Abstract
In this study, the effect of different defatting conditions on heat stability of confectionary sunflower protein isolate (SnPI) and the particle size of the produced nanoparticles was investigated. The evaluated factors included temperatures of defatting (40, 50, and 60 °C), time of defatting (2, 6, and 10 h), and the amount of activated carbon (0, 25, and 50% of sample weight). The results of the central composite design showed a significant effect (P < 0.05) among the studied factors, where denaturation temperature and particle size of SnPI nanoparticles were found to be in the ranges of 75.05–89.12 °C and 268–1594 nm, respectively. Moreover, the interaction of activated carbon with temperature and time of defatting proved to be influential factors for the heat stability of confectionary SnPI.
Keywords: Sunflower protein isolate, Heat stability, Defatting, Nanoparticle, DSC
Introduction
Sunflower seed (Helianthus annuus L.) is known to be one of the main sources of plant oils worldwide. According to FAO statistics, three countries of Ukraine, Russian Federation, and Argentina are considered as the major producers of sunflower seeds [1].
Lipids and proteins, which are the main components of sunflower seeds, constitute 47–65 and 20–40% of the dehulled seeds, respectively [2, 3], and globulins are known to be the major parts of sunflower proteins (40–90% of the whole proteins) [3, 4]. Developing sunflower seeds as a source of dietary proteins is mainly limited due to (1) the presence of phenolic compounds, mainly chlorogenic acid (CGA), which may form complexes with proteins and affect their functionality and (2) denaturation of proteins during the defatting process (up to 140 °C in the industry), whose degree is one of the determining parameters of the functional properties (solubility, emulsification, and foam and gel properties) of proteins [3, 5–11].
Therefore, removal of phenolic compounds, along with the production of protein isolate, can be considered as one of the main concerns. The suggested methods for the removal of phenolic compounds may commonly alter protein solubility, which in turn can lead to an increase in protein loss; accordingly, aqueous mixtures will act better than organic solvents. Based on the results of other studies, mixtures of methanol/water have high extraction efficiencies for phenolic compounds, which leads to low protein loss and denaturation [3]. On the other hand, due to the mentioned protein impurities and considering the significant role of activated carbon in the defatting process and removal of protein impurities [12], e.g., phenolic compounds [13], its application in producing a highly pure and functional isolate may be of great importance [14].
Differential scanning calorimetry (DSC) is known to be one of the methods by which heat changes of proteins and thermal properties of carbohydrates and lipids could be studied and information about primary and secondary transitions could be obtained [15–18]. Bruylants et al. [14] investigated protein stability by DSC and found that DSC acts as a powerful technique for determining protein stability. Platts and Falconer [19] and Wen et al. [20] also studied protein stability by DSC and introduced it as a promising technique to study protein stability and denaturation. DSC thermograms provide information on denaturation temperature (T m) and the amount of heat required for heat transition [21]; therefore, T m is shown to be a sign of protein stability, through which stable proteins would exhibit higher temperatures [14].
In recent years, nanoparticles have gained great interest in the fields of encapsulation and drug delivery research. Application of the biopolymer-based nanocarriers to encapsulate nutraceuticals for controlled delivery systems has increased over the past decades mainly due to the many advantages including biocompatibility and non-toxicity [22, 23]. Among the many techniques applied for the preparation of nano- and microparticles, including desolvation, emulsification, precipitation, thermal gelation, nano-spray drying, nab-technology,1 and self-assembly, desolvation has gained great interest, mostly due to its several advantages.
Developing a commercially feasible method for obtaining non-denatured proteins with low CGA content and high protein yield is still a challenging and important task. There have been numerous studies regarding the isolation of protein from oily sunflower seed meal [6, 24], while little information is found on the isolation of proteins from confectionary sunflower seeds. In this study, the feasibility of isolating nearly pure proteins from confectionary sunflower meal and its heat stability under different defatting conditions, along with the feasibility of producing nanoparticles from the obtained protein isolates, has been investigated.
Materials and methods
Materials
High-grade confectionary seeds of H. annuus were purchased from local market and dehulled manually. n-Hexane, ethanol, methanol, acetone, sodium carbonate, and chlorogenic acid were purchased from Merck Company (Darmstadt, Germany). Activated carbon was obtained from Erbeslöh Company (Germany). Folin–Ciocalteu reagent and glutaraldehyde were purchased from Sigma-Aldrich (Germany).
Preparation of defatted sunflower meal (DSM)
The dehulled seeds were ground in a laboratory scale grinder (Moulinex, R10, Ireland) and sieved through mesh No. 35 to remove larger particles and gain uniformity in the resulting flour. The ground seeds were defatted by n-Hexane at three temperature levels (40, 50, and 60 °C) during 2, 6, and 10 h with a meal-to-solvent ratio of 1:5 (w/v) using a magnetic stirrer. The DSMs were obtained by centrifuging the suspension at 8000×g by a Hettich universal 320r centrifuge at 4 °C for 15 min. The meal was left at ambient temperature overnight in order to completely remove Hexane from the sample. All of the obtained samples contained less than 1% fat. For further defatting of sunflower proteins, treatments with activated carbon at three levels (0, 25, and 50% of sample weight) were performed after complete removal of phenolic compounds from sunflower defatted meal at the protein extraction stage.
