Abstract
Twelve pieces of longissimus dorsi were processed into Chinese traditional dry-cured loins. The changes in the proteolylic enzymes activities, myofibrillar proteins degradation, and free amino acids content were investigated during processing. Compared with fresh piece (0 day), the cathepsin B + L and calpains activities decreased after dry-curing and maintained potential activities values of 23.25 and 15.04% in the final products, respectively. The myosin heavy chain (MHC) and C protein were intensely degraded at the dry-ripened stage; the 50 kDa desmin increased at day 2 and then disappeared at day 11. The total free amino acids content increased from 333.18 mg/100 g in the raw to 1096.54 mg/100 g at the end of the dry-ripening. This work provided a mechanism for the accumulation of free amino acids and predicted the proteolysis extent of myofibrillar proteins by monitoring the changes of three marker proteins (MHC, C protein and 50 kDa desmin) during Chinese traditional dry-cured loins processing.
Keywords: Dry-cured loins, Cathepsin B + L, Calpains, Proteolysis, Free amino acids
Introduction
Chinese traditional dry-cured loins, one popular dry-cured meat product, are considered as a high quality product, with an outstanding flavor parameter. It is highly popular in the southwest of China. Similar to dry-cured hams and bacons, Chinese traditional dry-cured loins are produced by dry-curing and dry-cured ripening; however, the period of their processing is shorter than those of dry-cured products. The flavor of dry-cured lions is often described as distinctive tangy, delicate, meaty, and salty. The formation of the flavor quality of the dry-cured meat products requires a long processing stage, particularly the long dry-ripening phase. The aromatic composition of dry-cured meat products was fairly complex, with numerous flavor compounds during processing [1]. Many efforts have been made to determine the volatile compounds of dry-cured products in recent years; a number of outstanding reviews on the main volatile compounds and the mechanism of formation in dry-cured products have been published [2, 3]. However, as far as we know, the accumulation of nonvolatile compounds is not evaluated for Chinese traditional dry-cured loins.
Taste is one of the main sensory impressions of food or other substance and is derived from nonvolatile compounds that stimulates the olfactory and taste receptors [4]. Free amino acids, nucleotides, and small peptides were the main nonvolatile compounds in the dry-cured meat products [5]. It is well known that during dry-ripening, endopeptidases (mainly cathepsins and calpains) are involved in the initial breakdown of sarcoplasmic and myofibrillar proteins and then in the proteolysis of fractions to small peptides or free amino acids [6]. Jurado et al. [7] demonstrated that proteolysis not only influenced texture but also affected flavor development as it could change the formation of low-molecular weight compounds. During recent decades, various studies have been focused on improving the taste of these meat products with low-salt content such as dry-cured bacon [8] and Valencia dry-cured loins [9]. Most of abovementioned dry-cured meat products were processed for a long processing period, even >2 months. Compared with these dry-cured products, Chinese traditional dry-cured loins have a relatively shorter processing (within 20 days). During the short processing, the contribution of endopeptidases and myofibrillar proteins degradation to the tasted compounds including free amino acids and peptides are still not clear. No literature is available on the development of quantity of tasted compounds during Chinese traditional dry-cured loins processing, and it is very necessary to make clear the mechanism of accumulation of free amino acids.
The objective of the present study was, therefore, to investigate the evolution of free amino acids, assess the myofibrillar proteins degradation, and determine proteolytic enzyme activities during the short processing of Chinese traditional dry-cured loins.
Materials and methods
Processing and sampling of Chinese traditional dry-cured loins
All the procedures described in this study were in accordance with the Operating Procedures of Animal Slaughter of Animal Experimentation of National Standards of P. R. China. Experimental pigs aged from 5 to 6 months (body weight 93 ± 15 kg) were slaughtered. A total of 12 fresh longissimus dorsis with an average weight of approximately 2 kg for each piece were purchased from a local commercial slaughterhouse and stored at 4 °C. After 48 h of storage, L. dorsis was cut to fresh pieces (about 8 × 10 × 25 cm for each). A total of 48 fresh pieces (4 pieces were taken from every L. dorsis) from 12 L. dorsis were cut. Thirty-five fresh pieces with standard sizes and invisible connective tissue were selected from the fresh pieces. Dry-cured loins were processed according to the traditional procedures. The fresh pieces were dry-cured directly for 1 day with curing agents (4% NaCl (w/w) and 50 mg nitrate per kg lions) at 4 °C and 90% relative humidity (RH). During this period, 1–2 turnovers were given. After dry-curing, the pieces were washed to remove excess salt on the surface at 4 °C. Finally, the pieces were dry-ripened for 14 days according to the procedures shown in Table 1. The total processing procedures took 15 days. The muscle samples were taken at seven different processing points: fresh piece (0 day), end of dry-curing (1 day), 1 day of dry ripening (2 day), 4 days of dry ripening (5 days), 7 days of dry ripening (8 days), 10 days of dry-ripened stage (11 days), and 14 day of the dry-ripened stage (15 days). At each processing point, five samples were randomly taken for analysis. After sampling, samples were wrapped in an aluminum foil, frozen, and stored in liquid nitrogen for further analysis.
Table 1.
