnes, Staphylococcus aureus, Pseudomonas aeruginosa, and Candida albicans are skin pathogens associated with the development of abnormal follicular keratinization and inflammation [4]. Among a variety of factors contributing to the development of acne vulgaris, bacterium P. acnes is the main factor in the pathogenesis of acne vulgaris [5]. In general, topical therapies for acne vulgaris, including retinoide, benzoyl peroxide, and antibiotics, have been used to improve the control of moderate acne. As described, an antibiotic application for treating acne vulgaris is usually used to kill acne-related bacteria. Among the various antibiotics, tetracycline, erythromycin, and lincomycin are commonly taken for the antibiotic therapy [6]. However, treatment by antibiotics and benzoyl peroxide, used to treat acne vulgaris, normally causes several side effects such as the emergence of resistant bacteria, immune hypersensitivity, and organ damage in the case of using those medicines for a long period; moreover, they are toxic to human cells [7, 8]. Therefore, recent researches have focused on the development of alternative therapeutic substance with less adverse and strong antibacterial effects [9].
Chitosan is a linear polysaccharide comprising randomly distributed β-(1 → 4)-linked N-acetyl-d-glucosamine and d-glucosamine and possess several bioactivities, including antioxidant, antitumor, and enzymatic inhibitory effect. Recently, considerable attention has been focused on its applications in the food and pharmaceutical industry [8, 10]. However, high-molecular weight chitosan has a limitation on practical applications due to its insolubility at above pH 6.3 [11]. To overcome these disadvantages, recent researches have been conducted focusing on the transformation of chitosan into oligochitosan with a molecular weight of less than 10 kDa [12]. Oligochitosan, obtained by hydrolysis or degradation of chitosan, is a water-soluble chitosan with a low molecular as well as is known to be non-toxic, biodegradable and biocompatible due to their shorter chain lengths [13]. Oligochitosan is well absorbed in the body and can be added to any food as the main ingredient because it is easily soluble in water [14]. It has also been reported to exhibit high medicinal and functional aspects involved in the hypocholesterolemic effect [12], antitumor effects [14], accelerating calcium and ferrum absorption [15], immune-enhancement effect, antibacterial effect, and cholesterol regulation [16]. However, limited information is available on the antibacterial activity of low-molecular weight oligochitosan against acne-related bacteria. Therefore, the object of this research was to evaluate the antibacterial effect of oligochitosan against acne-related bacteria.
Materials and methods
Materials
Chitosan (MW 250 kDa) (80% degree of deacetylation) prepared from crab shell chitin was provided by Kitto Life Co. (Seoul, Korea) and oligochitosan (MW 1 kDa, MW 3 kDa, MW 5 kDa, and MW 10 kDa) (80% degree of deacetylation) were manufactured as previously reported by Park et al. [17] and Jung et al. [18]. Antibiotics were purchased from Sigma-Aldrich (St. Louis, USA). All other reagents were of the highest grade available commercially.
Evaluation of antimicrobial activity
Strains and culture conditions
The following microbial strains were obtained from the Korean Collection for Type Cultures (KCTC; Daejeon, Korea) and Korean Culture Center of Microorganisms (KCCM, Seoul, Korea): S. aureus KCTC1927, S. epidermidis KCCM 40,416, P. aeruginosa KCTC 1637, and P. acnes KCTC 3314. Five P. acnes clinical isolates were provided by the Gyeongsang National University Hospital (Jinju, Korea), a member of the National Biobank of Korea. P. acnes strains were anaerobically cultivated in brain heart infusion broth (Difco Inc., Detroit, USA) supplemented with 1.0% glucose and incubated at 37 °C for 24 h in a CO2 incubator (NAPCO 5400; General Laboratory Supply, Pasadena, USA) in a 10% CO2 humidified atmosphere.