Preparation of defatted and dephenolized sunflower meal (DDSM)
DDSM was prepared from DSM by cold (4 °C) extraction using 80% (v/v) methanol and water mixture at a meal to solvent ratio of 1:20 (w/v) along with mixing for 4 h. The suspension was then centrifuged (8000×g, 4 °C for 15 min). The extraction procedure was continued as described above until the obtained supernatant developed no more yellow color upon NaOH addition [5], which was achieved after six times of extraction with the methanol mixture to obtain a completely defatted and dephenolized meal. In case of using activated carbon, the procedure was followed to dissolve DDSM in distilled water (10 times the initial weight of DDSM) at 23 °C. Afterward, activated carbon (0, 1.75, and 3.5 g) was added to the mixture and the pH was lowered to 3.0 by 0.2 N HCl. The suspension was mixed magnetically in an ice bath for 1 h. Finally, activated carbon was removed by centrifugation at 9000 rpm for 15 min in a Hettich universal 320r centrifuge at 4 °C. The pH of DDSM and water solution was then adjusted to 7.0 by adding 0.2 N NaOH [12], and the solution was kept at 4 °C until the protein isolation procedure.
Preparation of SnPI
Water dispersions of DDSM at a meal to solvent ratio of 1:10 (w/v) were mixed for 1 h at pH 9 (by 2 N NaOH) and centrifuged (9000 rpm, 20 °C for 15 min), and the supernatant was collected. The resulting precipitates were subjected to the abovementioned protein extraction procedure. The supernatants of both stages were piled up and subjected to isoelectric precipitation at pH 4.5 (by 2 N HCl) by mixing it for 30 min and then centrifuging (9000 rpm, 4 °C for 15 min). The resultant protein was again mixed with double distilled water at pH 4.5 to achieve a semi-pure protein, and then centrifuged. The final obtained protein was mixed with water at pH 9, deep frozen at −80 °C, and freeze dried by a freeze dryer (Operon, OPR-FDCF-12012, Korea) [6].
Protein content
The protein content of sunflower seeds was measured by the Kjeldahl method, AOAC 920.53 [25], by using a nitrogen to protein conversion factor of 5.55. Each experiment was performed in three replicates.
Fat content
Fat content of sunflower seeds was measured according to AOAC 920.39 [25] using n-Hexane as an extracting solvent by means of a full automatic Soxhlet apparatus (Gerhardt, Germany). Each experiment was performed in three replicates.
Total phenolic content
Total phenolic content was determined according to the method described by Singleton and Rossi [26]. Briefly, 0.1 mL of the extract (prepared by dissolving 0.15 g of the sample in 10 mL of 70% (v/v) acetone and stirring for 30 min at ambient temperature, and then centrifuging the solution at 20,000×g for 20 min) was mixed with 0.25 mL of Folin reagent and 1.25 mL of 20% sodium acetate, and subsequently, 0.4 mL deionized water was added to the mixture. After keeping the solution for 40 min at room temperature, absorbance was measured at 725 nm by a 4050 UV–Visible spectrophotometer (Sigma-Germany) against the blank sample. Total phenolic content was then calculated as chlorogenic acid equivalents from its calibration curve (R2 = 0.9965) drawn from standard solutions. The results were expressed as mg of chlorogenic acid per g of the dry matter. Each test was conducted in triplicate.
DSC
A commercial DSC (DSC-60, SHIMADZU, Japan) was used to determine the denaturation temperatures and enthalpies of the samples. Hermetically sealed aluminum pans containing 2 mg of samples were prepared. Scanning was performed at 10 °C/min over the temperature range of 20–200 °C for proteins. Peak temperatures (T m in °C) and enthalpies were taken from the obtained thermograms. Each test was conducted in duplicate.
Color
Color values (L, a, and b) of the sunflower protein isolates were measured using a Minolta colorimeter, Chroma Meter CR-410, Japan. Color parameters were obtained using a CIE Lab color scale. L (lightness) values varied from black (0) to white (100), a values varied from green (−60) to red (+60), and b values varied from blue (−60) to yellow (+60). To measure the color parameters, each protein sample was dispersed homogenously on a white surface. Each test was conducted in triplicate.
Preparation of SnPI nanoparticles
SnPI nanoparticles were prepared by the desolvation method using ethanol as a desolvating agent. For this purpose, a solution of 15 mg/mL of freshly prepared SnPI in deionized water was prepared. The pH of the solution was adjusted to 9 by 2 N NaOH, and 0.02% of sodium azide was added as a preservative to all samples. The protein solution was then equilibrated for 2 h at room temperature and stored overnight (8 h) in a refrigerator (4 °C) for hydration of protein molecules. The protein solution was passed through a syringe filter made of polyvinylidene fluoride (0.45 μm) before nanoparticulation. The nanoparticles were obtained upon the dropwise addition of the antisolvent agent (ethanol or methanol) via a syringe pump (JMS SP-500, Japan) at 1 mL/min under mild stirring condition (1200 rpm). The ratio of the desolvating agent to the protein solution was 4:1 in all experiments. After the desolvation process, 20 μL of 0.25% glutaraldehyde solution was added to 1 mL of the protein solution as a crosslinker and crosslinked overnight. Then, the suspension was diluted by distilled water at a ratio of 1:100 in order to eliminate the desolvating agent, and lyophilized (Operon, OPR-FDCF-12012, Korea) and stored at −19 °C.
Particle size distribution and zeta potential measurement
A commercial dynamic light scattering (DLS) apparatus (Nanotrac Wave, Microtrac) was applied for size distribution and zeta potential analysis of the nanoparticles. Sample dilutions (100 times) with distilled water were performed to eliminate the effect of the desolvating agents on the results. Samples were analyzed under a fixed angle of 90°, temperature of 25 °C, and a laser wavelength of 658 nm.
Particle morphology
The morphology of the particles was determined using a scanning electron microscopy (SEM) at an accelerating voltage of 26 kV (KYKY-EM3200, China). A thin layer of gold was coated on the nanoparticles prior to imaging (KYKY-SBC12, China).
