Abstract
This study aims to understand the biofilm formation abilities of eight Bacillus cereus strains under food-industry-related conditions. Biofilms were grown in microtiter plates in tryptic soy broth (TSB) or brain heart infusion (BHI) at 30 °C for 24 or 48 h and quantified via the crystal violet assay. A significantly larger of biofilm was formed in TSB than in BHI after 48 h. Selected strains were used to test biofilm formation under food-related conditions produced by different surfaces (e.g., stainless steel, plastic, or glass), temperatures (25 or 30 °C), carbon sources, (glucose or glycerol) and NaCl. Biofilm formation appeared to be affected by surface properties, temperature, and carbon sources. A larger biofilm was formed on stainless steel at 30 °C compared to plastic and glass surfaces at 25 and 30 °C. Moreover, addition of glucose in combination with NaCl in TSB produced significantly larger biofilm than glucose, glycerol and/or NaCl. These results indicate that food-industry-related conditions could promote B. cereus biofilm formation, which is relevant to food safety.
Keywords: Bacillus cereus, Biofilm formation, Glucose, Glycerol, NaCl
Introduction
Biofilm is a community of bacteria embedded in a hydrated matrix of extracellular polymeric substances (EPS) and attached to a surface [1]. Bacterial biofilm formation is a very complex and dynamic process. It occurs in three different stages: (1) initial attachment (planktonic cells attach to a surface), (2) maturation (microorganisms start producing adhesion proteins and other EPS), and (3) detachment and dispersal (bacteria or small parts of the biofilm detach and re-attach to a new surface) [2, 3]. Biofilm cells are highly resistant to adverse environmental conditions, including antimicrobials and sanitizers [4]. Therefore, bacterial biofilm formation is currently a topic of great interest in medical, environmental and food microbiology because it can cause serious public health problems [5, 6].
Bacillus cereus is a Gram-positive, spore-forming, and facultatively anaerobic bacterium that is widely distributed in the environment. Usually, B. cereus is a soil inhabitant and is commonly isolated from food and food products, including dairy products, vegetables, rice, and meat [7]. This organism is a human pathogen that causes two different types of gastrointestinal diseases: (1) diarrheal and (2) emetic [8]. It is a well-known cause of biofilm formation on many food contact surfaces such as conveyor belts, stainless steel pipes, and storage tanks [9]. B. cereus biofilm formation in food-industry settings is considered as a serious food safety concern because it can act as a potential source of product contamination and recontamination [10].
Biofilm formation can be influenced by several environmental factors such as nutrient availability, osmolality, and maturation time [11]. In a food-industry-related environment, different carbon sources (e.g., glucose, glycerol, and ethanol), minerals, and food residues are precipitated depending on the clean-in-place procedures (CIP) that can substantially affect biofilm formation [12]. For example, with respect to Staphylococcus aureus, environmental conditions related to the food industry, including temperature and glucose and ethanol contents, influence the biofilm formation behavior [13]. In a food-industry environment, different surfaces (e.g., stainless steel (SS), plastic, and glass) may be applied according to the design of the equipment; this can effect B. cereus biofilm formation [14]. B. cereus can metabolize a number of carbon sources, including glucose and glycerol [15]. NaCl is widely used in almost all food-processing industries, particularly for preservation or seasoning purposes, and the concentration of NaCl differs among foods. Controlling NaCl content can significantly affect biofilm formation by S. aureus [16]. Therefore, for the food industry, it is important to recognize the potential effect of food-processing-related conditions on B. cereus biofilm formation. This study aims to investigate the biofilm formation ability of B. cereus ATCC 14579 and seven food isolates grown under food-related environmental conditions, including temperature, surface properties, various carbons sources (glucose and glycerol), and/or NaCl.
Materials and methods
Strains and culturing conditions
A total of eight B. cereus strains, of which seven strains were previously isolated from the traditional Korean soybean paste, and a reference strain ATCC 14579 were used in this study (Table 1). Strains were streaked on brain heart infusion (BHI; Becton–Dickinson, France) agar plates using stocks stored at −80 °C in a BHI broth containing 15% (v/v) glycerol (Daejung, Korea). These strains were then incubated at 30 °C for 24 h. A single colony was used to inoculate 10 ml of the BHI broth and incubated overnight (18 h) at 30 °C without shaking.
Table 1.
B. cereus strains used in this study
| B. cereus strain | Source of isolation | Obtained from | References |
|---|---|---|---|
| GIHE 62-5 | Soybean paste | Gangwon Institute of Health and Environment | This study |
| GIHE 62-9 | Soybean paste | Gangwon Institute of Health and Environment | This study |
| GIHE 728-17 | Soybean paste | Gangwon Institute of Health and Environment | This study |
| GIHE 617-4 | Soybean paste | Gangwon Institute of Health and Environment | This study |
| GIHE 617-5 | Soybean paste | Gangwon Institute of Health and Environment | This study |
| GIHE 617-6 | Soybean paste | Gangwon Institute of Health and Environment | This study |
| GIHE 617-8 | Soybean paste | Gangwon Institute of Health and Environment | This study |
| ATCC 14579 | Air | American Type Culture Collection | [33] |
Biofilm formation and quantification
Effect of growth media and incubation time
A biofilm was grown on 96-well polystyrene microtiter plates (flat bottom) (SPL Life Sciences, Korea), as described in [17]. In summary, each well was filled with 200 µl of either BHI or tryptic soy broth (TSB) (MB cell, Korea) and inoculated with 1% (v/v) overnight-grown culture. Microtiter plates were then wrapped with parafilm to prevent evaporation during incubation. These plates were statically incubated at 30 °C for 24 or 48 h. Biofilm formation was quantified using the crystal violet (CV) assay for total biomass estimation, as described previously [18]. Briefly, after appropriate incubation, the media was removed from each well and the attached biofilms were washed thrice with 220 µl of phosphate buffered saline (PBS) (pH 7.4) (Gibco, USA). After washing with PBS, the attached biofilm was stained with (0.1% w/v) CV (Difco, USA) for 30 min. Subsequently, unbound CV was removed and the biofilm was washed again thrice with 220 µl of PBS. Then, 200 µl of 70% ethanol was added for 30 min to elute the CV dye attached to the biofilm. Two hundred microliters of dissolved CV was transferred to a new 96-well plate to measure the absorbance at 595 nm using a spectrophotometer (Spectramax Plus 384, Molecular Devices, UK).
