Abstract
Amorphous granular starches (AGS) and non-granular amorphous starches (non-AGS) of corn, tapioca and rice were prepared using high hydrostatic pressure (HHP) treatment with ethanol and water washing, respectively and their physicochemical properties were investigated. Water holding capacity and apparent viscosity of AGS and non-AGS were higher than those of native one in all starches. In RVA pasting properties, AGS and non-AGS showed higher pasting temperature and lower peak viscosity than those of native one. Furthermore, non-AGS showed distinctively lower peak viscosity compared to that of AGS possibly due to its non-granular structure. Apparent viscosity of non-AGS revealed relatively lower than commercial pre-gelatinized starch because of heat and pressure-induced gelatinization. Maintaining granular structure in HHP treated pre-gelatinized starch provide a distinctive physicochemical characteristics compared to native starch and preparation of gelatinized starch with different gelatinization and washing methods could cause big differences in their physicochemical properties.
Keywords: Amorphous granular starch (AGS), Non-granular amorphous starch (non-AGS), High hydrostatic pressure (HHP), Non-thermal starch modification, Physicochemical properties
Introduction
Starch is the second abundant biomass found in nature, next to cellulose [1] and occurs in discrete granules [2]. Starch is known as a semi-crystalline material consisted of two parts: amorphous and crystalline regions. Native starches have been widely used in diverse industries as thickener, colloid stabilizer, gelling agent, etc. However, native starch is limited for applying to food industry due to insolubility in cold water and unstable viscosity [3]. So, various physical and/or chemical methods were used for modifying the starch.
Starch has been traditionally gelatinized by various heat treatments such as spray-drying, extrusion and drum-drying etc. in food industry. Gelatinization has been defined as an irreversible melting phase transition of the starch granules from an ordered to a disordered state and it takes place in excess amount of water [4]. During gelatinization, the amorphous region in a starch granule is destabilized by water absorption and swelling, while the crystalline region in the granule melts simultaneously with progressively increasing hydration [5].
On the other hand, hydrostatic high pressure (HHP) is a non-thermal technique which can gelatinize starch at room temperature. Also, it is suitable to apply for food industry because HHP treatment is only physical means, not chemical method. The material can be minimally processed because HHP treatment breaks the secondary and tertiary structures but leave the covalent bonds intact [1]. So, it is leading to ‘fresh-like’ products that can satisfy the consumer demands with unique textures or tastes preserving flavor, color, and nutritional value [6]. The high-pressure technology is interesting because it can provide the possibility to produce foods with a novel texture and high nutritional and sensory qualities [7]. For this reasons, HHP technique have been expected as alternative of heat treatment to modify the starch. Also, various applications by HHP treatment were added to existing means to modify the starch, such as hydrolyzing with different conditions (e.g., pressure time, acid and starch concentration, HCl concentration) [8, 9], acetylating [10, 11], cross-linking [12, 13], and hydroxylpropylating of several types of starch [14]. Besides, applications about HHP in starch chemistry by changing the several conditions were introduced [15]. For example, the pressure range which can occur the starch gelatinization was applied depending on the botanical origin and partly dependent on the crystalline structure [4]. Therefore, understanding pressure-induced gelatinization of starch is essential to apply the high-pressure treatment with starch-containing products and to understand and achieve the desired product functionality. Pressure-induced starch gelatinization resulted in relatively low release of amylose compared with that from heat-induced gelatinization [2]. After pressurization, swelling index was also increased with increasing pressure level (~600 MPa) and apparent viscosity and swelling power were increased [16, 17]. Besides, HHP accelerated physical and chemical reaction, better water absorbing ability, increased enzyme reaction ability, etc. [16]. In our previous study, six types of commercial starches—corn, tapioca, rice, waxy rice, potato and sweet potato starches—were used for preparing amorphous granular starch (AGS) [18]. AGS is completely gelatinized starch by HHP treatment but has intact granular shape similar to its native starches. We found that washing step with ethanol was very important to make AGS. If HHP treated starches were washed with distilled water, AGS could not made. And in this case, we called them non-AGS. When giving pressure at same pressure level (550 MPa) for 30 min, AGS corn, tapioca and rice starches could be obtained via ethanol washing among six types of starches. Further, both microscopic and macroscopic properties of AGS and non-AGS were studied. Therefore, in this study, physicochemical properties of AGS and non-AGS corn, tapioca and rice starches were investigated to provide the fundamental information and future application of AGS and non-AGS in the food, textile and paper industries.
Materials and methods
Materials
Three types of commercial starches were used in this study. Corn starch was obtained from Daesang Co. (Seoul, Korea) and tapioca starch was purchased from Sing Song industrial Co. (Seoul, Korea). Rice starch was prepared in laboratory using alkaline steeping method by Choi et al. [19]. Rice powder and 0.4% NaOH solution were mixed to 1:1.5 ratio and ground using a Waring blender (51 BL 31, Torrington, CT, USA). Then it was held at room temperature for 24 h and supernatant was removed. This step was repeated 5 times until proteins were completely removed. Rice starch suspensions were washed using distilled water to neutralize. Sample was dried using dry oven at 35 °C and then dried starch was ground and kept in a deep freezer. Pre-gelatinized corn starch was purchased from local market.
