Abstract
The objective of this study was to investigate the sleep-inducing effect of lettuce varieties and the extracts derived from romaine lettuce on pentobarbital-induced sleep. Romaine lettuce had a higher lactucin content (red romaine: 361.50 µg/g of extract, green romaine: 1071.67 µg/g of extract) compared to the green and red lettuce varieties. The seed and leaf extracts derived from romaine lettuce induced an increase in the sleep duration at low and high doses respectively. The seed extract of romaine lettuce showed higher content of polyphenols, including caftaric acid, chlorogenic acid, and chicoric acid, compared to the leaf extract. The IC50 value of the leaf extract for its DPPH radical-scavenging was significantly less (0.11 mg/mL) than that of the seed extract (0.21 mg/mL) (p < 0.05). Romaine lettuce is an interesting and valuable source of sleep potentiating material and contains antioxidant phenolics that protect from the oxidant stress caused by sleep disturbance.
Keywords: Lettuce, Lactuca sativa, Sleep, Anti-radical
Introduction
Sleep is necessary in order to maintain good health and performance, yet many individuals are sleep deprived. Physical, psychological, physiological, and environmental factors all influence the quantity and quality of sleep. Ancoli-Israel and Roth [1] reported that approximately one-third of the population might be exposed to a chronic disorder related to sleep. Sleep disorder, a subjective complaint, is the one in which it is hard to start or maintain sleep, which is usually non-refreshing or of poor quality and quantity [2].
Benzodiazepine and non-benzodiazepine prescription drugs, which act as potentiators of the central nervous system by suppressing the activity of γ-aminobutyric acid A (GABAA) receptors in the brain, are widely used to treat patients with sleep disorder. However, it is concerning that people may experience residual daytime effects, development dependency and related withdrawal symptoms, all of which are related to use of hypnotic drugs [3].
Herbal and natural products are the most common forms of complementary and alternative medicine [4]. These medicines can be purchased at supermarkets, pharmacies, and health food stores, and used without supervision. Therefore, the products should be safe and risk-free [5]. According to the National Health Interview Survey dataset published in 2006, therapies based on herbal medicine and relaxation therapies were used as the most commonly used alternative medicine modality for insomnia [6]. Among herbs and dietary supplements, valerian root, St. John’s wort, kava, passionflower and melatonin have been promoted as natural sleep aids [3].
Lactuca sativa, a member of the Compositae family, is the most popular herb used in salads in Korea. In addition, lettuce seed oil has been used as a sleeping aid and for pain and inflammation relief in folk medicine since a long time [7, 8]. The extracts of L. sativa seeds with crude methanol and petroleum ether have been reported to have a time- and dose-dependent analgesic effect in the formalin test in addition to a dose-dependent anti-inflammatory activity in a carrageenan model of inflammation [7].
Therefore, in current study, the hydro-alcoholic was prepared from varieties of commercial lettuces, and its sleep-prolonging effect was examined with the analysis of the content of lactucin and lacucopicrin, which were known to be sleep-inducing compounds. Moreover, romaine lettuce extracts, which showed a shorter sleep latency time than other lettuce, were assessed for their sleep latency and sleep duration in a pentobarbital-induced sleep model, and for their radical-scavenging activity against oxidative stress.
Methods and materials
Materials
Four varieties of lettuce (L. sativa L.), including two green varieties (Sangnok and Chung romaine) and two red varieties (Hongbit and Juk romaine) were studied. Lettuce seeds were purchased from Danong Co. Ltd. (Namyangju, Korea) and fresh lettuce leaves were purchase from the local market. Fresh leaves of lettuce were transported to the laboratory in coolers.
For high performance liquid chromatography (HPLC), all solvents used were of HPLC-grade and purchased from Caledon (Caledon, Canada). Pentobarbital sodium was purchase from HYPHARM. Co., Ltd. (Gyeonggi-do, Korea).
Preparation of extracts
The fresh lettuce was trimmed, washed with tap water, and dried at 60°C. Subsequently, the dried lettuce was powdered and stored at 5°C. The dried lettuce leaves and seeds were extracted twice with 70% ethanol in a Soxhlet apparatus for 4 h. The extracts were dried in a water bath followed by dissolving the yield (37% w/w) in a 1% saline (v/v) solution of Tween 80.
Determination of polyphenols and flavonoids
Total polyphenol and flavonoid contents of the extracts were measured using Folin–Ciocalteu and p-dimethylamino-cinnamaldehyde reagents, respectively using the method described by Amous et al. [9]. The results were expressed as the equivalents of gallic acid and catechin.