Effect of surface properties and temperature
A biofilm was grown on SS coupons (AISI type 304L, surface finish 2B) (18 × 18 mm2), plastic slides (PS) (18 × 18 mm2), and glass slides (GS) (18 × 18 mm2) in 12-well microtiter plates (SPL Life Sciences, Korea), as described previously [18]. Briefly, previously treated SS, PC, and GS were vertically put into the wells of a 12-well polystyrene plate and filled with 3 ml of TSB. Each well was inoculated with 1.0% (v/v) overnight culture and then incubated at 25 or 30 °C for 48 h. After incubation, biofilm production was quantified using the CV assay, as described above.
Effect of glucose and glycerol
Minimal tryptic soy broth (mTSB) containing 1.7% tryptone (Daejung, Korea), 0.3% soy peptone (MB cell, Korea), 0.25% dipotassium phosphate (Sigma, USA), and 0.5% NaCl (Duksan Pure Chemicals, Korea) was prepared, and the pH was adjusted to 7.3. Then, mTSB was supplemented with glucose (Bio Basic, Canada) or glycerol (Daejung, Korea) concentrations to achieve final concentrations of 0.25, 0.5, 1, 2, 4, 6, and 8% (w/v).
To measure the effect of glucose and glycerol on biofilm formation, a biofilm was formed on 24-well polystyrene microtiter plates (SPL Life Sciences, Korea) by filling each well with 2 ml of mTSB supplemented with different concentrations of glucose or glycerol. It was inoculated with 1% overnight-grown culture. The plates were incubated at 30 °C for 48 h under a static condition. Subsequently, the biofilm was quantified using the CV assay. The pH of the biofilm culture after appropriate incubation was measured using a digital pH meter (Thermo Scientific Orion Star A211, USA) [19]. To perform the CV assay, the media were removed from each well and the biofilm was washed thrice with 2.5 ml of PBS. After washing, the attached biofilm was stained with 2 ml of 0.1% CV for 30 min. Then, the unattached CV dye was removed, and the biofilm was washed again thrice with 2.5 ml of PBS. 2 ml of 70% ethanol was added for 30 min to elute the CV dye attached to the biofilm. The absorbance of dissolved CV was measured using the method described in the previous section.
Effect of sodium chloride
Note that mTSB supplemented with 0.25% of glucose was prepared with different concentrations of NaCl to achieve final percentages (w/v) of 0.0, 0.25, 0.5, 1, 2, 4, 6, and 8%. The water activity (aw) of the biofilm culture was measured using a digital water activity meter (Aquaspector AQS-2-TC, Nagy, Germany). The biofilm was formed with/without NaCl, and it was quantified using the method described in the previous section.
Data analysis
The results represent the average of at least three independent experiments, and each experiment included three biological replicates. The effects of media, incubation time, and temperature on biofilm formation by B. cereus strains were compared using a one-sided t test. Meanwhile, the influence of surface properties, glucose, glycerol, or NaCl on biofilm formation was compared using one-way analysis of variance (ANOVA) with Tukey’s post hoc (IBM SPSS Statistics, Version 22, USA). Statistical significance was considered when the p value was less than 0.05.
Results and discussion
Effect of growth media and incubation time on biofilm formation
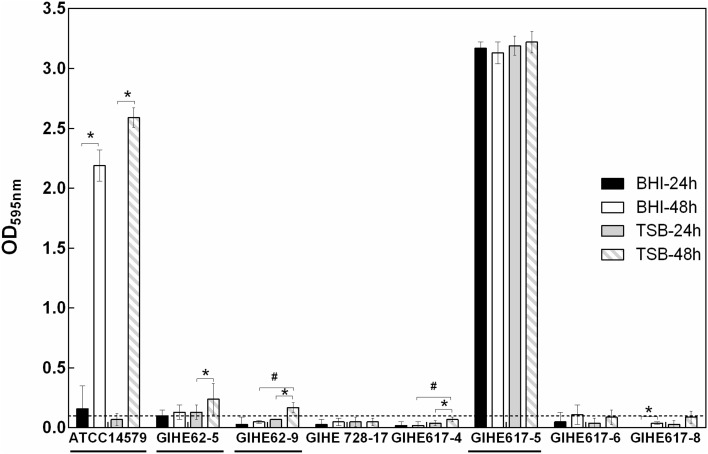
Biofilm formation can be influenced by many factors, including the composition of growth media and incubation time. In this experiment, in an initial attempt to select the appropriate growth media and incubation time that can trigger B. cereus biofilm formation, two types of media (TSB and BHI) and two incubation times (24 and 48 h) were used. Biofilm formation ability was estimated for seven strains isolated from soybean paste and for a reference strain (ATCC 14579) with a known biofilm-forming capacity [20]. A biofilm was formed on 96-well polystyrene plates at 30 °C. Growth media, incubation time, and the strains used greatly influenced B. cereus biofilm formation, as elucidated by the CV assay (Fig. 1). In BHI, after 24 h, two strains (ATCC 14579 and GIHE 617-5) out of the eight strains tested showed biofilm formation ability (OD595nm values > 0.1). After 48 h, two strains (GIHE 62-5 and GIHE 617-5) were capable of biofilm formation [21]. The density of CV staining significantly increased (p < 0.05) for only two strains (ATCC 14579 and GIHE 617-8) when the incubation time was increased from 24 to 48 h (Fig. 1). For the remaining six strains, no change in biofilm formation was observed during the increased incubation time under this growth condition.
