Abstract
With increasing application of yeast in fermented milk, in order to study the effect of yeast on milk protein during the fermentation process, the effects of the presence of Kluyveromyces marxianus in milk fermented by Streptococcus thermophilus and Lactobacillus bulgaricus were investigated. After fermentation, the amino acid, protein, and peptide contents were analyzed by ultra-performance liquid chromatography, two-dimensional gel electrophoresis, and liquid chromatography–mass spectrometry, respectively. After the addition of K. marxianus for fermentation, 25 protein spots changed significantly. These were mostly caseins and bovine serum proteins, and the content of total free amino acids increased by 16.30%; ten types of bioactive peptides were identified. Furthermore, the number of peptide types in milk fermented by K. marxianus increased significantly compared with milk fermented by Lactobacillus. K. marxianus is considered to promote proteometabolism in milk when added with Lactobacillus, generate flavor compounds, and improve the digestion and absorption character of milk.
Keywords: Kluyveromyces marxianus, Fermented milk, Protein, Peptide, Amino acid
Introduction
Fermented milk is a very popular dairy product consumed throughout the world. Proteolysis that occurs during the fermentation process is complex and varied; moreover, it has been verified as the most important biological mechanism that plays a vital role in determining the texture and flavor of milk [1]. The general process of proteolysis in milk starts with hydrolysis of caseins by proteinases that originate from rennet and lactic acid bacteria. This produces a series of intermediate-sized peptides, which are further hydrolyzed by peptidases into free amino acids (FAAs) [2]. A range of dairy products can be prepared by the action of different fermentation strains that release different proteases and peptidases. These enzymes degrade caseins into different peptides, FAAs, and flavor substances [1]. Lactobacillus bulgaricus and Streptococcus thermophilus are the most popular starters used to ferment dairy products and increase acidity, and their abilities to hydrolyze protein are well reported. Use of starters has great advantages in metabolizing beta-casein and alpha-S1-casein and increases the content of short-chain peptides and FAAs [2].
With continued research on starter cultures, yeast has emerged as a natural fermentation strain in milk fermentation processing and is one of the major dairy microflora after lactic acid bacteria [3]. Previously, it was believed that yeast could cause spoilage, undesirable odor, and defects [4]; however, with further research, scholars have found that some yeasts can be used as subsidiary starters to change the structure of dairy products during fermentation and maturation [4, 5]. Yeasts have protein hydrolytic activity and can generate aroma precursors that influence the flavor, texture, quality, and structure of dairy products. When used as a secondary starter culture, yeast can metabolize lactose and galactose and produce some flavor substances, such as ethanol and glycerol, to impart a pleasant flavor to fermented milk [6, 7]. At the same time, the production of alcohol and carbon dioxide can inhibit the growth of harmful bacteria and produce potential prebiotics for human use [8, 9]. As we all know, fermentation is used for milk protein degradation, and the degradation of milk proteins depends on the proteolytic pathway of the added lactic acid bacteria, which have been reported as an efficient and safe way to produce food-grade hydrolytic and bioactive peptides [10, 11]. Therefore, major starter cultures have been used to improve the fermentation process to alter the hydrolytic properties of proteins and increase peptide generation. Accordingly, there is an urgent need for a method to degrade milk proteins and to produce a novel dairy product rich in (bioactive) peptides for special populations.
Kluyveromyces marxianus is the main yeast group used in Kefir yogurt. It can metabolize lactose, citrate, protein, and fat [12], and its growth and milk composition changes have been studied [13]. Herein, we provide further discussion on the influence of K. marxianus in milk when fermented with S. thermophilus and L. bulgaricus, which are the two strains commonly used in yogurt. We focus on the changes in the amino acid, protein, and peptide content, which were analyzed by ultra-performance liquid chromatography (UPLC), two-dimensional electrophoresis (2-DE), and liquid chromatography–tandem mass spectrometry (LC–MS/MS). A number of bioactive peptides in fermented milk samples have been identified after comparison with other studies. We expect the use of yeast as a starter culture in milk fermentation with lactic acid bacteria to become a new research hotspot.
Materials and methods
Sample preparation
Streptococcus thermophilus (St), Lactobacillus bulgaricus (Lb), and Kluyveromyces marxianus (Km) were obtained from Beijing Sanyuan Food (Beijing, China). Reconstituted full cream milk (12%, w/v; Murray Goulburn, Melbourne, Australia) was heated at 110 °C for 15 min and used as a skimmed milk medium. The strains were stored at −80 °C and were activated before use. St and Lb were cultured in sterile milk at 37 and 42 °C, respectively. Then, they were subcultured in the same sterile milk in a ratio of 3% (v/v) for two or three generations to get activated. Km was cultured in Potato Dextrose Broth (PDB) medium (Beijing Land Bridge Technology Co., Ltd., Beijing, China) in a shaker with 180 rpm/min at 28 °C (2102c, Shanghai ZhiCheng analysis instrument manufacturing Co., Ltd., Shanghai, China) for 24; furthermore, it was subcultured 2–3 times in the same medium in a ratio of 3% (v/v) to get activated.
The concentrations of Lb, St, and Km in the medium were assessed by the dilution-plate method in de Man Rogosa and Sharpe medium (Beijing Land Bridge Technology, Beijing, China) and PDB medium. Lb, St, and Km were inoculated into sterile milk in the relative proportions of Lb:St:Km = 15:15:1, with a total concentration of bacteria in milk of 106 cfu/mL. This mixture was regarded as the mixed fermentation team. Lb and St were inoculated in the proportion of 1:1 under the same conditions as the Lactobacillus fermentation team. Raw milk was used as the negative control. All fermentations were incubated at 35 °C (LRH-250, Yiheng Technology Instrument, Shanghai, China) until pH 4.6 was attained.
Extraction of peptides and preparation for peptide analysis
The concentration of small peptides (<20 amino acids) and free amino groups in 12% (w/v) trichloroacetic acid (TCA) (12% TCA-SN) was determined as follows. The milk sample (3 g) was mixed with 3 mL of 24% (w/v) TCA, and the mixture was left for 30 min. The precipitated proteins were removed by centrifugation at 5000×g for 20 min at 4 °C in a refrigerated centrifuge (3K-15, Sigma, Osterode am Harz, Germany). The supernatant was filtered through a red ribbon filter paper (Xinxing, Hangzhou, China), and the filtrate was purified by Oasis® HLB extraction cartridges (Waters, Milford, MA, USA) after its protein concentration was analyzed by the Bradford method [14]. The eluent after extraction was collected and freeze-dried (Freeze Dry System, Labconco, Kansas City, MO, USA) into a powder. The powder was dissolved in ultrapure water containing 0.1% (v/v) formic acid (FA) before analysis by LC–MS/MS (solution concentration not more than 1 µg/µL). All reagents used in this step were of LC–MS-reagent grade.