Measurement of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
MIC is the method of quantitatively evaluating the antimicrobial activity. It is defined as the lowest concentration of antimicrobial agents that will inhibit the visible growth of a microorganism after 20–24 h of incubation [19]. The experiment procedures by the guideline of Clinical and Laboratory Standards Institute were followed [20]. MIC assay was performed using the serial two-fold dilution method with Mueller–Hinton broth (MHB; Difco Inc.) and 96-well microtiter plates (with clear flat bottoms). After incubation, MIC values were determined by reading the plates visually. This test was repeated three times.
MBC is the lowest concentration of an antibacterial agent required to kill a particular bacterium [21]. It can be determined from broth dilution MIC tests by sub-culturing to agar plates that do not contain the test agent. MBC is identified by determining the lowest concentration of antibacterial agent that reduces the viability of the initial bacterial inoculum by ≥99.9%.
Morphology assessment by scanning electron microscopy (SEM)
The morphological change of P. acnes cells by the treatment of 10 kDa oligochitosan was monitored using a SEM VEGA II LSU microscope (Tescan, Brno, Czech Republic). The specimens were primary fixed in 2.5% buffered glutaraldehyde (Sigma-Aldrich) for 2 h, rinsed in distilled water, and post-fixed in 1% buffered osmium tetroxide (Sigma-Aldrich) for 2 h. The fixed samples were then dehydrated stepwise with ethanol (25, 50, 70, 94, and 100%) and subsequently samples were freeze dried and observed by SEM.
Antibiotic susceptibility test (AST)
AST is used to determine whether an organism is susceptible or resistant to an antimicrobial agent [22]. The antibiotic resistance of the test strains was confirmed against commercial antibiotics by MIC assay. An antibiotic was serially diluted and then the bacterial growth was visually checked.
Determination of fractional inhibitory concentration (FIC)
FIC assay is widely used for evaluation of in vitro synergy for multiple agents [23]. This test method assesses interaction between antimicrobial agents by exposing bacteria to varying concentrations of the antimicrobial drugs [24]. In this study, the synergy effect between oligochitosan and antibiotics (tetracycline, erythromycin, and lincomycin) against pathogens of human skin was evaluated. FIC index was calculated using the following formula:
The interaction was defined as synergistic if the FIC index was <1; additive if the FIC index was 1.0; sub-additive if the FIC index was between 1.0 and 2.0; indifferent if the FIC index was 2, and antagonistic if the FIC index >2. Synergy was further sub-classified as marked (FIC index, ≤0.50) and weak (FIC index, between 0.50 and 1.0) [4].
Statistical analysis
In all cases, analyses were performed in triplicate and data were averaged over the three measurements. The standard deviation was also calculated. Significance of differences between the average MICs for each individual microorganism was determined by Student’s t test at the 95% significance level using SPSS version 12.0 (SPSS Inc., Chicago, USA).
Results and discussion
Determination of MIC and MBC of chitosan and oligochitosan
Acne vulgaris is one of the most typical skin diseases causing disfiguration and permanent scarring. According to Lee et al. [4], P. acnes appears to be the most probable organism to cause acne vulgaris and is therefore the target of topical antibiotic treatments. However, antibiotic therapies normally have several adverse effects such as organ damage, emergence of resistant bacteria, and immune hypersensitivity. To discover an alternative therapeutic agent with few side effects from marine resources, the antibacterial effects of chitosan and oligochitosan were quantitatively evaluated by the MIC assay. The MIC values of the chitosan and oligochitosan were 32–4096 μg/mL against Gram positive acne-related bacteria tested in this study. No antibacterial activity was observed against Gram negative acne-related bacterium, P. aeruginosa (data not shown). Interestingly, oligochitosan showed strong antibacterial activity against P. acnes strains in the MICs range of 16–512 μg/mL. Moreover, oligochitosan exhibits equal or lower MIC values than those of the chitosan against P. acnes. However, oligochitosan showed less antimicrobial effects against other acne-related bacteria, including P. aeruginosa, S. epidermidis and S. aureus. Oligochitosan (MW 1, 3, 5, and 10 kDa) showed stronger antibacterial effect on P. acnes than that of chitosan. Among oligochitosan, the highest antibacterial effect was observed in the treatment of 10 kDa oligochitosan. The MIC value of oligochitosan was 16–64 μg/mL against P. acnes strains.