The controlling procedures of dry-ripening during the processing
| The dry-ripening procedures of dry-cured lions | ||||||||
|---|---|---|---|---|---|---|---|---|
| Dry-ripening time (days) | 1 | 3 | 5 | 7 | 9 | 11 | 13 | 14 |
| RH (%) | 85 | 84 | 83 | 82 | 81 | 80 | 79 | 78 |
| Temperature (°C) | 13 | 16 | 19 | 22 | 25 | 28 | 31 | 34 |
During the processing, temperature firstly increased from 13 to 28 °C at 1.5 °C per day and then from 28 to 34 °C at 2 °C per day, progressively; RH% reduced from 85 to 79% at 0.5% per day and then from 79 to 78% at 1% per day, progressively
Extraction of a crude enzymes solution for cathepsin assays in vitro
The crude enzymes solution was extracted as described previously by Hernández et al. [10] with slight modification. Muscle sample (0.5 g) was homogenized in 2.5 ml of 50 mM sodium citrate buffers containing 1 mM EDTA and 0.2% (v/v) Triton X-100 at pH 5.0 with a DY89-I high speeds homogenizer (Scientz co., Ningbo, China) for 3 × 10 s at 10,000 rpm while cooled in ice and centrifuged at 12,000 g for 20 min at 4 °C with a refrigerated centrifuge (Hunan Xiangyi Laboratory Instrument Development Co., Changsha, China). The supernatant was filtered through a glass wool and used for enzyme assays. The protein content of supernatant was calculated using a bicinchoninic acid (BCA) protein assay kit (Thermo Scientific, MA).
Preparation of enzyme extracts for calpain assays in vitro
The crude enzymes solution was prepared as described previously by Toldrá and Flores [11] with slight modification. The muscle sample (1.0 g) was homogenized in 3 ml of 50 mM Tris buffers containing 3 mM EDTA and 10 mM mercaptoethanol, pH 8.5. The extract was homogenized (3 × 10 s at 10,000 rpm while cooled in ice) with a DY89-I high speeds homogenizer (Scientz co., Ningbo, China) and centrifuged at 12,000 g for 20 min at 4 °C with a refrigerated centrifuge; the supernatant was filtered through a glass wool and adjusted to pH 7.5, and then centrifuged again in the same conditions. The final supernatant was used for calpain assays. The protein content of supernatant was calculated using the protein assay kit mentioned above.
Cathepsin B + L activities determination
Cathepsin B + L activities were measured as described by Toldrá and Etherington [12] using N-CBZ-L-phenylalanyl-l-arginine-AMC as substrates. The reaction mixture comprised 50 μl of enzyme extract and 250 μl of reaction buffer, 40 mM sodium phosphate at pH 6.0, containing 0.4 mM EDTA, 10 mM cysteine, and 0.05 mM of the specific substrates. The reaction mixture was incubated for 30 min at 37 °C. The fluorescence was continuously monitored using a 96-Well Plate Reader M200 (Tecan, Austria) at 355 nm and 460 nm as the excitation and emission wavelengths. The assays of cathepsin B + L activity were done for five repeat. One unit (U) of cathepsin B + L activity was defined as the amount of enzyme hydrolyzes 1 μmol substrates per min at 37 °C; cathepsin B + L activity was expressed as U pre mg protein (U/mg protein).
Calpains activities determination
The measurement of calpains activities was conducted as described previously by Soh et al. [13] with slight modification. This assay was performed with succinyl- Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin (Suc- LLVY- AMC) substrates. The reaction mixture consisted of 50 μl of crude enzymes extract, 250 μl of reaction buffer, 100 mM Tris–HCl, and 1 mM DTT at pH 7.5, including 0.05 mM of the specific substrates. The reaction mixture was incubated at 37 °C. The fluorescence was continuously monitored with a 96-Well Plate Reader M200 (Tecan, Austria) at 355 nm and 460 nm as excitation and emission wavelengths, respectively. The assays of calpains activity were done for five repeat. One unit (U) of calpains activity was defined as the amount of enzyme that hydrolyzes 1 μmol substrates per min at 37 °C; calpains activity was expressed as U pre mg protein (U/mg protein).
Myofibrillar proteins preparation and sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE)
Myofibrillar proteins were prepared as described previously by Diaz et al. [14] with slight modification. SDS-PAGE was used to determine proteolysis of myofibrillar proteins by a Mini Protean II apparatus (Bio-Rad, CA) with 5 and 12.5% polyacrylamide gradient gels. Protein sample (50 µg) was loaded in per lane. The assays of SDS-PAGE were conducted for thrice repeat.
The fragments of myofibrillar proteins in gel were identified according to the molecular weight of standard proteins. The intensities of bands were quantified by Quantity One Software (Bio-Rad Laboratories, Philadelphia, PA, USA); Electrophoresis procedures were implemented by our previous work [15]. Actin was defined as the internal standard. The difference of protein bands was calculated by the relative ratio of the intensities of protein bands account for the intensity of actin.
Immunoblotting for desmin
The expression levels of desmin bands were assayed by SDS-PAGE and Western blotting according to our previous study [15] with slight modification. After electrophoresis, proteins were transferred to polyvinylidene fluoride membranes (Immobilon-P, Millipore, MA, USA). The membranes were blocked in TBST solution (50 mM Tris, 150 mM NaCl, 0.05% Tween-20, 5% dry milk, pH 7.4) for 1 h at room temperature and incubated with primary antibody for 24 h at 4 °C. The primary antibodies of rabbit anti-desmin (ab32363, abcam) and rabbit anti-β-actin (ab8227, abcam) were diluted 1:5000 with TBST solution, respectively. Membranes were incubated with secondary antibodies for 1 h at room temperature. The goat anti-rabbit HRP-conjugated secondary antibodies (ab205718, abcam) were diluted 1:1000 with TBST solution. The blot was incubated with DAB assay kit (sigma). Specialized Quantity One Software was used to quantify the blot. The assays of western blot were done for thrice repeat.
