Abstract
Here we investigated the effect of green lipped mussel oil complex (GLMOC) on inflammation and underlying mechanism in lipopolysaccharide stimulated RAW264.7 murine macrophage cells. GLMOC containing green lipped mussel oil (GLMO), olive oil, and vitamin E (10:20:1) can induce significant suppression of iNOS, leading to reduced nitric oxide synthesis, and cyclooxygenase-2, leading to reduced prostaglandin E2 synthesis. In addition, it down-regulated the release of pro-inflammatory cytokines, including tumor necrosis factor-α, interleukin (IL)-6, and IL-1β. Similar to upstream signaling mediators, GLMCO inhibited the degradation of inhibitory κB, nuclear translocation of NF-κB, and phosphorylation of mitogen activated protein kinases (MAPKs) in a dose-dependent manner. Among the components of GLMOC, GLMO was responsible for anti-inflammatory efficacy. Taken together, GLMOC induces anti-inflammatory activity via regulating NF-κB and MAPK signaling in lipopolysaccharide-induced RAW264.7 cells, providing underlying mechanisms that elucidate the anti-inflammatory efficacy of GLMOC.
Keywords: Green lipped mussel oil, Inflammation, Nuclear factor κB, Mitogen activated protein kinase, RAW264.7
Introduction
Green lipped mussel (Perna canaliculus, GLM) is a bivalve mussel mainly cultivated in New Zealand. Traditionally, the health beneficial effect of GLM has been known based on the finding that people living in the coastal area of New Zealand and consuming GLM in their diet developed less severe arthritis than those living in the inland area, wherein the oil fraction of GLM (GLMO) has been considered as a key fraction responsible for the physiological activity of GLM [1].
Many studies have demonstrated the green lipped mussel oil complex (GLMOC) containing GLMO, vitamin E, and olive oil shows health beneficial effect for treating arthritis, including osteoarthritis and rheumatoid arthritis [2–5], inflammation [6–8], and asthma [9, 10]. Lee et al. [5] reported that GLMOC relieved the pain and regulated cytokines in adjuvant-induced arthritis rat model. In clinical studies, GLMOC significantly improved the symptoms of osteoarthritis, including pain relief and joint function, in patients with osteoarthritis [2] and combination of GLMOC and fish oil ameliorated the joint pain in rheumatoid arthritis patients [3]. In Korea, GLMOC was approved as a raw material for functional foods that can improve joint health by the Korea Ministry of Food and Drug Safety in 2004. In inflammation, GLMOC was reported to significantly inhibit the enzymatic activity of cyclooxygenase (COX)-1 and -2 in vitro [7], as well as suppress the dextran sulfate sodium-induced symptoms of inflammatory bowel disease, including body weight loss, crypt area loss, and colon weight in C57BL/6 mice [8]. In addition, GLMOC reduced the expression of CD23 not only in leukotriene B4 (LTB4)-stimulated human monocytes but also in monocytes from allergic patients producing high level of LTB4 [11]. However, the mechanistic study underlying the anti-inflammatory efficacy of GLMOC was not clearly investigated.
Inflammation is a defense mechanism caused by physical or chemical injury and/or an infectious agent. Acute inflammation is an early beneficial response that helps eliminate pathogens and necrotic cells as well as initiates the healing process at the site of tissue injury [12]. Chronic inflammation refers to prolonged and/or repeated physiological processes of inflammation, which can develop into different chronic diseases such as cardiovascular diseases, diabetes, inflammatory bowel disease, and cancer [13, 14]. Inflammation can be mediated through toll-like receptors (TLRs) after recognizing the specific molecular patterns such as lipopolysaccharide (LPS) present in the gram-negative microbial compartment [15]. Downstream signaling induced by activation of TLRs is mediated through nuclear factor (NF)-κB, which can be activated through degradation of inhibitory-κB (IκB) by IκB kinase (IKK), and/or phosphorylation of histone H3 by p38 mitogen activated protein kinase (MAPK) [16]. The well-known target genes regulated by NF-κB-mediated inflammatory signal are inducible nitric oxide synthase (iNOS), cyclooxygenase (COX), and different cytokines, including tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and IL-1β [17], and many studies were performed to investigate the effect of natural compounds on them to elucidate the molecular mechanism of their anti-inflammatory efficacy [18].
To overcome the inconsistent efficacy of green lipped mussel extract on inflammation, GLMO was prepared by freeze-drying GLM (obtained from New Zealand) and extracted using CO2-supercritical fluid extraction (SFE), and GLMOC was formulated with vitamin E and olive oil as an antioxidant, which can achieve a consistently high inflammation regulatory activity [19]. In this study, we investigated the effect of freeze-dried and CO2-SFE extracted GLMOC on inflammation and the underlying mechanism of the action in LPS-stimulated RAW264.7 murine macrophage cells.
