Abstract
To develop natural antifungal agents against pathogenic dermal fungi, the antifungal activity of isothiocyanates (ITCs) extracted from horseradish (Armoracia rusticana) root was investigated. A paper disk diffusion assay showed that ITCs inhibited growth of the four pathogenic dermal fungi (Trichophyton rubrum, Trichophyton mentagrophytes, Microsporum canis, and Epidermophyton floccosum) at 5000 μg/mL, as well as perfectly inhibited the growth of the fungi at 10,000 μg/mL in a concentration-dependent manner. The minimum inhibitory concentrations of ITCs against T. rubrum, T. mentagrophytes, M. canis, and E. floccosum were 200, 200, 100, and 100 μg/mL, respectively. The minimum fungicidal concentrations of ITCs against the four pathogenic dermal fungi were 200 μg/mL. These results strongly suggested that ITCs extracted from horseradish root can be a candidate of natural antifungal agents against pathogenic dermal fungi, even though further study is needed to investigate how to use ITCs in clinical therapy.
Keywords: Antifungal activity, Isothiocyanates, Dermatophytes, MIC, MFC
Introduction
Onychomycosis is a chronic fungal infection of the nails and affects 20% of the world population under 40 years of age [1–3]. Various fungi can cause onychomycosis, the most frequently isolated pathogens are dermatophytes, especially Trichophyton rubrum and Trichophyton mentagrophytes, which cause approximately 71 and 20% of all case of onychomycosis, respectively. About 90% of all onychomycotic infections of the toenails in North American series arise from dermatophytes, and the infections affect the nail bed causing dystrophy and sometimes leading to complete nail loss [4–6].
The drugs, such as terbinafine tablets, itraconazole capsules, griseofulvin, and ciclopirox nail lacquer, have been proved to have good efficacy and reduced side-effect against dermatophytes, and they are approved by the Food and Drug Administration [7]. Although the effective medicine is available in the therapy of onychomycosis, there are still many unsolved problems, such as toxic side-effects, emergence of resistant strains, and high relapse rate [8–11].
Essential oils and essences of many herbs and spices are known to have antimicrobial activity. Treatment of strawberries [12], celery, and peppers [13] with vapors of methyl jasmonate, which occurs widely in the plant kingdom [14], extends their shelf life by inhibiting microbial growth. Isothiocyanates (ITCs), breakdown products of glucosinolates, are responsible for the pungent flavor of the plants in the Cruciferae family such as horseradish, mustard, broccoli, and wasabi, having the main component of allyl isothiocyanate (AITC). In addition, ITCs were shown to have strong antibacterial [15, 16], and antifungal [17] activities in liquid and vapor phases, such as Bacillus subtilis, Vibrio parahaemolyticus, Candida albicans, Aspergillus niger, and Penicillium citrinum. The antimicrobial activity of ITCs is believed to be due to the inactivation of extracellular enzymes through the cleavage of disulfide bonds [18]. Several mechanisms have been proposed for the antimicrobial activity of ITCs, including modulation of sulfhydryl enzymes, inhibition of ribonucleic acid (RNA) synthesis, partial inhibition of deoxyribonucleic acid (DNA) synthesis, and inhibition of protein synthesis by an action of the ITCs moiety (–N=C=S), [19]. From the above, it may be reasonable to explore the potential activity of ITCs for prevention of dermatophytes. In this study, therefore, the antifungal activity of ITCs extracted from the horseradish (Armoracia rusticana) root for development of natural antifungal agents against dermatophytes was investigated.
Materials and methods
Materials
Horseradish (A. rusticana) root powder obtained from Biocoats Co., Ltd. (Seoul, Korea) was used to extract ITCs. Allyl isothiocyanate (AITC, Wako, Tokyo, Japan), Phenylethyl isothiocyanate (PITC, Sigma-Aldrich, St. Louis, MO, USA), and 3-Butenyl isothiocyanate (BITC, Sigma-Aldrich) was prepared to measure the concentration in extracted ITCs.