Fig. 1.
Biofilm formation by B. cereus strains under different growth conditions. A total of eight strains were grown at 30 °C in either BHI or TSB on 96-well polystyrene microtiter plates for 24 or 48 h, and biofilm formation was quantified by the CV assay. The data represents the average of three independent biological experiments, each performed in triplicates (n = 9), and the standard deviations. The threshold for biofilm formation (solid line) is equal to the background signal plus two times the standard deviation (OD = 0.1). Values higher than the threshold level were considered positive for biofilm formation. The four selected strains used in further experiments are underlined. (asterisk) and (hash) indicate a significant difference (p < 0.05) compared with 24 h of incubation and BHI, respectively (t test, p < 0.05)
Meanwhile, in TSB, after 24 h, GIHE 62-5 and GIHE 617-5 were capable of biofilm formation, and after 48 h, ATCC 14579 and GIHE 62-9 also formed biofilms. These strains did not show biofilm formation ability after 24 h. In TSB, after 48 h, four strains (ATCC14579, GIHE 62-5, GIHE 62-9, and GIHE 617-4) showed significantly higher (p < 0.5) CV staining after 48 h, and for the other strains, no change in biofilm formation was observed.
The amounts of biofilms formed in TSB were significantly higher than that in BHI for two strains (GIHE 62-9 and GIHE 617-4) after 48 h; however, after 24 h, there was no difference in biofilm formation between BHI and TSB for all of the tested strains. Based on these results, TSB and 48 h were selected as the appropriate growth media and time point, respectively, for further biofilm experiments.
The obtained results reveal that growth media as well as incubation time could affect B. cereus biofilm formation. These results are in agreement with a recent study by Gao et al. [19], which showed that B. cereus forms larger biofilms (submerged) in TSB than in other growth media, including BHI. However, these results are contradictory to those reported by Hayrapetyan et al. [21], which described BHI as the optimum growth media for B. cereus biofilm formation. Noticeably, this result was based on a preliminary tests conducted using four different growth media for B. cereus biofilm formation on SS coupons, and the results were not presented. Wijman et al. [22] reported that the biofilm formation ability of B. cereus was largely dependent on the growth media. The superiority of TSB over BHI was also reported for other species, including Salmonella spp. [23]. In this study, we found that some of the tested strains, including the reference strain (ATCC 14579), formed a significantly higher amount of biofilm (p < 0.05) after 48 h than after 24 h in both BHI and TSB. However, there was no difference in the biofilm formation ability during this incubation period among some other strains. A similar result was reported previously, and it showed that some of the B. cereus strains, including ATCC 14579, formed significantly higher amounts of biofilms at 48 h than after 24 h, whereas some strains showed no change in biofilm formation [21, 22]. These results indicate that the biofilm formation ability of B. cereus is greatly affected by strain-specific features. The strain-specific behavior of B. cereus biofilm formation is in agreement with a number of recent studies [21, 22]. Biofilm formation can be largely affected by strain-specific features such as serotype, origin of isolation, and pathogenicity; however, it is largely dependent on the species [22, 23].