Materials and methods
Materials
Freeze-dried and CO2-SFE extracted GLMOC was from Gwanjeolpalpal (Syspang, Seoul). GLMO, vitamin E, and olive oil were also provided by Syspang (Seoul). LPS, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), and sulforaphane were purchased from Sigama (St. Louis, MO, USA).
Cell culture
RAW264.7 cell line was purchased from American Type Culture Collection (Manassas, VA, USA) and cultured in DMEM media (Life Technologies, Grand Island, USA) supplemented with 10% fetal bovine serum (Life Technologies), 1% penicillin/streptomycin (Life Technologies), and 1% HEPES (Life Technologies).
NO assay
The amount of NO released by RAW264.7 cells was determined using an NO assay kit (Promega, USA) following the manufacturer’s protocol. Briefly, 50 µL supernatant from different treatments was collected, and the enzymatic conversion of the supernatant nitrate to nitrite by nitrate reductase were determined by colorimetric assay at 540 nm.
Quantitative real time-PCR
Total mRNA was isolated using Trizol (Life Technologies) after 12 h treatments in RAW264.7 cells. cDNA was synthesized using a RevertAid strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA). The Taqman primers were used and the expression of the target RNA was determined with ABI PRISM 7900 (Life Technology). As an internal control, GAPDH was used.
PGE2 and cytokines assay
The supernatants after treatments for 24 h in RAW264.7 cells were used for the determination of the released PGE2 and cytokines. The concentrations of PGE2 were measured using the PGE2 competitive ELISA kit (Enzo Life Sciences Inc. America) and those of TNFα, IL-6, and IL-1β were by Sandwich ELISA kit(Ray Biotech, Inc. America) following the manufacturer’s protocol.
Western blot analysis assay
Cell lysates were isolated with radioimmunoprecipitation assay (RIPA) buffer (10 mM Tris–HCl, 5 mM EDTA, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 0.1 mM Na3VO4, 1% phenylmethylsulfonyl fluoride, and 1% protease inhibitor). The proteins were separated by 10% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes (Milipore, Billerica, MA, USA). The primary antibodies against iNOS, p-p65, p-ERK, p-p38, p-JNK (Cell signaling Inc., USA), COX-2, IκBα (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), and β-actin (Sigma) were probed. Secondary antibodies from Santa Cruz Biotechnology Inc. were used. Protein expression levels were visualized by a chemiluminescence reagent (GE healthcare, Little Chalfont, UK) in EZ capture MG (ATTO, Tokyo, Japan). The blots were quantified using EZ west Lumi plus (ATTO).
Cell proliferation assay
RAW264.7 cells were seeded into a 96 well plate and treated with different components after 24 h. Four hours before cell harvest, MTT solution (5 mg/mL) was added to each well. The dark blue formazan crystals formed were dissolved in DMSO and measured at 570 nm.
Statistical analysis
Statistical differences were determined using one-way ANOVA. Differences were considered as statistically significant at p < 0.05.
Results and discussion
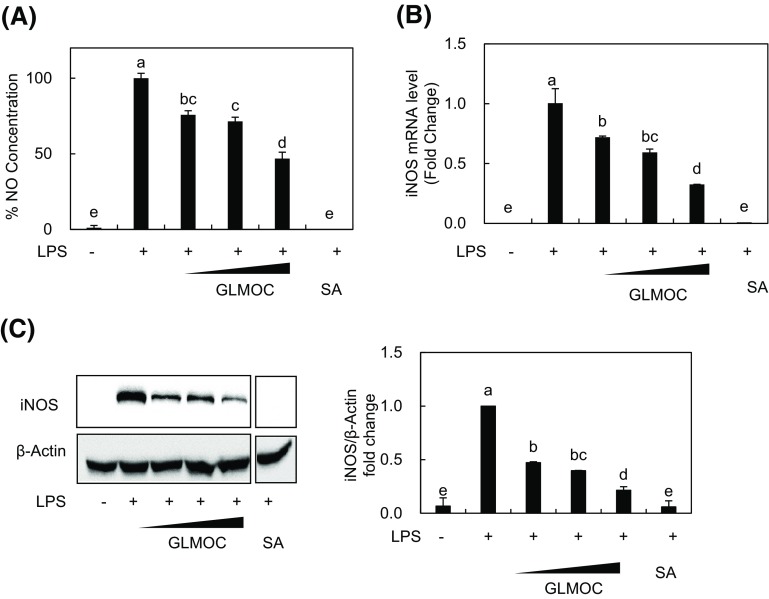
GLMOC suppressed iNOS expression and NO synthesis in LPS-induced RAW264.7 cells