Free amino acids determination
The extraction and derivatization of free amino acids were performed as described by Shim et al. [16] with slight modification. Eight hundred milligrams of sample were taken from liquid nitrogen and homogenized in 3 ml 3% 2-hydroxy-5-sulfobenzoic acid. After centrifugation at 15,000 g at 4 °C for 10 min, the supernatant was neutralized with 0.85 M K2CO3, centrifuged again, and filtrated by a 0.22 μm syringe filter (Millipore, Billerica, MA, USA). An automated amino acid analyzer (L-8900, Hitachi HighTechnologies Corp., Japan) was used to determine the content of free amino acids. The analytical procedures were conducted according to Shim et al. [16]. Absolute quantification was defined using peak area with external standards and an internal control. The assays of SDS-PAGE were done for thrice repeat.
Statistical analysis
Statistical analyzes were conducted by the one-way analysis of variance procedure (Duncan’s Multiple Range Test) of SAS 8.0 software (SAS Institute Inc., Cary, NC, USA) to analyze the changes of calpains, cathepsin activities, and free amino acids during processing. The significant difference was analyzed by Duncan’s Multiple Range Test model. The statistically significant level was set as 0.05.
Results and discussion
The changes of cathepsin B + L potential activities during processing
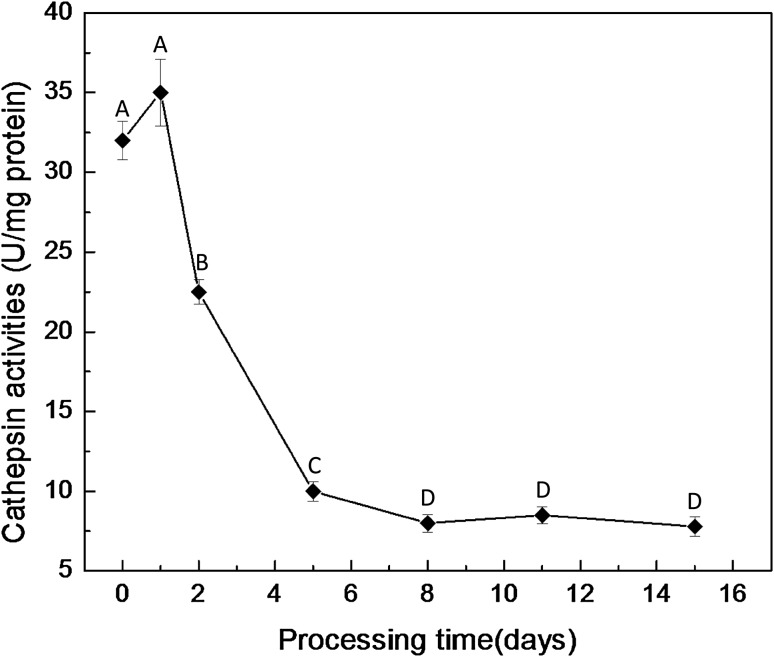
The changes of cathepsin B + L potential activities during the entire processing are shown in Fig. 1. The potential activities of cathepsin B + L decreased during the air dry-ripening stage, particularly at day 1 and day 4 of dry-ripening (p < 0.001). At the end of dry-ripening, about 23.25% activities of cathepsin B + L were retained, compared with the fresh piece.
Fig. 1.
Changes of the cathepsin B + L activities of dry-cured loins during processing. (A)–(D) Identical letters in the line indicate that there are no significant difference in different process (p > 0.05)
Endogenous enzymes are the key enzymes for the degradation of cytoskeletal proteins in meat tenderizing and dry-cured meat products [17]. Cathepsins and calpains have been indicated as crucial endopeptidases for the proteolysis of dry-cured meat products. However, overwhelming results demonstrated that cathepsin D lost its activity quickly, while cathepsin B, L, and H always maintained their activities during dry-cured ham processing [18, 19]. Ouali et al. [20] suggested that cathepsin H hardly hydrolyzed any myofibrillar proteins, whereas cathepsin B and L possessed extensive hydrolyzing activities for myofibrillar proteins. Cathepsin B and L are reported as the main endopeptidases responsible for muscle proteolysis and flavor formation in dry-cured meat products, since they could maintain stable activities during dry-cured meat processing [21]. Toldrá and Flores [11] reported that the activities of cathepsins were responsible for the generation of polypeptides, which could be further degraded to smaller peptides and free amino acids by exopeptidases. In our study, the cathepsin B + L potential activities could accelerate the proteolysis of Chinese traditional dry-cured loins during the processing.