Effect of surface properties and temperature on biofilm formation
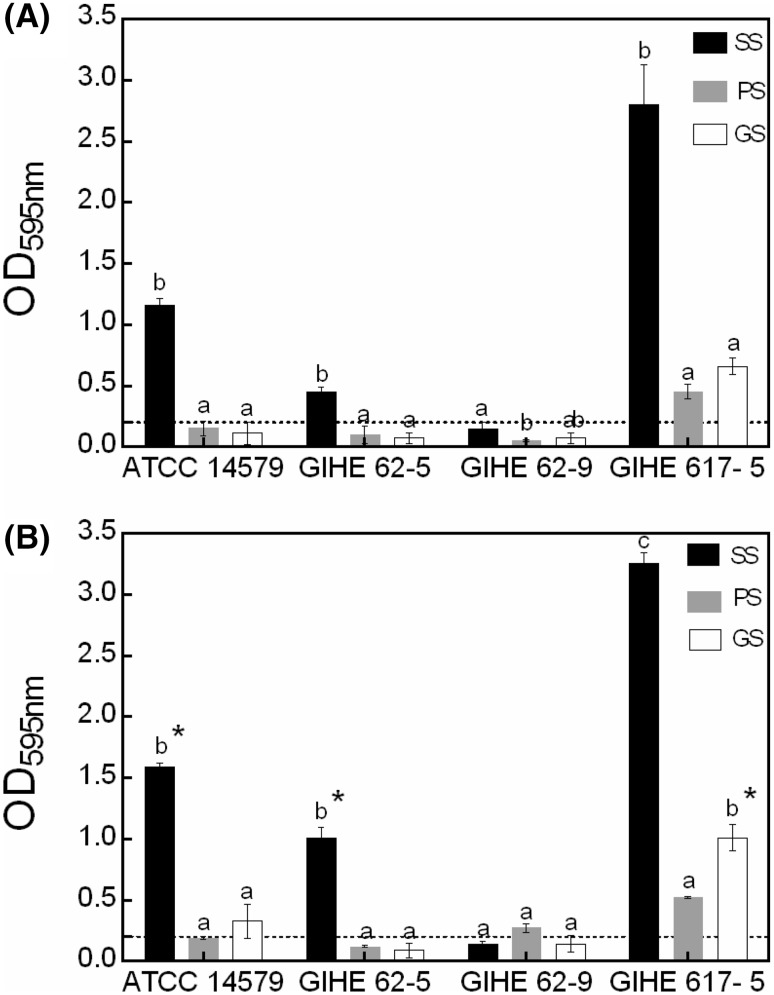
In a food-processing environment, different surfaces and temperatures can be used depending on the equipment design, which may affect B. cereus biofilm formation. Therefore, for selected strains, biofilm formation abilities were investigated for the most commonly used food-processing surfaces such as SS, PC, or GS at 25 or 30 °C. Based on the CV assay, all the tested strains except GIHE 62-9 were capable of biofilm formation at both 25 and 30 °C in TSB after 48 h of incubation [Fig. 2(A)]. Compared with SS, the amounts of biofilms formed on PS and GS were significantly lower (p < 0.05). These results are in agreement with a recent study by Hayrapetyan et al. [21], which reported a significantly higher amount of biofilm formation on SS when compared with PS for B. cereus food isolates. This higher amount of biofilm formation by B. cereus (capable of producing spores, which are hydrophobic) on SS (a hydrophobic surface) might be due to the differences in the thermodynamic properties that facilitate them to adhere together. These data indicate that B. cereus biofilm formation is substantially affected by surface properties. Next, the amount of biofilm formation at 30 °C was significantly higher (p < 0.05) for ATCC 14579, for GIHE 62-5 on SS, and for GIHE 617-5 on GS than that at 25 °C [Fig. 2(B)]. This data suggested that B. cereus biofilm formation is also affected by temperature. The temperature dependence of B. cereus biofilm formation has been reported previously by Wijman et al. [22].
Fig. 2.
Biofilm formation by B. cereus on different surfaces and at different temperatures. A biofilm was grown on stainless steel (SS), plastic slides (PS), or glass slides (GS) at 25 (A) or 30 °C (B) in TSB for 48 h and quantified using the CV assay. The data represents the average of two independent biological experiments, each performed in triplicates (n = 6), and the standard deviations. The threshold of biofilm formation (solid line) is equal to the background absorbance value plus three times the standard deviation (OD = 0.2). Groups with different alphabets within each strain indicate a significant difference (Tukey’s post hoc test p < 0.05). (asterisk) indicates a significant difference (p < 0.05) compared to incubation at 25 °C (t test, p < 0.05)
Effect of glucose and glycerol on biofilm formation
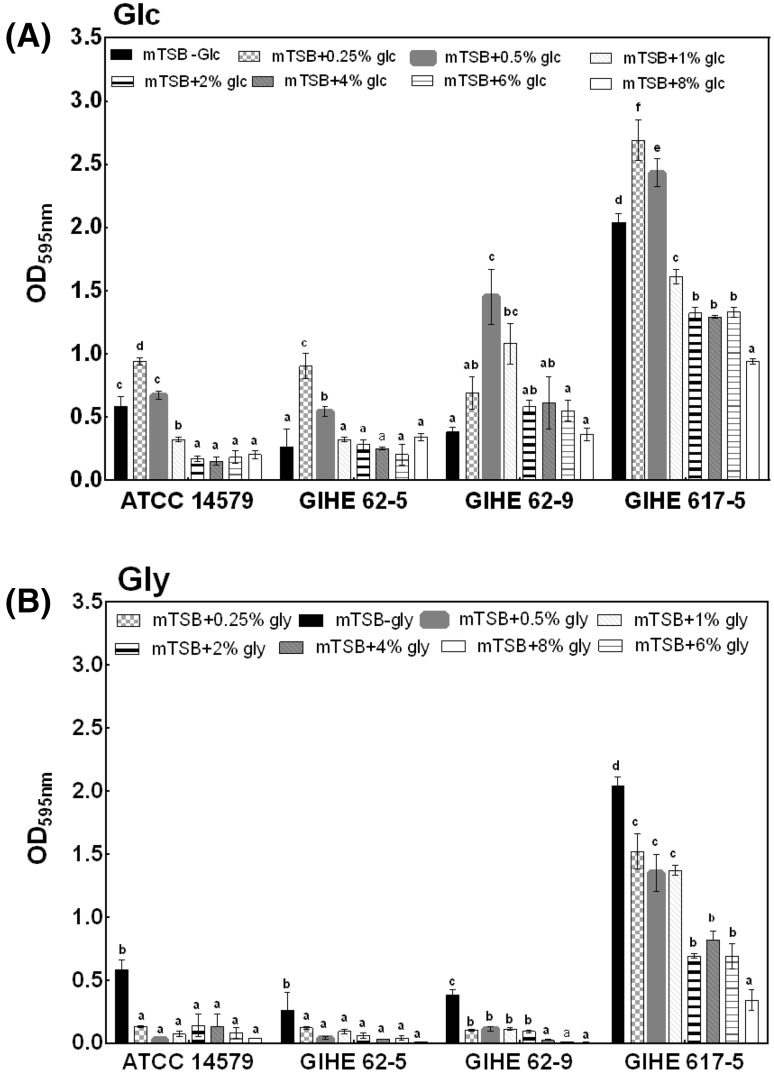
Biofilm formation was estimated for a total of four selected strains in mTSB with or without the addition of glucose or glycerol in the concentration range of 0.25–8%. These strains were selected based on their biofilm formation ability (OD595 nm value > 0.1) in TSB after 48 h of incubation at 30 °C (Fig. 1). The concentrations of glucose or glycerol chosen for this experiment are relevant to the food-industry environment [13]. For these experiments, a biofilm was grown on 24-well polystyrene plates, which have a larger surface area than the 96-well polystyrene plates used in the initial screening experiments described above. The individual effect of glucose or glycerol was compared in mTSB without their addition. The effect of glucose or glycerol on biofilm formation varied among the strains tested; however, for three of the four strains tested, the highest CV readings were obtained in mTSB supplemented with 0.5% glucose [Fig. 3(A)]. For only GIHE 62-9, biofilm formation appeared to increase with glucose concentrations of up to 0.5%. For GIHE 62-5 and GIHE 62-9, no change in biofilm formation was observed in a higher concentration range of glucose, i.e., from 2 to 8%. However, for the remaining two strains (ATCC 14579 and GIHE 617-5), the amount of biofilm formation dramatically decreased with an increasing concentration of glucose from 1 to 8%.