As mediators for inflammatory responses, NO synthesized by iNOS enzyme possesses cytotoxic property against not only exogenous substances but also host tissues and are considered as the targets for anti-inflammatory efficacy [20, 21]. Here we first investigated the effect of GLMOC on NO synthesis and expression of iNOS. As shown in Fig. 1(A), GLMOC at the concentration of 50, 100, and 300 μg/mL exerted a dose-dependent inhibition of NO synthesis induced by LPS in RAW 264.7 cells. Sulforaphane is a well-known phytochemical used as a positive control. The mRNA and protein expressions of iNOS were determined by quantitative real time PCR and western blot, respectively. GLMOC significantly suppressed the expression of iNOS stimulated by LPS, which were 28.4 ± 0.9 and 53.2 ± 0.1% inhibition of iNOS mRNA and protein at 50 μg/mL, 40.9 ± 2.8 and 61.0 ± 0.2% inhibition at 100 μg/mL, and 67.4 ± 0.3 and 78.9 ± 0.7% inhibition at 300 μg/mL, respectively [Fig. 1(B), (C)]. These results indicate that GLMOC possesses the significant regulatory efficacy on NO synthesis cascades during LPS-induced inflammatory process in RAW264.7 cells.
Fig. 1.
GLMOC suppressed iNOS expression and NO synthesis in LPS-induced RAW264.7 cells. (A) The produced NO was measured with NO assay. The cells (4 × 104/well in 96-well plate) were pretreated with GLMOC or SA for 2 h, and then LPS (100 ng/mL) for 22 h. (B) The relative amount of iNOS mRNA was measured with quantitative RT-PCR. The cells (8 × 105 cells in a 60 mm dish) were pretreated with GLMOC or SA for 2 h, and then LPS for 10 h. (C) The protein levels of iNOS and β-actin were determined by Western blot. The cells (8 × 105 cells in a 60 mm dish) were pretreated with GLMOC or SA for 2 h, and then LPS (100 ng/mL) for 22 h. Values represent the mean ± SD. Different lowercase letters indicate significant differences at p < 0.05. GLMOC (50, 100, and 300 μg/mL), SA (sulforaphane, 10 μM), LPS (100 ng/mL)
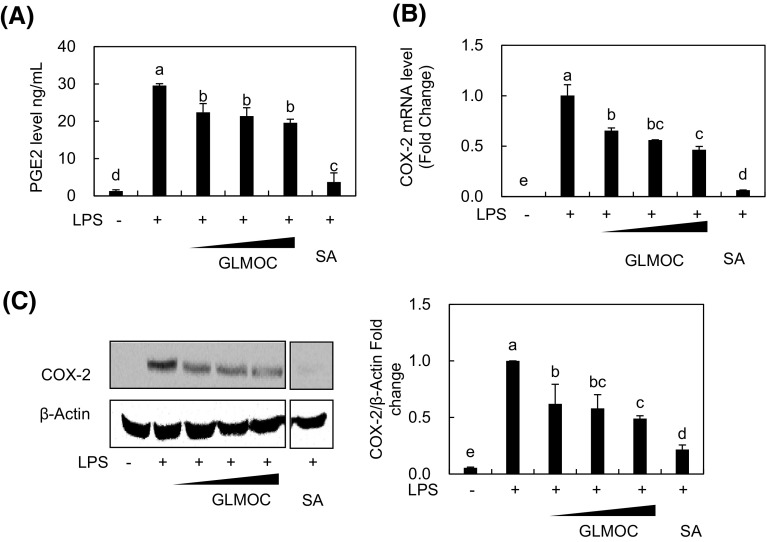
GLMOC inhibited the expression of COX-2 and prostaglandin E2 level in LPS-induced RAW264.7 cells
COX is the enzyme catalyzing the oxygenation of fatty acids, preferably arachidonic acid and producing different cell signaling mediators involved in the inflammatory processes [22], and many natural and synthetic agents have been studied to inhibit the specific COX, particularly COX-2 to suppress the inflammation and/or prevent cancer [18, 22, 23]. GLMOC significantly reduced the expression of COX-2 stimulated by LPS, which were 35.2 ± 2.6 and 38.0 ± 11% inhibition of mRNA and protein at 50 μg/mL, 43.8 ± 0.2 and 42.2 ± 10% inhibition at 100 μg/mL, and 54.2 ± 3.2 and 51.7 ± 1.4% inhibition at 300 μg/mL, respectively [Fig. 2(B), (C)]. In addition, the level of prostaglandin E2 (PGE2) produced by activated COX-2 was significantly decreased from 29.6 ± 0.5 ng/mL in LPS-stimulated cells to 19.5 ± 0.9 ng/mL after GLMOC treatment [Fig. 2(A)]. McPhee et al. [7] reported that GLMO inhibited the in vitro enzymatic activities of COX-1 and COX-2, wherein the activity was enhanced by hydrolysis of GLMO, supporting our results showing the significant regulation of COX-2 and related mediator of inflammation by GMLOC in RAW264.7 cells.