Determination of free radical-scavenging activity
In order to measure the free radical-scavenging activities of the extracts of the Korean lettuce (L. sativa), stable DPPH and ABTS were used. The method reported by Quang et al. [10] was slightly modified and used to measure the DPPH-scavenging activity. Similarly, the methods described by Wang et al. [11] and Almajano et al. [12] were slightly modified and used to measure the ABTS radical-scavenging activity. The IC50, defined as the amount of extract needed to decrease the absorbance of DPPH and ABTS radicals by 50%, was measured to estimate the antioxidant activity of the sample.
Animals
Korea University Animal Care Committee (Seoul, Korea) approved the protocols (KUIACUC-20160824-1) for the use of animals in the experiment. The animals (4-week-old ICR mice) used in the experiment were purchased from Orient Bio Inc. (Gyeonggi-do, Korea). The rodents were housed in acrylic cages supplied with food and water. The room was maintained at 24 °C, under the atmospheric humidity of 50–60%, and with a 12-h light/dark cycle. The mice were tested for pentobarbital-induced sleep after 1 week allowed for adaptation.
Pentobarbital-induced sleep test
The experiments were performed from 1:00 to 5:00 pm; the mice were not fed for 24 h. A 0.9% solution of physiological saline was used to suspend all the samples. The lettuce extract was orally administered in both the groups (80 mg and 160 mg/kg), such that the observers were blind to the individual treatment. The mice were injected with the pentobarbital (hypnotic dose: 42 mg/kg) 40 min later. Finally, the mice were housed individually and the sleep latency and duration were measured using the method described by Yang et al. [13]. Mice that did not sleep 15 min after the injection were excluded from the experiment.
Identification and quantification of lactucin and lactucopicrin
The Agilent HPLC series 1100 (Agilent, Waldbronn, Germany), equipped with a degasser, binary pump, autosampler, thermostat, and a photodiode array detector (DAD), was used to detect sesquiterpene lactones (STL) at 320 and 254 nm. The analytical conditions were the same as those described by Abu-Reidah et al. [14]. Briefly, a reversed-phase C18 analytical column (4.6 mm × 150 mm, 5 μm, Phenomenex) with the mobile phases consisted of acidified water (0.2% phosphoric acid, v/v) (A) and acetonitrile (B) was used. A linear gradient elution was programmed as follows: 0 min, 15% B; 5 min, 15% B; 35 min, 100% B; 45 min, 100% B; 46 min, 15% B; 60 min, 15. The flow rate was 0.7 mL/min, and the injection quantity was 20 μL.
Identification and quantification of phenolic compounds
Freeze-dried materials were ground and the resulting fine powder was extracted to identify and quantify the phenolic compounds using the method described by Ferreres et al. [15] with a little modifications. The seeds and leaves (0.4 g of freeze-dried) from each romaine sample were extracted twice with an 8 mL mixture of methanol, water and formic acid (25:24:3 v: v: v), and filtered using a 0.45 μm PVDF filter (Millex HV13, Millipore, Bedford, MA). The extracts (20 μL) were analyzed with an Agilent HPLC series 1100 equipped with a degasser, binary pump, autosampler, thermostat and a photodiode array detector (DAD). Separations were obtained on a LiChroCart C18 column (250 × 4 mm2; 5 μm particle size; Phenomenex). The mobile phases consisted of water with 5% formic acid (A) and methanol (B) at a solvent flow rate of 1 mL/min were applied in a gradient program starting with 5% B in A that reached 40% B at 25 min and then remained isocratic for 5 min. The UV chromatograms were read at 330 and 520 nm and the mean of three experiments with the standard error was used to express the results.
Statistical analysis
Statistical Package for Social Sciences version 12.0 (SPSS Inc., Chicago, IL, USA) was used to analyze the data. Data from the in vivo and in vitro tests are represented as mean ± standard error (SE), and mean ± standard deviation (SD), respectively. The results of the sleeping tests were analyzed by one-way analysis of variance (ANOVA), and Duncan’s multiple range tests using SPSS (version 12.0, SPSS Inc., Chicago, IL, USA). In order to compare the extraction yield, total polyphenols, and total flavonoids, between the seed and leaf extract, the Student’s t test was used. P values < 0.05 were considered statistically significant.