Fig. 3.
Biofilm formation by B. cereus in response to glucose and glycerol. A biofilm was grown on 24-well polystyrene microtiter plates for 48 h at 30 °C in mTSB supplemented with various concentrations of either A glucose or B glycerol broth and quantified using the CV assay. The data represents the average of three independent biological experiments, each performed in triplicates (n = 9), and the standard deviations. Groups with different alphabets within each strain indicate a significant difference (Tukey’s post hoc test p < 0.05)
Interestingly, when glucose was replaced by glycerol as an energy source in mTSB, the amount of biofilm formation significantly reduced (p < 0.5) for all of the tested strains [Fig. 3(B)]. For two strains (ATCC14579 and GIHE 62-5), no change in biofilm formation was observed with the addition of glycerol at concentrations ranging from 0.25 to 8%. However, two strains (GIHE 62-9 and GIHE 617-5) showed a decreased tendency for biofilm formation in mTSB supplemented with an increasing concentration of glycerol ranging from 0.25 to 8%.
The obtained results demonstrate that the addition of a low concentration of glucose (0.25–1%) in mTSB could significantly increase B. cereus biofilm formation; however, a higher concentration of glucose (>1%) inhibited biofilm formation. Gao et al. [19] reported that the addition of glucose (1%) in TSB can trigger B. cereus (905) biofilm formation (particularly, a submerged biofilm) as opposed to the absence of glucose in this growth media. Increased biofilm formation by B. cereus strains in a medium supplemented with a low concentration of glucose might be due to the decreased pH of the medium [19].
To verify whether the pH affects B. cereus biofilm formation during the addition of glucose in mTSB, the pH of the growth medium was measured, as described in the Materials and methods section. When B. cereus was grown in the absence of glucose in mTSB, the pH ranged from 7.84 ± 0.04 to 7.62 ± 0.28 for all of the tested stains (Table 1). In contrast, after addition of 0.25% glucose, the pH of the growth medium significantly decreased (ranging from 6.27 ± 0.07 to 6.78 ± 0.27; p < 0.05). Under this low-pH condition (addition of 0.25% glucose), all the strains formed a significantly higher amount of biofilms (p < 0.05) compared with the case when the pH was higher than 7.0 (absence of glucose in mTSB). However, the amount of biofilm formation increased in GIHE 62-5, GIHE 62-9, and GIHE 617-5 when 0.5% glucose was added (pH ranging from 5.04 ± 0.00 to 5.78 ± 0.01) [Fig. 3(A)]. These results clearly reveal that a low pH due to an increasing concentration of glucose contributes to B. cereus biofilm formation. B. cereus forms biofilms mainly at the air–liquid interface at the wall of the surface and produces pellicles that float on the liquid medium [21]. Additionally, B. cereus can also form submerged biofilms but to a lesser extent [22, 24]. The particular mechanism for different patterns of biofilm formation by B. cereus has not yet been revealed. This bacterium probably uses two distinct mechanisms for the formation of air–liquid biofilms (known to be dependent on SinI or TasA-like proteins) [25] and submerged biofilms (regulated by Spo0A) [19, 26]. B. cereus may use the second pathway for the formation of a biofilm grown under the addition of excess glucose in growth media; this decreases the pH of the biofilm culture and thereby facilitates a higher amount of biofilm formation [19]. A higher amount of biofilm formation during the addition of glucose to the growth media and the consequent decrease in pH has also been reported for other species, for example, S. aureus [27].
Importantly, upon the addition of a higher concentration of glucose (ranging from 0.5% to 8%), some strains, e.g., GIHE 617-5, showed decreased biofilm formation (Fig. 3) in this study even though the pH decreased (Table 2). Therefore, the addition of glucose decreased the pH of growth media; in addition, it might have contributed to other factors, including a decreased aw, which might substantially affect B. cereus biofilm formation [28]. A higher concentration of glucose can inhibit biofilm formation, and this result has also been reported previously for other species, including Aeromonas hydrophila [29].
Table 2.