Sample preparation for free amino acid analysis by UPLC
FAAs were extracted from milk as follows. Supernatant from the centrifugation described above was ultra-filtered in 3-kDa ultrafiltration centrifuge tubes (Millipore, Bedford, MA, USA) at 7000×g and 4 °C for 20 min. The samples and working amino acid standard were treated according to the method described in the AccQ-Tag Ultra Derivatization Kit Care and Use Manual (Waters) and Roucher et al. [15]. Briefly, the steps were as follows: 20 µL of derivatizing solution was added to the mixture containing 70 µL of buffer solution and 10 µL of sample. The mixture was vortexed for a few seconds, incubated for 1 min, and then at 55 °C for 10 min. As a control, 80 µL of buffer and 20 µL of derivation agent were mixed without heating. Samples were filtered through a 0.22-μm filter (Millipore) before injection.
FAAs were determined using the Waters UPLC® Amino Acid Analysis Application Solution (Waters, Milford, MA, USA), which used a Waters ACQUITY UPLC H-Class system, included derivatization chemistry, separation column, eluents, methods, and software. The Waters UPLC comprised a quaternary solvent management system (QSM), a sample manager–flow through needle (SM-FTN), a column oven (CH-A) that included a Waters C18 column (1.7 μm, 2.1 × 100 mm), and a photodiode array detector (250 nm). Chromatographic separation was achieved with solvent A (AccQ-Tag eluent A), solvent B (ultrapure water: AccQ-Tag eluent B = 9:1, v/v), solvent C (ultrapure water), and solvent D (AccQ-Tag eluent B) at a flow rate of 0.7 mL/min. The solvent gradient program is described in the Amino Acid Analysis Application Solution and not shown here. The column temperature was maintained at 49 °C, and the injection volume was 1 µL. All solvents used as reagents were from the Solution Kit or were of HPLC grade. The retention times of pure standards were compared with sample chromatograms to identify the amino acids.
Samples preparation for 2-DE
Milk samples were prepared as described by Goncalves et al. [16] with some modification. Acetone solution (containing 0.07% β-mercaptoethanol) was added to milk samples in a ratio of 3:1 (v/v). Each sample was shaken for 10 s, incubated 12 h at −20 °C, and centrifuged at 11,000×g at 4 °C for 5 min. The supernatant was discarded, whereas the protein was washed three times with 80% acetone solution (containing 0.07% β-mercaptoethanol) and then dried under a stream of ultra-high-purity nitrogen. Excess lysis buffer solution (containing 40 mM Tris, 7 M urea, 2 M thiourea, 4% CHAPS buffer, 1% dithiothreitol (DTT), 1 mM disodium EDTA) was added to achieve complete lysis of proteins; the mixture was sonicated for 5 min and incubated at 4 °C for at least 2 h to completely dissolve the precipitate. The concentration of protein was determined by the modified Bradford method with bovine serum albumin (BSA) as the standard [14]. Samples were stored at −80 °C until used.
Two-dimensional gel electrophoresis and image analysis
Protein sample was mixed with 450 µL of immobilized pH gradient (IPG) rehydration buffer containing 8 M urea, 2 M thiourea, 4% (w/v) CHAPS buffer, 0.5% (v/v) IPG buffer (pH 4–7) (GE Healthcare, Little Chalfont, UK) and trace amount of bromophenol blue. The mixture was vortexed for 1.5 min and the protein sample was added to 24 cm strips and placed in the Immobiline DryStrip Reswelling Tray (GE Healthcare) to rehydrate for 16 h at 20 °C.
After 23–24 h of isoelectric focusing (IEF) in the IPGphor II isoelectric focusing system (GE Healthcare) at 20 °C, samples were separated in IPG strips (GE Healthcare) in one dimension. The maximum current setting was 50 mA/strip and IEF was performed for a total voltage of 70 kVh. Following IEF, IPG strips were rinsed with ultrapure water and then equilibrated twice for 15 min with gentle stirring in a solution containing 50 mM Tris–HCl (pH 8.8), 6 M urea, 2% (w/v) sodium dodecyl sulfate (SDS), and 30% (v/v) glycerol. The protein was then added to 2% DTT and 2.5% iodoacetamide to equilibrate.
The second dimension of electrophoresis was conducted in an Ettan DALTtwelve electrophoresis system (GE Healthcare) with 13% polyacrylamide gels. Protein markers were applied in the range of 14.4–97.4 kDa (14.4, 20.1, 31, 43, 66.2, 97.4 kDa). After SDS-separation, proteins were fixed for 60 min in 10% (v/v) acetic acid and 40% (v/v) methanol, and the gel was stained 12 h with colloidal Coomassie brilliant blue G-250 [16]. The gel was gray-scanned with an UMAX Image Scanner (GE Healthcare) and the image was analyzed by ImageMaster 2D Platinum 7.0 software (GeneBio, Geneva, Switzerland). Background cutting, spot detection, and automatic matching were performed by the software, and detection point correction was performed manually. The software used relative quantitation when calculating the protein content of a point.
Protein spot picking and in-gel tryptic digestion
Selected protein spots were manually excised from the gels and cut into 1–2-mm colloidal particles; each of them was placed into a 1.5-mL Eppendorf safe-lock tube. The gel particles were soaked in ultrapure water for 30 min at 37 °C, and then washed with 100 µL of destaining solution (acetonitrile (ACN):100 mM NH4HCO3 = 3:7) for 30 min several times. The gels were washed twice with ultrapure water and dehydrated in ACN for 15 min. The ACN fraction was discarded and the residue dried for at least 5 min. The dried gel pieces were incubated with 10 µL of 25 ng/µL sequence-grade trypsin (TPCK Treated, Sigma, St. Louis, MO, USA) in 50 mM NH4HCO3 at 4 °C for 45 min. Redundant enzyme liquid was removed, and the remainder was incubated with 10 µL of working solution (10% ACN, v/v; 50 mM NH4HCO3) at 37 °C for 9–11 h. The working solution was removed and placed in new Eppendorf tubes. Extracting liquid (50% ACN and 0.1% FA) was added and the tubes were sonicated for 10 min, with the process repeated twice. The solutions were combined and freeze-dried to afford lyophilized peptide that was suspended in 0.1% FA solution with the peptide concentration below 1 µg/uL [17].