The cathepsin B + L activities has been extensively reported during dry-cured products processing such as for Chinese Jinhua ham [22]. Compared with dry-cured hams, the Chinese traditional dry-cured loins have a shorter processing time including dry-curing and air dry-cured ripening. Our results showed that the cathepsin B + L activities decreased during the entire air dry-ripened stage, which agreed with these reports by Zhao et al. [22]. Armenteros et al. [23] also reported that cathepsins B + L activities decreased and that NaCl inhibited their activities during the dry-cured ham processing. The decrease of cathepsin B + L activities can be attributed to the increase of salt content and the decrease of water activity as a result of water loss at the air dry-ripening stage. Sárraga et al. [24] reported that the salt concentrations of 8% intensely inhibited the cathepsins L activities. Toldrá [25] implied that proteolytic enzymes were still relatively active at the end of dry-ripening process. Based on our results that 23.25% values of cathepsin B + L were retained at the final products, we implied that cathepsin B + L could play an important role in degrading myofibrillar proteins during the Chinese traditional dry-cured loins processing.
The changes of calpains activities during processing
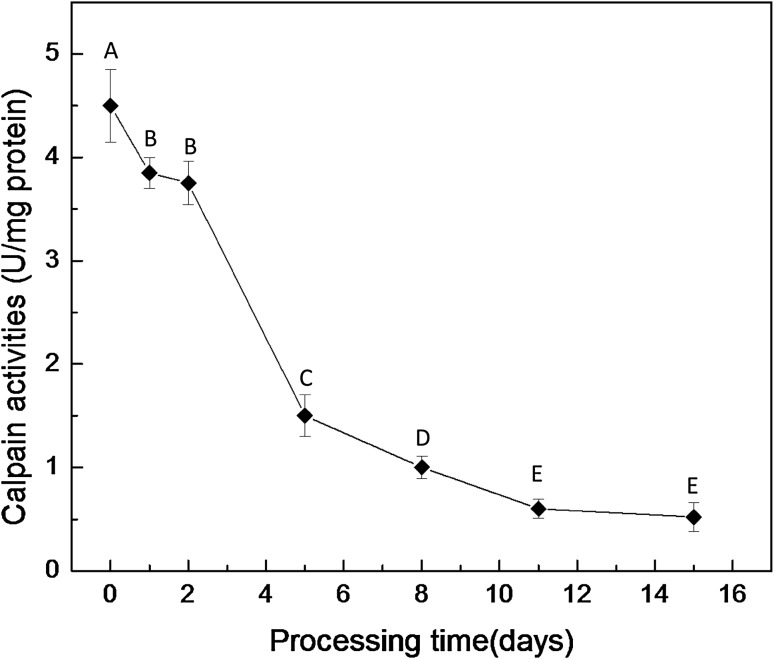
The changes in the calpains activities during process of Chinese traditional dry-cured loins are shown in Fig. 2. The activities of calpains significantly decreased at the dry-curing stage, at days 5, 8, and 11 of the dry-ripening stage (p < 0.001) and maintained certain potential activities (15.04%) at the end of dry-ripening compared with the fresh piece.
Fig. 2.
Changes of calpain activities of dry-cured loins during processing. (A)–(E) Identical letters in the line indicate that there are no significant difference in different process (p > 0.05)
Studies on the incidence of endogenous enzymes during the curing process of the dry-cured products have focused on cathepsins [12], whereas very few studies about the incidence of calpains had been reported so far. It is generally accepted that calpains play a key role in the meat tenderizing process, particularly in the degradation of myofibrillar proteins, which occurs during the first 24 h post mortem. It was difficult to extrapolate the information available during the dry-cured meat products processing. Sárraga et al. [18] suggested that calpains activities were not detected after 2.5 month of the dry-ripening processing; the activities were only detected in raw hams and at the first stage of the dry-cured processing wherein it was notably lower than that of the raw hams. There was a similarity in our results that the calpain activities intensively reduced at the dry-cured stage and the stage of dry-ripening from 2 days to 11 days. Dransfield et al. [26] implied that the disappearance of calpains activities during dry-cured products processing could be explained by the fact that calpains were quite unstable enzymes, particularly μ-calpain. Sárraga et al. [24] reported that the long dry-cured phase and the addition of curing salt made calpains unlikely to be involved in the entire Spanish dry-cured ham process. In our results, the retained calpains activities (15.04%) of the final products could be attributed to the shorter curing period of the entire dry-ripening stages compared with other dry-cured products. According to these results and those presented here, the major activities of calpains would take place at the dry-cured stage and the first steps of dry-ripening stage.
The changes of myofibrillar proteins during dry-cured loins processing
The degradation of myofibrillar proteins during processing
The electropherogram of myofibrillar proteins of dry-cured loins at different processing stages displayed significant difference (Fig. 3). The most striking cleaved bands at the end of the dry-ripening stage were in the 220 kDa myosin heavy chain (MHC), 140 kDa (C protein). On the other hand, a 50–65 kDa cleaved MHC bands increased through the drying-ripened processing. The relative intensities of MHC and C protein bands normalized with actin are shown in Fig. 3. The intensity of MHC decreased at days 8, 11, and 15 of dry-ripening (p < 0.01); the relative intensity of C protein reduced at dry-cured phase and day 15 of dry ripening (p < 0.01).
Fig. 3.
