Abstract
Uvaia (Eugenia pyriformis) frozen pulp processing generates a solid byproduct that can potentially contain important components of human nutrition. In this study, the drying of uvaia byproduct was studied. Two different drying treatments were tested: drying of wet waste and drying of waste with prior removal of water by centrifugation. Three drying temperatures were used: 40, 60, and 80 °C. Eight models were applied to fit the drying curves: Page, Lewis, Modified Page, Logarithmic, Midilli, Wang and Singh, Henderson and Pabis, and Weibull. Midilli presented an excellent fit to the curves. The effective moisture diffusivity of the uvaia byproduct ranged between 8.52 × 10−10 and 3.22 × 10−9 m2/s. The activation energy was 25.65 and 24.97 kJ/mol for non-centrifuged and centrifuged assays, respectively. The dried byproducts had a reduction of 3–21% of the total phenolic content against the control. The assay performed at 40 °C with centrifugation presented the lowest total color difference value.
Keywords: Uvaia, Byproduct, Brazilian native fruit, Solid waste, Drying mathematical model
Introduction
The fruit processing industry generates a lot of solid waste throughout its chain, comprising mostly peel, seeds, and pulp. In several cases, residues are factored into the operating costs for companies and are considered environmental contamination sources and as a waste [1, 2].
Fruit residues may contain numerous interesting compounds, such as nutrients (fibers, minerals, and carbohydrates) and bioactive compounds (polyphenolic compounds and fibers), which are important for human physiological functions. Therefore, it is desirable to reuse these residues in the food industry in order to minimize losses and add value to other products [3].
Many authors have studied ways to reuse the solid waste generated by the fruit industry. Examples of this application can be found in the ethanol, biogas, cosmetic and pharmaceuticals, animal [1, 4], and food industries. Some examples of food products that were formulated using fruit byproducts are bread formulation using orange pomace [5]; sweet formulation using passion fruit peel [6]; flour using apple pomace [7]; and cereal bars and biscuits using orange, passion fruit and watermelon residues [8].
Fruit byproducts generally present high moisture content, which makes them perishable. One way to facilitate their subsequent use is to dry them. In the drying process, water is removed using heat, reducing the water activity of the product and increasing its shelf life [9]. Attributes such as color and phenolic compounds can sometimes be altered after drying. Color is considered an important quality attribute to the consumers, and due to the antioxidant capacity of phenolic compounds present in fruits, they have been associated with benefits to human nutrition [10, 11].
Brazilian fruit production is quite diverse but few species are widely commercialized. There are many species still unknown that have great potential for exploitation. Many species have even aroused the domestic and foreign interest [12, 13]. Uvaia (Eugenia pyriformis) is a native fruit from the Myrtaceae family that has great potential to be exploited economically and technologically and is currently found in Brazil from the state of São Paulo to Rio Grande do Sul. The fruit is yellow or orange in color, 1.4–4 cm in length with a diameter of approximately 3 cm, pH of approximately 2.8, and acidity of 1.53–3.98% [11, 14].
No studies on the drying of uvaia solid waste have been reported. Thus, the objective of this work was to study mathematical modeling of the drying moisture data and quality parameters of uvaia byproduct dried in a conventional oven, under different conditions, for future application in the food industry.
Materials and methods
Materials
Uvaia byproduct sample was obtained from a native fruit producer, located in the city of Paraibuna (23° 27′53.94′′ South, 45º 42′31.88′′ West), in the state of São Paulo, Brazil, in October (2013). The byproduct was obtained after fresh fruit pulp processing, which was conducted using a pulper with nylon scraper blades (MS25 No. 56,032 model, Tortugan, Atibaia, Brazil). The byproduct comprised peels, bagasse, and seeds. The samples were stored at −18 °C until used. This study did not involve any experimentation with human or animal subjects performed by any of the authors.
Byproduct proximate composition
The proximate composition of uvaia byproduct was evaluated. AOAC methods [15] were used to determine nitrogen (method 920.152), ash (method 940.26), fiber (method 958.29), and moisture (method 920.151) contents. Protein content was calculated as the quantity of nitrogen multiplied by the nitrogen conversion factor of 6.25. The lipid content was determined according to the method described by Bligh and Dyer [16]. Carbohydrate content was estimated by difference.
Drying experiment
Drying experiments were performed in a convective dryer (MA035, Piracicaba, Marconi, Brazil) with dimensions of 80 cm × 100 cm × 61 cm located at the Department of Food Technology, State University of Campinas. The air velocity of 0.4 m/s was measured with an anemometer (AM 4202, Lutron, Taiwan). The byproduct sample (1 kg) at 25 °C was spread into a thin layer (approximately 1 cm) on a perforated tray with dimensions of 51 cm × 73 cm and mesh size of 0.3 cm × 0.3 cm. Three drying temperatures were tested: 40, 60, and 80 °C. Furthermore, two pretreatments were performed: drying with or without previous centrifugation of the sample. The assays without previous centrifugation received the following nomenclature: 40NC, 60NC, and 80NC (the number corresponds to the drying temperature, in °C). The assays with prior centrifugation received the following nomenclature: 40C, 60C, and 80C. For the 40C, 60C, and 80C assays, the byproduct was centrifuged for 5 min at 1036×g (Mueller Eletrodomésticos S.A, Timbó, Brazil). The temperature of the convective dryer was set 30 min before beginning each drying procedure. This period was necessary to achieve steady-state conditions. In order to obtain the drying curves, the byproduct samples were weighed every 30 min for the assays at 40 and 60 °C and every 15 min for the assays at 80 °C, until constant sample weight was achieved. The digital balance had a sensitivity of 0.01 g and was positioned as close to the dryer as possible (1.5 m). The assays were performed in triplicate and the averages were used for the data analysis.