Preparation of AGS and non-AGS
The method and background for preparation of AGS and non-AGS were introduced in detail in our previous study [18]. For preparation of AGS, 30% (w/v) suspensions of each starch were prepared using distilled water. Each starch suspensions was transferred to retortable pouch and then hermetically heat-sealed before pressure treatment. And then, it was put pressurized in HHP unit (C.I.P Process Controller, Ilshin Autoclave, Daejeon, Korea) using distilled water as a pressure medium. The HHP unit was maintained at ambient temperature (25 °C) during pressurization. For complete gelatinization of starch, the HHP treatment was achieved at 550 MPa for 30 min. After HHP treatment, to make AGS, ethanol (EtOH) which was solvents to support the preservation of morphology of gelatinized starch granule was used to wash the HHP-treated starches. Washing was conducted three times with sufficient EtOH (two times of amount of starch suspension) and centrifuged at 3000 rpm for 5 min at 4 °C. Starch samples washed with EtOH were dried at ambient temperature for overnight and then samples were grounded and sieved by 80 mesh sieve for further analysis. Otherwise, distilled water was used as a washing solvent to make non-AGS.
Water holding capacity (WHC)
For observing the water holding capacity of native starch, AGS and non-AGS, the methods of Lee and Moon [20] was used with slight modification. The starch suspensions were prepared by adding 12.5 mL of distilled water to 0.5 g of starch samples in conical tube. After complete mixing for 1 min by vortex mixer, the mixtures were placed at ambient temperature. The supernatant and precipitate of mixtures were separated by centrifuging at 3000 rpm for 15 min at 4 °C. After discarding the supernatant, the conical tube was placed upside down above paper tissue for 15 min to remove the remaining distilled water in precipitate. And then the precipitate was dried at 105 °C dry oven for overnight. The water holding capacity was calculated by differences of weight of precipitate between before and after drying.
Pasting properties
Pasting properties of native starch, AGS and non-AGS were measured by rapid visco analyzer (RVA) (RVA-3D, Newport Scientific Pty. LTD., Warriewood, NSW, Australia). The 25 mL of distilled water was added to 3 g (d.b) of samples of corn and tapioca starches in RVA canister, whereas 1.5 g (d.b) of rice starch was used for analysis because rice starch suspension had relatively high viscosity. The pasting properties were measured under the standard method-1 (STD-1) conditions. The conditions of time and temperature of analysis included an initial holding phase at 50 °C for 1 min, a heating phase from 50 to 95 °C at 12 °C/min, an intermediate holding phase at 95 °C for 2.5 min, linear cooling from 95 to 50 °C at 12 °C/min, and a final holding phase at 50 °C for 2 min. These were analyzed under constant rotating paddle (160 rpm).
Swelling power and solubility
Swelling power and solubility of native starch, AGS and non-AGS were evaluated using the following method with slight modification [21]. Starch (0.5 g, d.b) was suspended in 30 mL of distilled water and heated in a shaking water bath at 60 and 90 °C for 30 min. After heating, samples were centrifuged at 3000 rpm for 60 min and precipitates were separated from the supernatant and weighed. The supernatant was dried at 105 °C and weighed. Swelling power and solubility were calculated using the following equations:
Apparent viscosity
The apparent viscosity of native starch, AGS and non-AGS was measured by using a Brookfield viscometer (model RVDV-II + PRO, Brookfield Engineering Laboratories, Middleboro, MA, USA). Starch samples were suspended in 200 mL of distilled water to make 7% (w/v) starch suspension. After gentle stirring of starch suspension by spatula, the measurement of apparent viscosity of starch suspension was conducted using a No. 1 spindle at 200 rpm under ambient temperature.
Statistical analysis
All experiments were replicated three times by each method. Also, the data of physicochemical properties of starch were analyzed using Analysis of Variance (ANOVA), and expressed as mean value ± standard deviation. Among experimental mean values, significant differences were assessed by a Duncan’s multiple range test (p < 0.05). All statistical computations and analyses were conducted using SAS version 8.02 for Windows (SAS Institute, Inc., Cary, NC, USA).