SDS–PAGE results of myofibril proteins of dry-cured loins during processing. (A)–(D) Identical letters in the line indicate that there are no significant difference in different process (p > 0.05), respectively
Several protein fragments have been identified in dry-cured products by proteomic tools such as titin, MHC [8], and troponin T. Information derived from the identification of the naturally generated protein fragments could be very important to better understand the mechanisms of flavor development during processing. Toldrá et al. [19] observed that smaller fragments of the 50–100 kDa and 20–45 kDa regions in biceps femoris muscle significantly increased during dry-cured ham processing. Bermúdez et al. [27] reported that the myofibrillar fraction of the 66 kDa significantly decreased in semimembranosus muscles of Celta dry-cured ham; they implied that these proteins bands were degraded to peptides of smaller molecular weight during the ripening stage. Compared with other dry-cured meat products, we found that C protein was significantly hydrolyzed during the processing, which could be explained by the difference of muscle samples. Moreover, Wang et al. [28] reported that the C protein, β-tropomyosin, troponin I, and troponin-C were cleaved during the dry-cured duck processing and that there were significant correlations among the changes of the degraded bands of proteins, free amino acids content, and cathepsin activities. In our results, the main hydrolyzed proteins were considered as MHC and C protein, which agreed with the results obtained by Wang et al. [28]. The combined activities of calpains and cathepsin B and L could play a key role in the degradation of MHC and C protein during processing.
Degradation of desmin during processing
The evolution of desmin during the entire processing of dry-cured loins is shown in Fig. 4. There was a rapid degradation of desmin at the dry-ripening stage, particularly at the end of dry-ripening. Surprisingly, we found that there was a new fragment (about 50 kDa) appearance at the early drying-ripened stage and disappeared at the end of dry-ripened. The relative intensities of desmin bands normalized with actin are shown in Fig. 4. The relative intensity of 50 kDa fragment significantly increased at day 2 of air dry-ripening and then decreased at day 11 of air dry-ripening (p < 0.01). Desmin, with molecular weight of approximately 54 kDa, locates at the periphery of the Z discs. The network of desmin intermediate filaments is responsible for interlinking Z discs of adjacent myofibrils and seems to be associated with the nucleus, plasma membrane, and other membranous organelles. Due to its significance in maintaining the structural integrity of the muscle cell, desmin degradation is believed to have a significant impact on meat postmortem tenderization [29]. Christensen et al. [30] reported that desmin firstly resulted in the appearance of the fragment with molecular weights of approximately 50 kDa, and then the fragment was significantly degraded in L. dorsi. Schäfer et al. [31] suggested that aging accelerated the degradation of desmin post mortem to generate fragments with molecular weight ranging from approximately 50 to 39 kDa. In our results, we found that the desmin fragment did not significant change at the early days, and then the fragment was degraded at the dry-ripening stage; the protein with a molecular weight of 50 kDa appeared at day 2 of air dry-ripening and disappeared at day 11 of air dry-ripening during the dry-cured loins processing. Baron et al. [32] demonstrated that desmin was certainly degraded by cathepsin B, and that it sequentially cleaved into small fragments from the C-terminal end of the molecule, whereas calpains cleaved desmin at specific structural features. Three proteins (MHC, C protein, and 50 kDa desmin) were degraded at different stages respectively, whose cleavages could be attributed to corporate activities of calpains and cathepsin B and L.
Fig. 4.
The changes of desmin in myofibrillar proteins during dry-cured loins processing by western blot. (A)–(C) Identical letters in the line indicate that there are no significant difference in different process (p > 0.05)
The changes of free amino acids during processing
The evolution of 18 free amino acids (FAA) and the total free amino acids (TFAA) content during the entire processing of dry-cured loins are shown in Table 2. The significant increase of the TFAA content was observed throughout the processing in Chinese dry-cured loins, particularly at the dry-ripened stage. The TFAA content increased during dry-ripening (p < 0.001) and reached average values of 1096.54 mg/100 g at the end of dry-ripening, compared with the fresh piece (333.18 mg/100 g). The main FAA were tryptophane, alanine, glutamic acid, leucine, and lysine at the final products.
Table 2.