Results and discussion
Lactucin and lactucopicrin contents of seed extracts derived from lettuce varieties
Lactuca sativa (lettuce), an annual herb which belongs to the Compositae family, is known for its medicinal value. Traditionally, lettuce has been suggested to have a sedative-hypnotic property [16]. Lactucopicrin and lactucin are the major active compounds of lactucarium, and were reported to have analgesic activity equal to or greater than that of ibuprofen in mice. They also showed a sedative activity as revealed by measuring the spontaneous movement in mice. Lactucin, a sesquiterpene lactone of the Lactuca species, was reported to have a sedative property in the spontaneous locomotor activity test [17]. The lettuce opium is known to have these analgesic, antitussive, and sedative properties because of the lactucin; its ester lactucopicrin has been used in Europe for centuries [17]. However, no analgesic and sedative effect has been reported for lactucopicrin in the other report [18]. Unlike lactucopicrin, lactucin is presumed to be the predominant substance exhibiting the sedative property including sleep-promoting effect.
Table 1 shows the lactucin and lactucopicrin contents of seed extracts from four different types of lettuce. Lactucin contents of seed extracts ranged from 226.20 to 1071.67 µg/g of extract. Romaine lettuce had a higher lactucin content compared to green and red lettuce. The lactucin contents of red and green romaine lettuce were 361.50 and 1071.67 µg/g of extract, respectively. In contrast, green and red lettuce had a higher lactucopicrin content compared to green and red romaine lettuce. The lactucopicrin contents of green and red lettuce were 1448.08 and 1321.18 µg/g of extract respectively.
Table 1.
Lactucin and lactucopicrin contents of seed extract from different types of lettuces
| Sesquiterpene lactones | Lettuce (µg/g of extract) | Romaine (µg/g of extract) | ||
|---|---|---|---|---|
| Red | Green | Red | Green | |
| Lactucin | 226.20 ± 32.63 | 446.42 ± 15.81 | 361.50 ± 27.63 | 1071.67 ± 199.24 |
| Lactucopicrin | 1321.18 ± 180.93 | 1448.08 ± 1.48 | 226.20 ± 32.65 | 150.22 ± 1.79 |
The effect of lettuce seed extract on sleep latency and total sleep duration of pentobarbital-induced sleep in mice
Pentobarbital sodium, one of the barbiturate compounds, is known to induce sleep in both rodents and humans [19]. The pentobarbital-induced sleep in mice is a classic method used to screen sedative–hypnotic drugs. Therefore, we used to this method to evaluate the sleep-prolonging effect of various lettuce varieties.
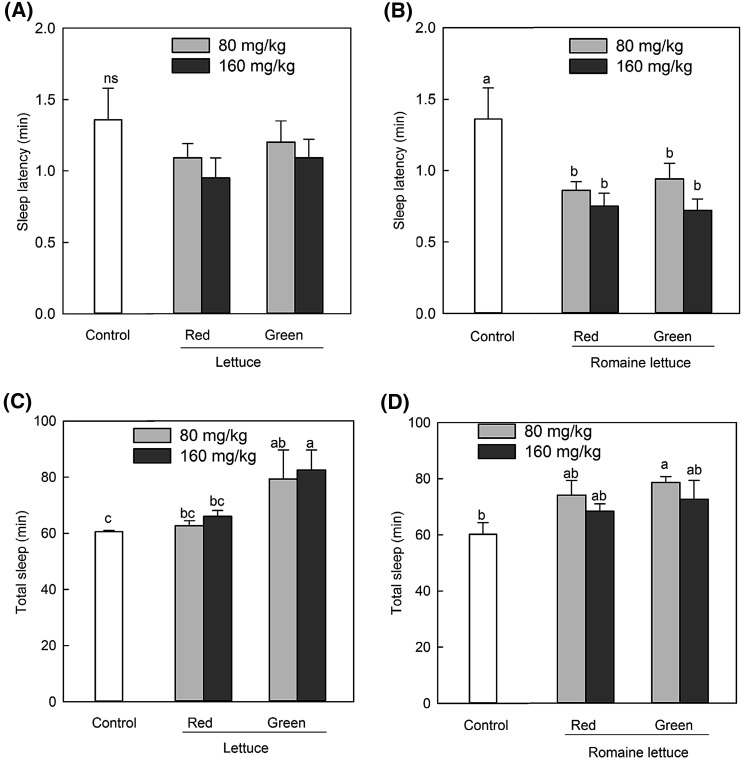
Seed extracts were administered 40 min prior to the pentobarbital-induced sleeping test. The seed extracts from lettuce and the vehicle group showed differences in their effect on the onset of sleep (Fig. 1). The sleep latency and the sleep duration in pentobarbital-treated mice were 1.36 ± 0.22 and 60.56 ± 0.43 min, respectively. The seed extracts derived from green and red lettuce at doses of 80 and 160 mg/kg did not decrease the sleep latency compared to the control [Fig. 1(A)]. However, the seed extracts derived from green and red romaine lettuce at doses of 80 and 160 mg/kg decreased the sleep latency significantly when compared to the control [p < 0.05, Fig. 1(B)].