Protein identification
The peptide sample was analyzed using an Ultimate3000 RSLCnano System coupled with a Thermo Scientific Q Exactive Quadrupole-Orbitrap mass spectrometer (Thermo Fisher Scientific, Waltham, MA USA) according to the methods of Holder et al. [18] and Jrad et al. [19] with some modifications. A 1 uL sample of protein was loaded onto an RP-HPLC enrichment C18 Acclaim PepMap100 column (100 µm × 2 cm, nanoViper, C18, 5 µm, 100 Å; Thermo Scientific) with a C18 Acclaim PepMap RSLC analytical column (75 µm × 15 cm, nanoViper, C18, 2 µm, 100 Å; Thermo Scientific). Chromatographic separation was performed with solvent A (water:ACN:FA = 98:2:0.1, v/v/v) and solvent B (water:ACN:FA = 20:80:0.1, v/v/v) at a flow rate of 0.25 µL/min. The gradient was as follows: 4% solvent B for 4 min, increased linearly to 35% solvent B in 95 min, then increased to 90% solvent B in 10 min. The column was re-equilibrated under the starting condition for 8 min before the next sample was injected. The total analysis time was 120 min per sample. The column temperature was maintained at 35 °C.
Ionization of peptides by electrospray ionization was performed in positive mode at a voltage of 2.5 kV. The ion source temperature was 300 °C. Each MS spectrum was acquired at the range of m/z values of 200–3000 and MS/MS fragmentation was performed on the ten most intense ions.
Peptide information from MS/MS spectra was analyzed by Thermo Proteome Discoverer 1.4 (PD 1.4, Thermo Scientific). The peptide identification database was composed of the bovine database downloaded from www.ncbi.org. The PD soft search parameters were specified as follows: an unspecific enzyme cleavage was selected, a 0.02-Da mass tolerance was allowed on fragment ions, and a 10-ppm precursor mass tolerance was allowed for each peptide identified. For valid peptide identification, the minimum score considered necessary for the q-value was below 0.05 [18, 19].
Sequence alignments with known peptides displaying any biological activity were performed with the NCBI database available at http://www.ncbi.nlm.nih.gov.
Statistical analysis
SPSS 13.0 (SAS Institute, Cary, NC, USA) was used for the statistical analysis of variance to determine the effects of starters on milk.
Results and discussion
Free amino acids in fermented milk
After milk fermentation, we detected and quantified 16 types of FAAs in the milk using an UPLC H-Class System, with an analysis time of 10 min. In the fermented milk, the contents of Pro, Thr, and total FAA were increased significantly, as shown in Table 1. In addition, the content of Cys increased significantly in the mixed-fermented milk compared with raw milk; the content of total FAA and Cys increased by 16.30 and 12.48%, respectively. This phenomenon was supported in part by the data of Roostita et al. [13], who compared general yeast species in milk and found that Km has weaker proteolytic reactions. After growth in milk, the dominant amino acids included Leu, Val, Ala, Ile, and Phe. Furthermore, the content of Arg, Asp, and Gly was significantly decreased (P < 0.01), particularly the content of Glu in the mixed fermentation team. As the precursors of flavors, such as alcohols, aldehydes, esters, carboxylic acids, thiols, and thioesters, the contents of those amino acids change would significantly affect the flavor of fermented milk [20].
Table 1.
The concentration of free amino acid in raw milk and milk after 6 h fermentation at 35 °C (× 10−6 M)
| Raw milk | Lactobacillus fermentation | Mixed fermentation | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Ala/Met | 34.75a | 1.06 | 27.67a | 5.66 | 55.69b | 7.07 |
| Arg | 26.52a | 0.71 | 21.38b | 2.83 | 25.46ab | 0.71 |
| Asp | 46.45a | 4.95 | 23.62ab | 8.49 | 19.70b | 4.24 |
| Cys | 381.25B | 9.19 | 413.19AB | 4.24 | 428.84A | 16.97 |
| Glu | 197.22A | 10.61 | 205.11A | 7.07 | 103.57B | 9.90 |
| Gly | 60.36A | 7.07 | 13.27B | 0.00 | 25.45B | 0.71 |
| His | 5.82a | 0.71 | 10.33a | 4.95 | 16.83a | 2.83 |
| Ile | 15.42a | 6.36 | 7.58a | 0.00 | 30.80a | 1.41 |
| Leu | 7.77a | 0.71 | 6.52a | 2.83 | 11.50a | 4.95 |
| Lys | 10.12a | 0.71 | 5.74a | 0.00 | 13.00a | 4.24 |
| Phe | 5.37a | 3.54 | 4.18a | 2.12 | 9.18a | 1.41 |
| Pro | 17.00C | 1.41 | 85.44B | 9.19 | 155.34A | 5.66 |
| Ser | 21.20a | 0.00 | 12.68a | 0.71 | 25.18a | 5.66 |
| Thr | 10.29a | 2.83 | 18.62a | 1.41 | 32.68a | 1.41 |
| Tyr | 1.54a | 0.71 | 10.23a | 0.71 | 8.50a | 2.12 |
| Val | 7.76a | 0.71 | 10.50a | 2.12 | 20.45a | 1.41 |
| Total | 848.84B | 7.42 | 876.06B | 21.21 | 982.17A | 42.43 |
a, bValues in table with different letters differ significantly (P ≤ 0.05)
A, BValues in table with different letters differ highly significantly (P ≤ 0.01)
Typically, various malty flavor compounds produced by branched-chain amino acids, such as acetaldehyde, as the major taste compounds in yogurt. It is generated from Thr, and some other flavor compounds are caused by Asn and Pro [4, 19]. Lactobacillus probably produces a malty flavor in fermented milk but below the taste threshold. This is usually because of the low proteolytic activity, which leads to low levels of essential amino acids in milk. When Km grows with Lactobacillus in milk, it either obtains sufficient energy-giving substrates from the milk or uses metabolites from Lactobacillus and produces compounds with a malty flavor [19] such Ala/Met, Thr, Pro, and Val (see Table 1). In other words, Km and Lactobacillus have a synergism in the metabolism [12] and produced a mild taste in fermented dairy products.