Fig. 2.
GLMOC inhibited the expression of COX-2 and prostaglandin E2 level in LPS-induced RAW264.7 cells. (A) The PGE2 amount in the media was determined by competitive ELISA. The cells (8 × 105 cells in a 60 mm dish) were pretreated with GLMOC or SA for 2 h, and then LPS for 22 h. (B) The relative amount of COX-2 mRNA was measured with quantitative RT-PCR. The experimental condition is same with Fig. 1(B). (C) The protein levels of COX-2 and β-actin were determined by Western blot. The experimental condition is same with Fig. 1(C). Values represent the mean ± SD. Different lowercase letters indicate significant differences at p < 0.05. GLMOC (50, 100, and 300 μg/mL), SA (10 μM), LPS (100 ng/mL)
GLMOC reduced the production of pro-inflammatory cytokines in LPS-stimulated RAW264.7 Cells
Pro-inflammatory cytokines, including TNF-α, IL-6, and IL-1β, play important role in the inflammatory process [24]. Previously, GLMO suppressed the expression of TNF-α and interferon (IFN)-γ in adjuvant-induced arthritis model in rats [5] and TNF-α and IL-12p40 in LPS-induced human THP-1 monocytes [25]. Therefore, we tested the effect of GLMOC on the expression levels of TNF-α, IL-6, and IL-1β in LPS-stimulated RAW264.7 cells. After the induction of mRNA expression of TNF-α, IL-6, and IL-1β with LPS, GLMOC treatment significantly inhibited up to 50.8 ± 0.2, 51 ± 10, 30 ± 11%, respectively, compared to the LPS-treated control [Fig. 3(A)]. The amount of cytokines released into the media was also measured by ELISA assay. As shown in Fig. 3(B), the concentrations of TNF-α, IL-6, and IL-1β were decreased from 2.58 ± 0.5, 1.02 ± 0.1 ng/mL, and 113 ± 15 pg/mL in LPS-treated control to 0.84 ± 0.1, 0.73 ± 0.0 ng/mL, and 24.9 ± 5.8 pg/mL in GLMOC treated groups, respectively, which showed similar pattern with mRNA regulation by GLMOC treatment. In line with previous studies, GLMOC strongly down-regulated the expression of pro-inflammatory cytokines in RAW264.7 cells.
Fig. 3.
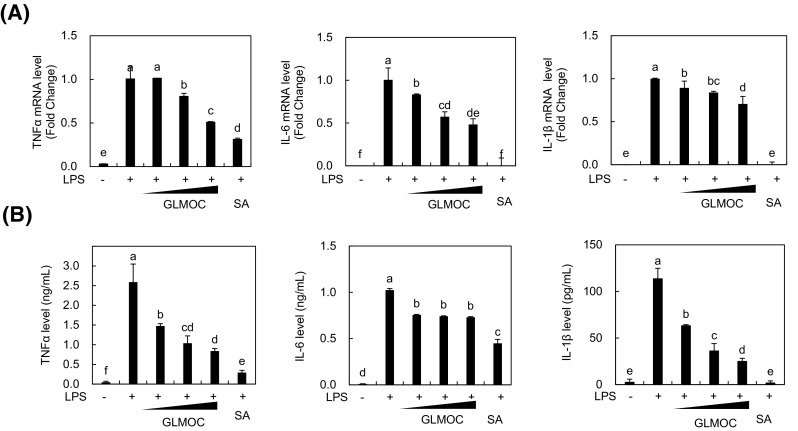
GLMOC reduced the production of pro-inflammatory cytokines in LPS-stimulated RAW264.7 cells. The cells (8 × 105 cells in a 60 mm dish) were pretreated with GLMOC or SA for 2 h. LPS was then treated for 10 h for mRNA measurement by quantitative real time PCR (A), and for 22 h for protein measurement of cytokines in the media by ELISA assay (B). Values represent the mean ± SD. Different lowercase letters indicate significant differences at p < 0.05. GLMOC (50, 100, and 300 μg/mL), SA (10 μM), LPS (100 ng/mL)
GLMOC blocked the NF-κB signaling pathway in LPS-stimulated RAW264.7 cells
NF-κB composed of p65 and p50 subunits is a well-known transcription factor that plays a key role in mediating inflammatory responses and is activated by inflammatory cytokines such as TNF-α and/or pathogen-associated molecular patters [26]. After LPS treatment, we found that inhibitor κB that can bind and sequester NF-κB in the cytoplasm was degraded, and then significantly recovered in the presence of GLMOC in a dose-dependent manner [Fig. 4(A)]. The phosphorylation of NF-κB subunit p65 activated by LPS stimulation was also suppressed by GLMOC maintaining the total form of p65 at certain level in RAW264.7 cells [Fig. 4(A)]. To confirm the effect of GLMOC on NF-κB signaling, we collected the nuclear fractions and checked the activated NF-κB localized in the nucleus. As shown in Fig. 4(B), upon exposure of RAW264.7 cells to LPS, both p65 and phosphorylated p65 were accumulated in the nuclear fraction, which were significantly inhibited by GLMOC treatment at 300 μg/mL. These results clearly indicate that GLMOC can modulate the NF-κB signaling activated by LPS, which might lead to down-regulation of iNOS, COX-2, and cytokines in RAW264.7 cells.