Fig. 1.
Effect of lettuce seed extract on sleep onset and sleep duration in mice treated with pentobarbital (42 mg/kg, i.p.). Control was just pentobarbital treated-mice group and pentobarbital was administered 40 min after oral administration of the lettuce seed extract. (A) Sleep latency of red and green lettuce seed extract. (B) Sleep latency of red and green romaine lettuce seed extract. (C) Sleep duration of red and green lettuce seed extract. (D) Sleep duration of red and green romaine lettuce seed extract. Each column represents the mean ± SE of the values obtained from 8 mice. Different letters mean significant differences (p < 0.05) among samples (Duncan’s multiple range test)
Oral administration (80 and 160 mg/kg) of the seed extracts derived from green and red lettuce showed a tendency to increase the sleep duration in pentobarbital-induced mice. Specifically, the seed extract derived from green lettuce significantly increased the sleep duration compared to the control at 160 mg/kg [p < 0.05, Fig. 1(C)] but not at 80 mg/kg [Fig. 1(C)]. While the seed extract derived from green romaine lettuce decreased the sleep latency at the 80 mg/kg concentration, it increased the duration of sleep significantly (p < 0.05). It has also been reported that both State-Trait Anxiety Inventory and sleep scores were significantly improved by L. sativa seed oil without any side effects at the dose strength used [20]. Ghorbani et al. [21] reported that the butanol fraction of the lettuce extract potentiates sleep parameters indicating that non-polar agents are responsible for these effects of lettuce.
Unlike green and red lettuce, red and green romaine lettuce showed a significant difference in sleep latency [Fig. 1(B)]. The content of lactucin, which was known as the active substance of lettuce, was higher in romaine lettuce, especially green romaine lettuce, than the other lettuces (Table 1). Therefore, romaine lettuces were selected for further experiments.
The effect of seed and leaf extracts derived from green romaine lettuce on sleep latency and total sleep duration of pentobarbital-induced sleep in mice
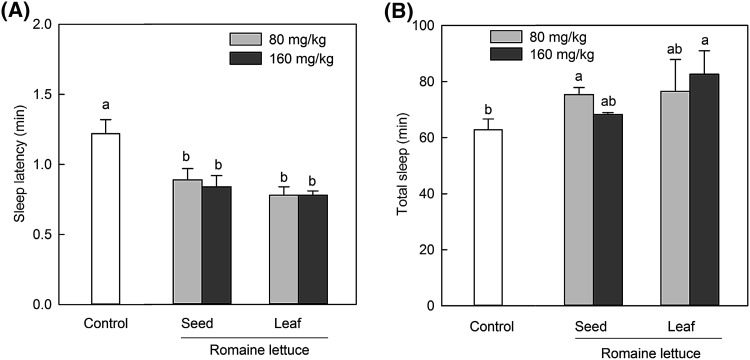
The seed extract derived from green romaine lettuce significantly reduced sleep latency and increased sleep duration in pentobarbital-induced mice when compared to other lettuce varieties (Fig. 1). Therefore, we compared the effect of seed and leaf extracts of green romaine lettuce on sleep latency and sleep duration (Fig. 2). Both the extracts at doses of 80 and 160 mg/kg caused a significant reduction in sleep latency (p < 0.05, Fig. 2).
Fig. 2.
Effects of the administration of seed and leaf extracts derived from green romaine lettuce on sleep onset and sleep duration in mice treated with pentobarbital (42 mg/kg, i.p.). Control was just pentobarbital treated-mice group and pentobarbital was administered 40 min after the oral administration of seed and leaf extracts derived from green romaine lettuce. (A) Sleep latency of green romaine seed and leaf extract. (B) Sleep duration of green romaine seed and leaf extract. Each column represents the mean ± SE values obtained from 8 mice. Different letters show significant differences (p < 0.05) among samples (Duncan’s multiple range test)
The seed extract had a significant effect on the sleep duration at a dose of 80 mg/kg compared to control, but not at 160 mg/kg. The leaf extract had a significantly effect on the sleep duration at 160 mg/kg compared to control. The effect of the seed extract at 80 mg/kg and the leaf extract at 160 mg/kg on the sleep duration did not show a significant difference. Thus, the seed and leaf extracts derived from romaine lettuce induced an increase in the sleep duration at low and high doses respectively. In addition, both low and high doses of the seed and leaf extracts derived from romaine lettuce caused a significant reduction in sleep latency (p < 0.05).