In fact, in the milk fermentation process, Km not only produces high amounts of FAAs but also consumes lactic acid produced by Lactobacillus. In the study by Akabanda et al. [20], a strain of Km was able to reduce the pH of fermented milk (as we also found in our study and data not shown) and produce appreciable amounts of acetaldehyde, ethanol, and carbon dioxide, thereby imparting unique flavor and taste to fermented milk. Mutualism of bacteria is a popular topic of research currently, and the catabolism of lactate and amino acid by Lactobacillus and Km confirms that these substrates complement each other and can be used to deacidify dairy products.
Analysis of fermented milk by 2-DE
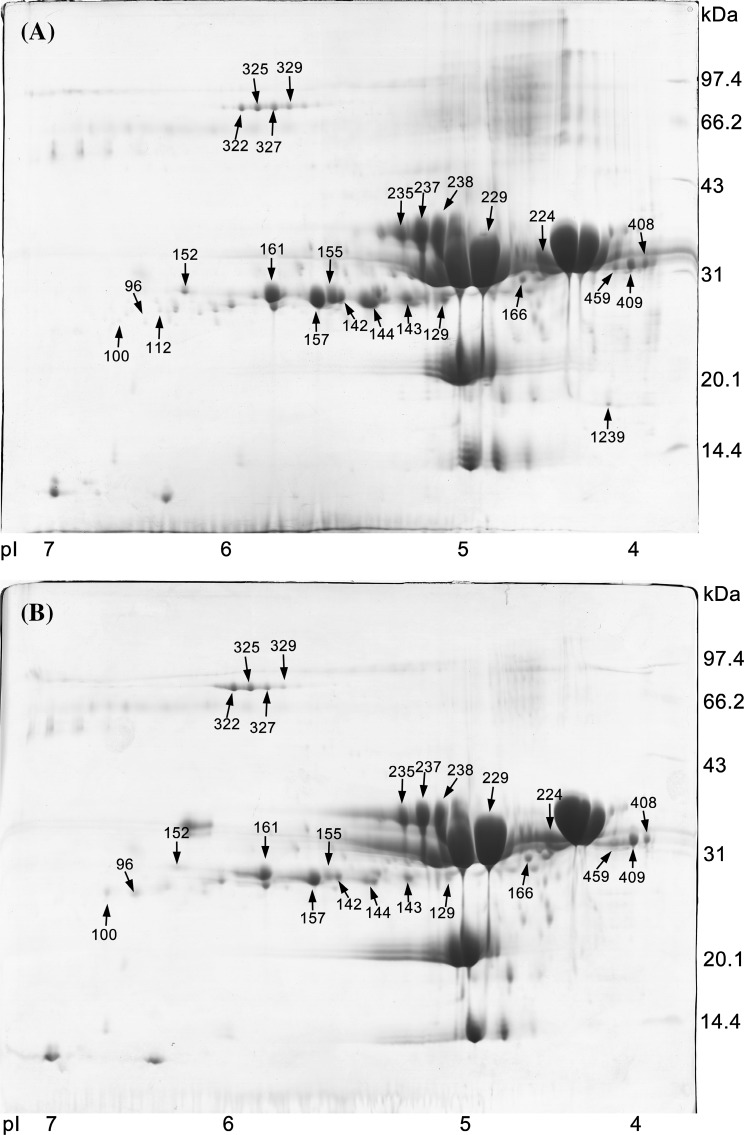
We prepared raw milk, Lactobacillus-fermented milk, and mixed-fermented milk for 2-DE analysis. The changes in protein content in the different samples were visualized on 2-DE gels (Fig. 1, the data of raw milk protein gel maps not shown). After image analysis, 25 of the most differentiated spots were selected for mass spectrometry analysis.
Fig. 1.
Coomassie stained 2D electropherograms of Lactobacillus fermentation (A) and mixed fermentation (B) (6 h fermentation at 35 °C) milk protein gel maps, (pH 4–7 NL). The major milk proteins are labeled; apparent pH and molecular mass are shown on the horizontal and vertical axes, respectively
The relative protein content of the different protein spots (% Vol × 1000) was calculated. The abundance of protein in the 25 spots was varied, and the mean content of each protein spot is not shown. The changes in protein content for protein spots across the different cultures show the variation in hydrolysis and indicate the different protease activity of the bacteria in the fermentation process.
In different environments, the metabolic activities of Km (e.g., proteolytic and lipolytic) and conditions such available oxygen may change. These factors would be expected to be very different depending on the presence or absence of Lactobacillus in fermented milk [21]. Our results showed that the protein content was significantly different between samples because of the interaction between Km and Lactobacillus in the fermentation process. In addition, it should be noted that Km fermentation can last 2–3 days but the short fermentation period used in this study may have resulted in weak interaction between Km and Lactobacillus and only slight changes in protein content [22].
Identification and change of protein spots after 2-DE separation
All the selected 25 protein spots were excised from the gel for further nano-LC–ESI–MS/MS detection and analysis. Protein identification data including accession number, description, experimental and theoretical pI value/MW, and three search result parameters are listed in Table 2. The search result parameters included sequence coverage (SC, %), number of matched peptides (NP), and score (S).
Table 2.