Mathematical modeling
The collected results were used to calculate the moisture ratio (MR) and the curves were constructed with the data of MR versus time.
MR was calculated according to Eq. (1).
| 1 |
Mt, Me, and M0 correspond, respectively, to the moisture contents at the time of weighing (t), at equilibrium, and at the beginning of the assay.
The drying rate (DR) of the byproducts was calculated according to Eq. (2) and expressed as kg water/(kg dry matter·min):
| 2 |
t2 and t1 are the drying times and Mt1 and Mt2 are the moisture contents at the time of weighting.
The mathematical models used to quantify the drying kinetics were Page [17]; Lewis [18]; Modified Page [19]; Logarithmic [20]; Midilli [21]; Wang and Singh [20]; Henderson and Pabis [22]; and Weibull [23]. The equations are presented below.
Page:
| 3 |
Lewis:
| 4 |
Modified Page:
| 5 |
Logarithmic:
| 6 |
Midilli:
| 7 |
Wang and Singh:
| 8 |
Henderson and Pabis:
| 9 |
Weibull:
| 10 |
In order to verify if the models had adjusted well to the drying curves, the following parameters were used: R2 (coefficient of determination), root mean square error (RMSE), and χ2 (reduced Chi square). The higher the R2 values and the lower the RMSE and χ2 values, the better the quality of the curves [17]. RMSE and χ2 formulas are presented in Eqs. (11) and (12), respectively. MRexp is the experimental value for the moisture ratio, MRpre is the predicted value for the moisture ratio, N is the number of observations, and z is the number of constants.
| 11 |
| 12 |
Effective moisture diffusivity
Fick’s second diffusion law (Eq. 13) is commonly used to calculate effective moisture diffusivity (Deff) [19].
| 13 |
Considering the thin layer of byproduct as an infinite slab, just the z axis can be considered. The solution for Eq. (13) is presented in the Eq. (14) [24]. For long drying periods, the equation can be simplified (Eq. 15), i.e., L being half the thickness (m) of the slab [25].
| 14 |
| 15 |
To calculate Deff for each assay, the slope of the plot of lnMR versus time was calculated.
Activation energy
The relationship between the effective moisture diffusivity and temperature can be represented by Arrhenius equation [26, 27].
| 16 |
Ea is the activation energy (kJ/mol), R is the gas constant (8.314 J/molK), and Do is the Arrhenius factor (m2/s). Ea and Do were obtained, respectively, from the slope and the interception of the plot of ln Deff versus 1/T.
The activation energy was calculated separately for the centrifuged assays (40C, 60C and 80C) and non-centrifuged assays (40NC, 60NC and 80NC).
Total phenolic content
Total phenolic content was estimated following the method of Singleton and Rossi (1965), described by Haminiuk et al. [28] using the Folin–Ciocalteu reagent. The byproduct samples were extracted with 40 mL of a 40% ethanol solution for 1 h. After the extraction, they were centrifuged at 1535×g and the supernatant filtered with a Whatman n°1 filter paper. The extracts were pipetted (100 µL) into 5 mL of distilled water and 500 µL of Folin–Ciocalteu reagent was added to the mixture. Three minutes later, 1.5 mL of a 15% sodium carbonate solution was added and finally, distilled water was added to complete a total volume of 10 mL. After a period of 2 h in the dark at room temperature, the absorbance of the mixture was measured at 765 nm using a spectrophotometer (Beckman DU70, Germany). Gallic acid was used as a standard. The results are expressed as gallic acid equivalents.
Color evaluation
The color of the byproduct powders was evaluated using a colorimeter (Mini Scan XE Hunter Associates Laboratory, Inc, Reston, Virginia, USA), calibrated against white pattern (x = 80.3; y = 85.1, z = 91.0). The color system used in the analysis was the CIELab. L* value represents the lightness/darkness of the sample, ranging from 0 to 100, being 0 for black and 100 for white. The “a*” value represents greenness and redness, ranging from −60 to 60. Finally, the “b*” value represents blueness and yellowness, ranging from −60 to 60 [29]. To compare the samples, the ratio of a*/b*, total color difference (ΔE), and chromaticity (C) were calculated, according to the following equations [30]:
| 17 |
| 18 |
L*0, a*0, and b*0 are the color values for the powder control sample (lyophilized byproduct). In order to compare the drying processes, a lyophilized control sample was obtained (Control). The lyophilization process was conducted in a lyophilizer (Alpha 2–4 LD plus model, Christ, Osterode am Harz Germany). The samples were frozen in trays and then lyophilized under the following conditions (in dark): 44 h, 0.12 mbar, T = −40 °C (main drying) and 4 h, 2.5 mbar, T = −10 °C (final drying).
Statistical analysis
Nonlinear regression of the drying curves was performed using the MATLAB R2016a software (The Mathworks, Inc, Natick, MA, USA).
The parameters of color and total phenolic content were analyzed by ANOVA test and Tukey’s test (p < 0.05) using the software XLSTAT (Addinsoft, New York, NY, 2016).
Results and discussion
Byproduct proximate composition
In order to characterize the raw material, the proximate composition analysis of the byproduct was performed. The results, in grams per 100 g (wet basis), were 89.20 ± 0.50 (moisture), 2.64 ± 0.03 (protein), 0.36 ± 0.02 (lipid), 0.25 ± 0.03 (ash), 4.72 ± 0.11 (fiber), and 2.8 ± 0.5 (carbohydrate).