Preparation of ITCs from horseradish root
ITCs were extracted by using a steam distillation method [16]. A mixture of the horseradish root powder (200 g) and distilled water (550 mL) was prepared to extract ITCs. For maximum production of ITCs by an enzyme (β-thioglucoside glucohydrolase) in the horseradish root, the mixture was shaken in a water bath at 40 °C for 2 h. The reacted mixture was distilled and concentrated at 120 °C in an oil bath for 120 min by rotary evaporator (Rotavapor R-200; BUCHI Labortechnik AG, Flawil, Switzerland). Essential oils (ITCs) were extracted from the concentrated solution using centrifugation (Bionova, Model Mega 17R; Hanil Science Industrial, Seoul, Korea) at 5000×g for 20 min. ITCs extracted from the horseradish root were analyzed for the identification of the main active components involved in antifungal activities using a 7890A GC system (Agilent Technologies, Santa Clara, CA, USA) equipped with a 5975C mass spectrometer (Agilent Technologies) according to a method by Choi et al. [20]. ITCs were prepared using dissolution in n-hexane (Showa Chemical Industry Co., Tokyo, Japan) at a 1:1 ratio. The hexane fraction was separated using an HP-5 column (30 m × 0.25 mm I.D., 0.25 μm film thickness; Agilent Technologies). The column temperature was maintained at 50 °C for 1 min, and then increased to 250 °C for 3 min. The inlet temperature was 250 °C for 3 min. Helium was used as a carrier gas at a flow rate of 1.0 mL/min. The extraction yield of ITCs was determined using standard concentrations of AITC, PITC, and BITC.
Preparation of fungal inoculum suspension
The four fungal strains were used for performing the antifungal activity assay of ITCs extracted from the horseradish root. Trichophyton rubrum ATCC 28188, Trichophyton mentagrophytes ATCC 18748, Microsporum canis ATCC 36299, and Epidermophyton floccosum ATCC 10227 obtained from the Korean Collection for Type Cultures (KCTC, Daejeon, Korea) were pre-cultured in Sabouraud’s dextrose broth (SDB, Difco Co., Detroit, USA) for 2 weeks at 28 °C. The inocula were then cultured on a Sabouraud’s dextrose agar (SDA, Difco Co.) placed slant at 28 °C for 7 days in order to produce conidia according to a method by Maria et al. [21]. Turbidity of the final conidia was adjusted to 70% transmission at a wavelength of 520 nm using a spectrophotometer, which is corresponding to 1.0 × 106 to 5.0 × 106 CFU/mL.
Paper disk diffusion assay
The antifungal activity of ITCs extracted from the horseradish root was measured using the paper disk diffusion assay. The culture of fungal spores was adjusted to 1.0 × 106 CFU/mL using the sterile culture medium described above, and 100 μL of each fungal culture was spread on a Sabouraud dextrose agar (SDA, Difco Co.) plate with a sterile glass rod. Each 100 μL of ITCs (10,000, 5000, and 2500 mg/L) diluted with sterilized SDB was absorbed into a 8-mm paper disk (Whatman No. 2) placed on the SDA plate and then incubated at 28 °C for 2 weeks.
Minimum inhibitory concentration (MIC) assay
The MICs of ITCs against fungi were determined by the standard M38-A approved by National Committee for Clinical Laboratory Standards (NCCLS, 22) with some modification. The conidia suspensions of the four fungal strains were diluted 1:50 in RPMI 1640 medium (Gibco, Thermo Fisher Scientific, MA, USA) to get 1.0 × 106 CFU/mL. The ITCs (800 μg/mL) extracted from the horseradish root was initially dissolved in ethyl alcohol and then serially diluted in a twofold series with the sterilized RPMI 1640 medium. Each 100 µL of fungus adjusted to 1.0 × 106 CFU/mL was inoculated into micro-tubes, which had been previously filled with 100 µL medium containing 100 µL of ITCs at different concentrations. The micro-tubes were incubated at 28 °C for 2 weeks. The growth of each fungus was judged by naked eye. MIC was defined as the lowest concentration that shows visible growth. The sterilized RPMI 1640 medium was used as the blank. The negative control was a mixture of 100 μL of the sterilized RPMI 1640 medium and 100 μL of each strain inoculum.