Fig. 4.
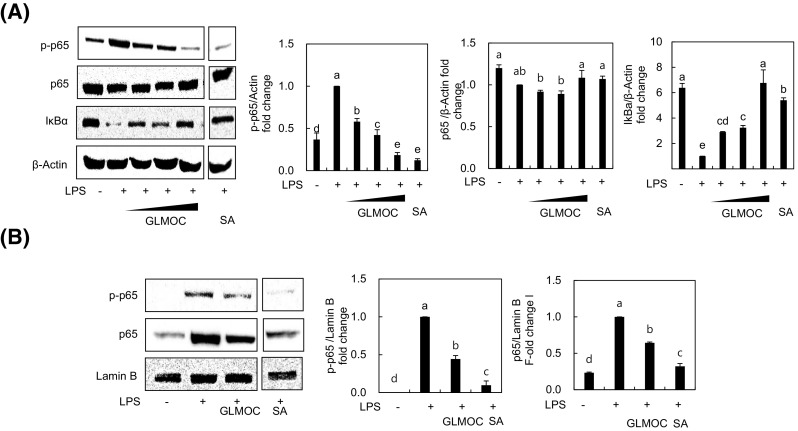
GLMOC blocked the NF-κB signaling pathway in LPS-stimulated RAW264.7 cells. The cells (8 × 105 cells in a 60 mm dish) were pretreated 2 h with GLMOC or SA, and then LPS was treated for 30 min. The protein levels of phosphorylated p65 (p-p65) and p-65, IκB, and β-actin (A), and the nuclear protein levels of p-p65, p-65, and lamin B (B) were determined by Western blot. Values represent mean ± SD. Different lowercase letters indicate significant differences at p < 0.05. GLMOC [50, 100, and 300 μg/mL (A) or 300 μg/mL (B)], SA (10 μM), LPS (100 ng/mL)
GLMOC regulated the MAPKs signaling pathway in LPS-induced RAW264.7 cells
In addition to NF-κB, MAPKs are also critical in mediating inflammation. For example, extracellular signal-regulated kinase (ERK) and p38 are known to be phosphorylated by LPS and involved in posttranscriptional regulation of TNF-α, which can result in inflammation driven by massive TNF-α [27, 28]. Here we also repeated the significant activation of p38, ERK, and c-Jun N-terminal kinase (JNK) by LPS stimulation and found that GLMOC at 50, 100, and 300 μg/mL showed the dose-dependent suppressions of phosphorylation on p38, ERK, and JNK of up to 56.5 ± 3.6, 52.2 ± 3.1, and 57.3 ± 7.8%, respectively, compared to LPS-treated control (Fig. 5). These results suggest that GLMOC provides the additional efficacy on inhibiting inflammation through MAPKs in macrophages.
Fig. 5.