Results and discussion
Water holding capacity (WHC)
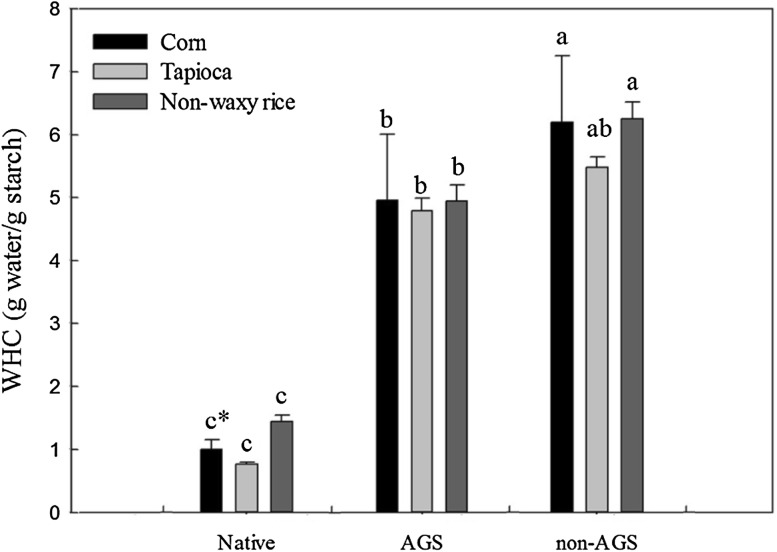
Water holding capacity (WHC) of native starch, AGS and non-AGS was shown in Fig. 1. In all three starches, non-AGS showed the highest WHC followed by AGS and native starches. After HHP treatment, WHC of all native starches were increased more than 4 times in both AGS and non-AGS. It has been reported that WHC of high pressure treated (600 MPa for 1 h) 10–70% suspensions of maize starch were relatively higher than that of native maize starch and they were increased with increasing both moisture content and degree of gelatinization [22]. This suggested that the hydroxyl groups in starch granules which can combine with water molecules were increased by gelatinization of starch induced by HHP treatment resulting in increase of WHC. Moreover, difference in WHC between AGS and non-AGS could be come from their intact or disintegrated granular structure. Non-AGS has disintegrated granular structure and release some amylose and amylopectin residues which promote starch gel networking resulting in increase in WHC.
Fig. 1.
Water holding capacity of native starch, amorphous granular starch (AGS) and non-AGS
Rapid visco analysis (RVA)
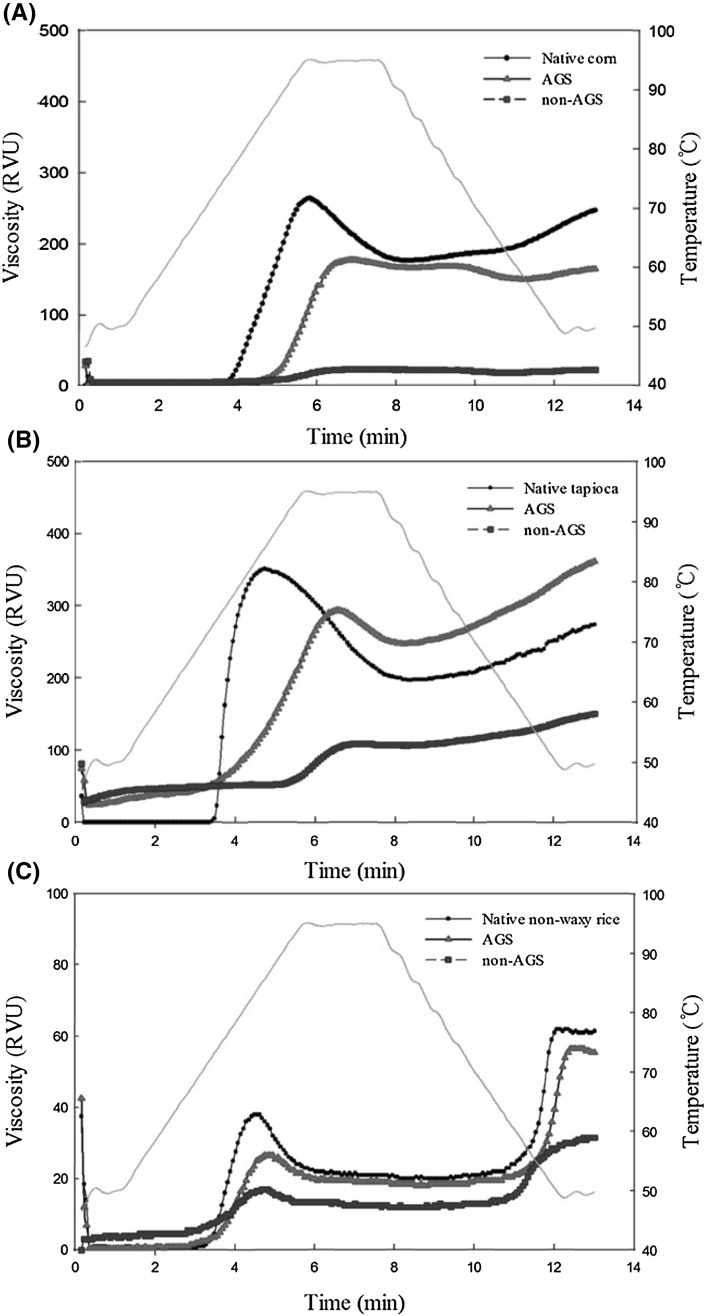
Figure 2 and Table 1 show the RVA pasting curves and pasting properties of native starch, AGS and non-AGS, respectively. Figure 2 clearly shows the difference between native starches, AGS and non-AGS in all samples. Native starches showed the highest peak viscosity (PV) followed by AGS and non-AGS suggesting that maintenance of granular structure greatly influence the peak viscosity of starch paste (Fig. 2; Table 1). It has been reported that the decrease of resistance on shear comes from the instability of starch granule structure. Thus, the collapse of granular structure of AGS by HHP treatment would arise prior to arrival at PV [16]. On the other hand, both pasting temperature (GT) and peak time (PT) of non-AGS were higher than those of AGS and native starches in all samples (Table 2) suggesting that disintegration of granular structure greatly influenced both GT and PT. Trough viscosity (TV) indicates the degree of collapse of starch granules at 95 °C. TV of native corn and rice starches were higher than those of their AGS and non AGS, whereas TV of AGS tapioca was higher than that of native tapioca starch (Table 1). This result indicated that AGS tapioca was relatively resistant to heat and shear compared to AGS corn and rice. Breakdown (BD) is a gap between PV and TV, and indicates the degree of collapse of