Minimum fungicidal concentration (MFC) assay
The MFCs of ITCs against the four fungal strains were determined according to a method by Bamba et al. [23]. A loopful of each fungal culture without visible growth in the micro-tube was inoculated onto the SDA plate and incubated under the same conditions described above. MFC is defined as the lowest concentration that show no growth on the SDA plates.
Results and discussion
Active components for antifungal activities of ITCs extracted from horseradish root
ITCs extracted from the horseradish root were analyzed using gas chromatograph-mass spectrometer (GC–MS) to identify the main components involved in antibacterial activities (data not shown) as detailed in a previous paper [20]. ITCs contained two major ITC derivatives, 59.995% AITC and 35.819% PITC, and one minor ITC derivative of 1.532% BITC. The concentrations of AITC and PITC in ITCs were calculated using a standard curve. The horseradish extract contained 208,766 µg/mL of AITC and 72,778 µg/mL of PITC [20].
Antifungal activity of ITCs extracted from horseradish root
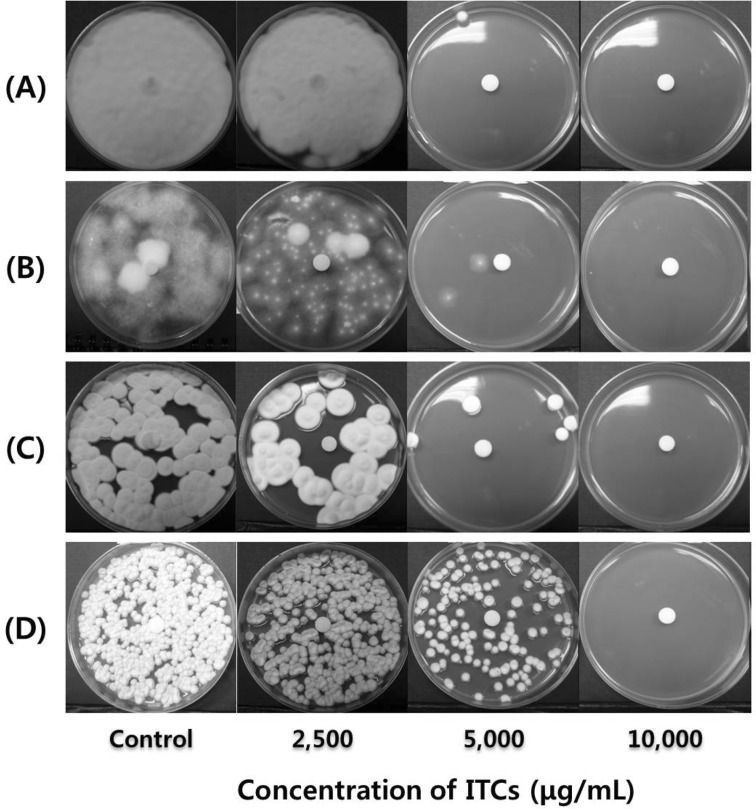
The antifungal activities of ITCs extracted from the horseradish root against the four fungal strains as analyzed by the paper disk diffusion assay are shown in Fig. 1. ITCs inhibited growth of the four fungal strains at 5000 μg/mL, and perfectly inhibited their growth at 10,000 μg/mL. Antifungal activities of ITCs against the four pathogenic dermal fungi were concentration dependent. The reason for the formation of a clear zone all over the agar plate, but not around paper disk, may be the volatile nature of ITCs.
Fig. 1.