Statistical analysis
A central composite design (CCD) with 19 experiments (including three factors at three levels [temperature (40, 50, and 60 °C), time (2, 6, and 10 h), and treatment with activated carbon (0, 25, and 50% of sample weight)] and five repetitions at the central point) was applied to evaluate the combined effect of different variables on the desired responses, for which the response surface methodology was used. The coded values designed by CCD and responses are given in Table 1. A full quadratic polynomial model with 10 terms (Eq. 1) was fitted in each response to study the effect of variables and to mathematically describe the process.
| 1 |
where a 0, a i, a ii, and a ij are the regression coefficients and x i and x j are the coded levels of independent variables of i and j.
Table 1.
Coded values designed by CCD and responses of protein isolates
| Run | Experimental values | Coded values | T m (°C) (denaturation temperature) | ∆H (J/g) | Total phenol content (mg/g) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Temp (°C) | Time (h) | Act. C (%) | Temp | Time | Act. C | ||||
| 1 | 50 | 6 | 25 | 0 | 0 | 0 | 81.30 ± 2.10cd | 6.50 ± 0.51cd | 0.78 ± 0.04b |
| 2 | 60 | 10 | 0 | 1 | 1 | −1 | 75.05 ± 0.50f | 4.47 ± 0.32e | 1.43 ± 0.07a |
| 3 | 50 | 6 | 0 | 0 | 0 | −1 | 81.20 ± 0.73cd | 7.00 ± 0.12bcd | 1.41 ± 0.08a |
| 4 | 50 | 6 | 25 | 0 | 0 | 0 | 82.30 ± 1.20bcd | 6.47 ± 0.51 cd | 0.80 ± 0.05b |
| 5 | 50 | 10 | 25 | 0 | 1 | 0 | 81.21 ± 1.30cd | 7.01 ± 0.41bcd | 0.78 ± 0.06b |
| 6 | 50 | 6 | 25 | 0 | 0 | 0 | 81.00 ± 0.51cd | 6.90 ± 0.32bcd | 0.80 ± 0.06b |
| 7 | 60 | 2 | 50 | 1 | −1 | 1 | 80.49 ± 0.80ed | 7.02 ± 0.20bcd | 0.58 ± 0.01c |
| 8 | 50 | 6 | 25 | 0 | 0 | 0 | 81.40 ± 0.30cd | 6.30 ± 0.21d | 0.82 ± 0.06b |
| 9 | 40 | 2 | 50 | −1 | −1 | 1 | 89.12 ± 1.50a | 8.20 ± 0.51a | 0.58 ± 0.02c |
| 10 | 40 | 2 | 0 | −1 | −1 | −1 | 83.34 ± 0.71bcd | 6.90 ± 0.26bcd | 1.41 ± 0.06a |
| 11 | 40 | 10 | 0 | −1 | 1 | −1 | 81.82 ± 0.80bcd | 6.70 ± 0.31bcd | 1.37 ± 0.03a |
| 12 | 50 | 6 | 25 | 0 | 0 | 0 | 82.40 ± 0.90bcd | 6.60 ± 0.41bcd | 0.84 ± 0.01b |
| 13 | 40 | 6 | 25 | −1 | 0 | 0 | 84.24 ± 1.41bc | 7.51 ± 0.36abc | 0.82 ± 0.06b |
| 14 | 60 | 2 | 0 | 1 | −1 | −1 | 77.60 ± 0.62ef | 6.07 ± 0.51d | 1.33 ± 0.07a |
| 15 | 50 | 2 | 25 | 0 | −1 | 0 | 83.50 ± 1.61bcd | 7.20 ± 0.45abcd | 0.82 ± 0.06b |
| 16 | 50 | 6 | 50 | 0 | 0 | 1 | 82.85 ± 1.20bcd | 7.10 ± 0.39abcd | 0.58 ± 0.02c |
| 17 | 60 | 6 | 25 | 1 | 0 | 0 | 76.17 ± 0.83f | 4.46 ± 0.25e | 0.86 ± 0.06b |
| 18 | 40 | 10 | 50 | −1 | 1 | 1 | 84.96 ± 0.92b | 7.71 ± 0.65ab | 0.60 ± 0.01c |
| 19 | 60 | 10 | 50 | 1 | 1 | 1 | 75.94 ± 0.40f | 4.38 ± 0.23e | 0.62 ± 0.01c |
Results are expressed as mean ± standard deviation (n = 2 for T m and ∆H and n = 3 for total phenol content)
a–zTukey Grouping, mean values following the same letter in the same column are not significantly different (p < 0.05)
Coding in MATLAB (release 7) was used to design the experiments and to model and analyze the results.
Test for normality of the data was performed in the SAS software version 9.1 Service Pack 4, and in cases of non-normality, the appropriate data transformation was applied. Mean comparison was performed using Tukey’s test at 5% level.
Results and discussion
According to the results of the chemical analysis, the investigated sunflower seeds contained 49.6% fat, 21.6% protein, and 18.43 mg/g (1.84%) total phenolic compounds, and it was found that the temperature and time for the defatting process influenced fat content in the final protein isolate (data not shown).
Color
The results of the Hunter Lab color parameters of sunflower protein isolates are given in Table 2. As apparent from the table, the obtained color parameters are dependent on the phenolic compound content and the extraction procedure of the protein isolates. Sample Nos. 4, 7, 9, 16, 18, and 19 show greenish color and Sample No. 4 shows a bluish color (with negative a and b values, respectively). The greenish color of the samples may be ascribed to the oxidation of phenolic compounds into o-quinones during the process of protein extraction in alkaline medium [6]. Activated carbon resulted in higher lightness values (L) and bright-colored proteins, which affirms the lower number of phenolic compounds remaining in the product.