starch granules as cooling through 95–50 °C. In case of tapioca and rice starches, non-AGS showed higher BD than that of AGS, whereas in case of corn starch opposite result was revealed (Table 1). This may come from different PV of those starches. Non-AGS of corn, tapioca and rice starches revealed 92, 70 and 55% reduction of PV compared to their native starches, respectively. This difference may cause the different BD in those starches. Final viscosities (FV) of native starches were higher than those of their AGS and non-AGS except for tapioca starch (Table 1). In case of corn and rice starches, setbacks (SB) of native starches were higher than AGS and non-AGS, whereas SB of AGS and non-AGS were higher than that of native starch in case of tapioca starch. It has been reported that SB of non-crystalline granular waxy corn starch was lower than that of native starch, whereas that of non-crystalline granular tapioca starch was higher than that of native starches [16].
Fig. 2.
RVA pasting curves of native starch, amorphous granular starch (AGS) and non-AGS from corn (A), tapioca (B), rice (C)
Table 1.
RVA pasting properties of native starch, amorphous granular starch (AGS) and non-AGS
| Samples | PV (RVU) | TV (RVU) | BD (RVU) | FV (RVU) | SB (RVU) | PT (min) | GT (°C) |
|---|---|---|---|---|---|---|---|
| Corn starch | |||||||
| Native | 267.5 ± 4.9c* | 180.5 ± 6.4c | 87.0 ± 1.4b | 252.0 ± 7.1c | 71.5 ± 0.7b | 5.8 ± 0.0c | 77.1 ± 0.5d |
| AGS | 177.0 ± 8.5d | 150.0 ± 7.1d | 27.0 ± 1.4d | 164.5 ± 7.8d | 14.5 ± 0.7d | 6.8 ± 0.1a | 85.8 ± 0.2c |
| Non-AGS | 23.5 ± 3.5g | 4.0 ± 0.0f | 19.5 ± 3.5de | 22.0 ± 4.2g | 18.0 ± 4.2d | 7.0 ± 0.2a | 90.8 ± 0.8b |
| Tapioca starch | |||||||
| Native | 355.5 ± 6.4a | 202.5 ± 7.8b | 153.0 ± 1.4a | 278.5 ± 6.4b | 76.0 ± 1.4b | 4.7 ± 0.0d | 72.5 ± 0.5f |
| AGS | 294.5 ± 14.8b | 246.5 ± 12.0a | 48.0 ± 2.8c | 362.0 ± 9.9a | 115.5 ± 2.1a | 6.5 ± 0.1b | 77.8 ± 0.3d |
| Non-AGS | 109.0 ± 12.7e | 101.5 ± 0.7e | 8.5 ± 12.0b | 150.0 ± 11.3e | 48.5 ± 10.6b | 7.0 ± 0.2a | 92.8 ± 1.2a |
| Rice starch | |||||||
| Native | 38.0 ± 2.8f | 20.0 ± 2.8e | 18.0 ± 0.0def | 61.5 ± 3.5f | 41.5 ± 0.7c | 4.4 ± 0.0d | 74.3 ± 0.5e |
| AGS | 26.5 ± 0.7 fg | 18.0 ± 0.0e | 8.5 ± 0.7f | 55.5 ± 0.7f | 37.5 ± 0.7c | 4.6 ± 0.1d | 77.6 ± 0.3d |
| Non-AGS | 17.0 ± 1.4g | 2.5 ± 0.7f | 14.5 ± 0.7ef | 12.0 ± 0.0h | 9.5 ± 0.7d | 4.7 ± 0.1d | 78.6 ± 0.7d |
* Means with different letter in the same column are significantly different (p < 0.05)
PV peak viscosity, TV trough viscosity, BD breakdown, FV final viscosity, SB, setback, PT peak time, GT pasting temperature
Table 2.