Changes in FAA content (mg/100 g) of Chinese traditional dry-cured loins during the processing
| Free amino acid | Fresh pieces | End of dry-curing | Dry-ripening stage | ||||
|---|---|---|---|---|---|---|---|
| 0 d | 1 d | 2 d | 5 d | 8 d | 11 d | 15 d | |
| Asp | 5.92 ± 0.47A | 1.54 ± 0.14C | 4.15 ± 0.36AB | 6.21 ± 0.87A | 3.93 ± 0.15B | 1.15 ± 0.36C | 2.24 ± 0.27BC |
| Thr | 26.32 ± ± 3.24D | 21.71 ± 2.14D | 36.83 ± 2.19C | 68.21 ± 3.46B | 81.92 ± 6.93A | 83.84 ± 5.14A | 84.85 ± 8.47A |
| Ser | 19.62 ± 1.25D | 26.72 ± 2.14C | 30.96 ± 1.17C | 59.68 ± 4.44CB | 71.52 ± 5.02B | 75.04 ± 6.28A | 86.48 ± 8.73A |
| Asn | 22.25 ± 1.67D | 22.20 ± 1.41D | 38.40 ± 1.20C | 60.05 ± 3.37B | 74.80 ± 2.03AB | 78.60 ± 3.39A | 83.50 ± 3.78A |
| Glu | 36.40 ± 3.39CD | 45.58 ± 4.55C | 63.30 ± 4.36B | 103.55 ± 6.83A | 105.55 ± 5.92A | 108.81 ± 5.96A | 117.25 ± 7.52A |
| Gly | 24.85 ± 1.15CD | 19.95 ± 1.17D | 28.41 ± 2.25C | 40.65 ± 1.31A | 38.75 ± 2.55AB | 39.45 ± 1.51A | 44.40 ± 2.71A |
| Ala | 56.45 ± 5.59E | 44.92 ± 4.41EF | 69.65 ± 3.25D | 107.81 ± 3.78C | 145.55 ± 2.01B | 150.45 ± 5.49B | 200.21 ± 7.05A |
| Val | 12.61 ± 1.24E | 18.65 ± 1.15D | 19.05 ± 1.52D | 35.85 ± 2.24C | 44.05 ± 2.96B | 50.07 ± 4.05AB | 57.33 ± 3.98A |
| Met | 5.11 ± 0.78D | 8.55 ± 0.89C | 8.55 ± 0.57C | 16.22 ± 0.68B | 18.75 ± 0.80B | 21.15 ± 1.35A | 21.43 ± 1.62A |
| Ile | 9.82 ± 0.79D | 9.15 ± 0.82D | 13.54 ± 1.78C | 26.24 ± 1.67B | 31.92 ± 2.54AB | 33.05 ± 1.45A | 35.85 ± 2.23A |
| Leu | 15.45 ± 0.84D | 25.21 ± 1.20C | 26.95 ± 1.16C | 41.76 ± 1.34B | 50.63 ± 2.83AB | 53.45 ± 2.99A | 58.69 ± 3.23A |
| Tyr | 9.63 ± 0.94C | 16.61 ± 1.83B | 14.55 ± 0.91B | 27.25 ± 1.21AB | 31.38 ± 1.40A | 30.95 ± 1.66A | 29.47 ± 3.91A |
| Phe | 13.64 ± 0.82C | 17.13 ± 1.40B | 18.32 ± 1.35B | 34.35 ± 1.02A | 30.85 ± 2.55A | 32.16 ± 1.67A | 32.68 ± 2.88A |
| Trp | 20.33 ± 1.53D | 20.51 ± 2.79CD | 24.37 ± 2.49C | 31.15 ± 2.56B | 43.83 ± 3.97A | 46.46 ± 3.78A | 41.73 ± 6.01A |
| His | 3.91 ± 0.39E | 6.52 ± 0.69D | 9.43 ± 0.82C | 16.85 ± 1.11B | 20.34 ± 1.36A | 23.67 ± 1.32A | 19.98 ± 1.00AB |
| Lys | 18.24 ± 1.45E | 24.76 ± 1.38D | 25.92 ± 1.36D | 58.62 ± 2.02C | 70.68 ± 2.06B | 79.62 ± 6.71AB | 91.56 ± 7.74A |
| Arg | 11.85 ± 1.24D | 18.85 ± 1.16C | 19.38 ± 0.87C | 43.17 ± 2.63B | 43.28 ± 2.45B | 51.05 ± 1.97A | 53.37 ± 3.25A |
| Tau | 20.78 ± 3.17B | 13.23 ± 3.32C | 19.37 ± 2.88B | 23.08 ± 3.86B | 28.40 ± 4.13AB | 28.35 ± 2.35AB | 35.52 ± 4.25A |
| Total | 333.18 ± 13.08F | 361.79 ± 16.73E | 471.13 ± 20.45D | 800.66 ± 12.73C | 936.13 ± 25.05B | 987.32 ± 22.79B | 1096.54 ± 25.69A |
A–FIdentical letters indicate that there is no significant difference in different processing points (p > 0.05)
FAA are very important not only for their contribution to specific taste but also for their involvement in degradation reactions that generate volatile compounds, which provide the flavor in dry-cured meat products. It has been reported that peptides and free amino acids from protein degradation products were the precursors of flavor compounds [33].The changes of FAA have been studied in dry-cured ham; the nonvolatile compounds generated from proteins decided the sensory quality at dry-cured products [27]. In our results, the increase in TFAA content could contribute to the sensory quality at the final product.