Table 2.
Hunter Lab color parameters of sunflower protein isolates
| Run | Hunter Lab color parameters | ||
|---|---|---|---|
| L | a | b | |
| 1 | 69.00 ± 0.20c | 1.60 ± 0.10 g | 13.01 ± 0.30hi |
| 2 | 42.31 ± 0.50k | 3.00 ± 0.10ef | 13.61 ± 0.24h |
| 3 | 43.50 ± 0.31jk | 3.61 ± 0.20de | 15.52 ± 0.41f |
| 4 | 61.51 ± 0.30d | −3.22 ± 0.21j | −16.60 ± 0.32k |
| 5 | 68.90 ± 0.51c | 3.90 ± 0.10d | 19.01 ± 0.31cd |
| 6 | 60.11 ± 0.42e | 0.82 ± 0.11h | 16.91 ± 0.23e |
| 7 | 77.60 ± 0.30a | −0.52 ± 0.10i | 13.90 ± 0.30gh |
| 8 | 55.30 ± 0.21f | 7.50 ± 0.30b | 19.62 ± 0.41bc |
| 9 | 77.33 ± 0.32a | −0.71 ± 0.10i | 13.60 ± 0.32h |
| 10 | 44.10 ± 0.54j | 4.00 ± 0.20d | 14.70 ± 0.50fg |
| 11 | 47.20 ± 0.61i | 9.90 ± 0.40a | 21.11 ± 0.42a |
| 12 | 54.50 ± 0.40f | 7.41 ± 0.30b | 19.33 ± 0.41cd |
| 13 | 55.51 ± 0.11f | 7.60 ± 0.21b | 20.30 ± 0.32ab |
| 14 | 50.21 ± 0.52h | 5.21 ± 0.10c | 14.90 ± 0.20f |
| 15 | 55.72 ± 0.60f | 2.54 ± 0.12f | 15.61 ± 0.32f |
| 16 | 77.01 ± 0.40a | −0.64 ± 0.10i | 13.00 ± 0.40hi |
| 17 | 53.22 ± 0.21g | 7.00 ± 0.41b | 18.51 ± 0.22d |
| 18 | 76.43 ± 0.30a | −0.78 ± 0.10i | 12.22 ± 0.10ij |
| 19 | 75.11 ± 0.50b | −0.60 ± 0.11i | 11.74 ± 0.32j |
Results are expressed as mean values ± standard deviations
a–zTukey Grouping, mean values following the same letter in the same column are not significantly different (p < 0.05)
Denaturation temperature (Tm)
According to the obtained thermograms of DSC, denaturation starts from the point where the curve deviates from baseline. Considering the difficulty in identifying the exact temperature of denaturation, the extrapolated slope was defined as the peak temperature and the baseline as the onset temperature of denaturation. Yet as biopolymers like proteins show a wide range of thermal transition temperatures, the peak temperature is commonly regarded as the temperature of denaturation [17]. According to the DSC thermograms of the extracted protein isolate samples, the endothermic peaks varied in the range of 75.05–89.12 °C, which is characteristic of the denaturation temperature of the isolates (Fig. 1; Table 1). The proteins differed in their thermic stability and enthalpy of denaturation. The lowest value of denaturation temperature (75.05 °C) was related to the treatment that was subjected to the highest temperature and time of defatting (60 °C and 10 h) and the lowest amount of activated carbon (0%). In contrast, the highest value (89.12 °C) was ascribed to the treatment that was subjected to the lowest temperature and time of defatting (40 °C and 2 h) and applied the highest amount of activated carbon (50%). Salgado et al. [6] reported the denaturation temperature of methanol-extracted SnPI at 110.7 °C. The higher amounts of this temperature, compared to the results of the current study, may be related to the variety of sunflower species, process of extracting proteins, and the related processes. According to the results of Gonzalez-Perez et al. [5], the denaturation temperature of SnPI was reported to be 99.7 °C. Monomeric forms of helianthinin have denaturation temperatures of about 65 and 90 °C, and sunflower albumins are reported to have denaturation temperatures of above 100 °C (up to 118 °C) [3]. Since the globulins contribute the most to the SnPI, the denaturation temperature of the whole protein isolate is close to that of the globulins.
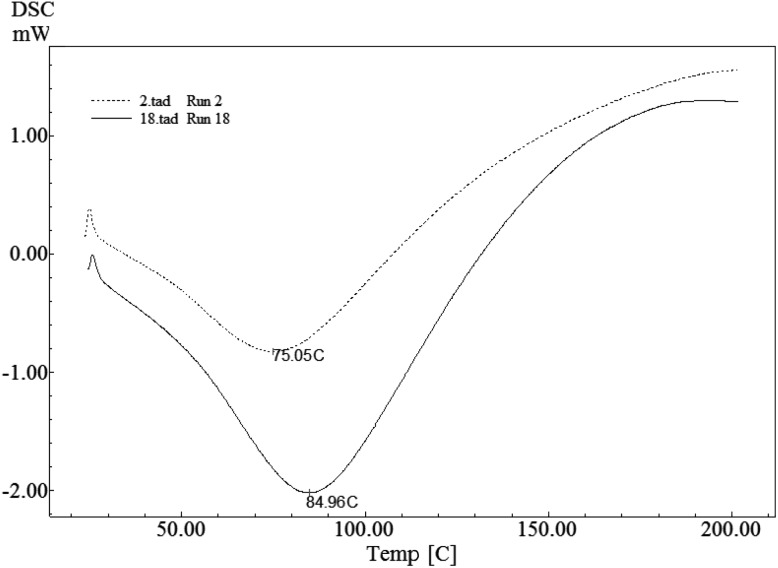
Fig. 1.