Effects of glucose on the pH of the B. cereus biofilm formed in mTSB at 30 °C after 48 h
| B. cereus | mTSB | mTSB + Glc (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.25% | 0.5% | 1% | 2% | 4% | 6% | 8% | ||
| 14579 | 7.67 ± 0.40c | 6.78 ± 0.27b | 5.29 ± 0.08a | 5.26 ± 0.01a | 5.22 ± 0.01a | 5.21 ± 0.01a | 5.20 ± 0.01a | 5.12 ± 0.02a |
| 62-5 | 7.62 ± 0.28c | 6.40 ± 0.04b | 5.09 ± 0.08a | 5.19 ± 0.2 a | 5.02 ± 0.01a | 5.05 ± 0.01a | 5.08 ± 0.02a | 4.95 ± 0.00a |
| 62-9 | 7.34 ± 0.16c | 6.27 ± 0.07b | 5.04 ± 0.00a | 5.21 ± 0.28a | 4.99 ± 0.01a | 4.96 ± 0.1 a | 4.94 ± 0.02a | 4.86 ± 0.01a |
| 617-5 | 7.84 ± 0.04d | 6.78 ± 0.08c | 5.78 ± 0.01b | 5.27 ± 0.02a | 5.22 ± 0.02a | 5.30 ± 0.1a | 5.27 ± 0.01a | 5.25 ± 0.06a |
Different alphabets in each row indicate significant differences (Tukey’s post hoc test, p < 0.05)
When we substituted glycerol for glucose in mTSB, the amount of B. cereus biofilm formation significantly decreased for all of the tested strains. Gao et al. [19] found similar results in B. cereus (905) biofilm formation. This could be explained by glycerol uptake or the metabolism mechanism used by Bacillus species. Deletion mutant studies and the addition of glycerol to a minimal growth medium in a previous study resulted in no growth of Bacillus subtilis, thereby causing no production of biofilms [30]. However, this organism grew well when glucose was added to the minimal growth media.
Influence of NaCl on biofilm formation
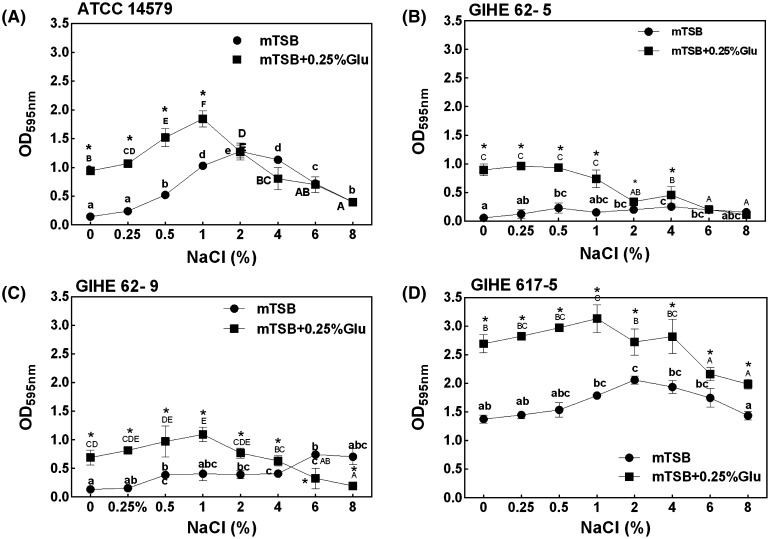
The influence of the four selected B. cereus strains on biofilm formation was investigated in mTSB supplemented with different concentrations of NaCl (ranging from 0.25 to 8%). For this experiment, strains were grown for biofilm formation on 24-well polystyrene microtiter plates in mTSB added with NaCl or a combination of various concentrations of NaCl and glucose (0.25%) for 48 h at 30 °C. The individual effects of NaCl were compared with those of the combination of glucose and NaCl. The concentrations of NaCl were selected from a previous study by Rode et al. [13], which was considered to be relevant to the food-industry-related environment. The effect of NaCl concentration on biofilm formation and the optimum concentration of NaCl widely varied among the tested strains. However, the combination of glucose with NaCl significantly (p < 0.05) increased biofilm formation for all the strains studied when compared to the addition of NaCl alone (Fig. 4). Three strains (ATCC 14579, GIHE 62-9, and GIHE 617-5) showed a higher tendency for biofilm formation when glucose was supplemented with an increasing concentration of NaCl (up to 2%). Meanwhile, for GIHE 62-5, biofilm formation did not change with these concentrations of NaCl combined with glucose. Additionally, all of the strains showed a sharp reduction in biofilm formation when the NaCl concentration increased above 2% with glucose supplementation.
Fig. 4.