In order to evaluate the bactericidal activity of chitosan and oligochitosan, MBC assay was performed. MBC values of chitosan and oligochitosan were 32–8192 μg/mL against acne-inducing bacteria associated with acne vulgaris. Similar to the MIC results obtained in Table 1, oligochitosan showed strong antibacterial activity against P. acnes strains in MBC range of 32–2048 μg/mL.
Table 1.
Minimum inhibitory concentration (MIC) of chitosan and oligochitosan against acne-related bacteria
| Strains | Oligochitosan (MW 1 kDa) | Oligochitosan (MW 3 kDa) | Oligochitosan (MW 5 kDa) | Oligochitosan (MW 10 kDa) | Chitosan (MW 250 kDa) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| S. aureus KCTC1927 | 4096 | 8192 | 4096 | 8192 | 2048 | 4096 | 4096 | 8192 | 128 | 512 |
| S. epidermidis KCCM 40416 | 4096 | 8192 | 4096 | 8192 | 2048 | 4096 | 4096 | 8192 | 512 | 1024 |
| P. acnes KCTC 3314 | 512 | 1024 | 32 | 64 | 32 | 64 | 16 | 32 | 512 | 2048 |
| P. acnes isolate 2874 | 512 | 1024 | 64 | 256 | 32 | 64 | 32 | 64 | 512 | 2048 |
| P. acnes isolate 2875 | 512 | 1024 | 128 | 512 | 64 | 128 | 64 | 128 | 512 | 2048 |
| P. acnes isolate 2876 | 512 | 1024 | 64 | 128 | 64 | 128 | 32 | 64 | 512 | 2048 |
| P. acnes isolate 2877 | 512 | 1024 | 64 | 256 | 32 | 128 | 32 | 64 | 512 | 2048 |
| P. acnes isolate 2878 | 512 | 1024 | 128 | 256 | 64 | 128 | 64 | 64 | 512 | 2048 |
The antibacterial activity of oligochitosan was superior to that of the chitosan against P. acnes. Among oligochitosan, 10 kDa oligochitosan showed the highest antibacterial activity in a range of 32–128 μg/mL against P. acnes. However, the MBC values of 10 kDa oligochitosan were two-folds increased compared to those of MIC values. Similar patterns between MIC and MBC values were reported by several studies [9, 25]. Considering both MIC and MBC results, 10 kDa oligochitosan exhibited the highest antibacterial activity against P. acne strains. As reported by Rabea et al. [26] and Champer et al. [27], chitosan with high-molecular weight exhibited strong antimicrobial activity against cutaneous pathogens, excluding P. acnes. Interestingly, however, 10 kDa oligochitosan showed superior antibacterial activity against P. acnes strains in the ranges of MIC values from 16 to 128 μg/mL.
The mechanism of antimicrobial activity of oligochitosan is still yet to be elucidated. However, chitosan molecules are reported to be stacked over the microbial cell surface, blocking the nutrients, or bind to DNA, as such inhibiting transcription or permeability of the microbial cell wall [28, 29]. Chitosan samples with a Mw <5 kDa induce filamentation in B. megaterium by blocking the synthesis of DNA. Thus, the molecular weight of chitosan can influence the permeability of chitosan through the cell membrane [30]. Therefore, further studies are necessary to address the detail antimicrobial mechanism of 10 kDa oligochitosan particularly against P. acnes.
According to the previous reports, chitosan and chitosan phytochemical conjugates showed MIC value of 128–512 μg/mL for P. acnes and isolated P. acnes [9], and n-hexane extract of Sargassum serratifolium C. Agardh showed MICs of 128 μg/mL [25]. Lee et al. [4] also reported that phlorotannins from Eisenia bicyclis showed strong antibacterial activity in the ranges of 64–1024 μg/mL against P. acnes strains. Considering these results, oligochitosan exhibiting MICs of 16–64 μg/mL possessed superior antibacterial activity than that of phlorotannins. In addition, these results strongly indicate that oligochitosan will be a potential candidate to develop an alternative therapeutic agent for the treatment of P. acnes infection.