Apparent viscosities of native starch, amorphous granular starch (AGS) and non-AGS
| Viscosity (cp) | |||
|---|---|---|---|
| Sample | Native | AGS | Non-AGS |
| Corn starch | 17.05 ± 0.07h* | 27.40 ± 0.99f | 30.06 ± 0.48e |
| Tapioca starch | 16.90 ± 0.14h | 44.15 ± 0.49c | 48.63 ± 0.87b |
| Rice starch | 18.80 ± 0.14g | 42.15 ± 0.49d | 52.34 ± 0.57a |
* Means with different letter are significantly different (p < 0.05)
It is interesting to note that AGS showed similar pasting pattern with native starch, whereas non-AGS did not showed clear pasting characteristics (Fig. 2). This maybe the main difference between AGS and non-AGS and it comes from their granular structure. When giving pressure at 690 MPa for 5 min to water/starch mixture at 1/1 (v/w) ratio of native starches, the pasting curves of treated starches showed the similar pasting results with our non-AGS [1]. RVA pasting properties are highly dependent on two factors, such as intact granule structure and integrity of granular structure. Therefore, it is very important to maintain its granular structure in case of amorphous starch in terms of pasting properties.
Solubility and swelling power
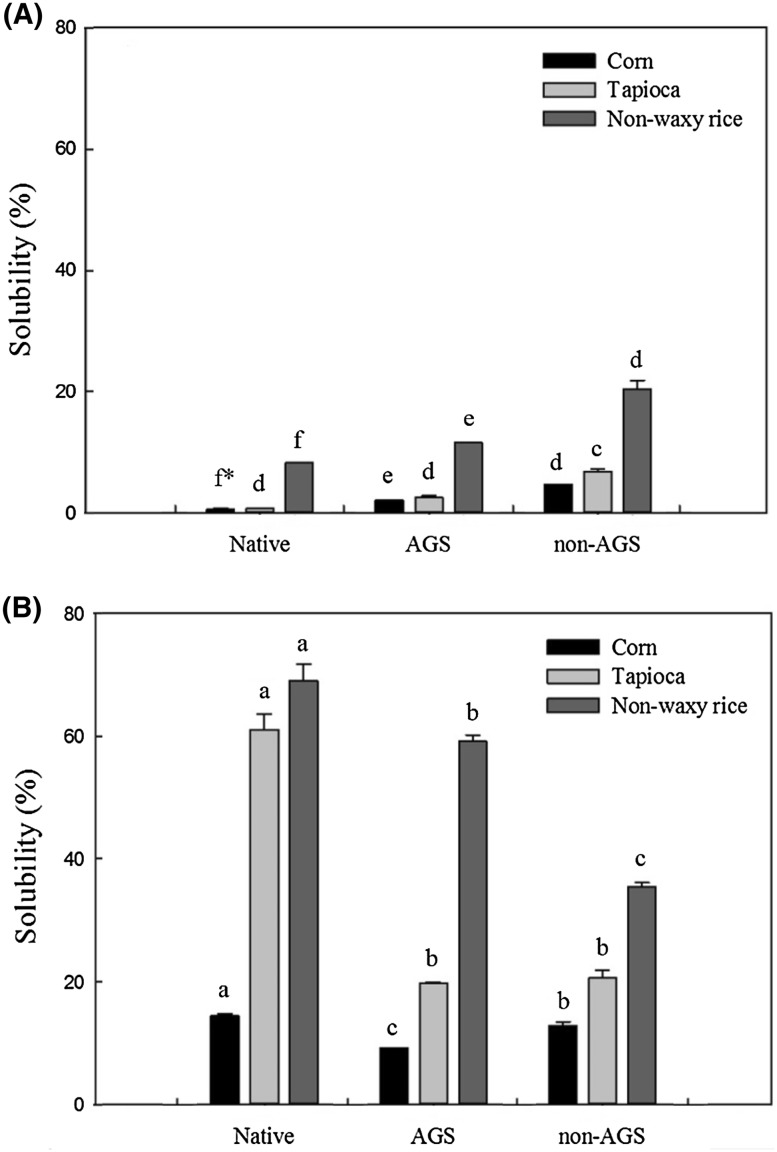
Solubility of native corn, tapioca, rice starches and their AGS and non-AGS at 60 and 90 °C are shown in Fig. 3. In all starches, native ones showed the lowest solubility and non-AGS revealed the highest solubility at 60 °C, which is just below gelatinization temperature of those starches. On the other hand, native starches showed the highest solubility and non-AGS revealed the lowest solubility at 90 °C, which is enough to disintegrate granular structure of those starches. AGS showed medium range of solubility at 60 and 90 °C except corn starch at 90 °C. In all starches, solubility of both native starches and AGS greatly increased with increasing temperature, whereas non-AGS did not show such a big increase. This result indicates that non-AGS is relatively less sensitive to temperature change because it already lost its granular structure and released its internal materials.