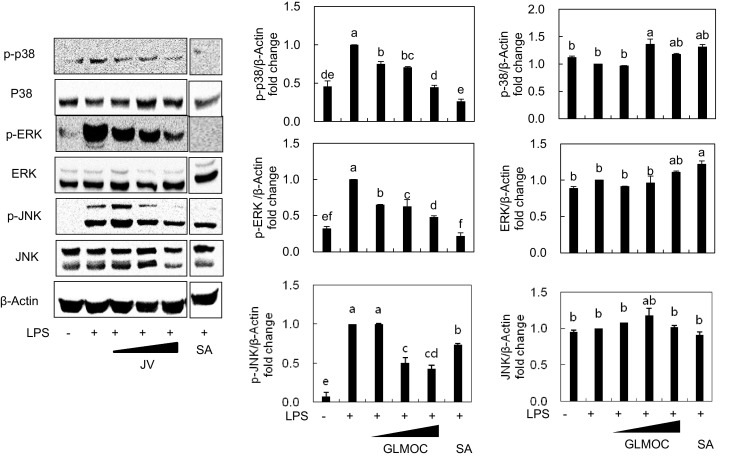
GLMOC regulated the MAPKs signaling pathway in LPS-induced RAW264.7 cells. The cells (8 × 105 cells in a 60 mm dish) were pretreated 2 h with GLMOC or SA, and then LPS was treated for 30 min. The protein levels of p-p38, p38, p-ERK, ERK, p-JNK, JNK, and β-actin were determined by Western blot. Values represent mean ± SD. Different lowercase letters indicate significant differences at p < 0.05. GLMOC (50, 100, and 300 μg/mL), SA (10 μM), LPS (100 ng/mL)
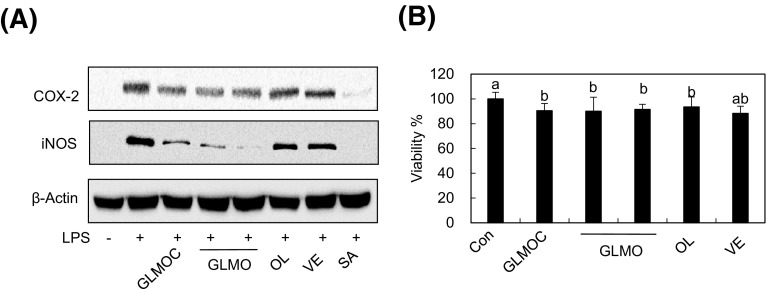
GLMO is the major components exerting anti-inflammatory effect without cytotoxicity in RAW264.7 cells
GLMOC is a mixture of GLMO with olive oil and vitamin E at a ratio of 10:20:1. To confirm that GLMO is the main constituents regulating inflammation, we compared GLMO (96.7 μg/mL), olive oil (193.5 μg/mL), and vitamin E (9.8 μg/mL) at the same concentration present in GLMOC (300 μg/mL). As shown in Fig. 6, two separate treatments of GLMO showed similar activity in regulating iNOS and COX-2 expression with GLMOC, whereas vitamin E and olive oil exerted marginal effect. To determine whether the effect of GLMOC and its constituents on inflammation induced by LPS is derived from cytotoxicity in RAW264.7 cells, we performed MTT assay and found that the viability of the cells was maintained above 90% after the treatment of GLMOC, GLMO, olive oil, or vitamin E. These results indicate that the anti-inflammatory effect of GLMOC is mainly derived from GLMO without inducing cytotoxicity.
Fig. 6.
GLMO is the major components exerting anti-inflammatory effect without cytotoxicity in RAW264.7 cells. (A) The cells (8 × 105 cells in a 60 mm dish) were pretreated with GLMOC (300 μg/mL), GLMO (96.7 μg/mL), olive oil (OL, 193.5 μg/mL), and vitamin E (VE, 9.8 μg/mL) for 2 h, and then LPS (100 ng/mL) for 22 h. The protein levels of iNOS, COX-2, and β-actin were determined by Western blot. (B) The cells (1 × 104 cells/well in 96-well plate) were treated with GLMOC (300 μg/mL), GLMO (96.7 μg/mL), olive oil (OL, 193.5 μg/mL), and vitamin E (VE, 9.8 μg/mL) for 24 h, and the cell viabilities were determined by MTT assay. Values represent the mean ± SD. Different lowercase letters indicate significant differences at p < 0.05
GLMO is known to contain different lipid components, including sterol esters, triglycerides, and free fatty acids, wherein ω-3 polyunsaturated fatty acids (PUFAs) are the major components, particularly eicosapentaenoic acid (EPA, 13%) and decosahexaenoic acid (DHA, 21%) [29]. McPhee et al. [7] reported that the increased amount of fatty acids by hydrolysis of triglycerides from GLMO and fish oils significantly increased the activity in suppressing the COX-1 and -2 activity, suggesting that the role of GLMO on inflammation is mainly due to the significant amounts of PUFAs. Interestingly, however, the administration of fish oil did not show beneficial efficacy in preventing the development of arthritis and ameliorating the symptoms of inflammatory bowel disease, whereas GLMO was found to be effective in both animal models [8, 30]. Although furan fatty acid that is unstable during oil extraction and analysis process was identified and suggested as a key component responsible for the anti-inflammatory activity of GLMO in adjuvant-induced arthritis rat model [31], more studies are necessary to identify the active ingredients and clarify the underlying mechanism of GLMO on inflammation related chronic diseases. In conclusion, GLMOC containing GLMO as the major active component showed the inhibition of iNOS expression, leading to the reduced production of NO, and COX-2 expression, leading to the reduced production of PGE2 as well as suppression of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β expression in LPS-stimulated RAW264.7 cells. The upstream signaling mediating the anti-inflammatory efficacy of GLMOC was suggested to be through the regulation of NF-κB signaling by recovering IκB and blocking nuclear translocation of NF-κB, and the MAPK signaling by inhibiting phosphorylation of p38, ERK, and JNK. Our findings in this study can provide the underlying mechanisms elucidating the anti-inflammatory efficacy of GLMOC in macrophage cells.