Effect of 10 kDa oligochitosan on P. acnes cells morphology
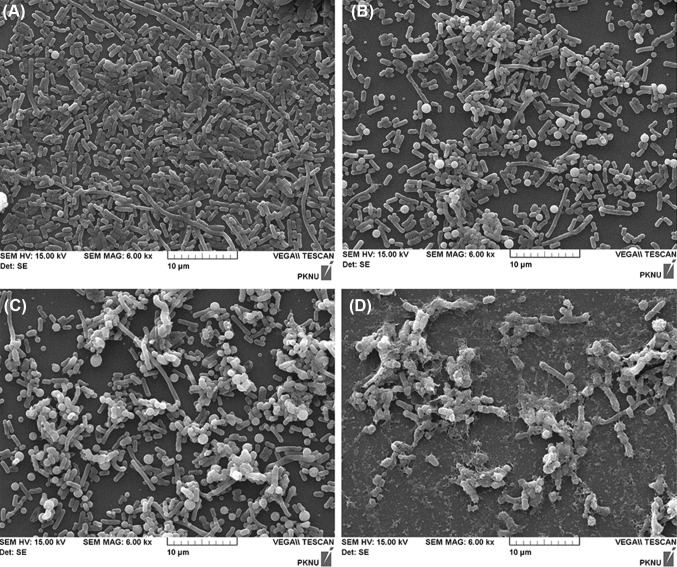
As described above, 10 kDa oligochitosan exhibited the highest antibacterial activity. To investigate the antibacterial effect of oligochitosan on P. acnes cells, changes in the cells morphology were monitored using SEM. Considering the MIC values of oligochitosan, P. acnes cells were treated by various concentrations of oligochitosan (0, 16, 32, and 64 μg/mL) and cells were cultured at 37 °C under anaerobic for a day. As shown in Fig. 1. SEM micrographs showed distorted and damaged cells by the treatment of 10 kDa oligochitosan above 32 μg/mL. In more detail, untreated P. acnes cells show normal cell surface architecture, which appears smooth and sharply layered [Fig. 1(A)]. In contrast, the bacterial cells treated by 10 kDa oligochitosan showed loosen integrity on their surface structure [Fig. 1(C), (D)]. These results coincide with the previous report of Champer et al. [27] that chitosan can bind to the surface of bacteria causing shrinkage of P. acnes cell surface structure. The antibacterial mechanism of chitosan has not been fully elucidated yet, but the most accepted antibacterial mechanism of chitosan is directly dependent on its de-acetylation and amino group [31]. Negatively charged bacterial cell surface interacts with positively charged chitosan, resulting in weakening of the cell wall either accompanied by cell lysis or cell wall damage [32]. Moreover, a higher molecular weight of chitosan is reported to have stronger antimicrobial activity than low-molecular chitosan. In this study, however, oligochitosan with low-molecular weight is more effective than chitosan with high-molecular weight for controlling P. acnes, suggesting that the different antibacterial mechanism against P. acne compared to chitosan with high-molecular weight. In order to address this issue in more detail, further study will be needed.
Fig. 1.
Scanning electron microscopy profiles on the antibacterial effect of 10 kDa oligochitosan against Propionibacterium acnes (×6000 K magnification) (A) control (B) treated with oligochitosan of 16 μg/mL (C) treated with oligochitosan of 32 μg/mL (D) treated with oligochitosan of 64 μg/mL
Antibiotics and benzoyl peroxide resistance of P. acnes strains
The antibiotic resistance profile of P. acnes and isolated P. acnes was determined based on the analysis of MIC breakpoint [33]. MIC breakpoint values of tetracycline, lincomycin, and erythromycin were 1–4 μg/mL, 2–8 μg/mL, and 4–8 μg/mL, respectively. The MIC values of lincomycin against P. acnes strains were in the range of the Soussy’s MIC breakpoint values, indicating susceptibility to the test antibiotic (Table 2). The MIC of P. acnes standard strain against tetracycline is 16 μg/mL, which was over the range of MIC breakpoint. Moreover, some P. acnes isolates were found to be resistant to erythromycin and tetracycline. However, P. acnes strains tested in this research were judged to be sensitive to benzoyl peroxide based on the analysis of the MIC values.