DSC thermograms for two SnPI samples (Run#2 and Run#18)
Based on the results of the other studies [16], protein transition from a native to a modified state is mainly due to the destruction of inter- and intra-molecular bonds and is known to be dependent on various factors, among which temperature and contact time are considered in the current study.
According to the results of Wolf and Tamura [27], 11S globulins convert to a fast sedimentation aggregate and a slow sedimenting fraction when subjected to heat treatment. The maximum rate of aggregation is observed at pH 4 to 6 during the heating process, and the combination of ionic and hydrophobic bonds has been suggested to form the aggregates. As shown in Fig. 1, with comparable sample weights and protein concentrations, the measured integrals of the peaks may be a direct measure of the specific reaction enthalpies. Sample No. 2 has the most temperature-sensitive proteins with the lowest denaturation temperature (75 °C) and lower enthalpy of denaturation. In contrast, Sample No. 18 proteins are already extensively denatured at 84.96 °C, which indicates that the main fraction of its protein is still native. That is, denaturation does not set in until 84.96 °C. The enthalpies of denaturation for the studied proteins lie in the range of 4.38–8.20 J/g protein. Accordingly, the amount of heat required to denature proteins decreases with decrease in denaturation temperature (similar to the findings of Arntfield and Murray [18]). Heat denaturation is always considered to be irreversible despite the applied pH and ionic strength [28]. Therefore, T m is a preferred parameter to evaluate thermal changes of proteins and their stability [20].
According to the results of the analysis of variance, which are not presented here, significant differences have been identified between the regression parameters, which suggests the meaningful effect of the studied parameters on the desired response (denaturation temperature). Lack of fit error was not found to be a significant parameter in this study, which indicates that the experimental data are well fitted to the polynomial model.
Equation 2 represents the significant studied terms in the current design after elimination of the non-significant ones. All of the investigated factors showed significant effects on the denaturation temperature, where linear and quadratic effects were shown to be powerful and an interaction was noticed between the amount of the activated carbon and the time of defatting in this polynomial model. Other obtained statistical parameters, i.e., coefficient of determination (R2), adjusted coefficient of determination (Adj-R2), prediction error sum of square (PRESS), and R2, were found to be 0.983, 0.972, 9.84, and 0.952, respectively. Therefore, by considering sufficiently high values of the mentioned parameters, the polynomial equation was fitted appropriately and could successfully cover more than 80% of the variations in the experimental data.
| 2 |
where T m, T, t, and C are denaturation temperature, temperature levels, time levels, and activated carbon levels, respectively.
With respect to the scatter plot of the values of predicted data versus experimental data, the suggested model could successfully cover the experimental data with coefficient of determination (R2) of 0.983. According to the normal percent probability plot of residuals in the studied design, the error distribution is approximately normal.
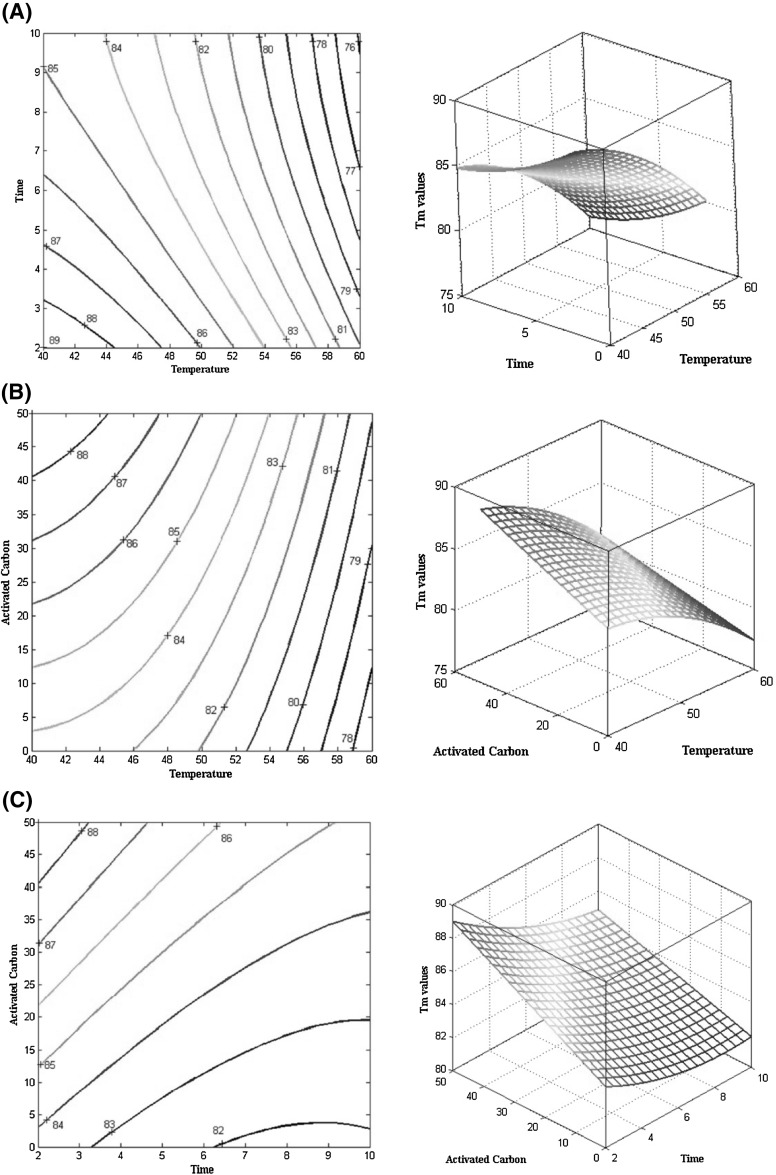
The contour plots of the effect of the studied parameters (temperature and time of defatting and different percentages of activated carbon) on the denaturation temperature of SnPI are illustrated in Fig. 2(A–C). As shown in Fig. 2A, the denaturation temperature shows an increasing trend with decreasing temperature and time of defatting. The unfavorable effects of isolation temperature on the stability of protein isolates are quite apparent, which has been noted in other studies as well [18, 29]. This is mainly because higher temperatures accelerate and facilitate weakening and destroying inter- and intra-molecular interactions, and the endothermic peaks in DSC thermograms often relate to the decomposition of hydrogen bonds [18]. In a study conducted on globular proteins, the contribution of hydrogen bonds was the same for all proteins and the differences in denaturation temperatures of proteins were mainly controlled by non-polar hydrophobic interactions and a type of cooperativity between the polar and non-polar groups [18]. The obtained different values of denaturation temperatures in this study may be related to different processing temperatures. As a result, different degrees of decomposition of hydrogen bonds and inter- and intra-molecular interactions would take place. Hence, samples treated at higher temperatures (60 °C) showed lower temperatures of denaturation than those treated at lower temperatures (40 °C). Moreover, increasing the solvent exposure time adversely affected the protein stability. With reference to the literature review, increasing the exposure temperatures leads to an increase in molar mass (due to the formation of protein polymeric particles) [30], decreased activity [31], and protein denaturation [30]. Long-term exposure to elevated temperatures (10 h at 60 °C) caused lower denaturation temperatures compared to slight treatments (2 h at 60 °C) in this study, which indicates the opposite effect of the contact time on protein isolate stability.