Extraction yield, total polyphenols, and total flavonoids in green romaine lettuce
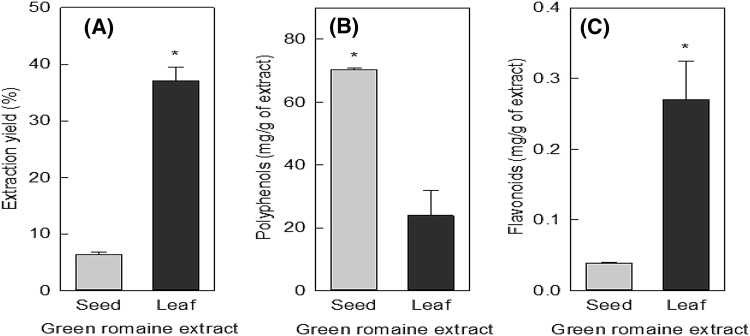
Good extraction is a crucial step for getting extracts with acceptable yields and strong antioxidant activity. The percentage yields of the ethanol extract, and the contents of polyphenols and flavonoids in green romaine lettuce are shown in Fig. 3.
Fig. 3.
Extraction yield (A), total polyphenols (B), and total flavonoids (C) of the green romaine lettuce extract. The seed and leaf extract were compared using the Student’s t test. Each value is represented as mean ± SD (n = 3). Values of p < 0.05 are considered statistically significant
The extraction yield (dry basis) of the seed and leaf were 6.41 and 37.13% respectively. The leaf extract had a higher extraction yield compared to the seed extract [Fig. 3(A)]. Being plant secondary metabolites, polyphenols are very important in judging the antioxidant activities of the extract. The total polyphenolic contents of the seed and leaf extracts were 70.37 and 23.84 mg/g, respectively [Fig. 3(B)]. However, the leaf extract showed a higher level of flavonoids compared to the seed extract [Fig. 3(C)]. The seed extract had a higher polyphenol content and a lower flavonoid content compared to the leaf extract (p < 0.05). Total polyphenols (885.18 mg) and flavonoid (10.02 mg) contents of leaf extract were higher than those of seed extract (451.07 and 0.25 mg, respectively). High yield of polyphenols and flavonoids derived from leaf extract might contribute to the enhancement of their medical applications. Therefore, leaf extract was selected for further studies.
The polyphenol contents of the green and red romaine lettuce were significantly different (Table 2). The major compounds obtained in the seed extract of romaine lettuce were more than those obtained from the leaf extract. The seed extract had a high level of chlorogenic acid (28.71 mg/g), while the leaf extract had a high level of chicoric acid (3.85 mg/g), among other phenolic components. The seed extract had a higher polyphenols content including caftaric acid, chlorogenic acid, and chicoric acid, than the leaf extract. However, the seed extract had a lower extraction yield than the leaf extract.
Table 2.
Main phenolic compounds in the leaf and seed extracts derived from romaine lettuce
| Romaine lettuce | Caftaric acid (mg/g of extract) | Chlorogenic acid (mg/g of extract) | Chicoric acid (mg/g of extract) | Isochlorogenic acid (mg/g of extract) |
|---|---|---|---|---|
| Leaf | 1.86 ± 0.35 | 1.22 ± 0.32 | 3.85 ± 0.48 | 0.33 ± 0.17 |
| Seed | 5.78 ± 1.07 | 28.71 ± 1.32 | 13.13 ± 0.76 | 4.94 ± 0.62 |
Lettuce provides many antioxidant compounds such as vitamin C and polyphenols in addition to the fiber [22]. Polyphenols, flavonols, and anthocyanins were shown to contain the higher antioxidant activities than vitamins C and E [23]. The genetic information of the plant determines the phenolic contents both qualitatively and quantitatively. As seen in Table 2, the seed extract shows a higher polyphenol content including the main phenolic compounds than the leaf extract derived from romaine lettuce. However, the leaf extract showed a higher content of flavonoids and a higher yield than the seed extract. The seed or leaf of romaine lettuce could prove to be good sources of antioxidants that would assist in the enhancement of the overall antioxidant capacity of an organism and protection against oxidative damage.