Acknowledgements
This research was supported by the Chung-Ang University research grant in 2016.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Doggrell SA. Lyprinol-is it a useful anti-inflammatory agent? Evid. Based Complement. Alternat. Med. 2011;2011:307121. doi: 10.1093/ecam/nep030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho SH, Jung YB, Seong SC, Park HB, Byun KY, Lee DC, Song EK, Son JH. Clinical efficacy and safety of Lyprinol, a patented extract from New Zealand green-lipped mussel (Perna canaliculus) in patients with osteoarthritis of the hip and knee: a multicenter 2-month clinical trial. Eur. Ann. Allergy. Clin. Immunol. 2003;35:212–216. [PubMed] [Google Scholar]
- 3.Gruenwald J, Graubaum HJ, Hansen K, Grube B. Efficacy and tolerability of a combination of Lyprinol and high concentrations of EPA and DHA in inflammatory rheumatoid disorders. Adv. Ther. 2004;21:197–201. doi: 10.1007/BF02850125. [DOI] [PubMed] [Google Scholar]
- 4.Hielm-Bjorkman A, Tulamo RM, Salonen H, Raekallio M. Evaluating complementary therapies for canine osteoarthritis part I: Green-lipped mussel (Perna canaliculus) Evid. Based Complement. Alternat. Med. 2009;6:365–373. doi: 10.1093/ecam/nem136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CH, Lum JH, Ng CK, McKay J, Butt YK, Wong MS, Lo SC. Pain controlling and cytokine-regulating effects of lyprinol, a lipid extract of Perna canaliculus, in a rat adjuvant-induced arthritis model. Evid. Based Complement. Alternat. Med. 2009;6:239–245. doi: 10.1093/ecam/nem100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halpern GM. Anti-inflammatory effects of a stabilized lipid extract of Perna canaliculus (Lyprinol) Allerg. Immunol. (Paris) 2000;32:272–278. [PubMed] [Google Scholar]
- 7.McPhee S, Hodges LD, Wright PF, Wynne PM, Kalafatis N, Harney DW, Macrides TA. Anti-cyclooxygenase effects of lipid extracts from the New Zealand green-lipped mussel, Perna canaliculus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007;146:346–356. doi: 10.1016/j.cbpb.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Tenikoff D, Murphy KJ, Le M, Howe PR, Howarth GS. Lyprinol (stabilised lipid extract of New Zealand green-lipped mussel): a potential preventative treatment modality for inflammatory bowel disease. J. Gastroenterol. 2005;40:361–365. doi: 10.1007/s00535-005-1551-x. [DOI] [PubMed] [Google Scholar]
- 9.Emelyanov A, Fedoseev G, Krasnoschekova O, Abulimity A, Trendeleva T, Barnes PJ. Treatment of asthma with lipid extract of New Zealand green-lipped mussel: a randomised clinical trial. Eur. Respir. J. 2002;20:596–600. doi: 10.1183/09031936.02.02632001. [DOI] [PubMed] [Google Scholar]
- 10.Mickleborough TD, Vaughn CL, Shei RJ, Davis EM, Wilhite DP. Marine lipid fraction PCSO-524 (lyprinol/omega XL) of the New Zealand green lipped mussel attenuates hyperpnea-induced bronchoconstriction in asthma. Respir. Med. 2013;107:1152–1163. doi: 10.1016/j.rmed.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Dugas B. Lyprinol inhibits LTB4 production by human monocytes. Allerg. Immunol. (Paris) 2000;32:284–289. [PubMed] [Google Scholar]
- 12.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 13.Laveti D, Kumar M, Hemalatha R, Sistla R, Naidu VG, Talla V, Verma V, Kaur N, Nagpal R. Anti-inflammatory treatments for chronic diseases: a review. Inflamm. Allergy Drug. Targets. 2013;12:349–361. doi: 10.2174/18715281113129990053. [DOI] [PubMed] [Google Scholar]