Fig. 3.
Solubility of native starch, amorphous granular starch (AGS) and non-AGS, determined at 60 °C (A) and at 90 °C (B)
It is interesting to note that all rice starches (native, AGS and non-AGS) showed relatively high solubility compared to those of corn and tapioca starches at both 60 and 90 °C suggesting that rice starch contains relatively large amount of soluble materials. Moreover, AGS corn revealed relatively lower solubility than non-AGS and in case of tapioca starch, AGS and non-AGS did not show significant (p < 0.05) difference at 90 °C. These results may come from different characteristics of different varieties.
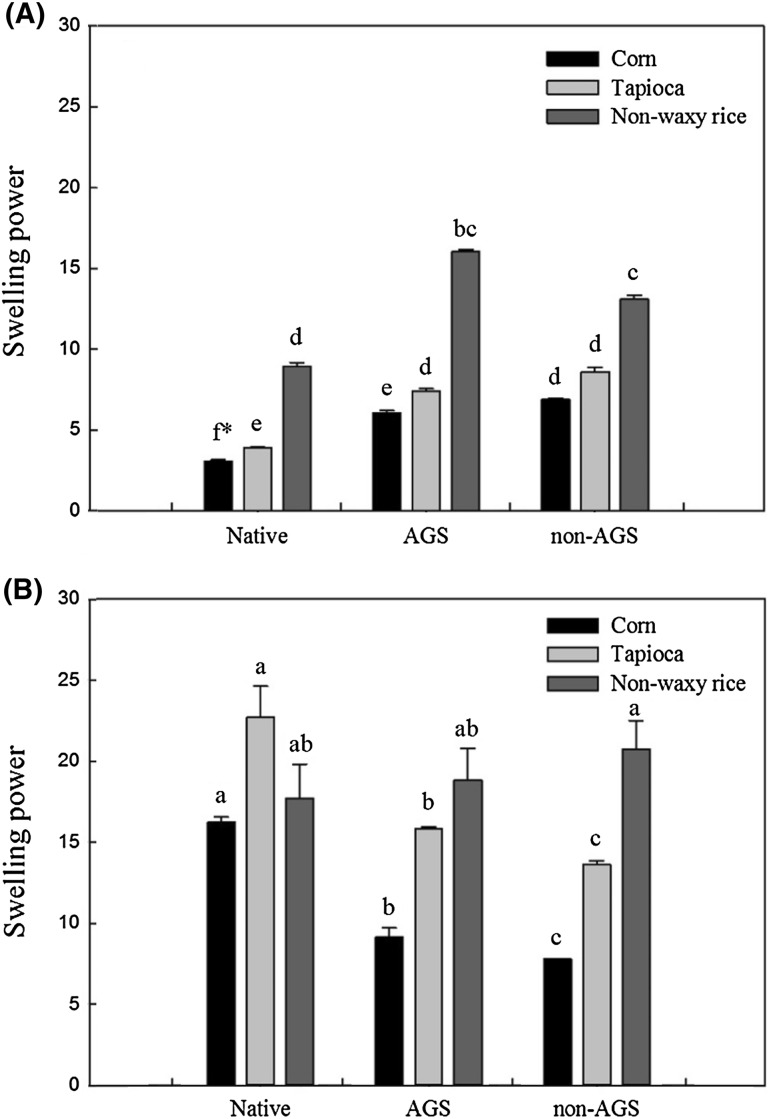
Figure 4 shows the swelling power of native corn, tapioca and rice starches and their AGS and non-AGS at 60 and 90 °C. Swelling power showed similar pattern with solubility at both 60 and 90 °C. Native starches showed the lowest and non-AGS revealed the highest at 60 °C, whereas native starches showed the highest and non-AGS revealed the lowest at 90 °C, in corn and tapioca starches. AGS also showed medium range of swelling power at both 60 and 90 °C in corn and tapioca starches. However, in case of rice starches, swelling power of AGS and non-AGS were not significantly different at 60 °C. Moreover, at 90 °C, native starches, AGS and non-AGS revealed no significantly different swelling power (p < 0.05). It is well known that rice starch granules are relatively smaller than other starches and weak at high temperature [23]. These distinctive characteristics may weaken the granular structure at relatively high temperature resulting in no difference in swelling power between granular starches (native and AGS) and non-granular starch (non-AGS) at 90 °C.
Fig. 4.