Antifungal activities of ITCs extracted from the horseradish root against the four fungal strains as analyzed by the paper disk diffusion assay. (A), Trichophyton rubrum; (B), Trichophyton mentagrophytes; (C), Microsporum canis; (D), Epidermophyton floccosum
Minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC)
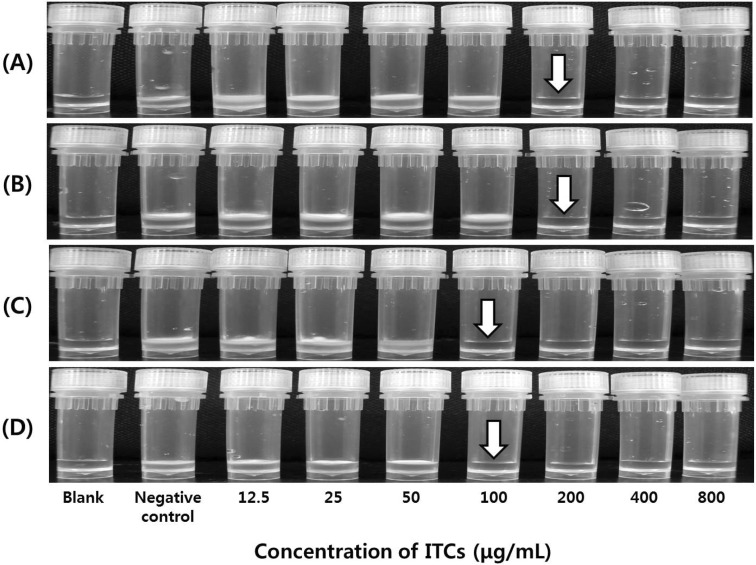
The analysis of MIC and MFC, revealed that the ITCs extracted from the horseradish root possessed potent antifungal activity against all the tested fungi. The MICs of ITCs against T. rubrum, T. mentagrophytes, M. canis, and E. floccosum were 200, 200, 100, and 100 μg/mL, respectively (Fig. 2 and Table 1). The MFCs of ITCs against the tested fungi were 200 μg/mL (Table 2).
Fig. 2.
MIC of ITCs extracted from the horseradish root against the four fungal strains. (A), Trichophyton rubrum; (B), Trichophyton mentagrophytes; (C), Microsporum canis; (D), Epidermophyton floccosum
Table 1.
MIC of ITCs extracted from horseradish root against four fungal strains
| Fungus | Minimum inhibitory concentration (μg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Blank | N.C. | 12.5 | 25 | 50 | 100 | 200 | 400 | 800 | |
| T. rubrum | –a | ++b | ++ | +c | + | + | – | – | – |
| T. mentagrophytes | – | ++ | ++ | + | + | + | – | – | – |
| M. canis | – | ++ | ++ | + | + | – | – | – | – |
| E. floccosum | – | ++ | ++ | + | + | – | – | – | – |
ano growth
bgood growth
cgrowth
Table 2.
MFC of ITCs extracted from horseradish root against four fungal strains
| Fungus | Minimum fungicidal concentration (μg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Blank | N.C. | 12.5 | 25 | 50 | 100 | 200 | 400 | 800 | |
| T. rubrum | –a | ++b | ++ | +c | + | + | – | – | – |
| T. mentagrophytes | – | ++ | ++ | + | + | + | – | – | – |
| M. canis | – | ++ | ++ | + | + | + | – | – | – |
| E. floccosum | – | ++ | ++ | + | + | + | – | – | – |
ano growth
bgood growth
cgrowth
The appearance of multidrug-resistant fungal strains and high relapse rate of onychomycosis makes it necessary to discover new classes of antifungal agent to solve the problem. For a long time, natural products, either as pure compounds or as extracts, are the focal point in the area of medicine research, and a series of molecules with antifungal activity against different fungal strains have been found, such as crude extracts, essential oils, terpenoids, saponins, phenolic compounds, alkaloids, peptides and proteins [24].