Drying curves
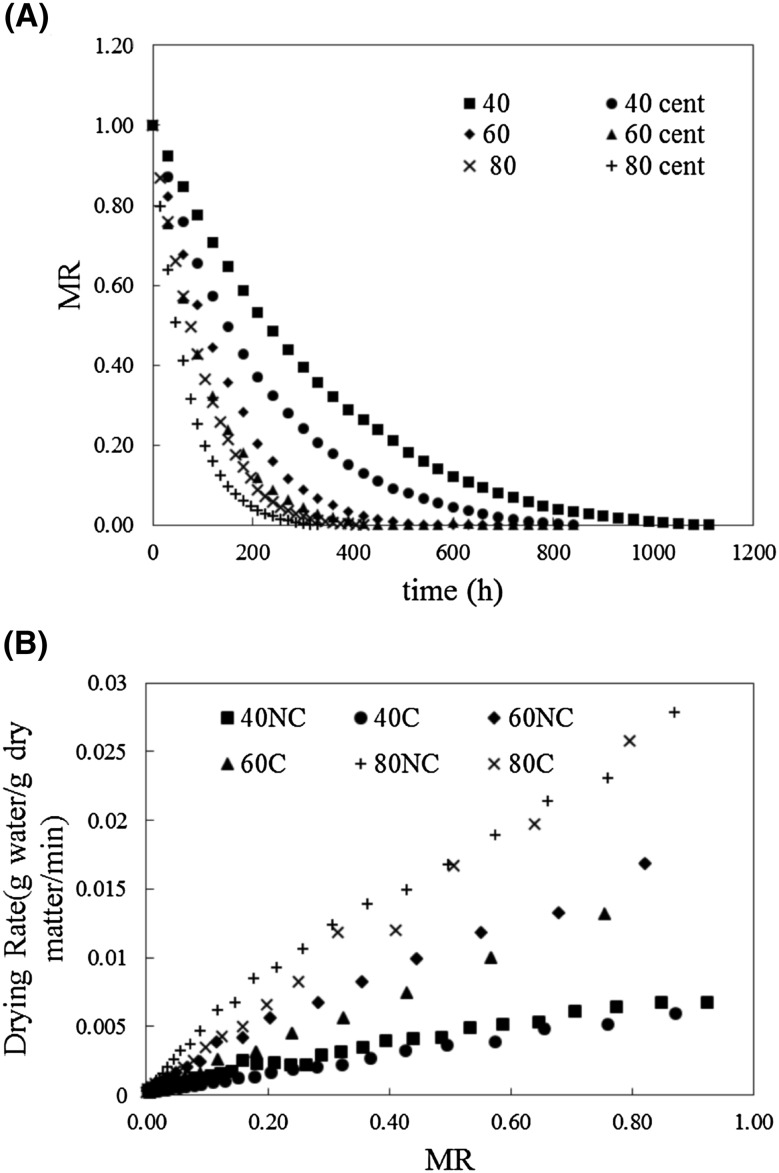
The drying curves (MR vs. time) are shown in Fig. 1(A) and the changes in the DR with MR during the drying of uvaia byproduct at the different studied conditions are shown in Fig. 1(B). As expected, the samples dried at higher temperatures presented shorter drying times. The times varied between 330 and 1110 min (80C and 40NC, respectively). Moreover, the samples that passed through the previous centrifuging process had lower drying times than that of the samples that were directly dried. The centrifugation process assists in the prior removal of water from the material, which leads to 30–44% reduction of the initial mass. This prior removal of the water was conducted in order to try and reduce the drying time and exposure of the waste to high temperatures and to minimize the loss of important bioactive compounds. When assays of same temperature were compared, the drying time of the samples with prior centrifugation was 24.5% lower for the conditions at 40 °C, 16.7% for 60 °C, and 21.4% for 80 °C. For example, samples 40NC and 40C had drying time of 1110 min and 840 min, respectively. Samples 60NC, 60C, 80NC, and 80C presented drying times (in minutes) of 540, 450, 420, and 330, respectively. The material that was prior centrifuged displays interesting results. The drying time of the residue at 60 °C with prior centrifugation was close to that of the residue without prior centrifugation at 80 °C. The fruit waste obtained from the mechanical pulping process of fruits underwent total disintegration and therefore exhibits a great surface area available for mass exchange, and consequently presents higher water loss during drying.
Fig. 1.
Drying curves (A) and DR curves (B) of uvaia byproducts at different conditions
It can be noted that the DR was not constant for any of the conditions. The lower temperature assays presented a lower variation between the initial and final DRs and presented a lower DR when compared to the higher temperature assays. The high rate of moisture loss when high temperatures were used was previously observed by other authors when drying vegetable byproducts such as pomegranate peels and lemon peels [17, 31]. In addition, when comparing the same temperatures, the assay with prior centrifugation presented lower DRs. The MR data were used to calculate the mathematical drying models (Table 1).
Table 1.