Listing of proteins identifications obtained from the 2-DE map of fermented milk (6 h fermentation at 35 °C) (after LC–MS/MS analysis)
| Spot | Accession | Description | Experimental pI/Mr(kDa) | Theoretical pI/Mr(kDa) | Search results | ||
|---|---|---|---|---|---|---|---|
| SC | NP | S | |||||
| 96 | 297474460 | PREDICTED: keratin, type II cytoskeletal 1 [Bos taurus] | 6.41/26.02 | 8.28/63.11 | 7.26 | 4 | 13.75 |
| 100 | 146186887 | KRT5 protein [Bos taurus] | 6.53/26.23 | 7.81/62.64 | 5.36 | 4 | 22.50 |
| 112 | 297474460 | PREDICTED: keratin, type II cytoskeletal 1 [Bos taurus] | 6.34/27.08 | 8.28/63.11 | 3.47 | 2 | 6.15 |
| 129 | 300459 | kappa-casein C [cattle, milk, Peptide Mutant, 169 aa] | 4.96/28.36 | 6.52/18.91 | 16.57 | 2 | 26.85 |
| 142 | 300459 | kappa-casein C [cattle, milk, Peptide Mutant, 169 aa] | 5.46/28.41 | 6.52/18.91 | 16.57 | 2 | 36.51 |
| 143 | 300459 | kappa-casein C [cattle, milk, Peptide Mutant, 169 aa] | 5.14/28.45 | 6.52/18.91 | 16.57 | 2 | 26.46 |
| 144 | 300459 | kappa-casein C [cattle, milk, Peptide Mutant, 169 aa] | 5.33/28.19 | 6.52/18.91 | 16.57 | 2 | 35.68 |
| 152 | 146186887 | KRT5 protein [Bos taurus] | 6.22/28.96 | 7.81/62.64 | 11.22 | 9 | 57.23 |
| 155 | 300459 | kappa-casein C [cattle, milk, Peptide Mutant, 169 aa] | 5.51/28.50 | 6.52/18.91 | 22.49 | 3 | 36.01 |
| 157 | 300459 | kappa-casein C [cattle, milk, Peptide Mutant, 169 aa] | 5.58/28.44 | 6.52/18.91 | 22.49 | 3 | 52.51 |
| 161 | 300459 | kappa-casein C [cattle, milk, Peptide Mutant, 169 aa] | 5.80/28.72 | 6.52/18.91 | 16.57 | 2 | 39.97 |
| 166 | 254169541 | CSN1S1, partial [Bos taurus] | 4.59/29.81 | 5.01/3.07 | 42.31 | 1 | 26.90 |
| 224 | 159793187 | alpha S1 casein, partial [Bos taurus] | 4.49/32.11 | 5.55/13.84 | 39.67 | 4 | 215.64 |
| 229 | 1680179 | beta-casein A2 variant [Bos taurus] | 4.79/32.07 | 6.30/13.91 | 22.31 | 2 | 166.08 |
| 235 | 27806963 | alpha-S2-casein precursor [Bos taurus] | 5.17/35.03 | 8.43/26.00 | 36.94 | 11 | 209.77 |
| 237 | 27806963 | alpha-S2-casein precursor [Bos taurus] | 5.08/35.00 | 8.43/26.00 | 48.20 | 17 | 348.08 |
| 238 | 27806963 | alpha-S2-casein precursor [Bos taurus] | 5.00/34.89 | 8.43/26.00 | 37.84 | 11 | 258.28 |
| 322 | 645985846 | Chain B, Crystal Structure Of BSA In Complex With Naproxen | 5.94/74.15 | 5.86/66.42 | 16.98 | 11 | 108.45 |
| 325 | 645985846 | Chain B, Crystal Structure Of BSA In Complex With Naproxen | 5.87/74.40 | 5.86/66.42 | 19.55 | 13 | 143.63 |
| 327 | 645985846 | Chain B, Crystal Structure Of BSA In Complex With Naproxen | 5.78/74.62 | 5.86/66.42 | 19.55 | 13 | 140.03 |
| 329 | 645985846 | Chain B, Crystal Structure Of BSA In Complex With Naproxen | 5.71/74.88 | 5.86/66.42 | 10.46 | 6 | 32.56 |
| 409 | 159793203 | Alpha S1 casein, partial [Bos taurus] | 4.07/31.61 | 4.84/11.51 | 37.25 | 3 | 73.52 |
| 459 | 254169541 | CSN1S1, partial [Bos taurus] | 4.14/31.20 | 5.01/3.07 | 42.31 | 1 | 39.52 |
| 1239 | 146186887 | KRT5 protein [Bos taurus] | 4.18/19.13 | 7.81/62.64 | 4.69 | 6 | 38.43 |
SC sequence coverage (%), NP no. of matched peptides, S score
Bacteria used in milk fermentation possess a complex enzyme system of proteinases, which hydrolyzes milk protein to provide a source of nitrogen for its growth [23]. However, different bacteria have different enzyme activities and lead to differences in protein content [24]. Analysis identified most of the proteins as caseins, including alpha-S1-casein, alpha-S2-casein, beta-casein, and kappa-casein (Table 2). As shown in Table 2 and the mean content for each protein spot for each culture, there is a significant difference in protein degradation after the addition of Km. As shown by the data for the kappa-casein spots 129, 142, 143, 144, 155, 157, 161, the content of kappa-casein in the Lactobacillus fermentation sample was significantly decreased compared with the negative control but was different from the mixed fermentation team. This indicates that after adding Km, the metabolic pathway of Lactobacillus may change, or both Lactobacillus and Km have some barrier to the use of kappa-casein.
Unlike the degradation of kappa-casein, the data for alpha-S2-casein spots 237 and 238 show that addition of Km significantly enhances (P < 0.01) the degradation of alpha-S2-casein. Casein is usually hydrolyzed into oligopeptides by proteases located in the cell wall, and then further degradation occurs through the action of peptidases [1]. The slow degradation of alpha-S2-casein by Lactobacillus may be caused by a deficiency in proteases that specifically attack alpha-S2-casein, and the growth of Km in milk results in the secretion of proteases that contribute to the hydrolysis of alpha-S2-casein. As reported, the auxiliary degradation of casein by yeast is the reason that there was a great effect on the concentration rather than the types of protein [5].
KRT5 protein (keratin 5) is produced in keratinocytes found in the epidermis and is a tough, fibrous protein. The keratin identified in Table 2 probably originates from the teat canal. It is difficult to hydrolyze and digest [25], and this is reflected in our observation of no noticeable change in KRT5 content (data not shown).
Interestingly, four protein spots were recognized as BSA combined with naproxen. Naproxen is a common anti-inflammatory drug used to reduce pain and fever [26] and binds to BSA with high affinity (binding constant of 0.035–0.83 μM) [27]. We speculate that this protein was collected from an animal that was treated with naproxen but complete metabolism was not achieved before the milk was collected. Fortunately, these proteins can be significantly degraded after fermentation, particularly with the help of Km (data not shown).