Fig. 2.
(A–C) Response surface and contour plot of the effect of activated carbon, time, and temperature of defatting on the denaturation temperature of SnPI
The contour plot of the effect of temperature and activated carbon on the denaturation temperature of SnPI (Fig. 2B) reveals that the denaturation temperature of sunflower protein isolates shows an increasing pattern by increasing the percent of activated carbon and decreasing the temperature of defatting. Based on the results obtained from the analysis of variance table and the suggested model, the significant effect of activated carbon on the denaturation temperature becomes clear. Increased stability of SnPI under the influence of activated carbon may be attributed to the removal of additional impurities (fat and phenolic compounds) of the isolate and resulting in an isolate with relatively higher purity. Protein impurities lead to decomposition, aggregation, and interaction [32]; for example, phenolic compounds as one such impurity affect the denaturation temperature of the protein isolate. As shown by Rawel et al. [33], binding of phenolic compounds to proteins changes their conformational structure and leads to denaturation. The amount of remaining total phenolic compounds after treating with 80% methanol and activated carbon for removing total phenols is given in Table 1. Treatments with activated carbon not only prevent protein denaturation [12] but also increase protein stability reasonably due to their purification effect [34]. As an example, activated carbon-treated samples showed higher stability compared to non-treated samples at the same time and temperature. This is quite obvious in Table 1, which indicates that the remaining amount of total phenol content decreases after treating with activated carbon. Among the treated samples, those with higher percentages of activated carbon showed higher denaturation temperatures than their counterparts. This proves that removing phenolic compounds renders proteins to be more stable. The proposed model also confirmed a significant interaction between activated carbon and the time of defatting.
The contour plot of the effect of time and activated carbon on the denaturation temperature of SnPI is shown in Fig. 2C. According to the discussed issues, the stability of SnPI increases with decreasing time of defatting and increasing percentage of activated carbon.
Nanoparticulation
The effect of different extraction factors on nanoparticulation parameters, i.e., particle size, polydispersity index, and zeta potential of SnPI nanoparticles, is given in Table 3. In the table, a significant difference is observed between the studied samples, which indicates that the procedure of defatting affects the resultant protein isolate stability and its functional properties. In this case, as expected, longer times of defatting and higher temperatures resulted in a more denatured protein, worsened its functional properties, and affected its stability; therefore, larger particles were formed. That is, Sample No. 2 produced larger particles than the others. On the other hand, the consumption of activated carbon could affect the proteins in removing impurities, and applying higher amounts of it produced smaller particles; however, the mentioned effect is more likely to be derived from the temperature and time of defatting, which may result in nanosized particles. Meanwhile, between the produced nanoparticles (Sample Nos. 4 and 9), Sample No. 9 proved to be lower in polydispersity index, which in turn indicates its uniformity.
Table 3.