Parameters of the mathematical models
| Model | Condition | Constants | R2 | RMSE | χ2 | |||
|---|---|---|---|---|---|---|---|---|
| A | k | |||||||
| Henderson and Pabis | 40NC | 1.0440 | 0.2042 | 0.9947 | 0.02154 | 0.000461 | ||
| 40C | 1.0130 | 0.2926 | 0.9987 | 0.01054 | 0.000078 | |||
| 60NC | 1.0310 | 0.4509 | 0.9954 | 0.02123 | 0.000450 | |||
| 60C | 1.0090 | 0.5860 | 0.9988 | 0.01082 | 0.000117 | |||
| 80NC | 1.0350 | 0.6332 | 0.9952 | 0.02054 | 0.000422 | |||
| 80C | 1.0060 | 0.9238 | 0.9997 | 0.00506 | 0.000026 | |||
| k | ||||||||
| Lewis | 40NC | 0.1959 | 0.9928 | 0.02482 | 0.000613 | |||
| 40C | 0.2888 | 0.9985 | 0.01104 | 0.000088 | ||||
| 60NC | 0.4382 | 0.9944 | 0.02279 | 0.000520 | ||||
| 60C | 0.5809 | 0.9987 | 0.01086 | 0.000118 | ||||
| 80NC | 0.6132 | 0.9941 | 0.02246 | 0.000504 | ||||
| 80C | 0.9186 | 0.9997 | 0.00519 | 0.000027 | ||||
| a | K | C | ||||||
| Logarithmic | 40NC | 1.0740 | 0.1743 | −0.0566 | 0.9994 | 0.00717 | 0.000047 | |
| 40C | 1.0250 | 0.2709 | −0.0247 | 1.0000 | 0.00150 | 0.000002 | ||
| 60NC | 1.0560 | 0.3997 | −0.0423 | 0.9988 | 0.01124 | 0.000126 | ||
| 60C | 1.0210 | 0.5506 | −0.0203 | 0.9998 | 0.00441 | 0.000019 | ||
| 80NC | 1.0530 | 0.5675 | −0.0365 | 0.9985 | 0.01183 | 0.000140 | ||
| 80C | 1.0090 | 0.9002 | −0.0077 | 0.9999 | 0.00273 | 0.000007 | ||
| A | k | B | n | |||||
| Midilli | 40NC | 0.9938 | 0.1537 | −0.0016 | 1.1020 | 0.9998 | 0.00429 | 0.000002 |
| 40C | 0.9997 | 0.2751 | −0.0016 | 1.0100 | 1.0000 | 0.00162 | 0.000003 | |
| 60NC | 0.9924 | 0.3732 | −0.0019 | 1.1200 | 0.9996 | 0.00706 | 0.000050 | |
| 60C | 0.9982 | 0.5559 | −0.0021 | 1.0230 | 0.9998 | 0.00476 | 0.000023 | |
| 80NC | 0.9858 | 0.5358 | −0.0021 | 1.1360 | 0.9994 | 0.00754 | 0.000057 | |
| 80C | 0.9990 | 0.9052 | −0.0012 | 1.0150 | 0.9999 | 0.00258 | 0.000007 | |
| A | K | N | ||||||
| Modified page | 40NC | 1.0440 | 2.2770 | 0.0896 | 0.9947 | 0.02184 | 0.000474 | |
| 40C | 1.0130 | 0.1886 | 1.5510 | 0.9987 | 0.01074 | 0.000064 | ||
| 60NC | 1.0310 | 0.5857 | 0.7700 | 0.9954 | 0.02188 | 0.000261 | ||
| 60C | 1.0090 | 0.7650 | 0.7660 | 0.9988 | 0.01123 | 0.000092 | ||
| 80NC | 1.0350 | 0.8071 | 0.7845 | 0.9952 | 0.02093 | 0.000288 | ||
| 80C | 1.0060 | 0.9945 | 0.9289 | 0.9997 | 0.00519 | 0.000016 | ||
| k | n | |||||||
| Page | 40NC | 0.1480 | 1.1520 | 0.9987 | 0.01083 | 0.000091 | ||
| 40C | 0.2669 | 1.0530 | 0.9993 | 0.00775 | 0.000019 | |||
| 60NC | 0.3761 | 1.1460 | 0.9990 | 0.00984 | 0.000023 | |||
| 60C | 0.5565 | 1.0550 | 0.9994 | 0.00778 | 0.000013 | |||
| 80NC | 0.5521 | 1.1450 | 0.9988 | 0.01037 | 0.000042 | |||
| 80C | 0.9107 | 1.0270 | 0.9998 | 0.00374 | 0.000007 | |||
| a | B | |||||||
| Wang and Singh | 40NC | −0.1357 | 0.0046 | 0.9885 | 0.03178 | 0.000926 | ||
| 40C | −0.1909 | 0.0090 | 0.9666 | 0.05285 | 0.002748 | |||
| 60NC | −0.2932 | 0.0211 | 0.9827 | 0.04132 | 0.001708 | |||
| 60C | −0.3689 | 0.0330 | 0.9616 | 0.06115 | 0.003739 | |||
| 80NC | −0.3973 | 0.0382 | 0.9752 | 0.04670 | 0.002181 | |||
| 80C | - 0.5402 | 0.0692 | 0.9245 | 0.07890 | 0.006225 | |||