Swelling power of native starch, amorphous granular starch (AGS) and non-AGS, determined at 60 °C (A) and at 90 °C (B)
Apparent viscosity
The apparent viscosity of native corn, tapioca, rice starches and their AGS and non-AGS are shown in Table 2. The apparent viscosities of native starches were from 16.90 to 18.80 cp, whereas those of AGS and non-AGS were from 27.40 to 44.15 cp and from 30.06 to 52.34 cp, respectively. In other words, native starches formed the suspension when mixing starches to water at ambient temperature, but AGS and non-AGS were instantly swelled and revealed pasting characteristics as soon as suspending to water. AGS was rapidly swelled compared to native starches because AGS is consisted of only amorphous region and water molecules easily penetrated to its granular structure followed by rising of viscosity. Non-AGS showed slightly higher apparent viscosities compared to AGS (Table 2).
Apparent viscosity of pre-gelatinized corn starch was 79.60 cp in the same experimental conditions, which was much higher than those of AGS and non-AGS corn. Commercial pre-gelatinized corn starch is made by general heat-treatment and AGS and non-AGS were made by non-thermal HHP treatment. Although commercial pre-gelatinized corn starch and AGS and non-AGS corn have different botanical source, AGS and non-AGS had distinguished apparent viscosity. It has been reported that 3% suspension of granular cold-water-soluble starch (GCWS) of high amylose corn starches (HA5 and HA7) treated by alcoholic-alkaline method was higher than native HA5 and HA7 [24]. Furthermore, 25% sorghum starch suspension treated at 300–600 MPa for 10 min had been reported the higher apparent viscosity than native sorghum starch [4]. Therefore, AGS and non-AGS made by HHP treatment were distinctively different with commercial pre-gelatinized starches and can be used in many areas in the food, cosmetic, textile and paper industries.
In this study, amorphous granular starch (AGS) and non-granular amorphous starch (non-AGS) of corn, tapioca and rice starches were prepared by high hydrostatic pressure (HHP) and investigated their physicochemical properties. Native starches, and their AGS and non-AGS revealed distinctively different physicochemical properties. AGS and non-AGS showed relatively high WHC and apparent viscosity compared to those of native starches. The main difference between native starches, AGS and non-AGS was revealed in RVA pasting properties. Peak viscosity decreased by HHP treatment (native > AGS > non-AGS) and maintaining granular structure (AGS) was helpful to retain peak viscosity. In case of non-AGS, its physicochemical characteristics were different with commercial pre-gelatinized starch possibly due to the difference between heat and pressure induced gelatinization. Native starch and AGS showed distinctively different properties because of their semi-crystalline and amorphous granular structure. AGS and non-AGS also did not reveal same characteristics due to their granular and non-granular structures. Moreover, non-AGS and commercial pre-gelatinized starch showed different apparent viscosity because of thermal and non-thermal gelatinization. Therefore, AGS and non-AGS prepared by HHP treatment implied possibilities to produce tailor made starch, which can be diversely used in many industries.
Acknowledgements
This research was supported by the High Value added Food Technology Development Program (314041033HD030) by the Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Katopo H, Song Y. Jane Jl. Effect and mechanism of ultrahigh hydrostatic pressure on the structure and properties of starches. Carbohydr. Polym. 2002;47:233–244. doi: 10.1016/S0144-8617(01)00168-0. [DOI] [Google Scholar]
- 2.Oh HE, Hemar Y, Anema SG, Wong M, Neil Pinder D. Effect of high-pressure treatment on normal rice and waxy rice starch-in-water suspensions. Carbohydr. Polym. 2008;73:332–343. doi: 10.1016/j.carbpol.2007.11.038. [DOI] [Google Scholar]
- 3.Yan H, Zhengbiao GU. Morphology of modified starches prepared by different methods. Food Rer. Int. 2010;43:767–772. doi: 10.1016/j.foodres.2009.11.013. [DOI] [Google Scholar]
- 4.Vallons KJR, Arendt EK. Effects of high pressure and temperature on the structural and rheological properties of sorghum starch. Innov. Food Sci. Emerg. 2009;10:449–456. doi: 10.1016/j.ifset.2009.06.008. [DOI] [Google Scholar]