Horseradish (A. rusticana) is a perennial plant of the Cruciferae family, both root and leaves were being used as a medicine during the middle ages and the root was used as a condiment on meats in Germany, Scandinavia, and Britain [25]. ITCs are breakdown products of sinigrin, which is the predominate glucosinolate presenting in horseradish, and is proved to be components responsible for the antimicrobial activities [26]. The antimicrobial activity of ITCs is attributed to its amphiphilic structure. Thiocyanate moiety (–N=C=S) is a key factor of the antimicrobial activity of ITCs. ITCs play a role mainly through inducing oxidative stress. [17, 27–29].
This study proved that ITCs have strong antifungal activities against the four pathogenic dermal fungi, with a MIC of 100–200 μg/mL (Table 1) and MFC of 200 μg/mL (Table 2). The antifungal activity of ITCs on Penicillium notatum [30], Alternaria alternate [31], Aspergillus flavus and Endomyces fibuliger [17] has been proven. Simultaneously, other essential oils have also been proved to have antifungal activities against dermatophytes. The MICs of onion oil against T. rubrum, T. mentagrophytes, M. canis, and E. floccosum were 2008 μg/mL [32]. Melaleuca alternifolia (tea tree) oil [33], and Moringa oleifera Lam oil [34] also have antifungal activities against Trichophyton sp. However, we did not compare our data with those results, because the method of measuring the antifungal activity is questionable, such as the initial concentration, exposure time and breakpoints, and is not standardized, although NCCLS methods are widely accepted for determining in vitro antifungal activity. In this study, the MIC (100–200 μg/mL) of ITCs against T. rubrum, T. mentagrophytes, M. canis, and E. floccosum was lower than those (2008 μg/mL) of onion oil. The MFC (200 μg/mL) of ITCs against the tested fungal strains was lower than MBC (208–2083 μg/mL) of ITCs against 5 dominant bacteria isolated from Jeotgal, Korean fermented sea food [16], and MBC (208.3–666.7 μg/mL) of ITCs against antibiotic-resistant bacteria [35]. These results indicate that ITCs are a potential antifungal agent, and the antimicrobial activity of ITCs against fungi is stronger than against bacteria.
From these results, ITCs extracted from horseradish root can be considered a candidate of natural antifungal agents against pathogenic dermal fungi. However, method of application of ITCs to clinical therapy needs further study.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Won CH, Lee JY, Li KS, Choi MR, Kim BJ, An JS, Kim KH, Cho SY, Moon SE, Kim JA, Eun HC. The long term efficacy and relapse rate of itraconazole pulse therapy verus terbinafine continuous therapy for toenail onychomycosis. Kor. J. Med. Mycol. 2007;12:139–147. [Google Scholar]
- 2.Park SH, Shin YM, Moon SK, Shin DH, Choi JS, Kim KH, Bang YJ. A clinical and mycological study of tinea pedis. Kor. J. Med. Mycol. 2006;11:123–131. [Google Scholar]
- 3.Pfaller MA, Sutton DA. Review of in vitro activity of sertaconazole nitrate in the treatment if superfical fungal infections. Diagnostic Microbio. Infect. Dis. 2006;56:147–152. doi: 10.1016/j.diagmicrobio.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Choi CP, Lee MH. Six cases of tinea capitis in asults. Kor. J. Med. Mycol. 2006;11:230–233. [Google Scholar]
- 5.Kim HJ, Lee WJ, Jun JB, Kim TH, Suh SB. A clinical, mycological and epidemiological study on tinea barbae during the last 24-year-period (1981-2004) Kor. J. Med. Mycol. 2006;11:64–70. [Google Scholar]