Table 2.
Minimum inhibitory concentration (MIC) of antibiotics against Propionibacterium acnes strains
| Strains | MIC (μg/mL) | |||
|---|---|---|---|---|
| Tetracycline | Lincomycin | Erythromycin | Benzoyl peroxide | |
| Break point | 4–8 | 2–8 | 1–4 | 250 |
| P. acnes KCTC 3314 | 16 | 4 | <1 | 64 |
| P. acnes isolate 2874 | 32 | 8 | <1 | 64 |
| P. acnes isolate 2875 | 2 | <1 | <1 | 64 |
| P. acnes isolate 2876 | <1 | <1 | 64 | 64 |
| P. acnes isolate 2877 | 32 | 8 | <1 | 64 |
| P. acnes isolate 2878 | 32 | 8 | <1 | 64 |
These antibiotic-resistant profiles against P. acnes strains are almost similar with that reported by Lee et al. [4], Kim et al. [9], and Kim et al. [25]. Likewise, the profiles of MICs to antibiotics were similar with the previous results [4, 9, 25]. The results obtained in this study indicated that some commercial antibiotics are no longer effective for treating bacterial infection related with P. acnes. Thus, alternative therapeutic agents for no longer effective antibiotics are required.
Synergistic effect between oligochitosan (MW 10 kDa) and antibiotics against P. acnes strains
With the emergence of multidrug resistant bacteria, the need for new antibiotics or therapeutic agents has increased [34]. It has been proved that one of the more effective approaches in developing alternative therapies or new drugs is the restoration of antibiotic effect in combination with antibacterial substances derived from natural products and traditional drug against drug-resistant bacteria [34]. Based on these reports, an interaction between 10 kDa oligochitosan and commercial antibiotics (or benzole peroxide) against P. acnes strains, antibiotic-resistant strains as reported previously using AST, was tested by the checkerboard method using FIC assay, as stated in “Materials and methods” section [24]. As shown in Table 1, 10 kDa oligochitosan was chosen for further study among the chitosan and its oligo since oligochitosan showed the highest antibacterial effect on P. acnes.
MICs of tetracycline against P. acnes strains combining with 10 kDa oligochitosan fairly decreased from 16 to 4 μg/mL. On comparing the MIC of the tetracycline alone, it can be observed that the MIC has decreased 2 to fourfolds in the combination of tetracycline and 10 kDa oligochitosan. In all test strains, the median ∑FIC indices were from 0.28 to 0.56, indicating a synergistic antibacterial effect between tetracycline and oligochitosan (Table 3).
Table 3.