| a | b | |||||||
| Weibull | 40NC | 1.1520 | 5.2520 | 0.9987 | 0.01083 | 0.000080 | ||
| 40C | 1.0530 | 3.5030 | 0.9993 | 0.00775 | 0.000019 | |||
| 60NC | 1.1460 | 2.3480 | 0.9990 | 0.00984 | 0.000023 | |||
| 60C | 1.0550 | 1.7430 | 0.9994 | 0.00778 | 0.000013 | |||
| 80NC | 1.1450 | 1.6800 | 0.9988 | 0.01037 | 0.006243 | |||
| 80C | 1.0270 | 1.0950 | 0.9998 | 0.00374 | 0.002542 | |||
Mathematical modeling
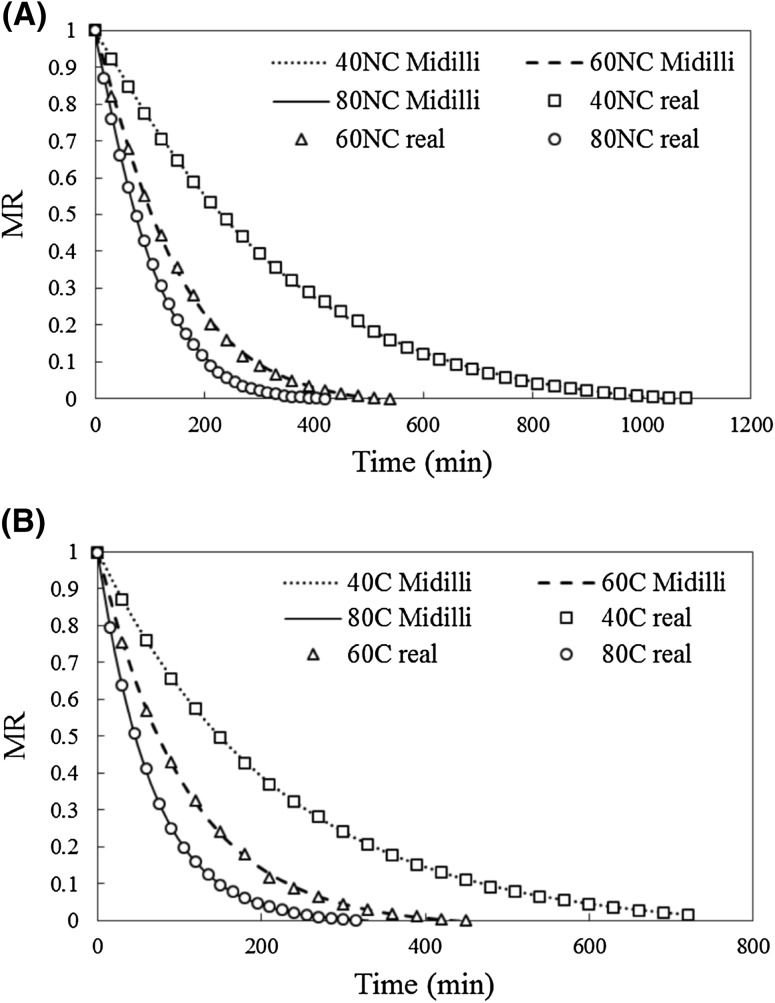
Eight different models were tested. Table 1 shows the constants, R2, RMSE, and χ2 for all the models and assays. The selection of the mathematical model that best fitted the curves was based on a high R2 and low RMSE and χ2 values. Almost all models, except for Wang and Singh, presented excellent R2 values, above 0.99. Midilli was considered the model that best fitted the majority of the experimental drying curves (40NC, 60NC, 80NC, and 80C), and Logarithmic was considered the model that best fitted 40C and 60C drying curves because of the high R2 values and low RMSE and χ2 values. In addition, for the 40C and 60C assays, Midilli was the second best model and R2, RMSE, and χ2 parameters were close to the ones calculated using the Logarithmic model. The plot of MR values predicted by Midilli and experimental values for all non-centrifuged and centrifuged assays are shown in Fig. 2(A), (B), respectively.
Fig. 2.
Experimental MR and predicted MR by Midilli model versus time. (A)—non-centrifuged assays. (B)—centrifuged assays
Effective moisture diffusivity
The effective moisture diffusivity values were calculated according to Eq. (13). Table 2 presents the results for both non-centrifuged (40NC, 60NC, and 80NC) and centrifuged (40C, 60C, and 80C) assays. As expected, higher temperatures resulted in higher effective moisture diffusivity. The values obtained in this study are comparable to the values obtained by other authors for other vegetable byproducts: 2.03 × 10−9 to 1.71 × 10−9 m2/s in the range of 50–90 °C for olive cake waste [25] and 4.02 × 10−9 to 5.31 × 10−9 m2/s for pomegranate peels at 50–70 °C [17].
Table 2.
Effective moisture diffusivity and activation energy for uvaia byproduct drying
| Drying condition | Deff (m2/s) | Activation energy (kJ/mol) |
|---|---|---|
| 40NC | 8.52 × 10−10 | 25.65 |
| 60NC | 1.75 × 10−9 | |
| 80NC | 2.59 × 10−9 | |
| 40C | 1.09 × 10−9 | 24.97 |
| 60C | 2.07 × 10−9 | |
| 80C | 3.22 × 10−9 |
Activation energy
According to Eq. (14), the values of lnDeff versus 1/T were plotted and the activation energy was calculated. For both non-centrifuged and centrifuged assays, the plot showed a linear tendency, with a R2 of 0.9817 and 0.9946, respectively. The non-centrifuged and centrifuged assays activation energies are presented in Table 2. These values are comparable to the activation energy obtained by other authors for vegetable byproducts: 39.66 kJ/mol for pomegranate byproducts [23]; 29.571 and 34.726 kJ/mol for 2.0 and 3.5 m/s (air drying velocity) for passion fruit peels [32].