Identification of peptides in fermented milk
Peptides derived from milk proteins during fermentation were analyzed every hour by nano-LC–ESI–MS/MS. For all identified peptides, we selected the molecular weight range under 3 kDa for analysis, and the number of peptide types is shown in Fig. 2. In the initial stage of fermentation, there was no significant difference between Lactobacillus-fermented milk and mixed-fermented milk. At this stage, the number of peptide types decreased rather than increased, possibly due to the exponential growth of bacteria. However, the number of peptide types increased significantly in the later stages of the mixed fermentation, and this was attributed to the growth of Km. At the end of fermentation, there were 1015.50 ± 7.78 and 1203.50 ± 34.65 different types of peptides identified in the Lactobacillus-fermented milk and mixed-fermented milk, respectively. The number of peptide types in the mixed fermentation was significantly higher than in the Lactobacillus fermentation (P < 0.01).
Fig. 2.
The number of peptide types change in the Lactobacillus fermentation team and mixed fermentation team along with fermentation time at 35 °C. The graph shows the mean ± standard deviation (n = 3)
At the end of fermentation, the relative content of peptides with molecular weight in the range of 0–1 kDa increased significantly (P < 0.05) in the mixed-fermented milk compared with the Lactobacillus fermentation, whereas the relative content of peptides with molecular weight over 3 kDa decreased significantly (P < 0.01) in the mixed-fermented milk (data not shown). Moreover, the content of caseins at the end of fermentation indicated that alpha-S1-casein and kappa-casein were degraded more in mixed-fermented milk than in Lactobacillus-fermented milk (data not shown). This indicates that the proteolytic enzymes from Km can assist to further degrade milk proteins and high molecular weight peptides to generate more small peptides [5]. Essentially, this makes fermented milk easier to digest and absorb after adding yeast as a starter [13].
Milk is an allergenic food, mostly because of the allergenic proteins, which are not easily removed [2]. The improvement of peptide degradation and further hydrolysis of proteins is expected to degrade protein allergenicity. As Shi et al. [2] have shown, the antigenicity of milk caseins (alpha-casein, beta-casein, kappa-casein) significantly decreases after fermentation by lactic acid bacteria. This is largely caused by changes in the protein structure brought about by microbial enzymes. As the above data show, Lactobacillus mixed with Km has obvious advantages in the hydrolysis of milk protein; it contributes to the degradation of milk protein and the generation of small peptides, which together help to reduce the allergenicity of milk.
Bioactivity of identified peptides
Among the identified peptides, many have various bioactivities (according to www.ncbi.nlm.nih.gov) such as angiotensin-converting-enzyme inhibitor (ACEI) activity, antihypertension activity, antibacterial activity, and immune activity. The bioactive regions from milk proteins were represented higher numbers in mixed fermentation team. There were ten peptides that have known, diverse biological activities from previous studies, and the amino acid sequences and biological activities of these bioactive peptides are shown in Table 3. For the ten listed bioactive peptides, nine have potential ACEI and antihypertension activity, two have antibacterial activity, and one has immune activity. In particular, a peptide derived from β-casein with the sequence YQEPVLGPVRGPFPIIV has multiple biological activities, including ACEI, antibacterial, and immunoregulation activity. It was first identified by Yamamoto et al. [34], and the concentration of an ACE inhibitor needed to inhibit 50% of the ACE activity (IC50) was measured, IC50 = 101 μg/mL. Subsequently, Sandré et al. [35] found its antimicrobial activity, and the minimal inhibition concentrations (MICs) against E. coli DPC6053 was measured by Birkemo et al. [36], MICs = 0.4 mg/mL. Moreover, the immunomodulating activity was found by Minkiewicz et al. [37]. Peptides with antihypertensive effects have become a well-known class of bioactive compounds, and have been researched by in vitro and in vivo studies. With further study, it is likely that more peptides with ACE inhibitory activity will be discovered.
Table 3.
Biological active peptide identified by LC–MS/MS in milk after 6 h fermentation at 35 °C
| m/z | Sequence of amino acid | MH+ | Charge | RT (min) | Origin | IPI accession | Biological activity (IC50) | References |
|---|---|---|---|---|---|---|---|---|
| 621.31 | DELQDKIHPF | 1241.62 | 2 | 51.65 | β-CN (f43-52) | IPI00697085.1; IPI00712994.3 |
ACEI | [28, 29] |
| 563.80 | ELQDKIHPF | 1126.59 | 2 | 42.72 | β-CN (f44-52) | IPI00697085.1; IPI00712994.3 |
ACEI | [28, 29] |
| 589.51 | QEPVLGPVRGPFPIIV | 1718.00 | 2 | 92.75 | β-CN (f194- 209) | IPI00697085.1; IPI00712994.3 |
ACEI | [30] |
| 541.82 | SQSKVLPVPQ | 1082.62 | 2 | 31.43 | β-CN (f166-175) | IPI00697085.1; IPI00712994.3 |
ACEI (92.μM) | [31] |
| 570.84 | RPKHPIKHQ | 1140.67 | 2 | 13.57 | α s1-CN (f1-9) | IPI00706094.1 | ACEI | [32] |
| 434.27 | SKVLPVPQ | 867.53 | 2 | 34.66 | β-CN (f168- 175) | IPI00697085.1; IPI00712994.3 |
ACEI and Antihypertension (39 mg L−1) | [30, 33] |