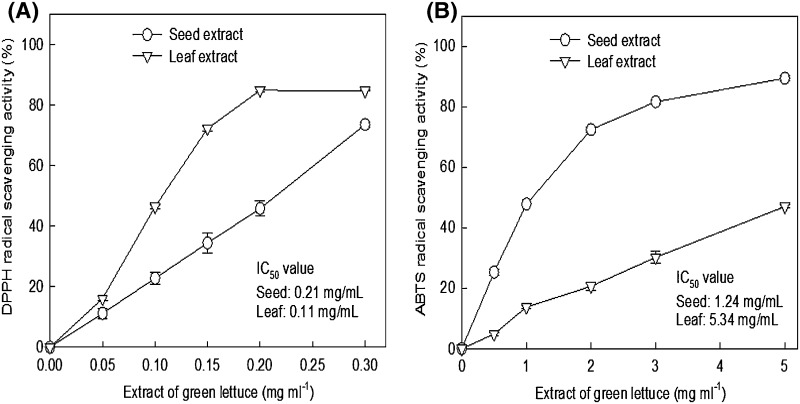
Radical-scavenging activity of green lettuce seed and leaf extract on DPPH and ABTS radicals
Methanol and water were used for the assay for DPPH· and ABTS·+. This method is useful in evaluating the free radical-scavenging activities of water- and non-water-soluble compounds. Figure 4 shows the DPPH and ABTS radical-scavenging activity of the seed and leaf extracts with different concentrations. As shown in Fig. 4(A), the IC50 value of the leaf extract for DPPH radical-scavenging was significantly lower (0.11 mg/mL) than that of the seed extract (0.21 mg/mL) (p < 0.05). However, ABTS radical-scavenging activity [Fig. 4(B)] of the leaf extract showed a significantly higher IC50 value (5.34 mg/mL) than that of the seed extract (1.24 mg/mL) (p < 0.05).
Fig. 4.
Radical scavenging activity of the seed and leaf extracts on DPPH (A) and ABTS radical (B). Each value represents mean ± SD
The lack of strong correlation between these two assays is likely attributable to the fact that every individual phenolic compound contained in lettuce causes a different response to each specific radical used in the assay. Antioxidant capacities by both assays were more strongly correlated with polyphenolics than with flavonoids content as reported previously [24, 25]. Kim et al. [25] found that antioxidant capacity measured by ABTS assay was highly correlated with polyphenolics content in plums, while its correlation with flavonoids contents was relatively weak. High ABTS radical scavenging activity of romaine leaf lettuce is presumably due to high content of polyphenols. And DPPH radical scavenging activity would be related to hydroxyl groups in the aromatic rings of flavonoids, which provide their hydrogen donating ability [26]. The structure-radical scavenging activity relationships of flavonoids demonstrated that the positions of phenolic OH groups could be more important for the radical scavenging activity than the number of phenolic OH groups [27]. The structural difference of flavonoids may be closely related to DPPH radical scavenging.
There are reports showing that oxidative stress to the brain might be induced by the sleep loss [28, 29]. It has been shown that sleep loss produces effects similar to those observed during the aging process [30]. It is thought that sleep plays a natural and important role in the amelioration of ROS generation during the sleep and wake cycle. Graves et al. [31] reported that both acute and chronic sleep deprivation increased the oxidative stress in the hippocampus and other areas of the brain. Zhang et al. [32] confirmed that significant changes in hippocampal and cortical oxidative stress resulted from acute sleep deprivation for 24 h or 72 h and that the changes were caused by the reduction of the superoxide dismutase activity and increase of the levels of malondialdehyde and nitric oxide. When the antioxidant defense mechanism fails, an increase in oxidative stress follows, which can explain the deficit in memory after sleep deprivation. It was reported that a higher metabolic rate resulted in the presence of abundant amount of polyunsaturated fatty acids, a deficiency in the antioxidant defense, and a high rate of the oxygen utilization. This situation made the brain more sensitive to the damage by oxidation [33]. Lettuce, especially romaine lettuce might play a potential neuroprotectants.
The results of this study show that lettuce, especially romaine lettuce, is an interesting and cheap source of sleep-potentiating material and antioxidant polyphenols. The seed and leaf extracts derived from romaine lettuce potentiates the pentobarbital-induced sleeping behavior in mice. Romaine lettuce could provide extracts with sleep-potentiating activity. In addition, the antioxidant polyphenols being used as natural antioxidants of the brain against the oxidative damage could be obtained from these extracts. Therefore, it is obvious that further study for the sleep structure and sleep mechanisms induced by romaine lettuce extracts is required before they can be used as dietary complements or as natural food antioxidants.