In our results, the final content of free amino acid was slightly lower than those reported by these authors of dry-cured hams [34]. The slightly lower total free amino acid content could be attributed to the short period of dry-ripening in Chinese traditional dry-cured loins compared with dry-cured hams. In agreement with the increase in the TFAA content, the content profile of each individual FAA was observed in our study. However, the increase was not the same for the different individual amino acids. The FAA that underwent the greatest increases were glutamic acid and alanine. The free amino acid profile is largely in accordance with those reports by different authors in dry-cured hams [5]. It implied that processing with low salts curing had no effect on the accumulation of free amino acid profiles. Furthermore, the fact that major myofibrillar proteins (such as nebulin, myosin heavy chains, and troponin I) were degraded into peptides or FAA has been reported during dry-cured products processing. Larrea et al. [35] showed that intense proteolysis occurred during the dry-cured ham processing, resulting in continuous accumulation of small peptides and FAA. Wang et al. [28] demonstrated that the increase of TFAA content was significantly and negatively correlated with activities of cathepsin B and L and with intensities of C protein, troponin I, and MLC2. Based on the fact and our results, the increase of TFAA could be attributed to the proteolysis of MHC, C protein, and desmin.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31471681), the Modern Agricultural Technical System Foundation (CARS-43-17), the National Agricultural Transformation of Scientific and Technological Achievements Projects of China (2013GB2C200191), the Scientific Research Foundation of Graduate School of Ningbo University (G16024), the Scientific and Technological program of Ningbo University (XKL14D2087 and 2013C910017) and the K.C. Wong Magna Fund at Ningbo University.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Chambers E, Koppel K. Associations of volatile compounds with sensory aroma and flavor: The complex nature of flavor. Molecules. 2013;18:4887–4905. doi: 10.3390/molecules18054887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.del Pulgar JS, García C, Reina R, Carrapiso AI. Study of the volatile compounds and odor-active compounds of dry-cured Iberian ham extracted by SPME. Food Sci. Technol. Int. 2013;19:225–233. doi: 10.1177/1082013212442199. [DOI] [PubMed] [Google Scholar]
- 3.Purriños L, Franco D, Carballo J, Lorenzo JM. Influence of the salting time on volatile compounds during the manufacture of dry-cured pork shoulder “lacón”. Meat Sci. 2012;92:627–634. doi: 10.1016/j.meatsci.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Khan MI, Jo C, Tariq MR. Meat flavor precursors and factors influencing flavor precursors-A systematic review. Meat Sci. 2015;110:278–284. doi: 10.1016/j.meatsci.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Garrido R, Domínguez R, Lorenzo JM, Franco I, Carballo J. Effect of the length of salting time on the proteolytic changes in dry-cured lacón during ripening and on the sensory characteristics of the final product. Food control. 2012;25:789–796. doi: 10.1016/j.foodcont.2011.11.036. [DOI] [Google Scholar]
- 6.Mora L, Sentandreu MA, Fraser PD, Toldrá F, Bramley PM. Oligopeptides arising from the degradation of creatine kinase in Spanish dry-cured ham. J. Agric. Food. Chem. 2009;57:8982–8988. doi: 10.1021/jf901573t. [DOI] [PubMed] [Google Scholar]
- 7.Jurado Á, García C, Timón ML, Carrapiso AI. Effect of ripening time and rearing system on amino acid-related flavour compounds of Iberian ham. Meat Sci. 2007;75:585–594. doi: 10.1016/j.meatsci.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Wu H, Zhang Y, Long M, Tang J, Yu X, Wang J, Zhang J. Proteolysis and sensory properties of dry-cured bacon as affected by the partial substitution of sodium chloride with potassium chloride. Meat Sci. 2014;96:1325–1331. doi: 10.1016/j.meatsci.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 9.Armenteros M, Aristoy MC, Barat JM, Toldrá F. Biochemical and sensory properties of dry-cured loins as affected by partial replacement of sodium by potassium, calcium, and magnesium. J. Agric. Food Chem. 2009;57:9699–9705. doi: 10.1021/jf901768z. [DOI] [PubMed] [Google Scholar]
- 10.Hernández P, Zomeño L, Ariño B, Blasco A. Antioxidant, lipolytic and proteolytic enzyme activities in pork meat from different genotypes. Meat Sci. 2004;66:525–529. doi: 10.1016/S0309-1740(03)00155-4. [DOI] [PubMed] [Google Scholar]
- 11.Toldrá F, Flores M. The use of muscle enzymes as predictors of pork meat quality. Food Chem. 2000;69:387–395. doi: 10.1016/S0308-8146(00)00052-2. [DOI] [Google Scholar]
- 12.Toldrá F, Etherington DJ. Examination of cathepsins B, D, H and L activities in dry-cured hams. Meat Sci. 1988;23:1–7. doi: 10.1016/0309-1740(88)90057-5. [DOI] [PubMed] [Google Scholar]
- 13.Soh BY, Song H-O, Lee Y, Lee J, Kaewintajuk K, Lee B, Choi Y-Y, Cho JH, Choi S, Park H. Identification of active Plasmodium falciparum calpain to establish screening system for Pf-calpain-based drug development. Malar. J. 2013;12:1–12. doi: 10.1186/1475-2875-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz O, Fernandez M, De Fernando GDG, de la Hoz L, Ordoñez JA. Proteolysis in dry fermented sausages: the effect of selected exogenous proteases. Meat Sci. 1997;46:115–128. doi: 10.1016/S0309-1740(97)00013-2. [DOI] [PubMed] [Google Scholar]