Total phenolic content
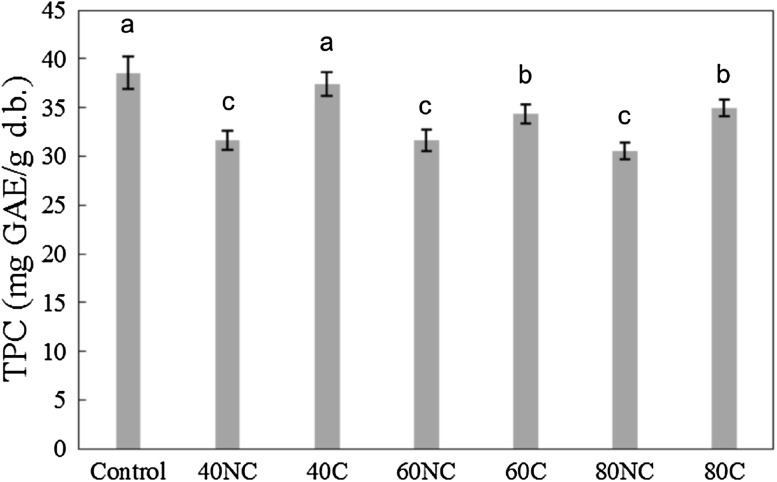
The results for total phenolic content for the dried byproduct in the different drying assays and for the lyophilized byproduct are presented in Fig. 3. It can be noted that the total phenolic content varied between the samples (p < 0.05). Lyophilization is a drying process that is recognized for maintaining and preserving the characteristics of the raw product. Therefore, in this study, a lyophilized product was used as a control sample for the drying process. On comparing the results, it was observed that the dried byproducts showed reductions of up to 21% (80NC) compared to the lyophilized byproduct. 40C did not present significant difference to the control (p > 0.05). Zillo et al. [33] compared the fresh fruit and the frozen pulp. They obtained 4.89 and 6.07 mg gallic acid per 100 g for the fruit and pulp, respectively, in wet basis. In this study, the values for the dried byproducts varied between 30.5 and 38.5 mg of gallic acid per g (dry basis). Considering the quantity of water of the wet byproduct (moisture of 89.2%), the quantity of total phenolic content of the wet byproduct was calculated, resulting in a maximum of 404 mg per 100 g.
Fig. 3.
Total phenolic content of uvaia control byproduct and uvaia dried under different conditions. Results with same letters indicate no significant statistical difference (p ≤ 0.05)
Haminiuk et al. [28] evaluated uvaia pulp and obtained 24.09 mg of gallic acid per gram (dry basis). In this study, the byproduct dried in hot air dryer showed a value 23–55% higher than that of the pulp evaluated by Haminiuk et al. [28]. Ramirez et al. [34] evaluated different uvaia genotypes, obtaining 373–652 mg per 100 g of lyophilized fruit. A record in literature for the phenolic compound evaluation of any uvaia byproduct was not found; only results for both pulp and entire fruit were found. Spoladore et al. [35] evaluated passion fruit peel drying and obtained 5.3–6.8 mg GAE/g (dry basis) for assays between 60 and 90 °C and the dry by-product lost from 67 to 75% of total phenolic content compared to the control. Considering the results obtained for total phenolic content of the uvaia byproduct, it was possible to observe that even after hot air drying, the byproducts retained their phenolic compounds. Therefore, there is a great potential to develop food products containing dried uvaia byproduct as a healthier ingredient.
Color evaluation
The values of L*, a*, and b* of the dried fruit residues were measured (Table 3). The lyophilized byproduct was used as the control. Significant variation (p < 0.05) in the color of the final product according to the different drying conditions was observed. It was possible to verify that previous centrifugation influenced the color of the dried product. It has been previously reported that dried products should exhibit high L* and low a*/b* values [10]. Samples with prior centrifugation showed statistical different (p < 0.05) and higher L* and lower a*/b* values than samples without prior centrifugation. As an example, the L* and a*/b* values for the 60NC treatment were 59.04 and 0.32 and for 60C, 62.25 and 0.28. The other temperature assays showed similar tendencies. Compared to the control sample, for all assays, a decrease in the luminosity value (L*) and yellow color (b*) and an increase in the red color (a*) were observed (p < 0.05).
Table 3.
Color parameters of uvaia dried byproduct at different conditions
| Sample | L*1 | a*1 | b*1 | ΔE1 | C1 | a*/b* 1 |
|---|---|---|---|---|---|---|
| Control | 76.79 ± 0.26a | 9.49 ± 0.09e | 48.66 ± 0.30a | 0 | 49.58 ± 0.30a | 0.1951 ± 0.0019f |
| 40NC | 58.41 ± 1.02d | 12.97 ± 0.34a | 42.03 ± 0.37c | 19.85 ± 1.10a | 43.99 ± 0.29c | 0.3085 ± 0.0105a |
| 40C | 66.84 ± 0.66b | 10.09 ± 0.11e | 43.64 ± 0.37b | 11.17 ± 0.67c | 44.79 ± 0.27b | 0.2312 ± 0.0033e |
| 60NC | 59.04 ± 1.14d | 12.62 ± 0.41a | 39.73 ± 0.28e | 20.1 ± 1.13a | 41.69 ± 0.26e | 0.3178 ± 0.0112a |
| 60C | 62.25 ± 0.50c | 11.60 ± 0.29c | 41.96 ± 0.33c | 16.16 ± 0.41b | 43.53 ± 0.39c | 0.2764 ± 0.0049c |
| 80NC | 59.03 ± 0.68d | 12.13 ± 0.33b | 40.76 ± 0.43d | 19.63 ± 0.48a | 42.53 ± 0.50d | 0.2977 ± 0.0054b |
| 80C | 61.09 ± 0.83c | 10.84 ± 0.38d | 42.19 ± 0.43c | 17.05 ± 0.64b | 43.56 ± 0.49c | 0.2569 ± 0.0073d |
1Different letters in the same column indicate significant statistical difference (p < 0.05)
According to ANOVA test, the ΔE values differed significantly (p < 0.05) between the different assays. For ΔE, there was no significant difference (p > 0.05) between the 40NC, 60NC, and 80NC assays. Among drying assays, 40C assay presented the lowest ΔE (p < 0.05), followed by 60C and 80C. In terms of color of the final product, the best treatment was 40C, showing low ΔE. Zillo et al. [33] obtained the following L* values for the fresh fruit and frozen pulp: 47.657 and 53.337. No records of uvaia byproduct color were found. While the ΔE values in this study ranged between 11 and 20, for passion fruit byproduct drying, the ΔE values for the external part of the peel ranged between 15 and 25 [35].