Acknowledgement
This research was supported by the Ministry of Trade, Industry and Energy (MOTIE) and the Korea Institute for Advancement of Technology (KIAT) through the Encouragement Program for The Industries of Economic Cooperation Region.
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;22(Suppl 2):S347–S353. [PubMed] [Google Scholar]
- 2.Richardson CE, Gradisar M, Barbero SC. Are cognitive “insomnia” processes involved in the development and maintenance of delayed sleep wake phase disorder? Sleep Med. Rev. 2016;26:1–8. doi: 10.1016/j.smrv.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Ramakrishnan K, Scheid DC. Treatment options for insomnia. Am. Fam. Physician. 2007;76:517–526. [PubMed] [Google Scholar]
- 4.Ni H, Simile C, Hardy AM. Utilization of complementary and alternative medicine by United States adults: results from the 1999 national health interview survey. Med. Care. 2002;40:353–358. doi: 10.1097/00005650-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Ernst E. When natural is not harmless. Int. J. Clin. Pract. 2006;60:380. doi: 10.1111/j.1368-5031.2006.00924b.x. [DOI] [PubMed] [Google Scholar]
- 6.Pearson NJ, Johnson LL, Nahin RL. Insomnia, trouble sleeping, and complementary and alternative medicine - Analysis of the 2002 National Health Interview Survey data. Arch. Intern. Med. 2006;166:1775–1782. doi: 10.1001/archinte.166.16.1775. [DOI] [PubMed] [Google Scholar]
- 7.Sayyah M, Hadidi N, Kamalinejad M. Analgesic and anti-inflammatory activity of Lactuca sativa seed extract in rats. J. Ethnopharmacol. 2004;92:325–329. doi: 10.1016/j.jep.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Said S, Kashef HE, Mazar ME, Salama O. Phytochemical and pharmacological studies on Lactuca sativa seed oil. Fitoterapia. 1996;67:215–219. [Google Scholar]
- 9.Arnous A, Makris DP, Kefalas P. Anthocyanin composition and colour characteristics of selected aged wines produced in Greece. J. Wine Res. 2002;13:23–34. doi: 10.1080/0957126022000004039. [DOI] [Google Scholar]
- 10.Quang DN, Hashimoto T, Nukada M, Yamamoto I, Tanaka M, Asakawa Y. Antioxidant activity of curtisians I-L from the inedible mushroom Paxillus curtisii. Planta Med. 2003;69:1063–1066. doi: 10.1055/s-2003-45159. [DOI] [PubMed] [Google Scholar]
- 11.Wang HC, Chen CR, Chang CMJ. Carbon dioxide extraction of ginseng root hair oil and ginsenosides. Food Chem. 2001;72:505–509. doi: 10.1016/S0308-8146(00)00259-4. [DOI] [Google Scholar]
- 12.Almajano MP, Carbo R, Delgado ME, Gordon MH. Effect of pH on the antimicrobial activity and oxidative stability of oil-in-water emulsions containing caffeic acid. J. Food Sci. 2007;72:C258–C263. doi: 10.1111/j.1750-3841.2007.00387.x. [DOI] [PubMed] [Google Scholar]
- 13.Yang H, Lee YC, Han KS, Singh H, Yoon M, Park JH, Cho CW, Cho S. Green and gold kiwifruit peel ethanol extracts potentiate pentobarbital-induced sleep in mice via a GABAergic mechanism. Food Chem. 2013;136:160–163. doi: 10.1016/j.foodchem.2012.07.111. [DOI] [PubMed] [Google Scholar]
- 14.Abu-Reidah IM, Arráez-Román D, Quirantes-Piné R, Fernández-Arroyo S, Segura-Carretero A, Fernández-Gutiérrez A. HPLC–ESI-Q-TOF-MS for a comprehensive characterization of bioactive phenolic compounds in cucumber whole fruit extract. Food Res. Int. 2012;46:108–117. doi: 10.1016/j.foodres.2011.11.026. [DOI] [Google Scholar]
- 15.Ferreres F, Gil MI, Castaner M, TomasBarberan FA. Phenolic metabolites in red pigmented lettuce (Lactuca sativa). Changes with minimal processing and cold storage. J. Agric. Food Chem. 1997;45:4249–4254. doi: 10.1021/jf970399j. [DOI] [Google Scholar]
- 16.Ceolin T, Heck R, Barbieri R, de Souza A, Rodrigues W, Vanini M. Medicinal plants used as sedative by ecological farmers from Southern Rio Grande do Sul State. Brazil. Rev. Enferm. UFPE On line. 2009;3:253–260. [Google Scholar]