- 14.Wang D, DuBois RN. Immunosuppression associated with chronic inflammation in the tumor microenvironment. Carcinogenesis. 2015;36:1085–1093. doi: 10.1093/carcin/bgv123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akira S, Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat. Rev. Immunol. 2008;8:837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 17.Hawiger J. Innate immunity and inflammation: a transcriptional paradigm. Immunol. Res. 2001;23:99–109. doi: 10.1385/IR:23:2-3:099. [DOI] [PubMed] [Google Scholar]
- 18.Murakami A, Ohigashi H. Targeting NOX, INOS and COX-2 in inflammatory cells: chemoprevention using food phytochemicals. Int. J. Cancer. 2007;121:2357–2363. doi: 10.1002/ijc.23161. [DOI] [PubMed] [Google Scholar]
- 19.Grienke U, Silke J, Tasdemir D. Bioactive compounds from marine mussels and their effects on human health. Food Chem. 2014;142:48–60. doi: 10.1016/j.foodchem.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 20.Shaw CA, Taylor EL, Megson IL, Rossi AG. Nitric oxide and the resolution of inflammation: implications for atherosclerosis. Mem. Inst. Oswaldo Cruz. 2005;100(Suppl. 1):67–71. doi: 10.1590/S0074-02762005000900012. [DOI] [PubMed] [Google Scholar]
- 21.Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75:639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 22.Echizen K, Hirose O, Maeda Y, Oshima M. Inflammation in gastric cancer: Interplay of the COX-2/prostaglandin E2 and toll-like receptor/MyD88 pathways. Cancer Sci. 2016;107:391–397. doi: 10.1111/cas.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esteve JB, Launay-Vacher V, Brocheriou I, Grimaldi A, Izzedine H. COX-2 inhibitors and acute interstitial nephritis: case report and review of the literature. Clin. Nephrol. 2005;63:385–389. doi: 10.5414/CNP63385. [DOI] [PubMed] [Google Scholar]
- 24.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat. Rev. Rheumatol. 2014;10:44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawson BR, Belkowski SM, Whitesides JF, Davis P, Lawson JW. Immunomodulation of murine collagen-induced arthritis by N,N-dimethylglycine and a preparation of Perna canaliculus. BMC Complement. Altern. Med. 2007;7:20. doi: 10.1186/1472-6882-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell S, Vargas J, Hoffmann A. Signaling via the NFkappaB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016;8:227–241. doi: 10.1002/wsbm.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA, Copeland NG, Kollias G, Tsichlis PN. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103:1071–1083. doi: 10.1016/S0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 28.Guma M, Stepniak D, Shaked H, Spehlmann ME, Shenouda S, Cheroutre H, Vicente-Suarez I, Eckmann L, Kagnoff MF, Karin M. Constitutive intestinal NF-kappaB does not trigger destructive inflammation unless accompanied by MAPK activation. J. Exp. Med. 2011;208:1889–1900. doi: 10.1084/jem.20110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy KJ, Mooney BD, Mann NJ, Nichols PD, Sinclair AJ. Lipid, FA, and sterol composition of New Zealand green lipped mussel (Perna canaliculus) and Tasmanian blue mussel (Mytilus edulis) Lipids. 2002;37:587–595. doi: 10.1007/s11745-002-0937-8. [DOI] [PubMed] [Google Scholar]
- 30.Whitehouse MW, Macrides TA, Kalafatis N, Betts WH, Haynes DR, Broadbent J. Anti-inflammatory activity of a lipid fraction (lyprinol) from the NZ green-lipped mussel. Inflammopharmacology. 1997;5:237–246. doi: 10.1007/s10787-997-0002-0. [DOI] [PubMed] [Google Scholar]
- 31.Wakimoto T, Kondo H, Nii H, Kimura K, Egami Y, Oka Y, Yoshida M, Kida E, Ye Y, Akahoshi S, Asakawa T, Matsumura K, Ishida H, Nukaya H, Tsuji K, Kan T, Abe I. Furan fatty acid as an anti-inflammatory component from the green-lipped mussel Perna canaliculus. Proc. Natl. Acad. Sci. USA. 2011;108:17533–17537. doi: 10.1073/pnas.1110577108. [DOI] [PMC free article] [PubMed] [Google Scholar]