To summarize, uvaia byproduct was dried in a convective laboratory dryer, and except for the Wang and Singh model, all the studied models exhibited an excellent fit to the drying data. Instrumental color and phenolic content were used as quality parameters of the dried byproduct. Considering both color and total phenolic content, 40C was the assay that better preserved the characteristics of the byproduct compared to the control. The dried fruit byproduct is a stable (low moisture) ingredient that could be of great interest to be added to other products formulations such as bakery and confectionery products. Studies on the application of dried byproducts in confectionery products are being conducted.
Acknowledgements
The present study was supported by the Cnpq (National Counsel of Technological and Scientific Development), process number 457190/2014-0. Cnpq also supported the first author with a doctorate scholarship and the third author with an undergraduate research scholarship.
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Djilas S, Canadanovic-Brunet J, Cetkovic G. By-products of fruits processing as a source of phytochemicals. Chem. Ind. Chem. Eng. Q. 2009;15:191–202. doi: 10.2298/CICEQ0904191D. [DOI] [Google Scholar]
- 2.Lousada Junior JE, Maria J, Neuman J, Neiva M. Caracterização físico-química de subprodutos obtidos do processamento de frutas tropicais visando seu aproveitamento na alimentação animal. Rev. Ciên. Agron. 2006;37:70–76. [Google Scholar]
- 3.Sousa M, Vieira L, da Silva M, de Lima A. Caracterização nutricional e compostos antioxidantes em resíduos de polpas de frutas tropicais. Ciênc. Agrotec. 2011;35:554–559. doi: 10.1590/S1413-70542011000300017. [DOI] [Google Scholar]
- 4.Górnaś P, Rudzińska M. Seeds recovered from industry by-products of nine fruit species with a high potential utility as a source of unconventional oil for biodiesel and cosmetic and pharmaceutical sectors. Ind Crops Prod. 2016;83:329–338. doi: 10.1016/j.indcrop.2016.01.021. [DOI] [Google Scholar]
- 5.O’Shea N, Rößle C, Arendt E, Gallagher E. Modelling the effects of orange pomace using response surface design for gluten-free bread baking. Food Chem. 2015;166:223–230. doi: 10.1016/j.foodchem.2014.05.157. [DOI] [PubMed] [Google Scholar]
- 6.Dias MV, Figueiredo LP, Valente WA, Ferrua FQ, Pereira PAP, Pereira AGT, Borges SV, Clemente PR. Estudo de variáveis de processamento para produção de doce em massa da casca do maracujá (passiflora edulis f. flavicarpa) Ciência e Tecnol. Aliment. 2011;31:65–71. doi: 10.1590/S0101-20612011000100008. [DOI] [Google Scholar]
- 7.Ktenioudaki A, O’Shea N, Gallagher E. Rheological properties of wheat dough supplemented with functional by-products of food processing: brewer’s spent grain and apple pomace. J Food Eng. 2013;116:362–368. doi: 10.1016/j.jfoodeng.2012.12.005. [DOI] [Google Scholar]
- 8.Ferreira MSL, Santos MCP, Moro TM, Basto GJ, Andrade RMS, Gonçalves ÉCB. Formulation and characterization of functional foods based on fruit and vegetable residue flour. J Food Sci Technol. 2015;52:822–830. doi: 10.1007/s13197-013-1061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madamba PS, Driscollb RH, Buckleb KA. The thin-layer drying characteristics of garlic slices. J Food Eng. 1996;29:75–97. doi: 10.1016/0260-8774(95)00062-3. [DOI] [Google Scholar]
- 10.Doymaz İ, Altıner P. Effect of pretreatment solution on drying and color characteristics of seedless grapes. Food Sci. Biotechnol. 2012;21:43–49. doi: 10.1007/s10068-012-0006-4. [DOI] [Google Scholar]
- 11.Pereira MC, Steffens RS, Jablonski A, Hertz PF, Rios ADO. Characterization and antioxidant potential of Brazilian fruits from the Myrtaceae family. J Agric Food Chem. 2012;60:3061–3067. doi: 10.1021/jf205263f. [DOI] [PubMed] [Google Scholar]
- 12.Barreto GPM, Benassi MT, Mercadante AZ. Bioactive compounds from several tropical fruits and correlation by multivariate analysis to free radical scavenger activity. J Braz Chem Soc. 2009;20:1856–1861. doi: 10.1590/S0103-50532009001000013. [DOI] [Google Scholar]
- 13.Clerici MTPS, Carvalho-Silva LB. Nutritional bioactive compounds and technological aspects of minor fruits grown in Brazil. Food Res Int. 2011;44:1658–1670. doi: 10.1016/j.foodres.2011.04.020. [DOI] [Google Scholar]
- 14.Moura MS. Propriedades funcionais de frutas tropicais brasileiras não tradicionais. Ph.D. Thesis, Universidade Federal Rural do Semi-Árido, Mossoró, RN, Brazil (2008)