Fractional inhibitory concentration (FIC) indices of 10 kDa oligochitosan in combination with antibiotics against Propionibacterium acnes
| Strains | Test compound | MIC (μg/mL) | ∑FICamax | ∑FICbmin | Median ∑FIC | Minimum concentration for observing synergy |
|---|---|---|---|---|---|---|
| P. acnes KCTC 3314 | Oligochitosan | 16 | 1.03 | 0.28 | 0.28 | 0.5 |
| Tetracycline | 16 | 4 | ||||
| Oligochitosan | 16 | 1.50 | 1.00 | 1.03 | 0.5 | |
| Benzoyl peroxide | 64 | 64 | ||||
| P. acnes isolate 2874 | Oligochitosan | 32 | 1.06 | 0.50 | 0.56 | 1 |
| Tetracycline | 32 | 16 | ||||
| Oligochitosan | 32 | 2.00 | 1.00 | 1.02 | 0.5 | |
| Benzoyl peroxide | 64 | 64 | ||||
| P. acnes isolate 2875 | Oligochitosan | 64 | 1.25 | 0.52 | 1.00 | 1 |
| Benzoyl peroxide | 64 | 64 | ||||
| P. acnes isolate 2876 | Oligochitosan | 32 | 0.02 | 0.02 | 0.02 | 0.02 |
| Erythromycin | 64 | 1 | ||||
| Oligochitosan | 32 | 1.50 | 1.00 | 1.03 | 1 | |
| Benzoyl peroxide | 64 | 64 | ||||
| P. acnes isolate 2877 | Oligochitosan | 32 | 1.25 | 0.25 | 0.34 | 1 |
| Tetracycline | 32 | 8 | ||||
| Oligochitosan | 32 | 1.50 | 1.00 | 1.03 | 1 | |
| Benzoyl peroxide | 64 | 64 | ||||
| P. acnes isolate 2878 | Oligochitosan | 64 | 1.03 | 0.50 | 0.52 | 2 |
| Tetracycline | 32 | 16 | ||||
| Oligochitosan | 64 | 2 | 2 | 2 | 64 | |
| Benzoyl peroxide | 64 | 64 |
The FIC index indicated synergistic effect: <0.5, marked synergy; 0.5 to <1.0, weak synergy; 1.0, additive; >1.0 to <2.0, subadditivie; 2.0, indifferent; >2.0, antagonistic
a∑FICmax, maximum FIC
b∑FICmin, minimum FIC
The highest synergistic antibacterial activity was observed in the combination with erythromycin and 10 kDa oligochitosan against P. acnes isolate 2876 strain. The MIC of erythromycin against the isolates combining with 32 μg/mL of 10 kDa oligochitosan dramatically decreased from 64 to 1 μg/mL. The median FIC index was 0.02, indicating very strong synergistic effect against P. acnes isolate. However, no synergistic antibacterial effect against P. acnes stains was observed in the combination of benzoyl peroxide and oligochitosan (Table 3).
It has been previously reported that phlorotannins of E. bicyclis showed the median ∑FIC indices ranging from 0.502 to 0.750 in combination with β-lactam antibiotics against P. acnes [4]. Kim et al. [25] also reported that a synergy effect between chitosan phytochemical conjugate and tetracycline with the median ∑FIC indices from 0.502 to 0.533 against P. acnes strains. Compared with these results, the synergistic effect of 10 kDa oligochitosan with antibiotics is remarkably high. Thus, the 10 kDa oligochitosan has a potential to restore the antibacterial activity of old-fashioned antibiotics such as tetracycline and erythromycin against P. acnes. It was anticipated that the 10 kDa oligochitosan would be a good candidate in the remedy of antibiotic-resistant P. acnes infection.
In conclusion, the oligochitosan possessed combined antibacterial effects on P. acnes. The finding obtained in this research strongly suggested that 10 kDa oligochitosan will be a potential alternative therapeutic substance for acne vulgaris treatment since it has strong antibacterial effect on acne-related bacteria.