Effect of different extraction parameters on particle size, polydispersity index, and zeta potential of SnPI nanoparticles
| Run | Size distributions | Particle size (nm) | Polydispersity index | Zeta potential |
|---|---|---|---|---|
| 2 |
|
1594.01 ± 200.41a | 0.03 ± 0.02c | −29.60 ± 2.01a |
| 4 |
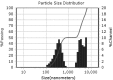
|
373.00 ± 30.15b | 2.16 ± 0.21a | −30.81 ± 3.01a |
| 9 |
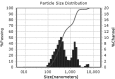
|
268.00 ± 25.20b | 1.22 ± 0.11b | −32.70 ± 3.22a |
Results are expressed as mean values ± standard deviations
a–zTukey Grouping, mean values following the same letter in the same column are not significantly different (p < 0.05)
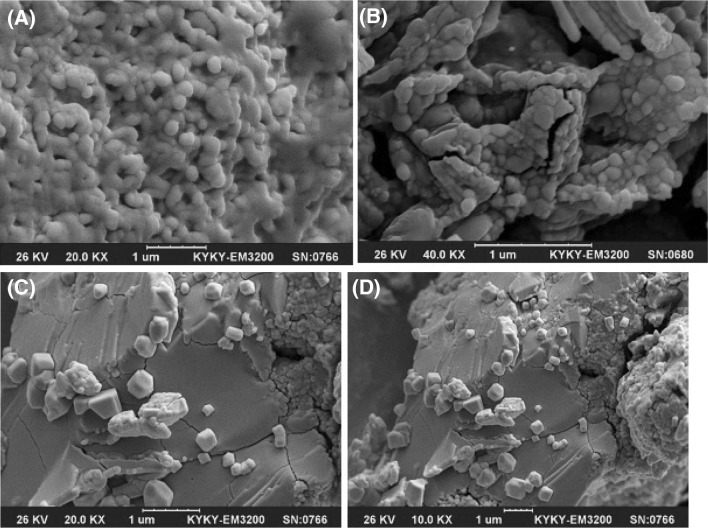
Teng et al. [35] studied nanoparticle formation from soy protein isolate and reported that the average size of the curcumin-loaded nanoparticles was in the range of 220.1–286.7 nm and their zeta potential was around −36 mV. As mentioned before, changes in secondary, tertiary, and quaternary structures of a protein molecule, which relates to denaturation, may affect nanoparticulation and change the formation of nanoparticles. The SEM images of the produced nanoparticles is presented in Fig. 3, which support the data obtained from DLS. According to the obtained images, the morphologies of the nanoparticles are similar to each other and show nearly spherical and smooth surface profiles. The SEM particle sizes were reported to be smaller than those in the DLS results, which is attributable to the reduction in size offered by the cast-drying process along with the vacuum environment in SEM imaging [35].
Fig. 3.
SEM images of (A) Run No. 4, (B) Run No. 9, (C, D) SnPI powder
The results of the current study revealed that the stability of confectionary SnPI can be affected under different conditions of the defatting process. Decreasing temperature and contact time during the defatting process led to higher temperatures of denaturation, mostly due to the less decomposition of hydrogen and hydrophobic bonds and smaller particle sizes. The activated carbon, as an effective factor in protein purification, not only maintained the protein nature but also led to more stable and smaller particle sizes of the protein isolate due to the removal of possible impurities.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Nanoparticle albumin-bound technology.
References
- 1.FAO. FAOSTATdata. Agriculture. Available from: http://faostat.fao.org Accessed Sep. 25, 2016.
- 2.Badwaik LS, Prasad K, Deka SC. Optimization of extraction conditions by response surface methodology for preparing partially defatted peanut. Int. Food Res. J. 19(1):(2012)
- 3.González-Pérez S, Vereijken JM. Sunflower proteins: overview of their physicochemical, structural and functional properties. J. Sci. Food Agr. 2007;87(12):2173–2191. doi: 10.1002/jsfa.2971. [DOI] [Google Scholar]
- 4.Pérez SG, Vereijken JM, Koningsveld GA, Gruppen H, Voragen AG. Physicochemical properties of 2S albumins and the corresponding protein isolate from sunflower (Helianthus annuus) J. Food Sci. 2005;70(1):C98–C103. doi: 10.1111/j.1365-2621.2005.tb09029.x. [DOI] [Google Scholar]
- 5.González-Pérez S, Merck KB, Vereijken JM, van Koningsveld GA, Gruppen H, Voragen AG. Isolation and characterization of undenatured chlorogenic acid free sunflower (Helianthus annuus) proteins. J. Agr. Food Chem. 2002;50(6):1713–1719. doi: 10.1021/jf011245d. [DOI] [PubMed] [Google Scholar]
- 6.Salgado PR, Ortiz SE, Petruccelli S, Mauri AN. Sunflower protein concentrates and isolates prepared from oil cakes have high water solubility and antioxidant capacity. J. Am. Oil Chem. Soc. 2011;88(3):351–360. doi: 10.1007/s11746-010-1673-z. [DOI] [Google Scholar]
- 7.Sastry MS, Subramanian N. Preliminary studies on processing of sunflower seed to obtain edible protein concentrates. J. Am. Oil Chem. Soc. 1984;61(6):1039–1042. doi: 10.1007/BF02636213. [DOI] [Google Scholar]
- 8.Xu L, Diosady LL. Removal of phenolic compounds in the production of high-quality canola protein isolates. Food Res. Int. 2002;35(1):23–30. doi: 10.1016/S0963-9969(00)00159-9. [DOI] [Google Scholar]
- 9.Gallo M, Vinci G, Graziani G, De Simone C, Ferranti P. The interaction of cocoa polyphenols with milk proteins studied by proteomic techniques. Food Res. Int. 2013;54(1):406–415. doi: 10.1016/j.foodres.2013.07.011. [DOI] [Google Scholar]
- 10.Shen F, Niu F, Li J, Su Y, Liu Y, Yang Y. Interactions between tea polyphenol and two kinds of typical egg white proteins—ovalbumin and lysozyme: Effect on the gastrointestinal digestion of both proteins in vitro. Food Res. Int. 2014;59:100–107. doi: 10.1016/j.foodres.2014.01.070. [DOI] [Google Scholar]
- 11.Sen M, Bhattacharyya DK. Nutritional quality of sunflower seed protein fraction extracted with isopropanol. Plant Food Hum. Nutr. 2000;55(3):265–278. doi: 10.1023/A:1008152201872. [DOI] [PubMed] [Google Scholar]