- 15.Cao J, Zhou G, Liu Y, Liao G, Zhang Q, Ye K, Pan D, Ou C. Activation of caspase-9 and its influencing factors in beef during conditioning. Animal. 2014;8:504–509. doi: 10.1017/S175173111300219X. [DOI] [PubMed] [Google Scholar]
- 16.Shim Y-S, Yoon W-J, Ha J, Seo D, Lee K-W, Lee W-Y, Kwon K-I, Kang T-S, Lee J-H, Kim H-J. Method validation of 16 types of structural amino acids using an automated amino acid analyzer. Food Sci. Biotechnol. 2013;22:1567–1571. doi: 10.1007/s10068-013-0252-0. [DOI] [Google Scholar]
- 17.Wang D, Dong H, Zhang M, Liu F, Bian H, Zhu Y, Xu W. Changes in actomyosin dissociation and endogenous enzyme activities during heating and their relationship with duck meat tenderness. Food Chem. 2013;141:675–679. doi: 10.1016/j.foodchem.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 18.Sárraga C, Gil M, García-Regueiro JA. Comparison of calpain and cathepsin (B, L and D) activities during dry-cured ham processing from heavy and light large white pigs. J. Sci. Food Agric. 1993;62:71–75. doi: 10.1002/jsfa.2740620110. [DOI] [Google Scholar]
- 19.Toldra F, Rico E, Flores J, Cathepsin B. D, H and L activities in the processing of dry-cured ham. J. Sci. Food Agric. 1993;62:157–161. doi: 10.1002/jsfa.2740620208. [DOI] [Google Scholar]
- 20.Ouali A, Garrel N, Obled A, Deval C, Valin C, Penny I. Comparative action of cathepsins D, B, H, L and of a new lysosomal cysteine proteinase on rabbit myofibrils. Meat Sci. 1987;19:83–100. doi: 10.1016/0309-1740(87)90014-3. [DOI] [PubMed] [Google Scholar]
- 21.Toldrá F, Flores M. The role of muscle proteases and lipases in flavor development during the processing of dry-cured ham. Crit. Rev. Food Sci. 1998;38:331–352. doi: 10.1080/10408699891274237. [DOI] [PubMed] [Google Scholar]
- 22.Zhao G, Zhou G, Wang Y, Xu X, Huan Y, Wu J. Time-related changes in cathepsin B and L activities during processing of Jinhua ham as a function of pH, salt and temperature. Meat Sci. 2005;70:381–388. doi: 10.1016/j.meatsci.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Armenteros M, Aristoy M-C, Barat JM, Toldrá F. Biochemical and sensory changes in dry-cured ham salted with partial replacements of NaCl by other chloride salts. Meat Sci. 2012;90:361–367. doi: 10.1016/j.meatsci.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 24.Sárraga C, Gil M, Arnau J, Monfort JM, Cussó R. Effect of curing salt and phosphate on the activity of porcine muscle proteases. Meat Sci. 1989;25:241–249. doi: 10.1016/0309-1740(89)90042-9. [DOI] [PubMed] [Google Scholar]
- 25.Toldrá F. The role of muscle enzymes in dry-cured meat products with different drying conditions. Trends Food Sci. Technol. 2006;17:164–168. doi: 10.1016/j.tifs.2005.08.007. [DOI] [Google Scholar]
- 26.Dransfield E, Etherington DJ, Taylor MA. Modelling post-mortem tenderisation—II: Enzyme changes during storage of electrically stimulated and non-stimulated beef. Meat Sci. 1992;31:75–84. doi: 10.1016/0309-1740(92)90073-D. [DOI] [PubMed] [Google Scholar]
- 27.Bermúdez R, Franco D, Carballo J, Sentandreu MÁ, Lorenzo JM. Influence of muscle type on the evolution of free amino acids and sarcoplasmic and myofibrillar proteins through the manufacturing process of Celta dry-cured ham. Food Res. Int. 2014;56:226–235. doi: 10.1016/j.foodres.2013.12.023. [DOI] [Google Scholar]
- 28.Wang D, Zhang M, Bian H, Dong H, Xu W, Xu X, Zhu Y, Liu F, Geng Z, Zhou G. Proteolysis and cathepsin activities in the processing of dry-cured duck. Poult. Sci. 2014;93:687–694. doi: 10.3382/ps.2013-03335. [DOI] [PubMed] [Google Scholar]
- 29.Taylor RG, Geesink G, Thompson V, Koohmaraie M, Goll D. Is Z-disk degradation responsible for postmortem tenderization? J. Anim. Sci. 1995;73:1351–1367. doi: 10.2527/1995.7351351x. [DOI] [PubMed] [Google Scholar]
- 30.Christensen M, Henckel P, Purslow PP. Effect of muscle type on the rate of post-mortem proteolysis in pigs. Meat Sci. 2004;66:595–601. doi: 10.1016/S0309-1740(03)00175-X. [DOI] [PubMed] [Google Scholar]
- 31.Schäfer A, Rosenvold K, Purslow PP, Andersen HJ, Henckel P. Physiological and structural events post mortem of importance for drip loss in pork. Meat Sci. 2002;61:355–366. doi: 10.1016/S0309-1740(01)00205-4. [DOI] [PubMed] [Google Scholar]
- 32.Baron CP, Jacobsen S, Purslow PP. Cleavage of desmin by cysteine proteases: Calpains and cathepsin B. Meat Sci. 2004;68:447–456. doi: 10.1016/j.meatsci.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 33.Toldra F. Proteolysis and lipolysis in flavour development of dry-cured meat products. Meat Sci. 1998;49:S101–S110. doi: 10.1016/S0309-1740(98)90041-9. [DOI] [PubMed] [Google Scholar]
- 34.Martın L, Antequera T, Ventanas J, Benıtez-Donoso R, Córdoba J. Free amino acids and other nonvolatile compounds formed during processing of Iberian ham. Meat Sci. 2001;59:363–368. doi: 10.1016/S0309-1740(01)00088-2. [DOI] [PubMed] [Google Scholar]
- 35.Larrea V, Hernando I, Quiles A, Lluch M, Pérez-Munuera I. Changes in proteins during Teruel dry-cured ham processing. Meat Sci. 2006;74:586–593. doi: 10.1016/j.meatsci.2006.05.009. [DOI] [PubMed] [Google Scholar]