- 5.Kawai K, Fukami K, Yamamoto K. Effect of temperature on gelatinization and retrogradation in high hydrostatic pressure treatment of potato starch–water mixtures. Carbohydr. Polym. 2012;87:314–321. doi: 10.1016/j.carbpol.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 6.Liu PL, Hu XS, Shen Q. Effect of high hydrostatic pressure on starches: A review. Starch/Starke. 2010;62:615–628. doi: 10.1002/star.201000001. [DOI] [Google Scholar]
- 7.Stolt M, Oinonen S, Autio K. Effect of high pressure on the physical properties of barley starch. Innov. Food Sci. Emerg. 2000;1:167–175. doi: 10.1016/S1466-8564(00)00017-5. [DOI] [Google Scholar]
- 8.Choi HW, Lee JH, Ahn SC, Kim BY, Baik MY. Effects of Ultra High Pressure, Pressing Time and HCl Concentration on Non-thermal Starch Hydrolysis Using Ultra High Pressure. Starch/Starke. 2009;61:334–343. doi: 10.1002/star.200800232. [DOI] [Google Scholar]
- 9.Lee JH, Choi HW, Kim BY, Chung MS, Kim DS, Choi SW, Lee DU, Park SJ, Hur NY, Baik MY. Nonthermal starch hydrolysis using ultra high pressure: I. Effects of acids and starch concentrations. LWT-Food. Sci. Technol. 2006;39:1125–1132. [Google Scholar]
- 10.Choi HS, Kim HS, Park CS, Kim BY, Baik MY. Ultra high pressure (UHP)-assisted acetylation of corn starch. Carbohydr. Polym. 2009;78:862–868. doi: 10.1016/j.carbpol.2009.07.005. [DOI] [Google Scholar]
- 11.Kim HS, Choi HS, Kim BY, Baik MY. Characterization of Acetylated Corn Starch Prepared under Ultrahigh Pressure (UHP) J. Agric. Food. Chem. 2010;58:3573–3579. doi: 10.1021/jf903939y. [DOI] [PubMed] [Google Scholar]
- 12.Hwang DK, Kim BY, Baik MY. Physicochemical Properties of Non-thermally Cross-linked Corn Starch with Phosphorus Oxychloride using Ultra High Pressure (UHP) Starch/Starke. 2009;61:438–447. doi: 10.1002/star.200800098. [DOI] [Google Scholar]
- 13.Kim HS, Hwang DK, Kim BY, Baik MY. Cross-linking of corn starch with phosphorus oxychloride under ultra high pressure. Food Chem. 2012;130:977–980. doi: 10.1016/j.foodchem.2011.07.104. [DOI] [Google Scholar]
- 14.Kim HS, Choi HS, Kim BY, Baik MY. Ultra high pressure (UHP)-assisted hydroxypropylation of corn starch. Carbohydr. Polym. 2011;83:755–761. doi: 10.1016/j.carbpol.2010.08.048. [DOI] [Google Scholar]
- 15.Kim HS, Kim BY, Baik MY. Application of Ultra High Pressure (UHP) in Starch Chemistry. Crit. Rev. Food Sci. Nutr. 2011;52:123–141. doi: 10.1080/10408398.2010.498065. [DOI] [PubMed] [Google Scholar]
- 16.Liu PL, Zhang Q, Shen Q, Hu XS, Wu JH. Effect of high hydrostatic pressure on modified noncrystalline granular starch of starches with different granular type and amylase content. LWT-Food Sci. Technol. 2012;47:450–458. doi: 10.1016/j.lwt.2012.02.005. [DOI] [Google Scholar]
- 17.Tan FJ, Dai WT, Hsu KC. Changes in gelatinization and rheological characteristics of japonica rice starch induced by pressure/heat combinations. J. Cereal Sci. 2009;49:285–289. doi: 10.1016/j.jcs.2008.11.006. [DOI] [Google Scholar]
- 18.Song MR, Choi SH, Kim HS, Kim BY, Baik MY. Efficiency of high hydrostatic pressure in preparing amorphous granular starch. Starch/Starke. 2015;67:790–801. doi: 10.1002/star.201500002. [DOI] [Google Scholar]
- 19.Choi HW, Chung KM, Kim CH, Moon TH, Kim DS, Park CS, Baik MY. Physicochemical Properties of Cross-linked Rice Starches. J. Korean Soc. Appl. Biol. Chem. 2006;49:49–54. [Google Scholar]
- 20.Lee YH, Moon TH. Composition, Water-Holding Capacity and Effect on Starch Retrogradation of Rice Bran Dietary Fiber. Korean J. Food Sci. Technol. 1994;26:288–294. [Google Scholar]
- 21.Koo HJ, Park SH, Jo JS, Kim BY, Hur NY, Baik MY. Physicochemical characteristics of 6-year-old Korean ginseng starches. LWT-Food Sci. Technol. 2005;38:801–807. doi: 10.1016/j.lwt.2004.10.009. [DOI] [Google Scholar]
- 22.Fukami K, Kawai K, Hatta T, Taniguchi H, Yamamoto K. Physical Properties of Normal and Waxy Corn Starches Treated with High Hydrostatic Pressure. J. Appl. Glycosci. 2010;57:67–72. doi: 10.5458/jag.57.67. [DOI] [Google Scholar]
- 23.Thomas DJ, Atwell WA. Starches. (2nd ed.). St. Paul: Eagan Press, (Chapter 1) (2008)
- 24.Chen J, Jane J. Properties of Granular Cold-Water-Soluble Starches Prepared by Alcoholic-Alkaline Treatments. Cereal Chem. 1994;71:623–626. [Google Scholar]