Biofilm formation by B. cereus in response to NaCl or a combination of glucose and NaCl. Strains A ATCC14579, B GIHE 62-5, C GIHE 62-9, and D GIHE 617-5 were grown on 24-well polystyrene microtiter plates in mTSB supplemented with different concentrations of NaCl, i.e., ranging from 0 to 8%, or in combination with glucose (0.25%) and NaCl; they were incubated at 30 °C for 48 h. Biofilm formation was quantified using the CV assay for absorbance at 595 nm. The data represents the average of three independent biological experiments, each performed in triplicates (n = 9), and the vertical bars indicate the standard deviations. Groups with different alphabets within each strain and each treatment indicate a significant difference (Tukey’s post hoc test, p < 0.05). Asterisk indicates a significant difference (p < 0.05) compared to NaCl
However, when only NaCl was added in mTSB, an increasing tendency for biofilm formation was noticed for ATCC 14579 and GIHE 617-5 up to a concentration of 2%. However, when the concentration of NaCl was further increased (from 2 to 8%), these two strains showed a reducing tendency for biofilm formation. Meanwhile, there was no dramatic change in the biofilm formation ability with an increasing concentration of NaCl for GIHE 62-5 and GIHE 62-9. These data indicate highly diverse and complex patterns of biofilm formation by the four selected B. cereus strains when they were exposed to different concentrations of NaCl. The combination of glucose with different concentrations of NaCl added to mTSB led to an extreme change in the biofilm formation behavior of all of the tested strains. The obtained results show that in mTSB, biofilm formation by B. cereus significantly increased at low NaCl concentrations in combination with glucose; however, at a high concentration of NaCl, biofilm formation significantly decreased. This might be due to the fact that high concentration of NaCl in the growth medium might result in a lower aw in the biofilm culture, which leads to disruption of normal cellular functions. Therefore, we tested the effect of aw with an increasing concentration of NaCl in the mTSB growth medium used for biofilm formation. The aw of mTSB considerably decreased with increasing concentration of NaCl (Table 3). The aw of mTSB without the addition of NaCl was found to be 0.998. When NaCl was added at an increasing concentration range (from 0.25 to 2%), there was no noticeable change in aw. However, when the NaCl concentration was increased from 4 to 8%, aw substantially changed from 0.96 to 0.94. These results confirm that osmotic drift due to the increasing concentration of NaCl in the growth medium inhibits B. cereus biofilm formation. A previous study on Salmonella enterica described a similar biofilm formation behavior under an osmotic stress condition produced by NaCl [31].
Table 3.
Effect of the increase in NaCl concentration on aw in mTSB
| B. cereus | mTSB | mTSB + NaCl (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.25% | 0.5% | 1% | 2% | 4% | 6% | 8% | ||
| aw | 0.998 | 0.997 | 0.995 | 0.992 | 0.99 | 0.977 | 0.964 | 0.939 |
In conclusion, this study described the physiological phenotypes of B. cereus biofilm formation under food-related conditions, including the presence of two carbon sources (glucose and glycerol) and NaCl. The obtained results showed a highly diverse behavior of B. cereus biofilm formation under food-related conditions. This study indicated that the presence of glucose and/or NaCl could promote B. cereus biofilm formation. A previous study showed that the B. cereus biofilm is highly resistant to disinfectants [32] and contains up to 90% of the spores of the total biofilm cells [22]. Therefore, a higher amount of biofilm formation under food-industry-related environmental conditions might be related to food safety issues because biofilms formed on industrial equipment surfaces can act as a source for spore formation, thereby contaminating the food products. However, as food is made of many components, before making general conclusions regarding B. cereus biofilm formation induced by glucose, glycerol, or NaCl, further studies are required to elucidate the effects of food-related environmental conditions.
Acknowledgements
This work was supported by a research grant from the Kangwon National University, 2015, and a Grant (Grant No. 22A20153713433) from the Brain Korea (BK) 21 Plus project funded by the Government of the Republic of Korea. The sponsor had no role in the study design, data collection, analysis, interpretation, decision to publish, or the preparation of this manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 2.Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 3.Stoodley P, Sauer K, Davies DG, Costerton JW. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002;56:187–209. doi: 10.1146/annurev.micro.56.012302.160705. [DOI] [PubMed] [Google Scholar]
- 4.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial Biofilms. Annu. Rev. Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez CJ, Mende KB, Miriam LA, Kevin SR, Desiree RW, Joseph CM, Clinton K. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect. Dis. 2013;13:47. doi: 10.1186/1471-2334-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall-Stoodley L, Costerton JW, Stoodley P, State M, Engineering B. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 7.Fratamico PM, Bhunia AK, Smith JL. Foodborne Pathogens: Microbiology and Molecular Biology. Norwich, England: Caister Academic Press; 2005. pp. 409–419. [Google Scholar]
- 8.Kotiranta A, Lounatmaa K, Haapasalo M. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes. Infect. 2000;2:189–198. doi: 10.1016/S1286-4579(00)00269-0. [DOI] [PubMed] [Google Scholar]
- 9.Christison CA, Lindsay D, von Holy A. Cleaning and handling implements as potential reservoirs for bacterial contamination of some ready-to-eat foods in retail delicatessen environments. J. Food Prot. 2007;70:2878–2883. doi: 10.4315/0362-028X-70.12.2878. [DOI] [PubMed] [Google Scholar]