- 15.AOAC. Official Method of Analysis of AOAC Association of Official Analytical Chemists, Gaithersburg, MD, USA (2006)
- 16.Bligh EG, Dyer WJ. A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 17.Doymaz İ. Experimental study on drying characteristics of pomegranate peels. Food Sci. Biotechnol. 2011;20:965–970. doi: 10.1007/s10068-011-0133-3. [DOI] [Google Scholar]
- 18.Falade KO, Solademi OJ. Modelling of air drying of fresh and blanched sweet potato slices. Int J Food Sci Technol. 2010;45:278–288. doi: 10.1111/j.1365-2621.2009.02133.x. [DOI] [Google Scholar]
- 19.Demiray E, Tulek Y. Drying characteristics of garlic (Allium sativum L.) slices in a convective hot air dryer. Heat Mass Transf. 2014;50:779–786. doi: 10.1007/s00231-013-1286-9. [DOI] [Google Scholar]
- 20.Wang Z, Sun J, Liao X, Chen F, Zhao G. Mathematical modeling on hot air drying of thin layer apple pomace. Food Res Int. 2007;40:39–46. doi: 10.1016/j.foodres.2006.07.017. [DOI] [Google Scholar]
- 21.Sadi T, Meziane S. Mathematical modelling, moisture diffusion and specific energy consumption of thin layer microwave drying of olive pomace. Int. Food Res. J. 2015;22:494–501. [Google Scholar]
- 22.Roberts J, Kidd D, Padilla-Zakour O. Drying kinetics of grape seeds. J Food Eng. 2008;89:460–465. doi: 10.1016/j.jfoodeng.2008.05.030. [DOI] [Google Scholar]
- 23.Kara C, Doymaz I. Thin layer drying kinetics of by-products from pomegranate juice processing. J Food Process Preserv. 2015;39:480–487. doi: 10.1111/jfpp.12253. [DOI] [Google Scholar]
- 24.Crank J. The Mathematics of diffusion. Oxford University Press: London (1975)
- 25.Vega-gálvez A, Miranda M, Puente L, Lopez L, Rodriguez K, Di K. Effective moisture diffusivity determination and mathematical modelling of the drying curves of the olive-waste cake. Bioresour Technol. 2010;101:7265–7270. doi: 10.1016/j.biortech.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 26.Alibas I. Mathematical modeling of microwave dried celery leaves and determination of the effective moisture diffusivities and activation energy. Food Sci. Technol. 2014;34:394–401. [Google Scholar]
- 27.Gokhale SV, Lele SS. Dehydration of red beet root (Beta vulgaris) by hot air drying: process optimization and mathematical modeling. Food Sci. Biotechnol. 2011;20:955–964. doi: 10.1007/s10068-011-0132-4. [DOI] [Google Scholar]
- 28.Haminiuk CWI, Plata-Oviedo MSV, Guedes AR, Stafussa AP, Bona E, Carpes ST. Chemical, antioxidant and antibacterial study of Brazilian fruits. Int J Food Sci Technol. 2011;46:1529–1537. doi: 10.1111/j.1365-2621.2011.02653.x. [DOI] [Google Scholar]
- 29.Hwa C, Lim C, Figiel A, Wojdyło A, Oziembłowski M. Colour, phenolic content and antioxidant capacity of some fruits dehydrated by a combination of different methods. Food Chem. 2013;141:3889–3896. doi: 10.1016/j.foodchem.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 30.De Azevêdo JCS, Fujita A, de Oliveira EL, Genovese MI, Correia RTP. Dried camu-camu (Myrciaria dubia H.B.K. McVaugh) industrial residue: a bioactive-rich amazonian powder with functional attributes. Food Res. Int. 2014;62:934–940. doi: 10.1016/j.foodres.2014.05.018. [DOI] [Google Scholar]
- 31.Romdhane NG, Bonazzi C, Kechaou N, Mihoubi NB. Effect of air-drying temperature on kinetics of quality attributes of lemon (Citrus limon cv. lunari) peels. Dry. Technol. 2015;33:1581–1589. doi: 10.1080/07373937.2015.1012266. [DOI] [Google Scholar]
- 32.Bezerra CV, Meller da Silva LH, Corrêa DF, Rodrigues AMC. A modeling study for moisture diffusivities and moisture transfer coefficients in drying of passion fruit peel. Int J Heat Mass Transf. 2015;85:750–755. doi: 10.1016/j.ijheatmasstransfer.2015.02.027. [DOI] [Google Scholar]
- 33.Zillo RR, Silva PPM, Zanatta S, Carmo LF, Spotto MHF. Qualidade físico-química da fruta in natura e da polpa de uvaia congelada. Rev. Bras. Prod. Agroindusriais. 2013;15:293–298. [Google Scholar]
- 34.Ramirez MR, Schnorr CE, Feistauer LB, Apel M, Henriques AT, Moreira JCF, Zuanazzi JA. Evaluation of the polyphenolic content, anti-inflammatory and antioxidant activities of total extract from Eugenia pyriformes cambess (uvaia) fruits. J Food Biochem. 2012;36:405–412. doi: 10.1111/j.1745-4514.2011.00558.x. [DOI] [Google Scholar]
- 35.Spoladore SF, Bissaro CA, Vieira TF, Silva MV, Haminiuk CWI, Demczuk B. Modelagem matemática da secagem de casca de maracujá e influência da temperatura na cor, compostos fenólicos e atividade antioxidante. Rev. Bras. Pesqui. em Aliment. 2014;5:17–25. doi: 10.14685/rebrapa.v5i2.163. [DOI] [Google Scholar]