Acknowledgements
This research was supported by the Marine Biotechnology Program (20150220) funded by the Ministry of Oceans and Fisheries, Republic of Korea. Moreover, this study was supported by the special fund of Pukyong National University, donated by the SKS Trading Co. in Lynnwood, WA, USA in memory of the late Mr. Young Hwan Kang for his inspiration and deep concern for fisheries science. The pathogen for this study was provided by the Gyeongsang National University Hospital Branch of National Culture Collection for Pathogens (GNUH-NCCP).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Uhlenhake E, Yentzer BA, Feldman SR. Acne vulgaris and depression: a retrospective examination. J. Cosmet. Dermatol. 2010;9:59–63. doi: 10.1111/j.1473-2165.2010.00478.x. [DOI] [PubMed] [Google Scholar]
- 2.Farrar MD, Ingham E. Acne: inflammation. Clin. Dermatol. 2004;22:380–384. doi: 10.1016/j.clindermatol.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Ash C, Harrison A, Drew S, Whittall R. A randomized controlled study for the treatment of acne vulgaris using high-intensity 414 nm solid state diode arrays. J. Cosmet. Laser Ther. 2015;17:170–176. doi: 10.3109/14764172.2015.1007064. [DOI] [PubMed] [Google Scholar]
- 4.Lee JH, Eom SH, Lee EH, Jung YJ, Kim HJ, Jo MR, Son KT, Lee HJ, Kim JH, Lee MS, Kim YM. In vitro antibacterial and synergistic effect of phlorotannins isolated from edible brown seaweed Eisenia bicyclis against acne-related bacteria. Algae. 2014;29:47–55. doi: 10.4490/algae.2014.29.1.047. [DOI] [Google Scholar]
- 5.Liu PF, Nakatsuji T, Zhu W, Gallo RL, Huang CM. Passive immunoprotection targeting a secreted CAMP factor of Propionibacterium acnes as a novel immunotherapeutic for acne vulgaris. Vaccine. 2011;29:3230–3238. doi: 10.1016/j.vaccine.2011.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han S, Lee K, Yeo J, Baek H, Park K. Antibacterial and anti-inflammatory effects of honeybee (Apis mellifera) venom against acne-inducing bacteria. J. Med. Plants Res. 2010;4:459–464. [Google Scholar]
- 7.Kim JY, Oh TH, Kim BJ, Kim SS, Lee NH, Hyun CG. Chemical composition and anti-inflammatory effects of essential oil from Farfugium japonicum flower. J. Oleo Sci. 2008;57:623–628. doi: 10.5650/jos.57.623. [DOI] [PubMed] [Google Scholar]
- 8.Lee SH, Ryu BM, Je JY, Kim SK. Diethylaminoethyl chitosan induces apoptosis in HeLa cells via activation caspase-3 and p53 expression. Carbohydr. Polym. 2011;84:571–578. doi: 10.1016/j.carbpol.2010.12.027. [DOI] [Google Scholar]
- 9.Kim JH, Je JY, Kim YM. Anti-inflammatory effects of chitosan-phytochemical conjugates against Propionibacterium acnes-induced inflammation. Korean J. Fish. Aquat. Sci. 2016;49:589–593. [Google Scholar]
- 10.Cho YS, Kim SK, Ahn CB, Je JY. Preparation, characterization, and antioxidant properties of gallic acid-grafted-chitosans. Carbohydr. Polym. 2011;83:1617–1622. doi: 10.1016/j.carbpol.2010.10.019. [DOI] [Google Scholar]
- 11.Seo S, King JM, Prinyawiwatkul W. Simultaneous depolymerization and decolorization of chitosan by ozone treatment. J. Food Sci. 2007;72:522–526. doi: 10.1111/j.1750-3841.2007.00563.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim SK, Rajapakse N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): a review. Carbohydr. Polym. 2005;62:357–368. doi: 10.1016/j.carbpol.2005.08.012. [DOI] [Google Scholar]
- 13.Lodhi G, Kim YS, Hwang JW, Kim SK, Jeon YJ, Je JY, Ahn CB, Moon SH, Jeon BT, Park PJ. Chitooligosaccharide and its derivatives: preparation and biological applications. BioMed Res. Int. Article ID 654913 (2014) [DOI] [PMC free article] [PubMed]
- 14.Xia WS. Physiological activities of chitosan and its application in functional foods. J. Chinese Inst. Food Sci. Technol. 2003;3:77–81. [Google Scholar]