- 6.Kim SM, Lee YW, Ahn KJ. A clinical and mycological study of tinea capitis. Kor. J. Med. Mycol. 2006;11:184–190. [Google Scholar]
- 7.diagnosis and treatment Aditya K, Gupta AK, Linh Q. Tu. Dermatophytes. J. Am. Acad. Dermatol. 2006;54:1050–1055. doi: 10.1016/j.jaad.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Tosti A, Piraccini MB, Stinchi C, Colombo MD. Relapses of onychomycosis after successful treatment with systemic antifungals: a three-year follow-up. Dermatol. 1998;197:162–166. doi: 10.1159/000017990. [DOI] [PubMed] [Google Scholar]
- 9.Hay RJ. The future of onychomycosis therapy may involve a combination of approaches. Br. J. Dermatol. 2001;145:3–8. doi: 10.1046/j.1365-2133.2001.145s60003.x. [DOI] [PubMed] [Google Scholar]
- 10.Mukherjee PK, Leidich SD, Isham N. Clinical Trichophyton rubrum strain exhibiting primary resistance to terbinafine. Antimicrob. Agents Chemo. 2003;47:82–86. doi: 10.1128/AAC.47.1.82-86.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin S, Lim S. Antifungal effects of herbal essential oils alone and in combination with ketoconazole against Trichophyton spp. J. App. Microbiol. 2004;97:1289–1296. doi: 10.1111/j.1365-2672.2004.02417.x. [DOI] [PubMed] [Google Scholar]
- 12.Fenwick GR, Heaney RK, Mullin WJ, VanEtten CH. Glucosinolates and their breakdown products in food and food plants. CRC Cr. Rev. Food Sci. 1982;18:123–201. doi: 10.1080/10408398209527361. [DOI] [PubMed] [Google Scholar]
- 13.Chadwick CI, Lumpkin TA, Elberson LR. The botany, uses and production of Wasabia japonica (Miq.) (Cruciferae) Matsum. Econ. Bot. 1993;47:113–135. doi: 10.1007/BF02862015. [DOI] [Google Scholar]
- 14.Sikkema J, de Bont JAM, Poolman B. Interactions of cyclic hydrocarbons with biological membranes. J. Biol. Chem. 1994;269:8022–8028. [PubMed] [Google Scholar]
- 15.Isshiki K, Tokuoka K, Mori R, Chiba S. Preliminary examination of allyl isothiocyanate vapor for food preservation. Biosci. Biotech. Biochem. 1992;56:1476–1477. doi: 10.1271/bbb.56.1476. [DOI] [Google Scholar]
- 16.Kim HY, Gornsawun G, Shin IS. Antibacterial activities of Isothiocyanates (ITCs) extracted from horseradish (Armoracia rusticana) root in liquid and vapor phases against 5 dominant bacteria Isolated from low-salt Jeotgal, a Korean salted and fermented seafood. Food Sci. Biotechnol. 2015;24:1405–1412. doi: 10.1007/s10068-015-0180-2. [DOI] [Google Scholar]
- 17.Nielsen PV, Rios R. Inhibition of fungal growth on bread by volatile components from spices and herbs, and the possible application in active packaging, with special emphasis on mustard essential oil. Int. J. Food Microbiol. 2000;60:219–229. doi: 10.1016/S0168-1605(00)00343-3. [DOI] [PubMed] [Google Scholar]
- 18.Kawakishi S, Kaneko T. Interaction of proteins with allyl isothiocyanate. J. Agr. Food Chem. 1987;35:85–88. doi: 10.1021/jf00073a020. [DOI] [Google Scholar]
- 19.Turgis M, Han J, Caillet S, Lacroix M. Antimicrobial activity of mustard essential oil against Escherichia coli O157:H7 and Salmonella typhi. Food Control. 2009;20:1073–1079. doi: 10.1016/j.foodcont.2009.02.001. [DOI] [Google Scholar]
- 20.Choi JK, Gornsawun G, Shin IS. Effect of a polypropylene (PP) patch containing isothiocyanates (ITCs) extracted from horseradish (Armoracia rusticana) root on the shelf-life of low-salt Myeong-ran Jeotgal. Food Sci. Biotechnol. 2015;24:1–12. doi: 10.1007/s10068-015-0001-7. [DOI] [Google Scholar]