- 10.Srey S, Jahid IK, Ha S. Do. Biofilm formation in food industries: A food safety concern. Food Control. 2013;31:572–585. doi: 10.1016/j.foodcont.2012.12.001. [DOI] [Google Scholar]
- 11.Garrett TR, Bhakoo M, Zhang Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008;18:1049–1056. doi: 10.1016/j.pnsc.2008.04.001. [DOI] [Google Scholar]
- 12.Chmielewski RAN, Frank JF. Biofilm Formation and Control in Food Processing Facilities. Compr. Rev. Food Sci. Food Saf. 2003;2:22–32. doi: 10.1111/j.1541-4337.2003.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 13.Rode TM, Langsrud S, Holck A, Møretrø T. Different patterns of biofilm formation in Staphylococcus aureus under food-related stress conditions. Int. J. Food Microbiol. 2007;116:372–383. doi: 10.1016/j.ijfoodmicro.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Hussain MS, Oh DH. Substratum attachment location and biofilm formation by Bacillus cereus strains isolated from different sources: Effect on total biomass production and sporulation in different growth conditions. Food Control. 2017;77:270–280. doi: 10.1016/j.foodcont.2017.02.014. [DOI] [Google Scholar]
- 15.Rosenfeld E, Duport C, Zigha A, Schmitt P. Characterization of aerobic and anaerobic vegetative growth of the food-borne pathogen Bacillus cereus F4430/73 strain. Can. J. Microbiol. 2005;51:149–158. doi: 10.1139/w04-132. [DOI] [PubMed] [Google Scholar]
- 16.Lim Y, Jana M, Luong TT, Lee CY. Control of Glucose- and NaCl-Induced Biofilm Formation by rbf in Staphylococcus aureus. J. Bacteriol. 2004;186:722–729. doi: 10.1128/JB.186.3.722-729.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merritt JH, Kadouri DE, O’Toole GA (2005) Growing and analyzing static biofilms. Curr Protoc Microbiol Chapter 1:Unit 1B.1. [DOI] [PMC free article] [PubMed]
- 18.Castelijn GAA, Parabirsing JA, Zwietering MH, Moezelaar R, Abee T. Surface behaviour of S. Typhimurium, S. Derby, S. Brandenburg and S. Infantis. Vet. Microbiol. 2013;161:305–314. doi: 10.1016/j.vetmic.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 19.Gao T, Foulston L, Chai Y, Wang Q, Losick R. Alternative modes of biofilm formation by plant-associated Bacillus cereus. Microbiology open. 2015;4:452–464. doi: 10.1002/mbo3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayrapetyan H, Tempelaars M, Nierop Groot M, Abee T. Bacillus cereus ATCC 14579 RpoN (Sigma 54) Is a Pleiotropic Regulator of Growth, Carbohydrate Metabolism, Motility, Biofilm Formation and Toxin Production. PLoS One. 2015;10:e0134872. doi: 10.1371/journal.pone.0134872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayrapetyan H, Muller L, Tempelaars M, Abee T, Groot MN. Comparative analysis of biofilm formation by Bacillus cereus reference strains and undomesticated food isolates and the effect of free iron. Int. J. Food Microbiol. 2015;200:72–79. doi: 10.1016/j.ijfoodmicro.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Wijman JGE, De Leeuw PPLA, Moezelaar R, Zwietering MH, Abee T. Air-liquid interface biofilms of Bacillus cereus: Formation, sporulation, and dispersion. Appl. Environ. Microbiol. 2007;73:1481–1488. doi: 10.1128/AEM.01781-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stepanović S, Ćirković I, Ranin L, Švabić-Vlahović M. Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett. Appl. Microbiol. 2004;38:428–432. doi: 10.1111/j.1472-765X.2004.01513.x. [DOI] [PubMed] [Google Scholar]
- 24.Majed R, Faille C, Kallassy M, Gohar M. Bacillus cereus Biofilms-same, only different. Front. Microbiol. 2016;7(July):1054. doi: 10.3389/fmicb.2016.01054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caro-Astorga J, Perez-Garcia A, de Vicente A, Romero D. A genomic region involved in the formation of adhesin fibers in Bacillus cereus biofilms. Front. Microbiol. 2015;5(January):1–11. doi: 10.3389/fmicb.2014.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan F, Yiyang WLL, Yuming G, Jian HC, Yunrong YF. The comER gene plays an important role in biofilm formation and sporulation in both Bacillus subtilis and Bacillus cereus. Front. Microbiol. 2016;7(June):1–16. doi: 10.3389/fmicb.2016.01025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foulston L, Elsholz AKW, DeFrancesco AS, Losick R. The extracellular matrix of Staphylococcus aureus biofilms comprises cytoplasmic proteins that associate with the cell surface in response to decreasing pH. MBio. 2014;5:e01667-14. doi: 10.1128/mBio.01667-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbosa-Cánovas GV, Fontana AJ, Schmidt SJ, Labuza TP. Water Activity in Foods: Fundamentals and Applications. Ames: Blackwell; 2007. pp. 229–272. [Google Scholar]
- 29.Jahid IK, Lee N-Y, Kim A, Ha S-D. Influence of glucose concentrations on biofilm formation, motility, exoprotease production, and quorum sensing in Aeromonas hydrophila. J. Food Prot. 2013;76:239–247. doi: 10.4315/0362-028X.JFP-12-321. [DOI] [PubMed] [Google Scholar]
- 30.Shemesh M, Chaia Y. A combination of glycerol and manganese promotes biofilm formation in Bacillus subtilis via histidine kinase KinD signaling. J. Bacteriol. 2013;195:2747–2754. doi: 10.1128/JB.00028-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lianou A, Koutsoumanis KP. Strain variability of the biofilm-forming ability of Salmonella enterica under various environmental conditions. Int. J. Food Microbiol. 2012;160:171–178. doi: 10.1016/j.ijfoodmicro.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Peng J. Sen, Tsai WC, Chou CC. Surface characteristics of Bacillus cereus and its adhesion to stainless steel. Int. J. Food Microbiol. 2001;65:105–111. doi: 10.1016/S0168-1605(00)00517-1. [DOI] [PubMed] [Google Scholar]
- 33.Lindbäck T, Mols M, Basset C, Granum PE, Kuipers OP, Kovács ÁT. CodY, a pleiotropic regulator, influences multicellular behaviour and efficient production of virulence factors in Bacillus cereus. Environ. Microbiol. 2012;14:2233–2246. doi: 10.1111/j.1462-2920.2012.02766.x. [DOI] [PubMed] [Google Scholar]