- 15.Bravo-Osuna I, Millotti G, Vauthier C, Ponchel G. In vitro evaluation of calcium binding capacity of chitosan and thiolated chitosan poly (isobutyl cyanoacrylate) core-shell nanoparticles. Int. J. Pharm. 2007;338:284–290. doi: 10.1016/j.ijpharm.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 16.Xia W, Liu P, Zhang J, Chen J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocolloid. 2011;25:170–179. doi: 10.1016/j.foodhyd.2010.03.003. [DOI] [Google Scholar]
- 17.Park PJ, Je JY, Jung WK, Byun HG, Kim SK. Free radical scavenging activities of chitooligosaccharides hydrolyzed from chitosan with high degree of deacetylation on 1,1-diphenyl-2-picrylhydrazyl radical., J. Chitin Chitosan 9: 108–113 (2004)
- 18.Jung WK, Moon SH, Kim SK. Effect of chitooligosaccharides on calcium bioavailability and bone strength in ovariectomized rats. Life Sci. 2006;78:970–976. doi: 10.1016/j.lfs.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Grierson DS, Afolayan AJ. Antibacterial activity of some indigenous plants used for the treatment of wounds in the Eastern Cape. South Africa. J. Ethnopharmacol. 1999;66:103–106. doi: 10.1016/S0378-8741(98)00202-5. [DOI] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: Approved standard, 8th ed. CLSI document M07-A8. CLSI, Wayne, PA, pp 68 (2006)
- 21.Amyes S, Miles RS, Thomson CJ, Tillotson G. Antimicrobial Chemotherapy: Pocketbook. CRC Press, Florida, pp 25 (1996)
- 22.Jenkins SG, Schuetz AN. Current concepts in laboratory testing to guide antimicrobial therapy. Mayo Clin. Proc. 2012;87:290–308. doi: 10.1016/j.mayocp.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003;52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh MH, Yu CM, Yu VL, Chow JW. Synergy assessed by checkerboard. A critical analysis. Diagn. Microbiol. Infect. Dis. 1993;16:343–349. doi: 10.1016/0732-8893(93)90087-N. [DOI] [PubMed] [Google Scholar]
- 25.Kim YH, Kim JH, Kim DH, Kim SH, Kim HR, Kim YM. Synergistic Antimicrobial Effect of Sargassum serratifolium (C. Agardh) C. Agardh extract against human skin pathogens. Korea. J. Food Sci. Technol. 2016;48:241–246. [Google Scholar]
- 26.Rabea EI, Badawy ME, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003;4:1457–1465. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- 27.Champer J, Patel J, Fernando N, Salehi E, Wong V, Kim J. Chitosan against cutaneous pathogens. AMB Express. 2013;3:37. doi: 10.1186/2191-0855-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shon DH. Chitosan oligosaccharides for functional foods and microbial enrichment of chitosan oligosaccharides in soy-paste. In: Proceedings of the International workshop on Bioactive Natural Products. The Committee on Science and Technology in Developing Countries (COSTED) and the Science Council of Japan, Tokyo, Japan: 56–66. (2001)
- 29.Tharanathan RN, Kittur FS. Chitin—The undisputed biomolecule of great potential. Crit. Rev. Food Sci. Nutr. 2003;43:61–87. doi: 10.1080/10408690390826455. [DOI] [PubMed] [Google Scholar]
- 30.Park SC, Nah JW, Park Y. pH-dependent mode of antibacterial actions of low molecular weight water-soluble chitosan (LMWSC) against various pathogens. Macromol. Res. 2011;19:853–860. doi: 10.1007/s13233-011-0812-1. [DOI] [Google Scholar]
- 31.Goy RC, Britto DD, Assis OB. A review of the antimicrobial activity of chitosan. Polimeros. 2009;19:241–247. [Google Scholar]
- 32.Eaton P, Fernandes JC, Pereira E, Pintado ME, Malcata FX. Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy. 2008;108:1128–1134. doi: 10.1016/j.ultramic.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Soussy CJ, Cluzel R, Courvalin P. Definition and Determination of in vitro antibiotic susceptibility breakpoints for bacteria in France. Eur. J. Clin. Microbiol. Infect. Dis. 1994;13:238–246. doi: 10.1007/BF01974543. [DOI] [PubMed] [Google Scholar]
- 34.Eom SH, Park JH, Yu DU, Choi JI, Choi JD, Lee MS, Kim YM. Antimicrobial activity of brown alga Eisenia bicyclis against methicillin-resistant Staphylococcus aureus. Fish. Aquat. Sci. 2011;14:251–256. [Google Scholar]