- 12.Chen RF. Removal of fatty acids from serum albumin by charcoal treatment. J. Biol. Chem. 1967;242(2):173–181. [PubMed] [Google Scholar]
- 13.Shawabkeh RA, Abu-Nameh ES. Absorption of phenol and methylene blue by activated carbon from pecan shells. Colloid J. 2007;69(3):355–359. doi: 10.1134/S1061933X07030143. [DOI] [Google Scholar]
- 14.Bruylants G, Wouters J, Michaux C. Differential scanning calorimetry in life science: thermodynamics, stability, molecular recognition and application in drug design. Curr. Med. Chem. 2005;12(17):2011–2020. doi: 10.2174/0929867054546564. [DOI] [PubMed] [Google Scholar]
- 15.Ellepola SW, Ma CY. Thermal properties of globulin from rice (Oryza sativa) seeds. Food Res. Int. 2006;39(3):257–264. doi: 10.1016/j.foodres.2005.07.015. [DOI] [Google Scholar]
- 16.del Angel SS. Martínez EM, López MA. Study of denaturation of corn proteins during storage using differential scanning calorimetry. Food Chem. 2003;83(4):531–540. doi: 10.1016/S0308-8146(03)00149-3. [DOI] [Google Scholar]
- 17.Koshiyama I, Hamano M, Fukushima D. A heat denaturation study of the 11S globulin in soybean seeds. Food Chem. 1981;6(4):309–322. doi: 10.1016/0308-8146(81)90004-2. [DOI] [Google Scholar]
- 18.Arntfield SD, Murray ED. The influence of processing parameters on food protein functionality I. Differential scanning calorimetry as an indicator of protein denaturation. Can. I. Food Sc. Tech. J. 14(4):289–294 (1981)
- 19.Platts L, Falconer RJ. Controlling protein stability: Mechanisms revealed using formulations of arginine, glycine and guanidinium HCl with three globular proteins. Int. J. Pharm. 2015;486(1):131–135. doi: 10.1016/j.ijpharm.2015.03.051. [DOI] [PubMed] [Google Scholar]
- 20.Wen J, Arthur K, Chemmalil L, Muzammil S, Gabrielson J, Jiang Y. Applications of differential scanning calorimetry for thermal stability analysis of proteins: qualification of DSC. J. Pharm. Sci. 2012;101(3):955–964. doi: 10.1002/jps.22820. [DOI] [PubMed] [Google Scholar]
- 21.Boye JI, Alli I. Thermal denaturation of mixtures of α-lactalbumin and β-lactoglobulin: a differential scanning calorimetric study. Food Res. Int. 2000;33(8):673–682. doi: 10.1016/S0963-9969(00)00112-5. [DOI] [Google Scholar]
- 22.Langer R, Chasin M. Biodegradable polymers as drug delivery systems. Health Care: Informa; 1990. [Google Scholar]
- 23.Elzoghby AO, El-Fotoh WS, Elgindy NA. Casein-based formulations as promising controlled release drug delivery systems. J. Control. Release. 2011;153(3):206–216. doi: 10.1016/j.jconrel.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Pickardt C, Hager T, Eisner P, Carle R, Kammerer DR. Isoelectric protein precipitation from mild-acidic extracts of de-oiled sunflower (Helianthus annuus L.) press cake. Eur. Food Res. Technol. 2011;233(1):31–44. doi: 10.1007/s00217-011-1489-6. [DOI] [Google Scholar]
- 25.AOAC. Official method of analysis of AOAC Intl. 16th ed. Method 991.43. Association of official analytical chemists, Arlington, VA, USA (1995)
- 26.Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16(3):144–158. [Google Scholar]
- 27.Wolf WJ, Tamura T. Heat denaturation of soybean 11S protein. Cereal Chem. 1969;46:331–344. [Google Scholar]
- 28.Giancola C, De Sena C, Fessas D, Graziano G, Barone G. DSC studies on bovine serum albumin denaturation effects of ionic strength and SDS concentration. Int. J. Biol. Macromol. 1997;20(3):193–204. doi: 10.1016/S0141-8130(97)01159-8. [DOI] [PubMed] [Google Scholar]
- 29.Graziano G, Catanzano F, Barone G. Prediction of the heat capacity change on thermal denaturation of globular proteins. Thermochim. Acta. 1998;321(1):23–31. doi: 10.1016/S0040-6031(98)00435-3. [DOI] [Google Scholar]
- 30.Roefs SP, De Kruif CG. Heat-induced denaturation and aggregation of β-lactoglobulin. Trend. Colloid Interf. Sci. VIII (pp. 262–266). Steinkopff (1994)
- 31.Pinto M, Morange M, Bensaude O. Denaturation of proteins during heat shock. In vivo recovery of solubility and activity of reporter enzymes. J. Biol. Chem. 1991;266(21):13941–13946. [PubMed] [Google Scholar]
- 32.Wang W, Ignatius AA, Thakkar SV. Impact of residual impurities and contaminants on protein stability. J. Pharm. Sci. 2014;103(5):1315–1330. doi: 10.1002/jps.23931. [DOI] [PubMed] [Google Scholar]
- 33.Rawel HM, Meidtner K, Kroll J. Binding of selected phenolic compounds to proteins. J. Agric. Food Chem. 2005;53(10):4228–4235. doi: 10.1021/jf0480290. [DOI] [PubMed] [Google Scholar]
- 34.Noritomi H, Kai R, Iwai D, Tanaka H, Kamiya R, Tanaka M, Muneki K, Kato S. Increase in thermal stability of proteins adsorbed on biomass charcoal powder prepared from plant biomass wastes. J. Biomed. Sci. Eng. 2011;4(11):692–698. doi: 10.4236/jbise.2011.411086. [DOI] [Google Scholar]
- 35.Teng Z, Luo Y, Wang Q. Nanoparticles synthesized from soy protein: preparation, characterization, and application for nutraceutical encapsulation. J. Agric. Food Chem. 2012;60(10):2712–2720. doi: 10.1021/jf205238x. [DOI] [PubMed] [Google Scholar]