- 17.Wesołowska A, Nikiforuk A, Michalska K, Kisiel W, Chojnacka-Wójcik E. Analgesic and sedative activities of lactucin and some lactucin-like guaianolides in mice. J. Ethnopharmacol. 2006;107:254–258. doi: 10.1016/j.jep.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Gromek D, Kisiel W, Klodzinska A, Chojnackawojcik E. Biologically-Active Preparations from Lactuca-Virosa L. Phytother. Res. 1992;6:285–287. doi: 10.1002/ptr.2650060514. [DOI] [Google Scholar]
- 19.Koch-Weser J, Greenblatt DJ. The archaic barbiturate hypnotics. N. Engl. J. Med. 1974;291:790–791. doi: 10.1056/NEJM197410102911512. [DOI] [PubMed] [Google Scholar]
- 20.Yakoot M, Helmy S, Fawal K. Pilot study of the efficacy and safety of lettuce seed oil in patients with sleep disorders. Int. J. Gen. Med. 2011;4:451–456. doi: 10.2147/IJGM.S21529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghorbani A, Rakhshandeh H, Sadeghnia HR. Potentiating effects of Lactuca sativa on pentobarbital-induced sleep. Iran J Pharm Res. 2013;12:401–406. [PMC free article] [PubMed] [Google Scholar]
- 22.Nicolle C, Cardinault N, Gueux E, Jaffrelo L, Rock E, Mazur A, Amouroux P, Remesy C. Health effect of vegetable-based diet: lettuce consumption improves cholesterol metabolism and antioxidant status in the rat. Clin. Nutr. 2004;23:605–614. doi: 10.1016/j.clnu.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Rice-Evans C, Miller NJ. Total antioxidant status in plasma and body fluids. Methods Enzymol. 1994;234:279–293. doi: 10.1016/0076-6879(94)34095-1. [DOI] [PubMed] [Google Scholar]
- 24.Chun OK, Kim DO, Moon HY, Kang HG, Lee CY. Contribution of individual polyphenolics to total antioxidant capacity of plums. J. Agric. Food Chem. 2003;51:7240–7245. doi: 10.1021/jf0343579. [DOI] [PubMed] [Google Scholar]
- 25.Kim DO, Chun OK, Kim YJ, Moon HY, Lee CY. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003;51:6509–6515. doi: 10.1021/jf0343074. [DOI] [PubMed] [Google Scholar]
- 26.Brandwilliams W, Cuvelier ME, Berset C. Use of a Free-Radical Method to Evaluate Antioxidant Activity. Food Sci Technol-Leb. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 27.Okawa M, Kinjo J, Nohara T, Ono M. DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity of flavonoids obtained from some medicinal plants. Biol. Pharm. Bull. 2001;24:1202–1205. doi: 10.1248/bpb.24.1202. [DOI] [PubMed] [Google Scholar]
- 28.Singh R, Kiloung J, Singh S, Sharma D. Effect of paradoxical sleep deprivation on oxidative stress parameters in brain regions of adult and old rats. Biogerontology. 2008;9:153–162. doi: 10.1007/s10522-008-9124-z. [DOI] [PubMed] [Google Scholar]
- 29.Hipolide DC. D’almeida V, Raymond R, Tufik S, Noberga JN. Sleep deprivation does not affect indices of necrosis or apoptosis in rat brain. Int. J. Neurosci. 2002;112:155–166. doi: 10.1080/00207450212022. [DOI] [PubMed] [Google Scholar]
- 30.Andersen ML, Martins PJ, D’Almeida V, Santos RF, Bignotto M, Tufik S. Effects of paradoxical sleep deprivation on blood parameters associated with cardiovascular risk in aged rats. Exp. Gerontol. 2004;39:817–824. doi: 10.1016/j.exger.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn. Mem. 2003;10:168–176. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Zhang HQ, Liang XY, Zhang HF, Zhang T, Liu FE. Melatonin ameliorates cognitive impairment induced by sleep deprivation in rats: role of oxidative stress. BDNF and CaMKII. Behav. Brain Res. 2013;256:72–81. doi: 10.1016/j.bbr.2013.07.051. [DOI] [PubMed] [Google Scholar]
- 33.Dringen R, Pawlowski PG, Hirrlinger J. Peroxide detoxification by brain cells. J. Neurosci. Res. 2005;79:157–165. doi: 10.1002/jnr.20280. [DOI] [PubMed] [Google Scholar]