- 21.Maria ESB, Daniel AS. JÚnia SH. In vitro methods for antifungal susceptibility testing of Trichophyton spp. Mycol. Res. 2006;110:1355–1360. doi: 10.1016/j.mycres.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 22.NCCLS. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: Approved standard. NCCLS document M38-A. National Committee for Clinical Laboratory Standards. Wayne, PA, USA (2002)
- 23.Bamba H, Kondo Y, Wong RM, Sekine S, Matsuzaki F. Evaluation of an assay method of the susceptibility of antimicrobial agents using a 96-well flat-bottom microplate and a microplate reader. Am. J. Gastroenterol. 1997;92:659–662. [PubMed] [Google Scholar]
- 24.Abad MJ, Ansuategui M, Bermejo P. Active antifungal substances from natural sources. ARKIVOC. 2007;VII:116–145. [Google Scholar]
- 25.Wikipedia. Horseradish. Available from: http://en.wikipedia.org/wiki/ Horseradish. Accessed Dec. 12, 2016
- 26.Depree JA, Howard TM, Savage GP. Flavour and pharmaceutical properties of the volatile sulphur compounds of wasabi (Wasabia japonica) Food Res, Inter. 1999;31:329–337. doi: 10.1016/S0963-9969(98)00105-7. [DOI] [Google Scholar]
- 27.Tunc S, Chollet E, Chalier P. Combined effect of volatile antimicrobial agents on the growth of Penicillium notatum. Inter. J. Food Microbiol. 2007;113:263–270. doi: 10.1016/j.ijfoodmicro.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Troncoso R, Espinoza C, Sánchez-Estrada A. Analysis of the isothiocyanates present in cabbage leaves extract and their potential application to control Alternaria rot in bell peppers. Food Res. Inter. 2005;38:701–708. doi: 10.1016/j.foodres.2005.02.004. [DOI] [Google Scholar]
- 29.Hammer KA, Carson CF, Riley TV. In vitro activity of Melaleuca alternifolia (tea tree) oil against dermatophytes and other filamentous fungi. J. Antimicro. Chemo. 2002;50:195–199. doi: 10.1093/jac/dkf112. [DOI] [PubMed] [Google Scholar]
- 30.Pinto E, Salgueiro LR, Cavaleiro C. In vitro susceptibility of some species of yeasts and filamentous fungi to essential oils of Salvia officinalis. Indus. Crops Products. 2007;26:125–141. doi: 10.1016/j.indcrop.2007.02.007. [DOI] [Google Scholar]
- 31.Chuang PH, Lee CW, Chou JY. Anti-fungal activity of crude extracts and essential oil of Moringa oleifera Lam. Bioresource. Technol. 2007;98:232–236. doi: 10.1016/j.biortech.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Kim HY, Sarinnart P, Shin IS. Antibacterial activities of isothiocyanates extracted from horseradish (Armoracia rusticana) root against antibiotic-resistant bacteria. Food Sci. Biotechnol. 2015;24:1029–1034. doi: 10.1007/s10068-015-0131-y. [DOI] [Google Scholar]
- 33.Kassie F, KnasmÜller S. Genotoxic effects of allyl isothiocyanate (AITC) and phenethyl isothiocyanate (PEITC) Chemico-Biological Interact. 2000;127:163–180. doi: 10.1016/S0009-2797(00)00178-2. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Li J, Tang L. Cancer-preventive isothiocyanates: dichotomous modulators of oxidative stress. Free Radical Biol. Med. 2005;38:70–77. doi: 10.1016/j.freeradbiomed.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 35.Sellam A, Dongo A, Guillemette T. Transcriptional responses to exposure to the brassicaceous defence metabolites camalexin and allyl-isothiocyanate in the necrotrophic fungus Alternaria brassicicola. Molecul. Plant Pathol. 2007;8:195–208. doi: 10.1111/j.1364-3703.2007.00387.x. [DOI] [PubMed] [Google Scholar]