| 941.04 | YQEPVLGPVRGPFPIIV | 1881.07 | 2 | 95.80 | β-CN (f193- 209) | IPI00697085.1; IPI00712994.3 |
ACEI/Antibacterial/Immunoregulation (101 mg L−1) |
[29, 30, 34–37] |
| 506.81 | RPKHPIKH | 1012.62 | 2 | 13.77 | αs1-CN (f1-8) | IPI00706094.1 | ACEI (Potential) | [38] |
| 576.35 | GPVRGPFPIIV | 1151.70 | 2 | 77.05 | β-CN (f199-204) | IPI00697085.1; IPI00712994.3 |
Antihypertensive | [34, 35] |
| 559.32 | VLNENLLRF | 1117.64 | 2 | 70.88 | αSl-CN (f30-38) | IPI00706094.1 | Antibacterial | [39] |
Acknowledgements
The authors gratefully acknowledge project support from the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period, China (2013BAD18B04) and the Science and Technology Program of Beijing, China (D141100004814001).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Shihata A, Shah NP. Proteolytic profiles of yogurt and probiotic bacteria. Int. Dairy J. 2000;10:401–408. doi: 10.1016/S0958-6946(00)00072-8. [DOI] [Google Scholar]
- 2.Shi J, Luo YK, Xiao Y, Li Z, Xu Q, Yao MJ. Effects of fermentation by Lactobacillus casei on the antigenicity and allergenicity of four bovine milk proteins. Int. Dairy J. 2014;35:75–80. doi: 10.1016/j.idairyj.2013.10.010. [DOI] [Google Scholar]
- 3.Lourens-Hattingh A, Viljoen B. Growth and survival of a probiotic yeast in dairy products. Food Res. Int. 2001;34(9):791–796. doi: 10.1016/S0963-9969(01)00085-0. [DOI] [Google Scholar]
- 4.De Freitas I, Pinon N, Maubois JL, Lortal S, Thierry A. The addition of a cocktail of yeast species to Cantalet cheese changes bacterial survival and enhances aroma compound formation. Int. J. Food Microbiol. 2009;129(1):37–42. doi: 10.1016/j.ijfoodmicro.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 5.Klein N, Zourari A, Lortal S. Peptidase activity of four yeast species frequently encountered in dairy products-comparison with several dairy bacteria. Int. Dairy J. 2002;12(10):853–861. doi: 10.1016/S0958-6946(02)00081-X. [DOI] [Google Scholar]
- 6.Grba S, Stehlik-Tomas V, Stanzer D, Vahcic N, Skrlin A. Selection of yeast strain Kluyveromyces marxianus for alcohol and biomass production on whey. Chem. Biochem. Eng. Q. 2002;16(1):13–16. [Google Scholar]
- 7.Zafar S, Owais M. Ethanol production from crude whey by Kluyveromyces marxianus. Biochem. Eng. J. 2006;27(3):295–298. doi: 10.1016/j.bej.2005.05.009. [DOI] [Google Scholar]
- 8.Narvhusa JA, Gadaga TH. The role of interaction between yeasts and lactic acid bacteria in African fermented milks: a review. Int. J. Food Microbiol. 2003;86:51–60. doi: 10.1016/S0168-1605(03)00247-2. [DOI] [PubMed] [Google Scholar]
- 9.Wang SY, Chen HC, Liu JR, Lin YC, Chen MJ. Identification of yeasts and evaluation of their distribution in Taiwanese kefir and viili starters. J. Dairy Sci. 2008;91(10):3798–3805. doi: 10.3168/jds.2007-0468. [DOI] [PubMed] [Google Scholar]
- 10.Elfahri KR, Vasiljevic T, Yeager T, Donkor ON. Anti-colon cancer and antioxidant activities of bovine skim milk fermented by selected Lactobacillus helveticus strains. J. Dairy Sci. 2016;99(1):31–40. doi: 10.3168/jds.2015-10160. [DOI] [PubMed] [Google Scholar]
- 11.Hafeez Z, Cakir-Kiefer C, Roux E, Perrin C, Miclo L, Dary-Mourot A. Strategies of producing bioactive peptides from milk proteins to functionalize fermented milk products. Food Res. Int. 63 (part A): 71–80 (2014)
- 12.Simova E, Beshkova D, Angelov A, Hristozova T, Frengova G, Spasov Z. Lactic acid bacteria and yeasts in kefir grains and kefir made from them. J. Ind. Microbiol. Biot. 2002;28(1):1–6. doi: 10.1038/sj/jim/7000186. [DOI] [PubMed] [Google Scholar]
- 13.Roostita R, Fleet GH. Growth of yeasts in milk and associated changes to milk composition. Int. J. Food Microbiol. 1996;31:205–219. doi: 10.1016/0168-1605(96)00999-3. [DOI] [PubMed] [Google Scholar]
- 14.Holland JW, Gupta R, Deeth HC, Alewood PF. UHT milk contains multiple forms of αS1-casein that undergo degradative changes during storage. Food Chem. 2012;133(3):689–696. doi: 10.1016/j.foodchem.2012.01.070. [DOI] [Google Scholar]
- 15.Roucher VF, Desnots E, Naël C, Agnoux AM, Alexandre-Gouabau MC, Darmaun D, Boquien CY. Use of UPLC-ESI-MS/MS to quantitate free amino acid concentrations in micro-samples of mammalian milk. SpringerPlus. 2013;2(1):622. doi: 10.1186/2193-1801-2-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goncalves A, Poeta P, Monteiro R, Marinho C, Silva N, Guerra A, Petrucci-Fonseca F, Rodrigues J, Torres C, Vitorino R, Domingues P, Igrejas G. Comparative proteomics of an extended spectrum β-lactamase producing Escherichia coli strain from the Iberian wolf. J. Proteomics. 2014;104:80–93. doi: 10.1016/j.jprot.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 17.Mamone G, Caira S, Garro G, Nicolai A, Ferranti P, Picariello G, Malorni A, Chianese L, Addeo F. Casein phosphoproteome: identification of phosphoproteins by combined mass spectrometry and two-dimensional gel electrophoresis. Electrophoresis. 2003;24(16):2824–2837. doi: 10.1002/elps.200305545. [DOI] [PubMed] [Google Scholar]
- 18.Holder A, Thienel K, Klaiber I, Pfannstiel J, Weiss J, Hinrichs J. Quantification of bio-and techno-functional peptides in tryptic bovine micellar casein and β-casein hydrolysates. Food Chem. 2014;158:118–124. doi: 10.1016/j.foodchem.2014.02.104. [DOI] [PubMed] [Google Scholar]
- 19.Jrad Z, Hatmi HE, Adt I, Girardet JM, Cakir-Kiefer C, Jardin J, Degraeve P, Khorchani T. Oulahal N. Effect of digestive enzymes on antimicrobial, radical scavenging and angiotensin I-converting enzyme inhibitory activities of camel colostrum and milk proteins. Dairy. Sci. Technol. 2014;94(3):205–224. [Google Scholar]
- 20.Mei J, Guo Q, Wu Y, Li Y, Yu H. Study of proteolysis, lipolysis, and volatile compounds of a Camembert-type cheese manufactured using a freeze-dried Tibetan kefir co-culture during ripening. Food Sci. Biotechnol. 2015;24(2):393–402. doi: 10.1007/s10068-015-0052-9. [DOI] [Google Scholar]
- 21.Gadaga TH, Mutukumira AN, Narvhus JA. The growth and interaction of yeasts and lactic acid bacteria isolated from Zimbabwean naturally fermented milk in UHT milk. Int. J. Food Microbiol. 2001;68(1):21–32. doi: 10.1016/S0168-1605(01)00466-4. [DOI] [PubMed] [Google Scholar]
- 22.Akabanda F, Owusu-Kwarteng J, Tano-Debrah K, Glover RLK, Nielsen DS, Jespersen L. Taxonomic and molecular characterization of lactic acid bacteria and yeasts in nunu, a Ghanaian fermented milk product. Food Microbiol. 2013;34:277–283. doi: 10.1016/j.fm.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 23.Kunji ERS, Mierau I, Hagting A, Poolman B, Konings WN. The proteotytic systems of lactic acid bacteria. Antonie van Leeuwenhoek. 1996;70(2–4):187–221. doi: 10.1007/BF00395933. [DOI] [PubMed] [Google Scholar]
- 24.Zhang DD, Chen LJ, Zhang M, Zhang Y, Fang GZ, Jiang TM. Improved peptide generation from milk fermented by heat-shocked Lactobacillus helveticus. Int. J. Food Sci. Tech. 2017;52(2):366–373. doi: 10.1111/ijfs.13289. [DOI] [Google Scholar]
- 25.Xie M, Shevchenko A, Wang B, Shevchenko A, Wang C, Yang Y. Identification of a dairy product in the grass woven basket from Gumugou Cemetery (3800 BP, northwestern China) Quatern. Int. 2016 [Google Scholar]
- 26.Bujacz A, Zielinski K, Sekula B. Structural studies of bovine, equine, and leporine serum albumin complexes with naproxen. Proteins. 2014;82(9):2199–2208. doi: 10.1002/prot.24583. [DOI] [PubMed] [Google Scholar]
- 27.Davies NM, Anderson KE. Clinical pharmacokinetics of naproxen. Clin. Pharmacokinet. 1997;32:268–293. doi: 10.2165/00003088-199732040-00002. [DOI] [PubMed] [Google Scholar]
- 28.Otte J, Lenhard T, Flambard B, Sørensen KI. Influence of fermentation temperature and autolysis on ACE-inhibitory activity and peptide profiles of milk fermented by selected strains of Lactobacillus helveticus and Lactococcus lactis. Int. Dairy J. 2011;21(4):229–238. doi: 10.1016/j.idairyj.2010.12.008. [DOI] [Google Scholar]
- 29.Robert MC, Razaname A, Mutter M, Juillerat MA. Identification of angiotensin-I-converting enzyme inhibitory peptides derived from sodium caseinate hydrolysates produced by Lactobacillus helveticus NCC 2765. J. Agr. Food Chem. 2004;52(23):6923–6931. doi: 10.1021/jf049510t. [DOI] [PubMed] [Google Scholar]
- 30.Chang OK, Roux É, Awussi AA, Miclo L, Jardin J, Jameh N, Dary A, Humbert G, Perrin C. Use of a free form of the Streptococcus thermophilus cell envelope protease PrtS as a tool to produce bioactive peptides. Int. Dairy J. 2014;38(2):104–115. doi: 10.1016/j.idairyj.2014.01.008. [DOI] [Google Scholar]
- 31.Hayes M, Stanton C, Slattery H, O’Sullivan O, Hill C, Fitzgerald G, Ross R. Casein fermentate of Lactobacillus animalis DPC6134 contains a range of novel propeptide angiotensin-converting enzyme inhibitors. Appl. Environ. Microb. 2007;73(14):4658–4667. doi: 10.1128/AEM.00096-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ong L, Henriksson A, Shah NP. Angiotensin converting enzyme-inhibitory activity in Cheddar cheeses made with the addition of probiotic Lactobacillus casei sp. Dairy Sci. Technol. 2007;87(2):149–165. doi: 10.1051/lait:2007004. [DOI] [Google Scholar]
- 33.Hernández-Ledesma B, Miralles B, Amigo L, Ramos M, Recio I. Identification of antioxidant and ACE-inhibitory peptides in fermented milk. J. Sci. Food Agr. 2005;85(6):1041–1048. doi: 10.1002/jsfa.2063. [DOI] [Google Scholar]
- 34.Yamamoto N, Akino A, Takano T. Antihypertensive effect of the peptides derived from casein by an extracellular proteinase from Lactobacillus helveticus CP790. J. Dairy Sci. 1994;77(4):917–922. doi: 10.3168/jds.S0022-0302(94)77026-0. [DOI] [PubMed] [Google Scholar]
- 35.Sandré C, Gleizes A, Forestier F, Gorges-Kergot R, Chilmonczyk S, Léonil J, Moreau MC, Labarre C. A peptide derived from bovine β-casein modulates functional properties of bone marrow-derived macrophages from germfree and human flora-associated mice. J. Nutr. 2001;131(11):2936–2942. doi: 10.1093/jn/131.11.2936. [DOI] [PubMed] [Google Scholar]
- 36.Birkemo GA, O’Sullivan O, Ross RP, Hill C. Antimicrobial activity of two peptides casecidin 15 and 17, found naturally in bovine colostrum. J. Appl. Microbiol. 2009;106(1):233–240. doi: 10.1111/j.1365-2672.2008.03996.x. [DOI] [PubMed] [Google Scholar]
- 37.Minkiewicz P, Slangen CJ, Dziuba J, Visser S, Mioduszewska H. Identification of peptides obtained via hydrolysis of bovine casein by chymosin using HPLC and mass spectrometry. Milchwissenschaft. 2000;55(1):14–17. [Google Scholar]
- 38.Silva SV, Pihlanto A, Malcata FX. Bioactive peptides in ovine and caprine cheeselike systems prepared with proteases from Cynara cardunculus. J. Dairy Sci. 2006;89(9):3336–3344. doi: 10.3168/jds.S0022-0302(06)72370-0. [DOI] [PubMed] [Google Scholar]
- 39.Somkuti GA, Paul M. Enzymatic fragmentation of the antimicrobial peptides casocidin and isracidin by Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus. Appl. Microbiol. Biot. 87(1): 235–242 (2010) [DOI] [PubMed]